Abstract
Early reperfusion after endovascular thrombectomy is associated with an improved outcome in ischemic stroke patients; however, the time dependency in elderly patients remains unclear. We investigated the time–outcome relationships in different age subgroups. Of 2420 patients enrolled in the RESCUE-Japan Registry 2 study, a study based on a prospective registry of stroke patients with acute cerebral large-vessel occlusion at 46 centers, we analyzed the data of 1010 patients with successful reperfusion after endovascular therapy (mTICI of 2b or 3). In 3 age subgroups (< 70, 70 to < 80, and ≥ 80 years), the mRS scores at 90 days were analyzed according to 4 categories of onset-to-reperfusion time (< 180, 180 to < 240, 240 to < 300, and ≥ 300 min). In each age subgroup, the distributions of mRS scores were better with shorter onset-to-reperfusion times. The adjusted common odds ratios for better outcomes per 1-category delay in onset-to-reperfusion time were 0.66 (95% CI 0.55–0.80) in ages < 70 years, 0.66 (95% CI 0.56–0.79) in ages 70 to < 80 years, and 0.83 (95% CI 0.70–0.98) in ages ≥ 80 years. Early reperfusion was associated with better outcomes across all age subgroups. Achieving early successful reperfusion is important even in elderly patients.
Subject terms: Neurology, Neurological disorders, Stroke
Introduction
Randomized clinical trials have demonstrated that endovascular thrombectomy for acute large-vessel occlusion has beneficial effects on 90-day outcomes1–6. Furthermore, a shorter time to successful reperfusion after endovascular thrombectomy has been demonstrated to be associated with better functional outcomes7–10. Although older age is a strong predictor of worse outcomes in stroke patients11, the treatment effect of endovascular thrombectomy has been proven to be consistent across all ages1; thus, guidelines recommend endovascular therapy even in octogenarians12. However, the effect of a shorter time to reperfusion in elderly patients remains controversial13,14. Therefore, we sought to identify the time–outcome relationship in elderly patients. For this purpose, we analyzed the association of onset-to-reperfusion time (ORT) with outcomes in different age subgroups using a large practice-based database.
Methods
Ethics statement
This study complied with the Declaration of Helsinki guidelines for investigations involving humans, and all methods were performed in accordance with relevant guidelines and regulations for observational studies. The study design and protocols were approved by the institutional review boards of all participating centers. Approving institutional review boards waived the need for informed consent because we used clinical information obtained in routine clinical practice. The institutional review boards of all participating centers approved the exemption in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. The full names of all institutional review boards are as follows: Institutional review boards of Red Cross Ise Hospital, Ube Industries Central Hospital, Ogaki Tokushukai Hospital, Osaka Medical College, Osaka University Hospital, Kagawa University, Kawasaki Medical School Hospital, Kanazawa Medical University Hospital, Kitasato University, Gifu University, Kyushu Medical Center, Red Cross Kyoto Daiichi Hospital, Kinki University, Kurashiki Central Hospital, Kurume University Hospital, Kannan Hospital, Kobe City Medical Center General Hospital, Kokura Memorial Hospital, National Cerebral and Cardiovascular Center, Saiseikai Toyama Hospital, Saiseikai Nagasaki Hospital, Saitama Medical University International Medical Center, Sapporo Medical University, Shimizu Hospital, Juntendo University Hospital, Seisho Hospital, National Hospital Organization Sendai Medical Center, Koseikai Takeda Hospital, Tanushimaru Central Hospital, Tama Medical Center, Tokushima University, Toranomon Hospital, Nagoya University, Red Cross Nagoya Daini Hospital, Nippon Medical School, Hakodate Shintoshi Hospital, Hakodate Neurosurgical Hospital, Hyogo College of Medicine, Hyogo Brain and Heart Center, Hirosaki University, Hiroshima University, Red Cross Fukui Hospital, Fukuoka University Chikushi Hospital, Mazda Hospital, Mie University Hospital, Miyakonojo Medical Association Hospital, Yamaguchi Prefectural Grand Medical Center, Yamaguchi University and Yokohamashintoshi Neurosurgical Hospital.
