Abstract
Indoleamine 2,3-dioxygenase 1 (IDO1) is an enzyme for tryptophan metabolism, involved in immune cell differentiation/maturation and cancer biology. IDO1 is also expressed in cardiomyocytes, but its roles in the cardiovascular system are not fully understood. Here, we reported the functions of IDO1 during cardiac hypertrophy. Quantitative real-time PCR and Western blot experiments demonstrated the upregulation of IDO1 mRNA and protein levels in human and hypertrophic mouse hearts, as well as in angiotensin II (Ang II)-induced hypertrophic rat cardiomyocytes. IDO1 activity and metabolite product kynurenine were upregulated in rodent hypertrophic hearts and cardiomyocytes. Inhibition of IDO1 activity with PF-06840003 reduced Ang II-induced cardiac hypertrophy and rescued cardiac function in mice. siRNA-mediated knockdown of Ido1 repressed Ang II-induced growth in cardiomyocyte size and overexpression of hypertrophy-associated genes atrial natriuretic peptide (Anp or Nppa), brain natriuretic peptide (Bnp or Nppb), β-myosin heavy chain (β-Mhc or Myh7). By contrast, adenovirus-mediated rat Ido1 overexpression in cardiomyocytes promoted hypertrophic growth induced by Ang II. Mechanism analysis showed that IDO1 overexpression was associated with PI3K-AKT-mTOR signaling to activate the ribosomal protein S6 kinase 1 (S6K1), which promoted protein synthesis in Ang II-induced hypertrophy of rat cardiomyocytes. Finally, we provided evidence that inhibition of PI3K with pictilisib, AKT with perifosine, or mTOR with rapamycin, blocked the effects of IDO1 on protein synthesis and cardiomyocyte hypertrophy in Ang II-treated cells. Collectively, our findings identify that IDO1 promotes cardiomyocyte hypertrophy partially via PI3K-AKT-mTOR-S6K1 signaling.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12012-021-09657-y.
Keywords: Cardiac hypertrophy, IDO1, MTOR, AKT, S6K1, Protein synthesis
Introduction
Cardiovascular diseases have already been the leading cause of death in humans worldwide. Among cardiovascular diseases, cardiac remodeling is common in human patients [1]. Cardiac remodeling could lead to arrhythmia, myocardial infarction, and heart failure. Cardiac hypertrophy is one of the hallmarks of cardiac remodeling and participates in various cardiac diseases [2]. Cardiomyocytes are terminus-differentiated cells and rarely proliferate. Under injury stress, cardiomyocytes cannot proliferate to respond to cardiac injury. Instead, the cardiomyocytes undergo hypertrophic growth. These cells express fetal markers, such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and β-myosin heavy chain (MYH7) [3]. Hypertension, neuroendocrine factors, and myocardial infarction can induce hypertrophic growth of cardiomyocytes to support the increased demand of the cardiac tissues [4]. Importantly, cardiac hypertrophy has been considered a promising target for the treatment of cardiac diseases such as heart failure [2–4]. However, our understanding of the mechanisms underlying cardiac hypertrophy is still not complete. This fact may delay the development of therapeutic drugs.
The heart is an organ with high energy demand, and the metabolic pattern of cardiomyocytes is much different from other cell types [5, 6]. The hypertrophic growth of cardiomyocytes is critically regulated by metabolic regulators, such as AMP-dependent protein kinase (AMPK) [7, 8], NAD+-dependent Sirtuins [9, 10], mammalian target of rapamycin (mTOR) [11, 12], and FOXOs [13, 14]. For instance, the AKT-mTOR signaling is an essential regulator for cardiac hypertrophy [15]. Various stimuli can activate the phosphoinositol 3-kinase (PI3K)-AKT singling. Mechanic stress of the extracellular matrix, aging, endocrine factors, insulins can trigger PI3K, leading to the activation of the kinase AKT [16]. AKT is critical for cardiac physiological and pathological progress. During cardiac hypertrophy, activation of AKT can result in the hyperactivation of the downstream mTOR, subsequently activates the p70 ribosomal protein S6 kinase 1 (S6K1) [17]. mTOR-S6K1 signaling is a crucial regulator for controlling organ size and participates in cardiac hypertrophy via its effects on de novo protein synthesis, the essential progress involved in cardiomyocyte hypertrophy [18]. Thus, the AKT-mTOR signaling pathway is considered a promising target for treating cardiac hypertrophy. But the regulation of the AKT-mTOR signaling pathway upon hypertrophic stress is not fully understood.
Indoleamine 2,3-Dioxygenase 1 (IDO1) participates in immunometabolism and inflammatory programming via its biochemical function in tryptophan catabolism [19]. Accumulating studies have investigated the roles of IDO1 in immune cells. IDO1 participates in potent immunosuppression in various preclinical models of human cancer. For instance, infiltrating T cells promoted the expression of IDO1 in glioblastoma cells, which contributed to the decline in patient survival [20]. IDO1 and the kynurenine pathway metabolites promoted the activation of the PI3K-AKT signaling pathway in the neoplastic colon epithelium, which inhibited apoptosis and promotes cancer cell proliferation [21]. Inhibition of IDO1 with PD-1 (programmed death 1) blockade and radiation led to durably increase survival in advanced glioblastoma [20, 22]. Some studies also reported the roles of IDO1 in the cardiovascular system. For example, exosomes derived from IDO1-overexpressing bone marrow mesenchymal stem cells (BMMSCs) facilitated the immunotolerance in rat cardiac allografts [23]. Besides, IDO1 fine-tuned immune homeostasis during atherosclerosis partially via repressing the production of interleukin-10 (IL-10) via regulating ERK1/2 kinase [24]. Furthermore, IDO1 deficiency in macrophages induced an anti-inflammatory response to chronic viral myocarditis of mice [25]. Eicosapentaenoic acid repressed atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice through IDO1 [26].. Furthermore, IDO1-expressing aortic plasmacytoid dendritic cells protected against atherosclerosis by introducing regulatory T cells [27]. Bedsides, endothelial cell IDO1 altered cardiac function after myocardial infarction through kynurenine [28]. These previous findings suggested the critical roles of IDO1 in cardiovascular tissues. However, it remains unknown whether IDO1 participates in cardiac hypertrophy and the underlying mechanisms.
Materials and Methods
Human Samples
Fresh human heart samples were obtained at the First Affiliated Hospital of Jiamusi University from Jan 2011 to Dec 2017. Five cases of cardiac hypertrophy and five controls were recruited in this study. The control samples were obtained intraoperatively from non-failing hearts undergoing ventricular corrective surgery or from dysfunctional donor hearts. The fresh heart tissues were harvested and stored in liquid nitrogen before use. Every patient or donor has signed a written form of informed consent for the research-only use of their tissues. The design and experimental protocols of this study were approved by the Clinical Study Ethic Community of the First Affiliated Hospital of Jiamusi University. The information of patients is shown in Table 1.
Table 1.
