Abstract
Introduction
Multiple myeloma is a progressive and incurable hematological disease characterized by disordered and clonal multiplication of plasmacytes in the bone marrow. The main clinical manifestations are caused by the presence of neoplastic cells in bone tissue, as well as the excessive production of immunoglobulins and normal humoral immunity suppression. Daratumumab is an anti-CD38 monoclonal antibody that has promising results in managing the multiple myeloma disease.
Objective
This study aimed to investigate the scientific evidence concerning the impact of the cytomegalovirus infections in the daratumumab treatment course in extensively pretreated multiple myeloma patients.
Method
To this end, an integrative literature review was performed in different databases, comprising a 5-year period.
Results
The studies analysis revealed that the cytomegalovirus infection reactivation can occur during the use of daratumumab in multiple myeloma patients previously treated, which led to treatment discontinuation, compromised the drug efficacy and favored the disease progression. Moreover, it was observed that even with prophylactic antiviral therapy there was an infection reactivation in some cases, as well as deaths, in more severe situations.
Conclusion
Thus, even considering that few reports on such a topic are available in the scientific literature, the present review showed that cytomegalovirus reactivation can impair daratumumab therapy, mainly in multiple myeloma patients heavily pretreated. In addition, this study could contribute as a tool for the clinical decision and management of adverse effects in medical practices, demonstrating the importance of patient monitoring for the possibility of cytomegalovirus reactivation in heavily pretreated myeloma patients.
Keywords: Hematologic cancer, Monoclonal antibody, Viral infection, Immune system, Immunotherapy
Introduction
Multiple myeloma (MM) is an immune system disease characterized as a progressive and incurable hematological neoplasia of B lymphocytes caused by an unregulated and clonal proliferation of plasmacytes in the bone marrow (BM). These cells produce and secrete monoclonal immunoglobulin (Ig) or its fragment, the M-protein.1, 2 Clinical manifestations arise from the infiltration of neoplastic plasma cells into the organs (mainly pelvic bones, spine and ribs), excess immunoglobulin production, kidney injury and suppression of normal humoral immunity.1, 3 In the last decade, the MM diagnostic was based on clinicopathological evidence with severe manifestations of organ damage, such as osteolytic bone lesions and renal failure. However, the International Myeloma Working Group review these criteria and included to existing requirements some validated biomarkers that can be associated with common MM symptoms. Thus, the diagnosis of MM should be made through the assay of excess plasmacytes in BM (about 10%) and bone lesions evaluated by imaging technology, such as magnetic resonance imaging (MRI) and positron emission tomography–computed tomography (PET–CT), to identify the presence of osteolytic bone lesions or osteoporosis with compression fractures. In addition, the serum quantification of light immunoglobulin chains that circulate unbound to heavy chains and renal damage, indicated by a 40% decrease from the lower limit of the normal glomerular filtration were also reported. A great number of patients that present these parameters evolve to MM in a period of approximately 2 years.4
Infections are a significant cause of morbidity and the main cause of death in MM patients, which is associated with B cell dysfunction, such as hypogammaglobulinemia, as well as abnormalities in T, dendritic and NK cells.5, 6 Moreover, some studies demonstrate the impact that anti-MM therapies have on the patient immune system, leading to increased susceptibility to infections and expanding pathogens.5, 6, 7, 8 The MM therapies have been improved over the past 10 years and include the combination of alkylating agents (melphalan and cyclophosphamide), protease inhibitors (bortezomib and carfilzomib) and immunomodulators (lenalidomide and thalidomide), whether or not associated with corticosteroids (prednisone and dexamethasone).9, 10, 11, 12, 13 Despite the advances in MM treatment, almost all patients eventually relapse or become refractory to drugs, leading to prolonged treatments, which increases the risk for infections.14, 15