Subjects
This study is a post-hoc analysis of RESCUE-Japan Registry 215, which was a prospective multicenter registry that enrolled 2420 patients with acute cerebral large-vessel occlusion at 46 centers in Japan between October 1, 2014 and September 30, 2016. RESCUE-Japan Registry 2 was designed to clarify the generalizability of the effectiveness of endovascular therapy in real-world patients. We enrolled consecutive patients aged ≥ 20 years who were hospitalized within 24 h of the onset of acute cerebral large-vessel occlusion.
Imaging and endovascular therapy
The diagnostic and treatment modalities were not unified in the RESCUE-Japan Registry 2. The Alberta Stroke Program Early Computerized Tomography Score (ASPECTS) was derived from computed tomography (CT) or magnetic resonance diffusion-weighted imaging (DWI)16,17. In patients with stroke in the posterior circulation, we measured the posterior circulation ASPECTS (pc-ASPECTS) using DWI18. The treatment modalities were determined by the attending physician. In this study, endovascular therapy included thrombectomy using stent retrievers and/or aspiration catheters, balloon angioplasty, stenting, intra-arterial fibrinolysis, piercing using guidewires and/or microcatheters, or a combination of these treatments, all of which have been approved in Japan. The stent retrievers used in this study were the Solitaire™ 2 revascularization device (Covidien, Irvine, CA), the Trevo® ProVue retriever/Trevo® XP ProVue retriever (Stryker, Fremont, CA), and the Revive® retriever (Codman, Raynham, MA). The aspiration catheter used was the Penumbra® system (Penumbra, Alameda, CA). Other devices for endovascular therapy procedures such as stenting or angioplasty were selected by the physicians in charge.
Variables and measurements
We obtained clinical information of patients from hospital charts. Follow-up information up to 90 days was collected primarily through a review of the hospital charts or by contacting the patients, relatives, and/or physicians. We used the following clinical data for the analyses in the current study: age, sex, vascular risk factors (i.e., atrial fibrillation, hypertension, and diabetes mellitus), pre-stroke modified Rankin Scale (mRS) score19, National Institute of Health Stroke Scale (NIHSS) score20, blood glucose level on admission, systolic blood pressure on admission, location of occlusion, use of intravenous recombinant tissue plasminogen activator (IV-rtPA), ASPECTS, onset-to-puncture time (OPT), ORT, modified thrombolysis in cerebral infarction (mTICI) score (0, no perfusion; 1, minimal perfusion; 2a, reperfusion of less than half of the previously occluded territory; 2b, reperfusion of more than half of the previously occluded territory; 3, complete reperfusion)21, symptomatic intracranial hemorrhage (sICH), and mRS score at 90 days after stroke. sICH was defined as intracranial hemorrhage within 72 h after stroke with neurological worsening of ≥ 4 points in the NIHSS score22. OPT and ORT were defined as the duration from the time that the patient was last seen to be well to the groin puncture and the end of the endovascular therapy, respectively. We defined successful reperfusion as an mTICI score of ≥ 2b.
We divided the patients into 3 age subgroups: < 70, 70 to < 80, and ≥ 80 years. Since the time–outcome relationship was demonstrated in patients with OPT of 6 h or less7–10, but not in patients with OPT of more than 6 h23,24, we focused on the early time window and divided ORT into 4 categories with thresholds of 180, 240, and 300 min. We compared the clinical background characteristics and outcome measurements according to the ORT categories in each age subgroup.
The primary outcome was set as the ordinal score on the mRS score at 90 days after stroke. The secondary outcomes were set as a good outcome (defined as mRS score ≤ 2) and mortality at 90 days after stroke. The safety outcome was defined as the occurrence of an sICH within 72 h after stroke.
Statistical analyses
We analyzed the database of patients with successful reperfusion after endovascular therapy (mTICI score 2b or 3). We analyzed continuous variables using the Mann–Whitney U test or Kruskal–Wallis test, and expressed them as median values and interquartile ranges. We analyzed categorical data using the chi-square test, and expressed them as numbers and percentages. In each age subgroup, the trends in the distributions of mRS scores were analyzed according to the ORT categories using the Jonckheere–Terpstra test. We developed multivariate logistic regression models to assess the association between ORT and outcomes by adjusting for the following clinically relevant factors: ASPECTS (≥ 6 or < 6), NIHSS score, pre-stroke mRS score, use of IV-rtPA, site of the main occlusions (anterior or posterior circulation), and vessels of the main occlusions (internal carotid artery [ICA] and M1 segment of the middle cerebral artery occlusion or other arteries). ORT was entered as a categorical or a continuous variable. Because the threshold of 6 for both anterior circulation ASPECTS and pc-ASPECTS was reported to show good discrimination ability25,26, we dichotomized both scores according to whether they were above or below 6. If both CT and DWI were performed before endovascular therapy, we used ASPECTS on DWI in patients with anterior circulation occlusion. We used pc-ASPECTS in patients with stroke in the posterior circulation. To assess potential selection bias, we performed sensitivity analyses. First, we developed multivariate logistic regression models using the multiple imputation method (Supplementary Methods). Second, we analyzed the database of patients with an OPT of ≤ 6 h. Third, we developed multivariate logistic regression model using ASPECTS on CT in patients with both ASPECTS on DWI and CT.