Patient information
| Patient | Age | Gender | Diagnosis | β-adrenergic blocker | ACE inhibitor |
|---|---|---|---|---|---|
| Control #1 | 48 | Male | Mitral valve defect | − | − |
| Control #2 | 37 | Female | Mitral valve defect | − | − |
| Control #3 | 51 | Female | Ventricular septal defect | − | − |
| Control #4 | 62 | Male | Mitral valve defect | − | − |
| Control #5 | 48 | Female | Ventricular septal defect | − | − |
| HCM #1 | 57 | Female | Hypertrophic obstructive cardiomyopathy | + | + |
| HCM #2 | 68 | Female | Hypertrophic obstructive cardiomyopathy | − | + |
| HCM #3 | 71 | Male | Hypertrophic obstructive cardiomyopathy | + | + |
| HCM #4 | 68 | Female | Hypertrophic obstructive cardiomyopathy | − | + |
| HCM #5 | 58 | Male | Hypertrophic obstructive cardiomyopathy | + | + |
Mouse Model of Cardiac Hypertrophy
All animals were raised under SPF-condition and had free access to food and water. The animals were maintained on a regular 12 h light/dark cycle. Cardiac hypertrophy in mice was induced by subcutaneously chronic treatment of Ang II (1.3 mg/kg/day for 14 or 28 days; Sigma) using a previously described protocol [10]. For PF-06840003 (Selleckchem) treatment, i.p. PF-06840003 (200 mg/kg/day) was applied daily for two weeks. Mice were anesthetized with isoflurane, and body temperature was maintained on a circulating heated water pad. The hypertrophic growth of the myocardial tissues was confirmed by the echocardiography test, increased heart weight, and overexpression of hypertrophy-associated fetal genes. Echocardiography was performed as described previously to determine fraction shortening and ejection fraction [29]. The mice sacrificed were with the cervical dislocation method, and H&E staining was performed as described previously [10]. The animal experiments were performed in the Animal Center of Hebei Medical University. The design and protocol for animal studies were approved by the Animal Study Ethic Community of Jiamusi University (#JMSU2018006).
Isolation and Culture of Neonatal Rat Cardiomyocytes
The neonatal rat cardiomyocytes are isolated from 1 to 3-day old neonatal Sprague Dawley rats using a previously described protocol [30]. The design and protocol for animal studies were approved by the Animal Study Ethic Community of Jiamusi University (#JMSU2017011). Rats were anesthetized with isoflurane and were sacrificed with the cervical dislocation method. Briefly, the left ventricle cardiac tissues were cut and digested with collagenase (Sigma) and trypsin (Gibco) to prepare single cells. The single cells were then pre-plated for 2 h to remove fibroblasts at 5% CO2 and 37 °C. Then, the cardiomyocytes were cultured in Dulbecco's modified eagle medium (DMEM) medium (Gibco), supplemented with 10% fetal bovine serum (FBS, Gibco), and 1% penicillin–streptomycin (Gibco) at 5% CO2 and 37 °C for 48 h before further use.
Cell Model of Cardiomyocyte Hypertrophy
Cardiomyocyte hypertrophy was induced by treatment with Ang II, isoproterenol (ISO, Sigma), and phenylephrine (PE, Sigma). Briefly, cardiomyocytes were cultured in DMEM medium for 24 h with 1% FBS, then Ang II (1 μM), PE (50 μM), ISO (30 μM) was added into the medium and treated the cells for additional 48 h to induce cardiomyocyte hypertrophy. The hypertrophic growth of cardiomyocytes was confirmed by the increase in cardiomyocyte size and overexpression of hypertrophy-associated fetal genes. Cardiomyocytes were stained with alpha-actinin (Sigma), and cell size was analyzed with ImageJ software. Pictilisib (PI3K inhibitor; 100 nM), perifosine (AKT inhibitor; 1 μM), rapamycin (mTOR inhibitor; 10 nM), MHY1485 (mTOR activator; 10 μM) were purchased from Selleck and used to treat cardiomyocytes as described in the figure legends.
Gene Silence and Overexpression
For gene silence, siRNA was used to target Ido1 in rat cardiomyocytes. The si-Ido1 (5ʹ-GGGCTTTGCTCTACCACAT-3ʹ) and negative control siRNA (si-NC, 5ʹ-CAGUUGCGCAGCCUGAAUG-3ʹ) were transfected into cells with Lipofectamine RNAiMax (Invitrogen) for 24 or 48 h before any other treatment. For gene overexpression, rat Ido1 expressing construct was cloned into the adenovirus system vectors. The adenovirus was produced in HEK293A cells using a protocol described previously [31]. The cardiomyocytes were infected with adenovirus expressing Ido1 (Ad-Ido1) or control adenovirus (Ad-Ctrl) for 24 or 48 h before any other treatment.
Quantitative Real-Time PCR
Cultured cells or heart tissues were extracted for total RNA with the TRIzol reagent (Byeotime). Then, the first strain cDNA was synthesized with one μg total RNA using the cDNA synthesis kit (ThermoFisher). Next, we performed quantitative real-time PCR (RT-qPCR) experiment to test the expression levels of target mRNAs with the SYBR Green II kit (TaKaRa). The RT-qPCR primers used in this study are shown in Table 2.
Table 2.
RT-qPCR primers used in this study
| Gene symbol | Forward primer (5ʹ–3ʹ) | Reverse primer (5ʹ–3ʹ) |
|---|---|---|
| Human ANP | GATGGTGACTTCCTCGCCTC | AAGAAAGCACACCAACGCAG |
| Human BNP | TGGAAACGTCCGGGTTACAG | CTGATCCGGTCCATCTTCCT |
| Human MYH7 | AGTGGCAATAAAAGGGGTAGC | CCAAGTTCACTCACATCCATCA |
| Human IDO1 | GCGCTGTTGGAAATAGCTTC | ATGTCCTCCACCAGCAGTC |
| Human GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
| Mouse Anp | TCTTCCTCGTCTTGGCCTTT | CCAGGTGGTCTAGCAGGTTC |
| Mouse Bnp | TGGGAGGTCACTCCTATCCT | GGCCATTTCCTCCGACTTT |
| Mouse Myh7 | CGGACCTTGGAAGACCAGAT | GACAGCTCCCCATTCTCTGT |
| Mouse Ido1 | AGGATCCTTGAAGACCACCA | CCAATAGAGAGACGAGGAAG |
| Mouse Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| Rat Anp | AGAGCCCTCAGTTTGCTTTTC | GAAGATGCCGGTAGAAGATGAG |
| Rat Bnp | GGTGCTGCCCCAGATGATT | CTGGAGACTGGCTAGGACTTC |
| Rat Myh7 | CGCTCAGTCATGGCGGAT | GCCCCAAATGCAGCCAT |
| Rat Ido1 | GTTCTTCGCATATATTTGTCCGG | CAGGGGGCAGTGCAGGCCA |
| Rat Gapdh | TGACAACTCCCTCAAGATTGTCA | GGCATGGACTGTGGTCATGA |
Western Blot
Cultured cells or heart tissues were used to extract total protein with RIPA lysis buffer (Millipore) with protease inhibitor cocktail (Biomake). Next, standard Western blot was performed to determine the levels of interested proteins according to a protocol modified from previous studies [31, 32]. The primary antibodies used for Western blot assay are :
Anti-IDO1 antibody (Abcam; 1:1000), anti-GAPDH antibody (Proteintech; 1:2000), anti-pAKT antibody (Cell Signaling Technology; 1:1000), anti-AKT antibody (Cell Signaling Technology; 1:1000), anti-pmTOR antibody (Cell Signaling Technology; 1:1000), anti-mTOR antibody (Cell Signaling Technology; 1:1000), anti-pS6K1 antibody (Cell Signaling Technology; 1:1000), anti-S6K1 antibody (Cell Signaling Technology; 1:1000). The secondary antibodies (1:5000) and ECL kit were purchased from Servicebio.
Protein Synthesis Assay
Protein synthesis assay was performed by determining [+H]-leucine incorporation into cardiomyocytes described in previous work [33].
IDO1 Activity Assay
IDO1 activity was measured with IDO1 Activity Assay Kit (Abcam, ab235936) according to the operation manual.
Kynurenine Level
Kynurenine (KYN) level was measured with Kynurenine (KYN) ELISA Kit (Abbexa, abx585209) according to the operation manual.
Statistical Analysis
All the values are shown as the mean ± SD of at least three independent repeated experiments. For analyzing the difference between the two groups, the standard Students’ t test was used. For analysis of the difference in more than two groups, one-way or two-way Analysis of Variance (ANOVA) was performed, followed by Turkey post-hoc test. All the statistical analysis was performed with the GraphPad Prism Software version 8, and P values less than 0.05 were considered as statistically significant.