Since some tumor-associated antigens have been identified in MM cells, immunotherapy is a promising approach for treatment of MM and relapsed/refractory MM, acting on repair, stimulation, and/or enhancement of the body's natural immune responses to reduce tumor development.14 Daratumumab (Dara) is a monoclonal antibody IG1κ that binds to the CD38 protein, which is significantly expressed on the MM tumor cell surface. The CD38 is a type II membrane glycoprotein that plays an important role in signaling, receptor-mediated cell adhesion, regulation of cytoplasmic calcium flux and enzymatic activity.16, 17, 18 Its action is due to the induction of tumor cell lysis by complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity and cellular phagocytosis in CD38-expressing neoplasms and, to a lesser extent, by inhibition of enzyme activity.16, 17, 19, 20 The Dara immunotherapy was effective as a single agent or in combination with protease inhibitors and immunomodulating agents against MM in newly diagnosed and relapsed/refractory patients.16, 17, 21 Regarding the adverse effects of this drug, Dara infusion may trigger anaphylactic reactions, especially in the first administrations, which demand pre- and post-infusion patient monitoring.17, 22 In addition, NK, regulatory B- and T cells also express CD38 on their surfaces, so they may be decreased during treatment with Dara, which may lead to the onset or reactivation of infections.17, 22
Cytomegalovirus (CMV) is a common pathogen in immunocompromised individuals and is associated with morbidity and mortality in hematopoietic transplant patients.23 In addition, studies have reported that the use of immunomodulatory agents and protease inhibitors is a risk factor for the development of symptomatic CMV reactivation infection in MM patients.23 Therefore, considering the relevance of infections during the treatment of MM patients and the wide use of Dara as a drug of choice due to its effectiveness, this study aims to conduct a literature review to find studies presenting the development or reactivation of CMV infections in patients receiving Dara and to report the impact of the infections in the course of the treatment.
Materials and methods
This study consists of an integrative literature review, which enables the search and analysis of studies already published on a predefined topic. This method allows the synthesis of available evidence, which may guide health care interventions or instigate new research.24, 25 The review was achieved by the following steps: identification of the research theme and elaboration of the guiding question, definition of the inclusion and exclusion criteria, selection of articles by reading the title and abstract and, subsequently, by reading them in full, collecting the main information from the selected studies, analysis of the results obtained and synthesis of the integrative review.24, 25
From the theme delimitation, the guiding question was formulated: “Do cytomegalovirus infections affect the daratumumab treatment course in multiple myeloma patients?”. The following inclusion criteria were listed: (I) studies addressing the issue of the occurrence of CMV infection during the Dara treatment in MM patients; (II) publications resulting from original articles, integrative and systematic literature reviews, case reports, randomized controlled trials and cohort studies published over the past five years; and (III) studies published in Portuguese and English. The updated package insert for Dara was also used as reading material.17 Publications that did not respect the delimitation of the theme and the objective of the study, resulting from opinions or reflections on articles, editorials and book chapters, were excluded.
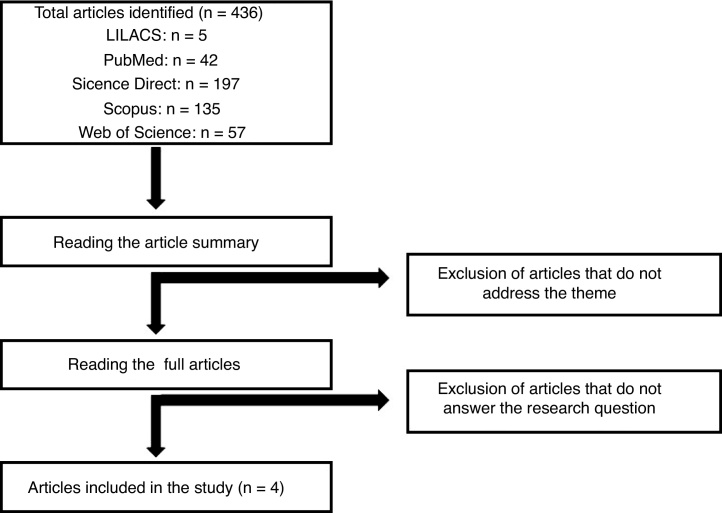
The data were collected from the LILACS (Latin American and Caribbean Health Sciences Literature), PubMed, Science Direct, Scopus and Web of Science databases. The search engine search used the terms “daratumumab and cytomegalovirus” and “multiple myeloma and cytomegalovirus”. The same terms were also used in the Portuguese language. Initially, articles were selected by reading the title and abstract, and after the articles were eligible, according to the inclusion and exclusion criteria, the articles were read in full. In total, 436 articles were found with the keywords used, according to the flowchart presented in Figure 1. After evaluating the articles regarding the inclusion and exclusion criteria mentioned for this study, 4 publications were elected for full reading and the development of the article discussion. The selected studies were further classified, based on the methodological design, according to the level of scientific evidence, following the Melnyk and Fineout-Overholt (2005) proposal.26
Figure 1.