Statistical significance was set at P < 0.05. We conducted all analyses with R software using the rms package (version 3.3.3; F Foundation for Statistical Computing, Vienna, Austria)27.
Results
Baseline characteristics
Among 2420 enrolled patients, 21 patients were excluded because 12 patients did not meet the eligibility criteria and 9 patients were lost to follow-up. Of the remaining 2399 patients, 1278 patients underwent endovascular therapy. Among them, 177 patients with unsuccessful reperfusion (mTICI 0–2a) and 7 patients without reperfusion time data were excluded. Patients without data of ASPECTS, NIHSS score, or mRS score before stroke were also excluded. Thus, 1010 patients were included in the analysis (Fig. 1).
Figure 1.
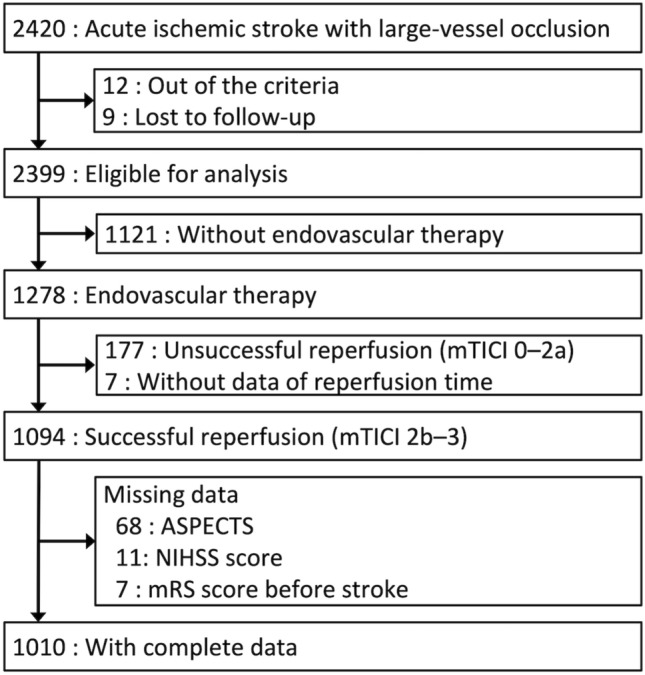
Flowchart of the study population. ASPECTS Alberta Stroke Program Early Computed Tomography Score, mTICI modified thrombolysis in cerebral infarction, NIHSS National Institutes of Health Stroke Scale.
Clinical characteristics according to the age subgroups are shown in Table 1. The proportions of patients with female sex, hypertension, atrial fibrillation, and pre-stroke mRS score > 2 were higher in the elderly subgroups. The NIHSS score and systolic blood pressure were higher in elderly patients. The proportion of patients with diabetes mellitus, IV-rtPA use, and ASPECTS ≥ 6 were not different among the age subgroups. The blood glucose level, location of the occlusion, OPT, and ORT were not different among the age subgroups.
Table 1.
Clinical characteristics according to age.