Results
IDO1 Expression and Activity are Upregulated in Hypertrophic Hearts
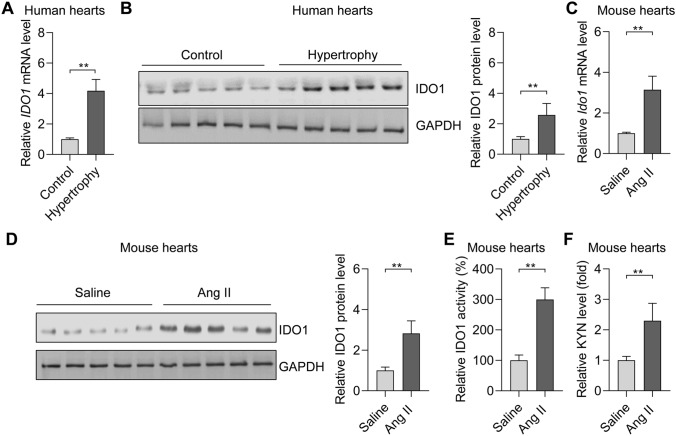
IDO is an enzyme for tryptophan catabolism and contributes to immune response and cancer biology. The roles of IDO1 in cardiac hypertrophy remain unknown. We monitored the expression of IDO1 in hypertrophic cardiac tissues in humans and rodents. Five human hearts with hypertrophy and five control hearts were included in this study. The hypertrophic phenotype of the human hearts was confirmed by the overexpression of hypertrophy-associated fetal genes, including ANP, BNP, and MYH7 (Supplementary Fig. 1). Of note, qRT-PCR and Western blot analyses revealed that the expression of the mRNA and protein levels of IDO1 was markedly upregulated in hypertrophic hearts compared with the controls (Fig. 1a, b). Therefore, IDO1 is overexpressed in human hypertrophic hearts.
Fig. 1.
The expression and activity of IDO1 are upregulated in the hypertrophic heart in humans and mice. a RT-qPCR analysis of the expression of IDO1 mRNA in control and hypertrophic hearts in humans. n = 5 in each group. **p < 0.01 by Student’s t test. IDO1, indoleamine 2, 3-dioxygenase 1. b Western blot analysis of the expression of IDO1 protein in control and hypertrophy hearts in humans. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. n = 3 in each group. **p < 0.01 by Student’s t test. c qRT-PCR shows the expression of Ido1 in control and hypertrophic hearts in mice. Cardiac hypertrophy was induced by subcutaneously chronic infusion of Ang II (1.3 mg/kg/day) for 28 days. n = 5 in each group. **p < 0.01 by Student’s t test. d Western blot shows the expression of Ido1 protein level in control and hypertrophic hearts in the mouse. Cardiac hypertrophy was induced by subcutaneously chronic infusion of Ang II (1.3 mg/kg/day) for 28 days. n = 5 in each group. **p < 0.01 by Student’s t test. e IDO1 activity increases in hypertrophic hearts in (c). n = 5 in each group. **p < 0.01 by Student’s t test. f Level of IDO1 downstream metabolite kynurenine (KYN). n = 5 in each group. **p < 0.01 by Student’s t test
We also induced cardiac hypertrophy in mice with the subcutaneously chronic infusion of Ang II for 28 days. The cardiac hypertrophy phenotype in the mouse was confirmed by the decrease in cardiac function and an increase in heart weights and cardiomyocyte size (Supplementary Fig. 2a–c). Besides, the overexpression of hypertrophy-associated fetal genes (Anp, Bnp, and Myh7) was also confirmed in a mouse model of cardiac hypertrophy (Supplementary Fig. 2d). Consistently, we observed the overexpression of the mRNA and protein levels of Ido1 in hypertrophic mouse hearts with RT-qPCR and Western blot (Fig. 1c, d). Besides, we also tested the activity of IDO1 and its metabolite product kynurenine. The results showed that IDO1 activity and kynurenine level were significantly upregulated in hypertrophic mouse hearts (Fig. 1e, f).
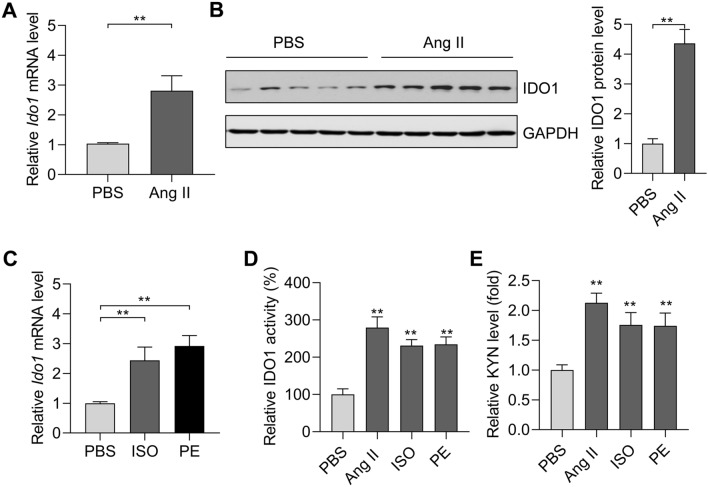
We isolated neonatal rat cardiomyocytes and induced cardiomyocyte hypertrophy with Ang II. Ang II treatment (1 μM for 48 h) significantly upregulated the expression of hypertrophy-associated fetal genes (Anp, Bnp, and Myh7) in rat cardiomyocytes (Supplementary Fig. 2e). Notably, Ang II treatment increased the mRNA and protein levels of Ido1 in rat cardiomyocytes (Fig. 2a, b). Additionally, the overexpression of Ido1 was also verified in hypertrophic cardiomyocytes induced by isoproterenol (ISO, 30 μM for 48 h) and phenylephrine (PE, 50 μM for 48 h) (Fig. 2c). Besides, we also tested the activity of IDO1 and its metabolite product kynurenine. The results showed that IDO1 activity and kynurenine level were significantly upregulated in hypertrophic cardiomyocytes (Fig. 2d, e). Therefore, overexpression of IDO1 in cardiac hypertrophy occurred in cardiomyocytes.
Fig. 2.
The expression and activity of IDO1 are increased in hypertrophic cardiomyocytes. a RT-qPCR analysis of Ido1 mRNA in hypertrophic cardiomyocytes. Rat Cardiomyocytes were treated with Ang II (1 μM) for 48 h to induce cardiomyocyte hypertrophy. n = 3 in each group. **p < 0.01 by Student’s t test. b Western blot analysis of the expression of IDO1 protein level in hypertrophic rat cardiomyocytes. n = 3 in each group. **p < 0.01 by Student’s t test. c qRT-PCR shows the expression of Ido1 mRNA level in hypertrophic cardiomyocytes induced by isoproterenol (ISO) and phenylephrine (PE). Cardiomyocyte hypertrophy was induced by ISO (30 μM) or PE (50 μM) for 48 h. n = 3 in each group. **p < 0.01 by one-way ANOVA followed by Turkey post-hoc test. d IDO1 activity increases in hypertrophic cardiomyocytes in. n = 3 in each group. **p < 0.01 vs PBS by one-way ANOVA followed by Turkey post-hoc test. e Level of IDO1 downstream metabolite kynurenine (KYN). n = 3 in each group. **p < 0.01 by one-way ANOVA followed by Turkey post-hoc test
Collectively, these findings demonstrated the overexpression of IDO1 in hypertrophic hearts across species, implicating that IDO1 may participate in cardiac hypertrophy.