Publication selection flowchart.
Results and discussion
The final sample resulted in four publications27, 28, 29, 30 to compose this integrative review. Data from the publications were analyzed and characterized in relation to the publication year, journal in which it was published, study design, level of evidence and main findings, as can be seen in Table 1. The publications are recent, being from 201827, 28 and 2019,29, 30 all of which are case reports on MM patients who had developed or reactivated CMV infections during the Dara treatment. Importantly, the total number of patients involved in the four studies were 40 and 12 of them had a reactivated CMV infection (Table 1). Furthermore, patients with a reactivated CMV infection were using Dara to treat relapsed/refractory MM and all studies reported 2–8 range lines of prior treatment. The details of the selected studies are described below.
Table 1.
Characterization of articles elected for the review.
| Author | Publication year | Journal | Study design | Level of evidence | Clinical findings | Total number of patients/CMV-infected |
|---|---|---|---|---|---|---|
| 1. Frerichs et al. | 2018 | Clinical Lymphoma, Myeloma and Leukemia | Case Report | VI | CMV reactivation during Dara treatment in refractory MM patient | 1/1 |
| 2. Lavi et al. | 2018 | European Journal of Hematology | Case Report | VI | CMV infections in MM patients on Dara therapy combined with other drugs | 3/3 |
| 3. Nahi et al. | 2019 | PLoS ONE | Case Report | VI | Occurrence of infections during treatment with Dara in MM patients | 23/3 |
| 4. Nakagawa et al. | 2019 | Annals of Hematology | Case Report | VI | CMV reactivation during Dara treatment in refractory MM patient | 13/5 |
Frerichs et al. (2018) presented a case report showing CMV reactivation in a 57-year-old female patient with MM, who was receiving Dara monotherapy. The case is a relapsing MM, refractory to immunomodulatory agents (thalidomide, lenalidomide and pomalidomide) and bortezomib and alkylating agents (melphalan and cyclophosphamide), as well as the signaling lymphocytic activation molecule family member 7 (SLAMF7)-targeting antibody elotuzumab. The patient then started, as line 7 of the treatment, Dara at a dose of 16 mg/kg and, after two infusions of the drug, episodes of fever were observed. After several exams for the cause, the polymerase chain reaction (PCR) test was performed, which showed the presence of CMV deoxyribonucleic acid (DNA), indicating a symptomatic infection caused by the reactivation of the virus in the patient. With antiviral therapeutic regimen, the patient's body temperature stabilized, platelet and hemoglobin levels returned to normal values and the CMV DNA quantification was below detection limits. Considering the patient's extensive MM treatment history, alternative treatment options were limited and would have all induced additional immunosuppression. Therefore, Dara therapy was reestablished and the patient had a good 7-month partial response, in which the CMV DNA load remained negative.27
Dara has also been used in combination with other drugs to increase treatment efficacy, as shown mainly in the CASTOR (Dara, bortezomib and dexamethasone),31 POLLUX (Dara, lenalidomide and dexamethasone),32 ALCYONE (Dara, bortezomib, melphalan and prednisone)33 and CASSIOPEIA (Dara, bortezomib, thalidomide and dexamethasone)34 studies. Despite the pharmacological benefits, the anti-MM treatment regimens can contribute to the patients’ immunosuppressive status, once these patients already have increased susceptibility to infections, as a result of myeloma-induced immune dysfunction, including impaired T- and NK-cell activity and decreased polyclonal immunoglobulin production.8, 27