| Total (N = 1010) | Age (years) | P value | |||
|---|---|---|---|---|---|
| < 70 (N = 303) | ≥ 70 to < 80 (N = 337) | ≥ 80 (N = 370) | |||
| Age, median (IQR), y | 76 (68–82) | 64 (55–67) | 75 (73–77) | 85 (82–87) | < 0.0001 |
| Male sex, No. (%) | 603 (60%) | 226 (75%) | 219 (65%) | 158 (43%) | < 0.0001 |
| Hypertension, No. (%) | 584 (58%) | 140 (46%) | 204 (61%) | 240 (64%) | < 0.0001 |
| Diabetes mellitus, No. (%) | 193 (19%) | 63 (21%) | 71 (21%) | 69 (16%) | 0.15 |
| Atrial fibrillation, No. (%) | 533 (53%) | 119 (39%) | 182 (54%) | 232 (63%) | < 0.0001 |
| Pre-stroke mRS score ≥ 2, No. (%) | 897 (89%) | 291 (96%) | 310 (92%) | 296 (80%) | < 0.0001 |
| NIHSS score, median (IQR) | 18 (13–23) | 16 (12–22) | 18 (13–23) | 18 (14–23) | 0.0021 |
| Blood glucose, median (IQR), mg/mL (N = 984) | 128 (110–156) | 126 (111–156) | 129 (110–156) | 127 (110–154) | 0.98 |
| Systolic blood pressure, median (IQR), mmHg (N = 981) | 154 (135–170) | 150 (128–167) | 152 (136–170) | 160 (140–173) | < 0.0001 |
| Location of occlusion | |||||
| ICA, No. (%) | 346 (34%) | 89 (29%) | 123 (36%) | 134 (36%) | 0.101 |
| M1 segment MCA, No. (%) | 424 (42%) | 129 (43%) | 140 (42%) | 155 (42%) | 0.97 |
| M2 segment MCA, No. (%) | 177 (18%) | 62 (20%) | 50 (15%) | 65 (18%) | 0.17 |
| M3 segment MCA, No. (%) | 6 (1%) | 3 (1%) | 2 (1%) | 1 (0%) | 0.48 |
| A1 segment ACA, No. (%) | 3 (0%) | 0 (0%) | 2 (1%) | 1 (0%) | 0.38 |
| A2 segment ACA, No. (%) | 12 (1%) | 3 (1%) | 4 (1%) | 5 (1%) | 0.91 |
| BA, No. (%) | 177 (8%) | 27 (9%) | 29 (9%) | 21 (6%) | 0.21 |
| VA, No. (%) | 8 (1%) | 6 (2%) | 1 (0%) | 1 (0%) | 0.020 |
| P1 segment PCA, No. (%) | 5 (0%) | 3 (1%) | 0 (0%) | 1 (0%) | 0.044 |
| P2 segment PCA, No. (%) | 2 (0%) | 0 (0%) | 1 (0%) | 1 (0%) | 0.65 |
| IV-rtPA therapy, No. (%) | 482 (48%) | 158 (52%) | 163 (48%) | 161 (44%) | 0.080 |
| ASPECTS on CT, median (IQR) (N = 638) | 10 (7–10) | 10 (8–10) | 10 (8–10) | 10 (8–10) | 0.82 |
| ASPECTS on DWI, median (IQR) (N = 788) | 7 (6–8) | 7 (6–8) | 8 (6–9) | 7 (6–9) | 0.12 |
| pc-ASPECTS, median (IQR) (N = 80) | 7 (6–8) | 7 (6–8) | 7 (6–8) | 7 (6–8) | 0.98 |
| ASPECTSa ≥ 6, No. (%) | 843 (83%) | 250 (83%) | 27 (82%) | 317 (86%) | 0.35 |
| Onset-to-puncture time, median (IQR), minutes | 205 (135–355) | 205 (135–320) | 200 (135–345) | 215 (140–400) | 0.11 |
| Onset-to-reperfusion time, median (IQR), minutes | 270 (195–435) | 265 (185–385) | 260 (185–410) | 280 (205–475) | 0.060 |
ACA anterior cerebral artery, ASPECTS Alberta Stroke Program Early Computed Tomography Score, BA basilar artery, CT computed tomography, DWI diffusion-weighted imaging, ICA internal carotid artery, IV-rtPA intravenous recombinant tissue plasminogen activator, IQR interquartile range, MCA middle cerebral artery, mRS modified Rankin Scale, NIHSS National Institutes of Health Stroke Scale, PCA posterior cerebral artery, VA vertebral artery.
aASPECTS was derived from CT or DWI. If both CT and DWI were performed before endovascular therapy, ASPECTS on DWI was used for the analysis. In patients with stroke in the posterior circulation, posterior circulation ASPECTS on DWI was used for the analysis.
The clinical characteristics according to the ORT categories in each age subgroup are shown in Supplementary Tables S1–S3. The proportion of patients with atrial fibrillation and an NIHSS score were higher in the shorter ORT categories in patients aged ≤ 80 years. The proportion of patients with IV-rtPA use was lower in those with an ORT > 300 min. The proportion of patients with ASPECTS ≥ 6 in patients aged < 70 years and the proportion of patients with ICA or M1 segment occlusion in patients aged 70 to < 80 years was different among the ORT categories.