IDO1 Promotes Cardiomyocyte Hypertrophy In Vivo and In Vitro
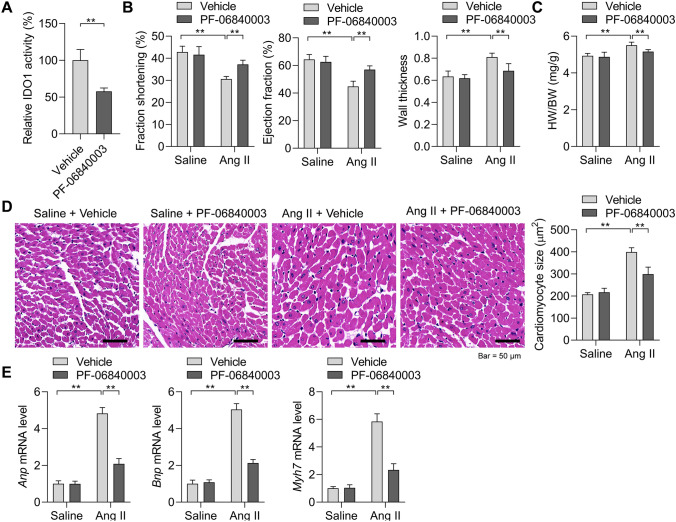
The above results revealed that IDO1 overexpression during cardiac hypertrophy might regulate the hypertrophic growth of cardiomyocytes. We first tested the effects of IDO1 in cardiac hypertrophy induced by Ang II via inhibiting IDO1 activity with PF-06840003. Cardiac hypertrophy was induced in mice with two weeks of Ang II in the presence or absence of PF-06840003. PF-06840003 treatment significantly reduced the activity of IDO1 in murine cardiac tissues (Fig. 3a). We observed that Ang II-induced decline in fraction shortening and ejection fraction were reversed by IDO1 inhibition, whereas wall thickness was reduced by IDO1 inhibition (Fig. 3b). The increase in heart weight induced by Ang II was also repressed by PF-06840003 treatment (Fig. 3c). Histological analysis revealed that PF-0680003 significantly reduced Ang II-induced increase in cardiomyocyte size and inhibited the expression of hypertrophic fetal genes (Fig. 3d, e). Collectively, IDO1 inhibition suppressed pathological cardiac hypertrophy.
Fig. 3.
Inhibition of IDO1 represses cardiac hypertrophy in vivo. a PF-06840003 reduces IDO1 activity in mouse hearts. Mice were treated with IDO1 inhibitor PF-06840003 (200 mg/kg/day, i.p.) for 14 days. n = 5 in each group. **p < 0.01 by Student’s t test. b Fraction shortening, ejection fraction, and left wall thickness of mice. Cardiac hypertrophy was induced by subcutaneously chronic infusion of Ang II (1.3 mg/kg/day) for 14 days in the presence or absence of IDO1 inhibitor PF-06840003 (200 mg/kg/day, i.p.). Ang II, angiotensin II. n = 5 in each group. **p < 0.01 by Student’s t test. n = 5 in each group. **p < 0.01 by one-way ANOVA followed by Turkey post-hoc test. c Heart weight-to-body weight ratio of mice with/without cardiac hypertrophy (b). n = 5 in each group. **p < 0.01 by one-way ANOVA followed by Turkey post-hoc test. d Haematoxylin and eosin (H&E) staining shows the increased cardiomyocyte size in mice with cardiac hypertrophy. n = 5 in each group. **p < 0.01 by one-way ANOVA followed by Turkey post-hoc test. e qRT-PCR shows the expression of hypertrophic genes in control and hypertrophic hearts in mice. n = 5 in each group. **p < 0.01 by one-way ANOVA followed by Turkey post-hoc test
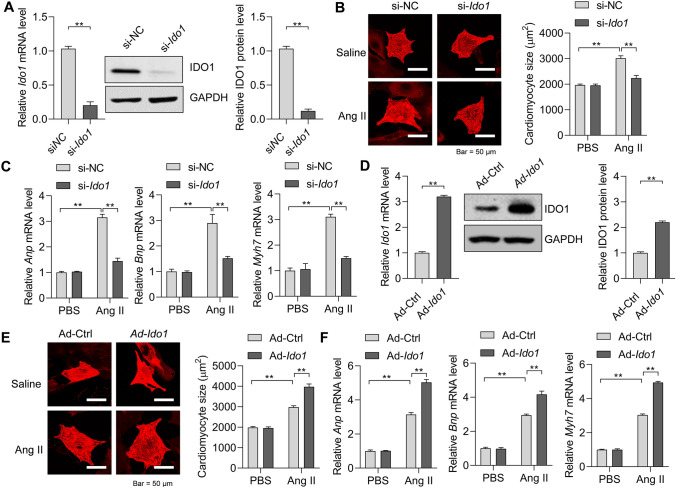
To test whether Ido1 regulates cardiomyocyte hypertrophy directly, we first knocked down Ido1 with siRNA in cardiomyocytes (Fig. 4a). Interestingly, Ido1 knockdown repressed the increase in cardiomyocyte size as induced by Ang II treatment (Fig. 4b). Besides, Ido1 deficiency also reduced the expression of Anp, Bnp, and Myh7 triggered by Ang II in cardiomyocytes (Fig. 4c). Thus, loss of Ido1 leads to the repression of cardiomyocyte hypertrophy. To study whether Ido1 overexpression contributes to cardiomyocyte hypertrophy, we generated adenovirus carrying rat Ido1. Adenovirus-mediated overexpression of rat Ido1 in cardiomyocytes was evidenced by qRT-PCR and Western blot (Fig. 4d). We found that Ido1 overexpression promoted the effects of Ang II on the increase in cardiomyocyte size (Fig. 4e). Besides, the overexpression of Ido1 in cardiomyocytes facilitated the overexpression of Anp, Bnp, and Myh7 (Fig. 4f). Therefore, Ido1 overexpression promotes cardiomyocytes hypertrophy.
Fig. 4.
IDO1 promotes cardiomyocyte hypertrophy in vitro. a siRNA-mediated knockdown of Ido1 in neonatal rat cardiomyocytes. Cardiomyocytes were transfected with siRNA targeting Ido1 (si-Ido1) or negative control siRNA (si-NC) for 48 h, then the RT-qPCR and Western blot were performed to analyze the knockdown efficiency. n = 3 in each group. **p < 0.01 by Student’s t test. b Ido1 deficiency represses Ang II-induced increase in cardiomyocytes. Cardiomyocytes were transfected with si-Ido1 or si-NC for 24 h, followed by Ang II (1 μM) induction for an additional 48 h. Then, cardiomyocyte size was analyzed with ImageJ. n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. c Ido1 deficiency reduces Ang II-induced overexpression of hypertrophic genes. The cardiomyocytes were treated as in (b). n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. d Adenovirus-mediated overexpression of Ido1 in cardiomyocytes. Cardiomyocytes were infected with adenovirus carrying rat Ido1 (Ad-Ido1) or control adenovirus (Ad-Ctrl) for 48 h, and then RT-qPCR and Western blot were performed to determine the overexpression efficiency. n = 3 in each group. **p < 0.01 by Student’s t test. e Ido1 overexpression promotes Ang II-induced increase in cardiomyocyte size. Cardiomyocytes were infected with Ad-Ido1 or Ad-Ctrl for 24 h, followed by Ang II (1 μM) induction for an additional 48 h. Then, cardiomyocyte size was analyzed with ImageJ. n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. f Ido1 overexpression facilitates Ang II-induced overexpression of hypertrophic genes. Cardiomyocytes were treated as in (e). n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test
Collectively, these findings revealed that IDO1 is a positive regulator for cardiomyocyte hypertrophy in vivo and in vitro.