Thus, Lavi et al. (2018) presented a case report of three relapsed/refractory MM patients, who were receiving immunotherapy with Dara associated with other drugs, and subsequently developed a severe CMV infection. Patient 1 was a 74-year-old man diagnosed with MM 7 years ago and previously treated with 7 lines of drugs. At present, the patient was receiving treatment with the combination Dara + pomalidomide + dexamethasone. The patient was hospitalized for diarrhea, fever and neutropenia and the PCR test indicated the presence of CMV in the blood. Patient 2 was a 64-year-old man who was diagnosed with MM 4 years ago (4 lines of prior treatment) and was being treated with Dara + carfilzomib + dexamethasone as therapy. Patient 3, a 36-year-old male with MM for 6 years (6 lines of prior treatment), undergoing a combined Dara + bortezomib + dexamethasone + venetclax therapy, was diagnosed with CMV colitis. In patients 1 and 2, anti-myeloma therapy was discontinued and, in addition, patient 1 died due to the severity of the infection.28 The case reports demonstrated the influence of CMV infections in patients who are in therapy with Dara for relapsed/refractory MM, which may lead to treatment interruption and, in more severe cases, cause patient's death.
As described in some studies, the European Society for Clinical Microbiology and the Infectious Disease Study Group recommend herpes zoster prophylaxis in patients receiving Dara, however the CMV reactivation has not been mentioned.35 Nakagawa et al. (2019) conducted a study of 13 patients undergoing MM treatment with Dara, in which five of them had CMV reactivation. There was no need to discontinue the Dara treatment during antiviral therapy, but one patient postponed Dara infusion. The patients received antiviral therapy for 13 days and there were no reports of the development of serious infections.30 As the Dara infusion should be discontinued if CMV disease develops, it is important to monitor the disease and perform prophylactic therapy in patients at risk.23
Infections are the major cause of mortality in MM patients.5 It has been reported that after Dara infusion patients show a decrease in the leukocyte count, which is due to the CD38 expression in these cells, leaving patients susceptible to infections.16 Nahi and colleagues conducted a study on 23 MM patients previously treated with 2–6 lines, who were receiving Dara monotherapy during the study. Interestingly, nine of them had viral and/or bacterial infections and of extreme relevance, 3 of these patients revealed a CMV reactivation after PCR analysis. The first patient was a 65-year-old man who developed fever and upper respiratory tract infection. The PCR test showed a CMV loading of 26,000 IU/mL, and Dara was discontinued during antiviral treatment with valganciclovir. The CMV loading decreased to 500 IU/mL after ten days and the patient was restarted on Dara. A second patient with CMV reactivation, an 82-year-old man, developed liver failure after receiving two doses of Dara and was hospitalized. The CMV viral load at admission was 2600 IU/mL. The patient died and the autopsy revealed severe heart failure as a possible cause of death. The PCR test of the CMV DNA and leukocyte levels showed a significant increase before the CMV reactivation. The third patient, an 80-year-old man, contracted an upper respiratory tract infection after the 6th dose of Dara, while increasing the CMV loading to 800 IU/mL. The respiratory tract infection was resolved spontaneously two weeks after Dara withdrawal (without CMV treatment) and the patient can be restarted on Dara.29
Based on the evaluation of the four studies, it is possible to note that, for most of the patients, it was necessary to interrupt the infusion until patient recovery, which may have compromised the efficacy of the Dara treatment, as well as caused disease progression.27, 28, 29, 30 In the more severe cases, the patient died due to the delay in diagnosing viral infection and the severe state of immunosuppression.28, 29 In the studies conducted by Lavi et al. (2018) and Nakagawa et al. (2019), Dara was combined with protease inhibitors (bortezomib and carfilzomib) or immunomodulators (lenalidomide and pomalidomide) drugs, plus dexamethasone.28, 30 It is known that bortezomib causes CMV reactivation.27, 36 Thus, the combination of these drugs with Dara can increase the risk of viral reactivation. However, in the other two studies reported in this review, the CMV reactivation was observed during treatment with Dara monotherapy.27, 29 In addition, it is relevant to note that the patients enrolled in the studies were heavily pretreated, which could have already depressed their immune systems. Thus, the treatment with immunosuppressive drugs, followed by replacement treatment with Dara monotherapy, can cause a cumulative effect on T, as well as NK, cell depletion.