Primary outcomes
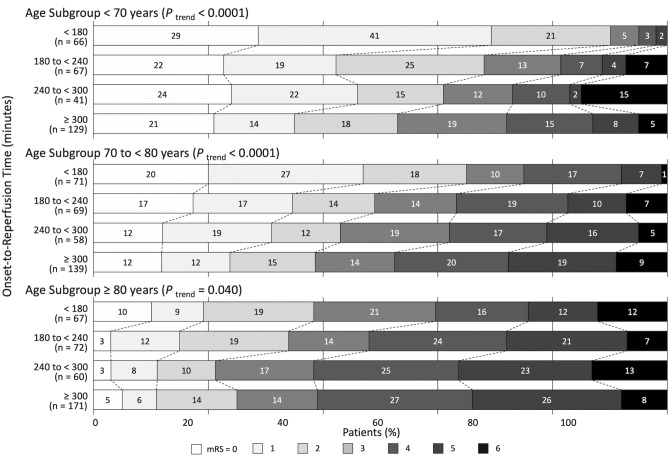
The shorter ORT categories were associated with better distributions of mRS scores at 90 days in each age subgroup (Fig. 2). The adjusted common odds ratios for better outcomes per 1-category delay in ORT were 0.66 (95% confidence interval [CI], 0.55–0.80) in patients aged < 70 years, 0.66 (95% CI 0.56–0.79) in patients aged 70 to < 80 years, and 0.83 (95% CI 0.70–0.98) in patients aged ≥ 80 years (Table 2). These adjusted common odds ratios were significantly different among age subgroups (interaction P = 0.031).
Figure 2.
Distributions of the modified Rankin Scale scores at 90 days according to onset-to-reperfusion time categories in each age subgroup. In each age subgroup, onset-to-reperfusion time was associated with better outcomes.
Table 2.
Adjusted odds ratios for outcomes according to ORT.
| Outcome | Subjects | Effect variable | Age subgroups | Adjusted valuesa (95% CI) | P for interaction |
|---|---|---|---|---|---|
| Primary outcome | |||||
| mRS score at 90 days | 1010 patients with mTICI scores ≥ 2b | Common odds ratios per 1-category delay in ORTb | < 70 years (N = 303) | 0.66 (0.55–0.80) | |
| 70 to < 80 years (N = 337) | 0.66 (0.56–0.79) | 0.031 | |||
| ≥ 80 years (N = 370) | 0.83 (0.70–0.98) | ||||
| Secondary outcomes | |||||
| mRS score ≤ 2 at 90 days | 897 patients with mTICI scores ≥ 2b and pre-stroke mRS score ≤ 2 | Odds ratios per 1-category delay in ORTb | < 70 years (N = 291) | 0.52 (0.39–0.68) | |
| 70 to < 80 years (N = 310) | 0.62 (0.49–0.78) | 0.016 | |||
| ≥ 80 years (N = 296) | 0.76 (0.60–0.96) | ||||
| Mortality at 90 days | 1010 patients with mTICI scores ≥ 2b | Odds ratios per 1-category delay in ORTb | < 70 years (N = 303) | 1.36 (0.82–2.26) | |
| 70 to < 80 years (N = 337) | 1.59 (1.01–2.50) | 0.054 | |||
| ≥ 80 years (N = 370) | 0.88 (0.63–1.22) | ||||
| Safety outcome | |||||
| sICH within 72 h | 1010 patients with mTICI scores ≥ 2b | Odds ratios per 1-category delay in ORTb | < 70 years (N = 303) | 0.98 (0.57–1.69) | |
| 70 to < 80 years (N = 337) | 1.21 (0.72–2.03) | 0.75 | |||
| ≥ 80 years (N = 370) | 0.90 (0.58–1.41) | ||||
CI confidence interval, mRS modified Rankin Scale, mTICI modified thrombolysis in cerebral infarction, ORT onset-to-reperfusion time, sICH symptomatic intracranial hemorrhage.