IDO1 Promotes AKT-mTOR Signaling to Facilitate Protein Synthesis
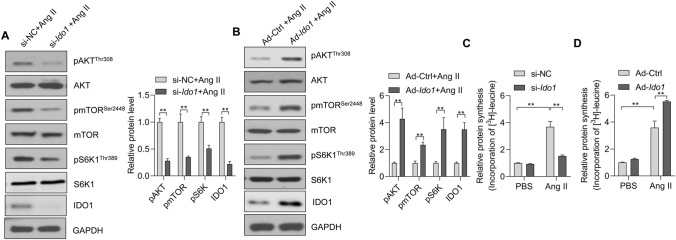
Next, we attempted to determine the potential mechanism by which IDO1 participated in cardiomyocyte hypertrophy. AKT-mTOR is a crucial regulator for cardiomyocyte hypertrophy, and various hypertrophic stimuli can increase the activation of AKT-mTOR signaling [2, 11, 17]. mTOR is a pivotal controller of organ size and hypertrophic growth of cells [11]. To test whether IDO1 regulated cardiomyocyte hypertrophy through the AKT-mTOR signaling pathway, we tested the effects of IDO1 on the activation (phosphorylation) of AKT and mTOR in Ang II-treated cardiomyocytes. The Western blot results showed that siRNA-mediated Ido1 knockdown reduced the phosphorylation levels of AKT and mTOR (Fig. 5a). By contrast, adenovirus-mediated overexpression of Ido1 increased phosphorylation of AKT and mTOR in cardiomyocytes treated with Ang II (Fig. 5b). Besides, IDO1 also promotes the phosphorylation of mTOR downstream effector p70 ribosomal protein S6 kinase 1 (S6K1) (Fig. 5a, b).
Fig. 5.
IDO1 promotes mTOR signaling in cardiomyocytes. Ido1 knockdown represses AKT-mTOR-S6K1 signaling in Ang II-treated cardiomyocytes. Cardiomyocytes were transfected with si-Ido1 and si-NC for 24 h, followed by Ang II treatment for an additional 24 h. mTOR, mammalian target of rapamycin; S6K1, p70 ribosomal protein S6 kinase 1. n = 3 in each group. **p < 0.01 by Student’s t test. a Ido1 overexpression facilitates AKT-mTOR-S6K1 signaling in Ang II-treated cardiomyocytes. Cardiomyocytes were infected with Ad-Ido1 and Ad-Ctrl for 24 h, followed by Ang II treatment for an additional 24 h. n = 3 in each group. **p < 0.01 by Student’s t test. b Ido1 overexpression facilitates AKT-mTOR-S6K1 signaling in Ang II-treated cardiomyocytes. Cardiomyocytes were infected with Ad-Ido1 and Ad-Ctrl for 24 hours, followed by Ang II treatment for an additional 24 hours. n=3 in each group. **p <0.01 by Student’s t test. c Ido1 knockdown reduces protein synthesis in cardiomyocytes. Cardiomyocytes were transfected with si-Ido1 and si-NC for 24 h, followed by Ang II treatment for an additional 24 h in [3H]-leucine-containing medium. Protein synthesis was determined by [3H]-leucine incorporation. n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. d Ido1 overexpression promotes protein synthesis in cardiomyocytes. Cardiomyocyte cells were infected with Ad-Ido1 or Ad-Ctrl for 24 h and treated as in (c). n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test
The ribosomal protein S6K1 is critical for de novo protein synthesis and contributes to the function of mTOR in cell size controlling [11]. Accumulating evidence has reported that increased protein synthesis is an essential feature of cardiomyocyte hypertrophy in vitro and cardiac hypertrophy in vivo [33, 34]. Besides, the AKT-mTOR-S6K1 signaling axis is a critical controller in protein synthesis and, subsequently, cardiac hypertrophy [18, 35, 36]. Thus, we tested the effects of IDO1 on Ang II-induced protein synthesis in cardiomyocytes using a [3H]-leucine incorporation assay. We observed that Ang II treatment significantly increased the protein synthesis levels in cardiomyocytes, which was repressed by siRNA-mediated Ido1 deficiency and enhanced by adenovirus-mediated Ido1 overexpression (Fig. 5c, d).
Therefore, these results demonstrated that IDO1 facilitates Ang II-induced de novo protein synthesis via activating the AKT-mTOR-S6K1 signaling axis.
AKT-mTOR Signaling is Critically Involved in the Function of IDO1 During Cardiac Hypertrophy
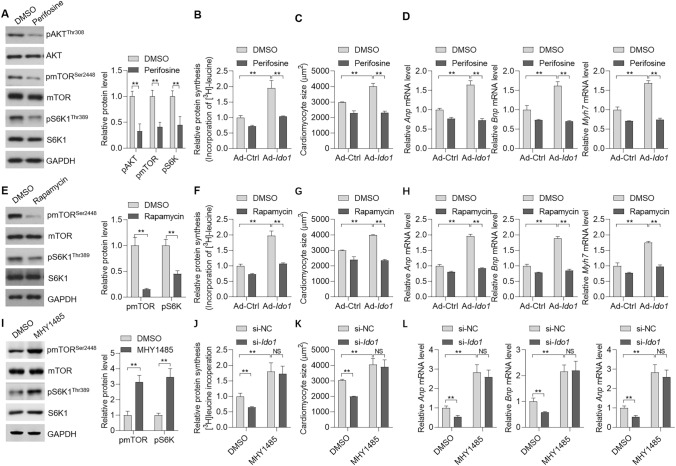
Next, we tested whether the AKT-mTOR signaling was critically involved in IDO-mediated protein synthesis and subsequently hypertrophic growth of cardiomyocytes. To this end, we treated the cells with AKT inhibitor perifosine (Fig. 6a). AKT inhibitor perifosine significantly blocked the effects of Ido1 on protein synthesis in cardiomyocytes treated with Ang II (Fig. 6b). Besides, the promoting roles of Ido1 in an increase of cardiomyocyte size and the hyperexpression of hypertrophic genes (Anp, Bnp, and Myh7) were also repressed by AKT inhibitor perifosine (Fig. 6c, d). As thus, the kinase AKT is critical for IDO1 function in protein synthesis and cardiomyocyte hypertrophy.
Fig. 6.