Immunotherapy with Dara may contribute to the immunosuppressed status of patients, however, the mechanism involved is still poorly understood. It is probably due to the binding to the CD38 protein, expressed on the surface of other cells, such as NK, B and regulatory T cells.16 These cells are responsible for playing an important role in the immunological response and are often decreased in patients receiving Dara infusions, mainly in patients heavily treated with prior lines, leading to greater susceptibility to infections.8 Indeed, both studies using Dara monotherapy showed a drastic reduction in CD4+ T and NK cells after starting treatment with this monoclonal antibody. The CD8+ T cells remained normal or increased, leading to a low CD4+/CD8+ ratio, which favors the occurrence of viral reactivations.
Few studies have reported on the CMV infection or reactivation in patients receiving Dara. However, as can be seen in our review, viral infections have a great impact on the treatment course, as they can be severe if they are uncontrolled. Thus, it is important that patients on Dara therapy be closely monitored for the viral load of different pathogens, including CMV, especially those who have already received other lines of treatment with immunosuppressant agents. Moreover, it is important to work in conjunction with the multi-professional team on antineoplastic therapy (MTAT) to observe early signs of CMV infection in patients receiving Dara in order to rapidly initiate treatment to control the infection and to prevent the interruption or discontinuation of the anti-myeloma treatment.
Conclusion
Review studies are extremely important in finding data that can help guide therapeutic approaches and are also useful in managing adverse effects caused by current treatments that have not yet been studied. In this sense, it was possible to observe the impact of CMV infection in MM patients receiving Dara, which can be discontinued during the antiviral management and patient recovery. Considering this, our study is useful to alert clinicians to the possibility of CMV reactivation in heavily pretreated myeloma patients, who are in treatment with Dara for relapsed/refractory MM.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Silva R.O., Brandão K.M., Pinto P.V., Faria R.M., Clementino N.C., Silva C.M. Mieloma múltiplo: características clínicas e laboratoriais ao diagnóstico e estudo prognóstico. Rev Bras Hematol Hemoter. 2009;31(2):63–68. [Google Scholar]
- 2.Holstein S.A., Liu H., McCarthy P.L. Multiple Myeloma. Hematol Oncol Clin North Am. 2014;28(6):1113–1129. doi: 10.1016/j.hoc.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Von Sucro L., Silva J.C., Gehlen G.W., Eldin J.F., Amaral G.A., Santana M.A. Mieloma múltiplo: diagnóstico e tratamento. Rev Med Minas Gerais. 2009;19(1):58–62. [Google Scholar]
- 4.Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.-V. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 5.Blimark C., Holmberg E., Mellqvist U.H., Landgren O., Bjorkholm M., Hultcrantz M. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2014;100(1):107–113. doi: 10.3324/haematol.2014.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garnica M. Multiple myeloma and infection: this association is still close. Hematol Transf Cell Therap. 2019;41(4):281–282. doi: 10.1016/j.htct.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zahid M.F., Ali N., Nasir M., Baig M.H., Iftikhar M., Bin Mahmood S.U. Infections in patients with multiple myeloma treated with conventional chemotherapy: a single-center, 10-year experience in Pakistan. Hematol Transf Cell Therap. 