aAdjustment was made for the Alberta Stroke Program Early Computed Tomography Score (ASPECTS) (≥ 6 or < 6), National Institutes of Health Stroke Scale score, pre-stroke modified Rankin Scale score, use of intravenous recombinant tissue plasminogen activator, site of the main occlusions (anterior or posterior circulation), and vessels of the main occlusions (internal carotid artery and M1 segment of the middle cerebral artery occlusion or other arteries). ASPECTS was derived from computed tomography (CT) or magnetic resonance diffusion-weighted imaging (DWI). If both CT and DWI were performed before endovascular therapy, ASPECTS on DWI was used for the analysis. In patients with stroke in the posterior circulation, posterior circulation ASPECTS on DWI was used for the analysis.
bORT was divided into four categories: < 180, 180 to < 240, 240 to < 300, and ≥ 300 min.
Secondary outcomes
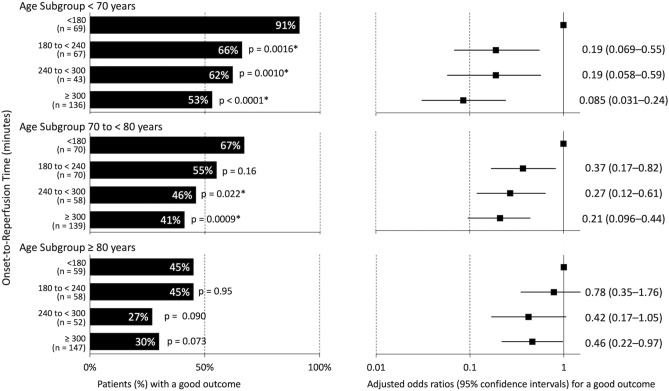
Of the 1010 patients with successful reperfusion, we analyzed 897 patients with pre-stroke mRS scores ≤ 2. In patients aged < 70 years and 70 to < 80 years, the proportion of good outcomes was higher in the shorter ORT categories (Fig. 3). The adjusted odds ratios for a good outcome per 1-category delay in ORT were 0.52 (95% CI 0.39–0.68) in patients aged < 70 years, 0.62 (95% CI 0.49–0.78) in patients aged 70 to < 80 years, and 0.76 (95% CI 0.60–0.96) in patients aged ≥ 80 years (Table 2). Using an ORT of < 180 min as a reference, the adjusted odds ratios for a good outcome in patients with an ORT of 180 to < 240, 240 to < 300, and ≥ 300 min were as follows: in patients aged ≤ 70 years, 0.19 (95% CI 0.069–0.55), 0.19 (95% CI 0.058–0.59), and 0.085 (95% CI 0.031–0.24); in patients aged 70 to < 80 years, 0.37 (95% CI 0.17–0.82), 0.27 (95% CI 0.12–0.61), and 0.21 (95% CI 0.096–0.44); and in patients aged ≥ 80 years, 0.78 (95% CI 0.35–1.76), 0.42 (95% CI 0.17–1.05), and 0.46 (95% CI 0.22–0.97), respectively (Fig. 3).
Figure 3.
Proportions of a good outcome and adjusted odds ratios for a good outcome according to onset-to-reperfusion time (ORT) categories in each age subgroup. The proportion of a good outcome (defined as mRS score ≤ 2) was lower in the delayed ORT categories than in those with ORT < 180 min in each age subgroup, although the association was marginal in patients aged ≥ 80 years.
Although a marginal association between delayed ORT and mortality was observed in patients aged 70 to < 80 years, no associations between ORT and mortality were found in the other age subgroups. The adjusted odds ratios for mortality per 1-category delay in ORT were 1.36 (95% CI 0.82–2.26) in patients aged < 70 years, 1.59 (95% CI 1.01–2.50) in patients aged 70 to < 80 years, and 0.88 (95% CI 0.63–1.22) in patients aged ≥ 80 years (Table 2). There was no heterogeneity with respect to the age subgroups (interaction P = 0.054). Differences in the mortality rate among the ORT categories were found in patients aged < 70 years, but not in patients aged 70 to < 80 or ≥ 80 years (Supplementary Table S4).
Safety outcomes
The adjusted odds ratios for sICH per 1-category delay in ORT were 0.98 (95% CI 0.57–1.69) in patients aged < 70 years, 1.21 (95% CI 0.72–2.03) in patients aged 70 to < 80 years, and 0.90 (95% CI 0.58–1.41) in patients aged ≥ 80 years. (Table 2). There was no heterogeneity with respect to age subgroups (interaction P = 0.75). No difference in the rate of sICH among the ORT categories was observed in any age subgroup (Supplementary Table S4).