mTOR signaling is involved in IDO1-mediated effects in cardiomyocyte hypertrophy. a Cardiomyocyte cells were with/without AKT inhibitor Perifosine (1 μM) for additional 48 h. Then, Western blot was performed to test the effects. n = 3 in each group. **p < 0.01 by Student’s t test. b Inhibition of AKT blocks IDO1 effects on protein synthesis in cardiomyocytes. Cardiomyocyte cells were infected with Ad-Ido1 or Ad-Ctrl for 24 h. The cells were treated with Ang II (1 μM), with/without AKT inhibitor perifosine (1 μM) for additional 24 h in [3H]-leucine-containing medium. n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. c Inhibition of AKT blocks IDO1 effects on cell size in Ang II-induced hypertrophic cardiomyocytes. Cardiomyocyte cells were infected with Ad-Ido1 or Ad-Ctrl for 24 h, then the cells were treated with Ang II (1 μM), with/without AKT inhibitor perifosine (1 μM) for additional 48 h. Cardiomyocytes were analyzed by ImageJ. n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. d The inhibition of AKT blocks IDO1 effects on the expression of hypertrophic markers. n = 3 in each group. Cardiomyocytes were treated as in (b). **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. e Cardiomyocyte cells were with/without mTOR inhibitor rapamycin (10 nM) for additional 48 h. Then, Western blot was performed to test the effects. n = 3 in each group. **p < 0.01 by Student’s t test. f Inhibition of mTOR blocks the effects of IDO1 on protein synthesis in cardiomyocytes. Cardiomyocyte cells were infected with Ad-Ido1 or Ad-Ctrl for 24 h. Then the cells were treated with Ang II (1 μM), with/without mTOR inhibitor rapamycin (10 nM) for additional 24 h in [3H]-leucine-containing medium. n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. g Inhibition of mTOR blocks IDO1 effects on cell size in Ang II-induced cardiomyocytes. n = 3 in each group. Cardiomyocyte cells were infected with Ad-Ido1 or Ad-Ctrl for 24 h. Then the cells were treated with Ang II (1 μM), with/without mTOR inhibitor rapamycin (10 nM) for additional 48 h. Cardiomyocyte size was analyzed by ImageJ **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. h Inhibition of mTOR blocks the effects of IDO1 on hypertrophic markers. The cells were treated as in (f). n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. i Cardiomyocyte cells were with/without mTOR activator MHY1485 (10 μM) for 48 h. Then, Western blot was performed to test the effects. n = 3 in each group. **p < 0.01 by Student’s t test. j Activation of mTOR blocks the effects of IDO1 knockdown on protein synthesis in cardiomyocytes. Cardiomyocyte cells were infected with si-Ido1 or si-NC for 24 h. Then the cells were treated with Ang II (1 μM), with/without mTOR activator MHY1485 (10 μM) for additional 24 h in [3H]-leucine-containing medium. n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. k Activation of mTOR blocks IDO1 knockdown effects on cell size in Ang II-induced cardiomyocytes. n = 3 in each group. Cardiomyocyte cells were infected with si-Ido1 or si-NC for 24 h. Then the cells were treated with Ang II (1 μM), with/without mTOR activator MHY1485 (10 μM) for additional 48 h. Cardiomyocyte size was analyzed by ImageJ **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. l Activation of mTOR blocks the effects of IDO1 knockdown on hypertrophic markers. The cells were treated as in (k). n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test
Finally, we also inhibited mTOR with rapamycin (Fig. 6e). Rapamycin treatment reduced the promoting effects of Ido1 overexpression on protein synthesis (Fig. 6f). Moreover, rapamycin also repressed the impact of Ido1 on cardiomyocytes size and the expression of hypertrophy genes (Anp, Bnp, and Myh7) (Fig. 6g, h). Interestingly, activation of mTOR with MHY1485 promoted Ang II-induced cardiomyocyte hypertrophy and blocked the effects of Ido1 knockdown (Fig. 6i–l). Therefore, these findings demonstrated that the AKT-mTOR signaling was critically involved in IDO1-mediated controlling protein synthesis and cardiomyocyte hypertrophy.
IDO1 Promotes PI3K Activation to Facilitate AKT-mTOR Signaling and Cardiomyocyte Hypertrophy
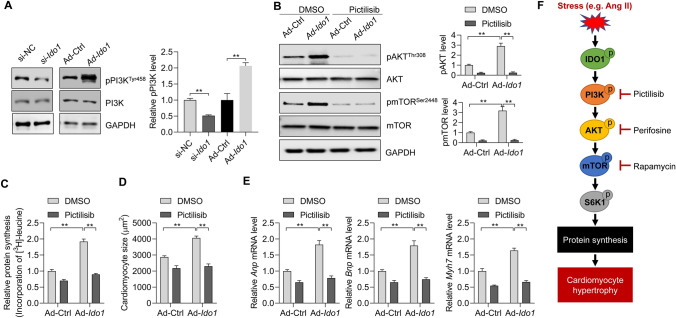
Finally, we analyzed the mechanism by which IDO1 promotes AKT-mTOR activation. PI3K is a critical upstream activator of AKT [4]. We explored whether PI3K was involved in IDO1-mediated activation of AKT. The effects of IDO1 knockdown and overexpression on PI3K phosphorylation were analyzed. We found that IDO1 knockdown repressed whereas IDO1 overexpression increased the phosphorylation of PI3K, indicating that IDO1 activated PI3K (Fig. 7a). Next, we inhibited PI3K with its inhibitor pictilisib (Supplementary Fig. 3), and we observed that pictilisib treatment significantly blocked IDO1-induced AKT-mTOR activation (Fig. 7b). We also analyzed the effects of pictilisib on protein synthesis. The results showed that pictilisib treatment repressed IDO1-mediated upregulation of protein synthesis in Ang II-treated cardiomyocytes (Fig. 7c). Finally, we tested the effects of pictilisib on cardiomyocyte hypertrophy. We found that pictilisib blocked the impact of IDO1 on cardiomyocyte size and expression of hypertrophy-associated fetal genes Anp, Bnp, and Myh7 (Fig. 7d, e). Therefore, these findings demonstrated that PI3K mediated the effects of IDO1 on AKT-mTOR and cardiomyocyte hypertrophy.
Fig. 7.
IDO1 activates PI3K to promote AKT-mTOR and cardiomyocyte hypertrophy. a Ido1 knockdown represses Ido1 overexpression activates PI3K signaling in Ang II-treated cardiomyocytes. Cardiomyocytes were transfected with si-Ido1 and si-NC or infected with Ad-Ido1 and Ad-Ctrl for 24 h, followed by Ang II treatment for an additional 24 h. PI3K, phosphoinositide 3-kinase. n = 3 in each group. **p < 0.01 by Student’s t test. b Inhibition of PI3K blocks IDO1-mediated activation of AKT-mTOR signaling. Cardiomyocyte cells were infected with Ad-Ido1 or Ad-Ctrl for 24 h, then the cells were treated with Ang II (1 μM), with/without PI3K inhibitor pictilisib (100 nM) for additional 24 h. n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. c Inhibition of PI3K blocks IDO1 effects on protein synthesis in cardiomyocytes. Cardiomyocyte cells were infected with Ad-Ido1 or Ad-Ctrl for 24 h, then the cells were treated with Ang II (1 μM), with/without PI3K inhibitor pictilisib (100 nM) for additional 24 h in [3H]-leucine-containing medium. n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. d Inhibition of PI3K blocks IDO1 effects on cell size in Ang II-induced hypertrophic cardiomyocytes. Cardiomyocyte cells were infected with Ad-Ido1 or Ad-Ctrl for 24 h, then the cells were treated with Ang II (1 μM), with/without PI3K inhibitor pictilisib (100 nM) for additional 48 h. Cardiomyocytes were analyzed by ImageJ. n = 3 in each group. **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. e The inhibition of PI3K blocks IDO1 effects on the expression of hypertrophic markers. n = 3 in each group. Cardiomyocytes were treated as in (c). **p < 0.01 by two-way ANOVA followed by Turkey post-hoc test. f A schematic diagram shows the IDO1 function in cardiac hypertrophy. IDO1 expression is upregulated during cardiac hypertrophy induced by stress such as Ang II. Increased IDO1 promotes AKT-mTOR signaling to facilitate protein synthesis and cardiomyocyte hypertrophy in a PI3K-dependent manner
Discussion
Herein in the present study, we identified IDO1 as an essential regulator for cardiac hypertrophy. IDO1 expression and activity were upregulated in hypertrophic hearts of humans and mice as well as hypertrophic rat cardiomyocytes. Gene silence and overexpression experiments demonstrated that IDO1 promotes cardiomyocyte hypertrophy through activating the protein synthesis pathway via the AKT-mTOR-S6K1 signaling axis. And pharmacological evidence indicated that AKT and mTOR were essentially contributed to the functions of IDO1 in protein synthesis and cardiomyocyte hypertrophy (Fig. 7f).
IDO1 is a rate-limiting enzyme involved in tryptophan catabolism and participates in immune activation and cancer development, and drug resistance [19, 22, 37]. The roles of IDO1 in the cardiovascular system are also recently identified. Overexpression of IDO1 in bone marrow mesenchymal stem cells regulated exosome components to promote cardiac allografts' immunotolerance in rats [23]. In chronic viral myocarditis of mice, Ido1 knockout induced an anti-inflammatory response in macrophages [25]. Besides, IDO1 is reported to regulate the production of IL-10 in macrophages and participates in atherosclerosis. The IDO1-derived metabolite, kynurenic acid, is responsible for reduced IL-10 production by activating a cAMP-dependent pathway and ERK1/2 inhibition [24]. The previous studies mainly focus on the roles of IDO1 in stem cells and immunes cells in the cardiovascular system. But the functions of IDO1 in cardiomyocytes remained unknown.