2019;41(4):292–297. doi: 10.1016/j.htct.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schütt P., Brandhorst D., Stellberg W., Poser M., Ebeling P., Müller S. Immune parameters in multiple myeloma patients: influence of treatment and correlation with opportunistic infections. Leukem Lymphom. 2009;47(8):1570–1582. doi: 10.1080/10428190500472503. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos M., Spencer A., Attal M., Prince H.M., Harousseau J.-L., Dmoszynska A. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 10.San Miguel J.F., Schlag R., Khuageva N.K., Dimopoulos M.A., Shpilberg O., Kropff M. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 11.Morgan G.J., Davies F.E., Gregory W.M., Russell N.H., Bell S.E., Szubert A.J. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood. 2011;118(5):1231–1238. doi: 10.1182/blood-2011-02-338665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimopoulos M.A., Moreau P., Palumbo A., Joshua D., Pour L., Hájek R. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos M.A., Goldschmidt H., Niesvizky R., Joshua D., Chng W.J., Oriol A. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(10):1327–1337. doi: 10.1016/S1470-2045(17)30578-8. [DOI] [PubMed] [Google Scholar]
- 14.Sun J., Muz B., Alhallak K., Markovic M., Gurley S., Wang Z. Targeting CD47 as a novel immunotherapy for multiple myeloma. Cancers. 2020;12(2):305. doi: 10.3390/cancers12020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Offidani M., Corvatta L., Polloni C., Gentili S., Brioni A., Visani G. Infectious complications in patients with multiple myeloma treated with new drug combinations containing thalidomide. Leukem Lymphom. 2011;52(5):776–785. doi: 10.3109/10428194.2011.555027. [DOI] [PubMed] [Google Scholar]
- 16.Lokhorst H.M., Plesner T., Laubach J.P., Nahi H., Gimsing P., Hansson M. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 17.Anvisa . Janssen-Cilag Farmacêutica Ltda; 2020. Dalinvi - Bula atualizada 09/03/2020. [Google Scholar]
- 18.Konopleva M., Estrov Z., Zhao S.R., Andreeff M., Mehta K. Ligation of cell surface CD38 protein with agonistic monoclonal antibody induces a cell growth signal in myeloid leukemia cells. J Immunol. 1998;161(9):4702–4708. [PubMed] [Google Scholar]
- 19.Overdijk M.B., Verploegen S., Bögels M., van Egmond M., van Bueren J.J.L., Mutis T. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. mAbs. 2015;7(2):311–320. doi: 10.1080/19420862.2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deneys V., Thiry C., Frelik A., Debry C., Martin B., Doyen C. Daratumumab: therapeutic asset, biological trap! Transfus Clin Biol. 2018;25(1):2–7. doi: 10.1016/j.tracli.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Salomon-Perzyński A., Walter-Croneck A., Usnarska-Zubkiewicz L., Dytfeld D., Zielińska P., Wojciechowska M. Efficacy of daratumumab monotherapy in real-world heavily pretreated patients with relapsed or refractory multiple myeloma. Adv Med Sci. 2019;64(2):349–355. doi: 10.1016/j.advms.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 22.McCullough K.B., Hobbs M.A., Abeykoon J.P., Kapoor P. Common adverse effects of novel therapies for multiple myeloma (MM) and their management strategies. Curr Hematol Malignan Rep. 2018;13(2):114–124. doi: 10.1007/s11899-018-0443-0. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa T., Aisa Y., Shimazaki K., Ito C., Nakazato T. Cytomegalovirus reactivation in patients with multiple myeloma. Eur J Haematol. 2016;96(1):78–82. doi: 10.1111/ejh.12551. [DOI] [PubMed] [Google Scholar]
- 24.Mendes K.D., Silveira R.C., Galvão C.M. Revisão integrativa: método de pesquisa para incorporação de evidências na saúde e na enfermagem. Text Cont Enferm. 2008;17(4):758–764. [Google Scholar]
- 25.Bordignon J.S., Rocha B.D., Prevedello B.P., Beck E.Q., Castro P., Smeha L.N. Hipoglicemia Neonatal: Revisão Integrativa. Disciplinar Scient. 2018;19(3):349–639. [Google Scholar]
- 26.Melnyk B.M., Foneout-Overholt E. Evidence-based practice in nursing & healthcare: a guide to best practice. Lippincot Williams & Wilkins; Philadelphia: 2005. Making the case for evidence-based practice; pp. 3–24. [Google Scholar]
- 27.Frerichs K.A., Bosman P.W.C., Nijhof I.S., Zweegman S., van de Donk N. Cytomegalovirus reactivation in a patient with extensively pretreated multiple myeloma during daratumumab treatment. Clin Lymphom Myelom Leukem. 2018;19(1):e9–e11. doi: 10.1016/j.clml.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavi N., Okasha D., Sabo E., Oren I., Benyamini N., Bar-Yoseph H. Severe cytomegalovirus enterocolitis developing following daratumumab exposure in three patients with multiple myeloma. Eur J Haematol. 2018;101:699–702. doi: 10.1111/ejh.13164. [DOI] [PubMed] [Google Scholar]
- 29.Nahi H., Chrobok M., Gran C., Lund J., Gruber A., Gahrton G. Infectious complications and NK cell depletion following daratumumab treatment of multiple myeloma. PLoS ONE. 2019;14(2):e0211927. doi: 10.1371/journal.pone.0211927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa R., Onishi Y., Kawajiri A., Onodera K., Furukawa E., Sano S. Preemptive therapy for cytomegalovirus reactivation after daratumumab-containing treatment in patients with relapsed and refractory multiple myeloma. Ann Hematol. 2019;98(8):1999–2001. doi: 10.1007/s00277-019-03645-7. [DOI] [PubMed] [Google Scholar]
- 31.Palumbo A., Chanan-Khan A., Weisel K., Nooka A.K., Masszi T., Beksac M. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 32.Dimopoulos M.A., Oriol A., Nahi H., San-Miguel J., Bahlis N.J., Usmani S.Z. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 33.Mateos M.V., Dimopoulos M.A., Cavo M., Suzuki K., Jakubowiak A., Knop S. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518–528. doi: 10.1056/NEJMoa1714678. [DOI] [PubMed] [Google Scholar]
- 34.Moreau P., White D., Benboubker L., Cook G., Leiba M., Morton J. Updated efficacy and safety analysis of daratumumab, lenalidomide, and dexamethasone (DRd) versus lenalidomide and dexamethasone (Rd) for relapsed or refractory multiple myeloma (RRMM; Pollux) Haematologica. 2018;103:31–40. doi: 10.3324/haematol.2018.194282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drgona L., Gudiol C., Lanini S., Salzberger B., Ippolito G., Mikulska M. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid or myeloid cells surface antigens [II]: CD22, CD30, CD33, CD38, CD40, SLAMF-7 and CCR4) Clin Microbiol Infect. 2018;24:S83–S94. doi: 10.1016/j.cmi.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Marchesi F., Mengarelli A., Giannotti F., Tendas A., Anaclerico B., Porrini R. High incidence of post-transplant cytomegalovirus reactivations in myeloma patients undergoing autologous stem cell transplantation after treatment with bortezomib-based regimens: a survey from the Rome Transplant Network. Transplant Infecti Dis. 2014;16(1):158–164. doi: 10.1111/tid.12162. [DOI] [PubMed] [Google Scholar]