Here, we identified IDO1 as a pivotal regulator of cardiac hypertrophy. The mRNA and protein expressions of IDO1 were overexpressed in hypertrophic hearts and cardiomyocytes. Of note, this increase in IDO1 expression in hypertrophic hearts was conserved across species (human, mouse, and rat). Significantly, our results demonstrated that IDO1 promoted the development of Ang II-induced cardiomyocyte hypertrophy in vivo and in vitro. Therefore, our findings revealed that IDO1 is critical for cardiomyocyte function and overexpression of IDO1 is a contributor to cardiomyocyte hypertrophy. IDO1 dysregulation contributes to the development of cardiomyocyte hypertrophy in vitro under the condition of Ang II treatment, but IDO1 overexpression was alone was unable to drive cardiomyocyte hypertrophy. This result suggests the IDO1 is a passenger factor but not a driving factor for cardiomyocyte hypertrophy.
IDO1 functions as a rate-limiting enzyme that catalyzes tryptophan degradation along the kynurenine pathway [21]. Tryptophan is essential for protein synthesis [37]. One of the hallmarks of cardiomyocyte hypertrophy is the increased de novo synthesis of proteins [2]. Various stimuli (e.g., Ang II) of hypertrophy can induce protein synthesis. Here we showed that increased protein synthesis was observed in Ang II-induced cardiomyocyte hypertrophy. Interestingly, our data revealed that Ido1 knockdown repressed while Ido1 overexpression promoted Ang II-increased protein synthesis. Therefore, IDO1 may play a complex role in regulating tryptophan degradation and protein synthesis, and the mechanisms of these two pathways are much different.
IDO1 can directly catalyze the degradation of tryptophan along the kynurenine pathway [19]. By contrast, the roles of IDO1 in regulating protein synthesis may be indirect. IDO1 promotes the activation of the AKT-mTOR signaling pathway along with cardiomyocyte hypertrophy. AKT-mTOR is a critical responder to nutrients and critically participates in protein synthesis via the ribosomal protein S6K1. We observed that S6K1 was also regulated by IDO1 in cardiomyocytes. Of note, we observed that inhibition of AKT with perifosine or inhibition of mTOR with rapamycin blocked the effects of IDO1 in cardiomyocyte protein synthesis and hypertrophic growth. Therefore, the AKT-mTOR-S6K1 signaling axis is essential for the function of IDO1 in regulating protein synthesis in hypertrophic cardiomyocytes. We also provided evidence that the effects of IDO1 on AKT activation depend on PI3K activation, which has been demonstrated to be a target of IDO1 metabolic products such as KYN [21]. Therefore, IDO1 may activate AKT-mTOR signaling via KYN-PI3K signaling pathway.Interestingly, this is also the first study that showed the notion that AKT inhibitor perifosine can serve as a potential drug for cardiac hypertrophy treatment. Besides, our findings and previous studies have revealed that IDO1 regulates the PI3K-AKT-mTOR signaling in cancer cells [21, 38]. Thus, IDO1 may participate in other biological progress, such as insulin resistance that involves the PI3K-AKT-mTOR signaling. Further studies are needed to validate this hypothesis.
In summary, IDO1 is a contributor to cardiomyocyte hypertrophy across species partially via the PI3K-AKT-mTOR-S6K1 signaling axis and protein synthesis. This study also implicates that IDO1 may participate in cardiac hypertrophy and heart failure, and IDO1 can serve as a promising therapeutic target for the treatment of cardiac hypertrophy and related cardiac failure.
Limitation of this Study
The human samples used were based on mixed-gender and in patients on different treatments, which could be confounding factors to the suggested findings. Besides, we did not test the roles of IDO1 in cardiac hypertrophy in vivo. Further studies are needed to test the in vivo function of IDO1 in mouse cardiac hypertrophy in vivo by using IDO1 knockout mice or IDO1 inhibitors.
Supplementary Information
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (PDF 271 kb)
Author Contributions
Yang Liu and Shuang Li performed most of the experiments; Zhanqun Gao collected human samples, Shuangjia Li and Qingyun Tan isolated cardiomyocytes, Yanmei Li isolated RNA and protein, Qingdong Wang and Dongwei Wang designed the study and analyzed the data.
Funding
This study was supported by The Health Commission of Heilongjiang Province (2019–314).
Declarations
Conflict of interest
All the authors declare that we have no conflict of interest to report.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s12012-023-09779-5
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Liu and Shuang Li have contributed equally to this work.
Change history
1/24/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s12012-023-09779-5
Contributor Information
Dongwei Wang, Email: mhnhua@163.com.
Qingdong Wang, Email: 3190013@qq.com.
References
- 1.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. Journal of the American College of Cardiology. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nature Reviews Cardiology. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. Journal of Molecular and Cellular Cardiology. 2016;97:245–262. doi: 10.1016/j.yjmcc.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacological Research. 2010;61:269–280. doi: 10.1016/j.phrs.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Tang X, Luo Y-X, Chen H-Z, Liu D-P. Mitochondria, endothelial cell function, and vascular diseases. Frontiers in Physiology. 2014;5:175. doi: 10.3389/fphys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang X, Chen X-F, Chen H-Z, Liu D-P. Mitochondrial sirtuins in cardiometabolic diseases. Clinical Science. 2017;131:2063–2078. doi: 10.1042/CS20160685. [DOI] [PubMed] [Google Scholar]
- 7.Gélinas R, Mailleux F, Dontaine J, Bultot L, Demeulder B, Ginion A, Daskalopoulos EP, Esfahani H, Dubois-Deruy E, Lauzier B. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nature Communications. 2018;9:1–17. doi: 10.1038/s41467-017-02795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan AY, Soltys C-LM, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. Journal of Biological Chemistry. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y-X, Tang X, An X-Z, Xie X-M, Chen X-F, Zhao X, Hao D-L, Chen H-Z, Liu D-P. Sirt4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. European Heart Journal. 2017;38:1389–1398. doi: 10.1093/eurheartj/ehw138. [DOI] [PubMed] [Google Scholar]
- 10.Tang X, Chen X-F, Wang N-Y, Wang X-M, Liang S-T, Zheng W, Lu Y-B, Zhao X, Hao D-L, Zhang Z-Q, Zou M-H, Liu D-P, Chen H-Z. SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation. 2017;136:2051–2067. doi: 10.1161/CIRCULATIONAHA.117.028728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Brink M. mTOR, cardiomyocytes and inflammation in cardiac hypertrophy. Biochimica et Biophysica Acta. 2016;1863:1894–1903. doi: 10.1016/j.bbamcr.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Morales CR, Li DL, Pedrozo Z, May HI, Jiang N, Kyrychenko V, Cho GW, Kim SY, Wang ZV, Rotter D. Inhibition of class I histone deacetylases blunts cardiac hypertrophy through TSC2-dependent mTOR repression. Science Signaling. 2016;9:ra34. doi: 10.1126/scisignal.aad5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin Z, Ma Z, Jiang S, Wang D, Fan C, Di S, Hu W, Li T, She J, Yang Y. FOXOs in the impaired heart: New therapeutic targets for cardiac diseases. Biochimica et Biophysica Acta. 1863;2017:486–498. doi: 10.1016/j.bbadis.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Ronnebaum SM, Patterson C. The FoxO family in cardiac function and dysfunction. Annual Review of Physiology. 2010;72:81–94. doi: 10.1146/annurev-physiol-021909-135931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clemente C, Xavier-Neto J, Dalla Costa A, Consonni S, Antunes J, Rocco S, Pereira M, Judice C, Strauss B, Joazeiro P. Focal adhesion kinase governs cardiac concentric hypertrophic growth by activating the AKT and mTOR pathways. Journal of Molecular and Cell Cardiology. 2012;52:493–501. doi: 10.1016/j.yjmcc.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Aoyagi T, Matsui T. Phosphoinositide-3 kinase signaling in cardiac hypertrophy and heart failure. Current Pharmaceutical Design. 2011;17:1818–1824. doi: 10.2174/138161211796390976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, Condorelli G, Ellingsen O. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. Journal of Cellular Physiology. 2008;214:316–321. doi: 10.1002/jcp.21197. [DOI] [PubMed] [Google Scholar]
- 18.Sciarretta S, Forte M, Frati G, Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circulation Research. 2018;122:489–505. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 inhibitors: From bench to bedside. Cancer Research. 2017;77:6795–6811. doi: 10.1158/0008-5472.CAN-17-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhai L, Ladomersky E, Lauing KL, Wu M, Genet M, Gritsina G, Győrffy B, Brastianos PK, Binder DC, Sosman JA, Giles FJ, James CD, Horbinski C, Stupp R, Wainwright DA. Infiltrating T cells increase IDO1 expression in glioblastoma and contribute to decreased patient survival. Clinical Cancer Research. 2017;23:6650–6660. doi: 10.1158/1078-0432.CCR-17-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishnupuri KS, Alvarado DM, Khouri AN, Shabsovich M, Chen B, Dieckgraefe BK, Ciorba MA. IDO1 and kynurenine pathway metabolites activate PI3K-Akt signaling in the neoplastic colon epithelium to promote cancer cell proliferation and inhibit apoptosis. Cancer Research. 2019;79:1138–1150. doi: 10.1158/0008-5472.CAN-18-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladomersky E, Zhai L, Lenzen A, Lauing KL, Qian J, Scholtens DM, Gritsina G, Sun X, Liu Y, Yu F. IDO1 inhibition synergizes with radiation and PD-1 blockade to durably increase survival against advanced glioblastoma. Clinical Cancer Research. 2018;24:2559–2573. doi: 10.1158/1078-0432.CCR-17-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He J-G, Xie Q-L, Li B-B, Zhou L, Yan D. Exosomes derived from IDO1-overexpressing rat bone marrow mesenchymal stem cells promote immunotolerance of cardiac allografts. Cell Transplantation. 2018;27:1657–1683. doi: 10.1177/0963689718805375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metghalchi S, Ponnuswamy P, Simon T, Haddad Y, Laurans L, Clément M, Dalloz M, Romain M, Esposito B, Koropoulis V, Lamas B, Paul J-L, Cottin Y, Kotti S, Bruneval P, Callebert J, den Ruijter H, Launay J-M, Danchin N, Sokol H, Tedgui A, Taleb S, Mallat Z. Indoleamine 2,3-dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of interleukin-10 production. Cell Metabolism. 2015;22:460–471. doi: 10.1016/j.cmet.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Guo G, Sun L, Yang L, Xu H. IDO1 depletion induces an anti-inflammatory response in macrophages in mice with chronic viral myocarditis. Cell Cycle. 2019;18:2598–2613. doi: 10.1080/15384101.2019.1652471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima K, Yamashita T, Kita T, Takeda M, Sasaki N, Kasahara K, Shinohara M, Rikitake Y, Ishida T, Yokoyama M, Hirata K. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:1963–1972. doi: 10.1161/ATVBAHA.111.229443. [DOI] [PubMed] [Google Scholar]
- 27.Yun TJ, Lee JS, Machmach K, Shim D, Choi J, Wi YJ, Jang HS, Jung IH, Kim K, Yoon WK, Miah MA, Li B, Chang J, Bego MG, Pham TN, Loschko J, Fritz JH, Krug AB, Lee SP, Keler T, Guimond JV, Haddad E, Cohen EA, Sirois MG, El-Hamamsy I, Colonna M, Oh GT, Choi JH, Cheong C. Indoleamine 2,3-dioxygenase-expressing aortic plasmacytoid dendritic cells protect against atherosclerosis by induction of regulatory T cells. Cell Metabolism. 2016;23:852–866. doi: 10.1016/j.cmet.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Melhem NJ, Chajadine M, Gomez I, Howangyin KY, Bouvet M, Knosp C, Sun Y, Rouanet M, Laurans L, Cazorla O, Lemitre M, Vilar J, Mallat Z, Tedgui A, Ait-Oufella H, Hulot JS, Callebert J, Launay JM, Fauconnier J, Silvestre JS, Taleb S. Endothelial cell indoleamine 2, 3-dioxygenase 1 alters cardiac function after myocardial infarction through kynurenine. Circulation. 2021;143:566–580. doi: 10.1161/CIRCULATIONAHA.120.050301. [DOI] [PubMed] [Google Scholar]
- 29.Schafer S, Viswanathan S, Widjaja AA, Lim WW, Moreno-Moral A, DeLaughter DM, Ng B, Patone G, Chow K, Khin E, Tan J, Chothani SP, Ye L, Rackham OJL, Ko NSJ, Sahib NE, Pua CJ, Zhen NTG, Xie C, Wang M, Maatz H, Lim S, Saar K, Blachut S, Petretto E, Schmidt S, Putoczki T, Guimaraes-Camboa N, Wakimoto H, van Heesch S, Sigmundsson K, Lim SL, Soon JL, Chao VTT, Chua YL, Tan TE, Evans SM, Loh YJ, Jamal MH, Ong KK, Chua KC, Ong BH, Chakaramakkil MJ, Seidman JG, Seidman CE, Hubner N, Sin KYK, Cook SA. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552:110–115. doi: 10.1038/nature24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X, Ma H, Han L, Zheng W, Lu Y-B, Chen X-F, Liang S-T, Wei G-H, Zhang Z-Q, Chen H-Z, Liu D-P. SIRT1 deacetylates the cardiac transcription factor Nkx2.5 and inhibits its transcriptional activity. Science Report. 2016;6:36576. doi: 10.1038/srep36576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia Y-Y, Lu J, Huang Y, Liu G, Gao P, Wan Y-Z, Zhang R, Zhang Z-Q, Yang R-F, Tang X, Xu J, Wang X, Chen H-Z, Liu D-P. The involvement of NFAT transcriptional activity suppression in SIRT1-mediated inhibition of COX-2 expression induced by PMA/Ionomycin. PLoS One. 2014;9:e97999. doi: 10.1371/journal.pone.0097999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li N, He Y, Wang L, Mo C, Zhang J, Zhang W, Li J, Liao Z, Tang X, Xiao H. d-galactose induces necroptotic cell death in neuroblastoma cell lines. Journal of Cellular Biochemistry. 2011;112:3834–3844. doi: 10.1002/jcb.23314. [DOI] [PubMed] [Google Scholar]
- 33.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. The Journal of Clinical Investigation. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, Pillai VB, Ravindra PV, Gupta M, Jeevanandam V, Cunningham JM, Deng CX, Lombard DB, Mostoslavsky R, Gupta MP. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nature Medicine. 2012;18:1643–1650. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao QD, Viswanadhapalli S, Williams P, Shi Q, Tan C, Yi X, Bhandari B, Abboud HE. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFκB signaling pathways. Circulation. 2015;131:643–655. doi: 10.1161/CIRCULATIONAHA.114.011079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 37.Cheong JE, Ekkati A, Sun L. A patent review of IDO1 inhibitors for cancer. Expert Opinion on Therapeutic Patents. 2018;28:317–330. doi: 10.1080/13543776.2018.1441290. [DOI] [PubMed] [Google Scholar]
- 38.Folgiero V, Miele E, Carai A, Ferretti E, Alfano V, Po A, Bertaina V, Goffredo BM, Benedetti MC, Camassei FD. IDO1 involvement in mTOR pathway: A molecular mechanism of resistance to mTOR targeting in medulloblastoma. Oncotarget. 2016;7:52900. doi: 10.18632/oncotarget.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (PDF 271 kb)