Sensitivity analysis
After the imputation of missing data using the multiple imputation method, the adjusted common odds ratios for better outcomes per 1-category delay in ORT were 0.66 (95% CI 0.55–0.79) in patients aged < 70 years, 0.67 (95% CI 0.56–0.79) in patients aged 70 to < 80 years, and 0.79 (95% CI 0.67–0.93) in patients aged ≥ 80 years (Supplementary Table S5), which also showed a significant time–outcome relationship in all age subgroups. These adjusted common odds ratios were not different among the age subgroups (interaction P = 0.087).
Of the 1010 patients, we analyzed 700 patients with an OPT of ≤ 6 h. Obvious time–outcome relationships were observed across all age subgroups. The adjusted common odds ratios for better outcomes per 1-category delay in ORT were 0.64 (95% CI 0.52–0.79) in patients aged < 70 years, 0.62 (95% CI 0.51–0.75) in patients aged 70 to < 80 years, and 0.74 (95% CI 0.60–0.91) in patients aged ≥ 80 years (Supplementary Table S5). These adjusted common odds ratios were not different among the age subgroups (interaction P = 0.24). The adjusted common odds ratios for better outcomes per 30-min delay in ORT were 0.89 (95% CI 0.82–0.97) in patients aged < 70 years, 0.85 (95% CI 0.79–0.92) in patients aged 70 to < 80 years, and 0.89 (95% CI 0.82–0.96) in patients aged ≥ 80 years (Supplementary Table S5). These adjusted common odds ratios were not different among the age subgroups (interaction P = 0.90).
In patients who underwent both CT and DWI before endovascular therapy in patients with anterior circulation occlusion, we used ASPECTS on CT for sensitivity analysis instead of ASPECTS on DWI. The adjusted common odds ratios for better outcomes per 1-category delay in ORT were 0.64 (95% CI 0.54–0.78) in patients aged < 70 years, 0.66 (95% CI 0.56–0.79) in patients aged 70 to < 80 years, and 0.83 (95% CI 0.70–0.99) in patients aged ≥ 80 years (Supplementary Table S5). These adjusted common odds ratios were significantly different among the age subgroups (interaction P = 0.015).
Discussion
In this current post-hoc analysis of a large clinical registry of patients with acute cerebral large-vessel occlusion, earlier reperfusion was associated with better outcomes across all age subgroups. To the best of our knowledge, this is the first report to show the time–outcome relationship in elderly patients using a large clinical registry. Our findings confirmed that achieving early successful reperfusion is important in patients of all ages.
Previous studies have investigated the association between ORT and outcomes in different age subgroups; however, the results in the elderly subgroup differed between studies13,14. One of two studies showed that ORT was independently associated with a favorable outcome at 90 days in 34 patients aged ≥ 80 years13, whereas the other study did not find an association between ORT and a good functional outcome in 78 patients aged > 80 years with mTICI of 2b or 314. In elderly patients, because of the severe stroke outcome11, it is difficult to show a small difference according to ORT. Given the retrospective nature of the study, an age-related selection bias for endovascular therapy may have influenced the difficulty in demonstrating the differences. The present study is important in that it showed the time–outcome relationship in patients of all ages, although the relationship was small and partial in elderly patients. Logistic regression models for primary outcome (mRS at 90 days) and good outcome (mRS ≤ 2 at 90 days) showed heterogeneity among age subgroups; however, the time–outcome relationship existed across all age subgroups (Table 2). In patients aged ≥ 80 years, the proportion of good outcomes according to ORT categories was not significantly different, but was significantly smaller in patients with ORT ≥ 300 min after adjustment for confounders (Fig. 3). In patients with OPT of 6 h or less, shorter time to reperfusion was consistently associated with better outcomes across all age subgroups without heterogeneity (Supplementary Table S5). Thus, a shorter time to reperfusion is important for all ages.
We assessed the effects of early reperfusion in consecutive patients who were hospitalized within 24 h of the onset of acute large-vessel occlusion. However, randomized clinical trials using imaging-based selection criteria showed that there was no time–outcome relationship in patients with late hospital arrival23,24. Furthermore, a large database showed that the time–outcome relationships were stronger in patients with an OPT of ≤ 270 min than in those with an OPT of > 270 min10. Based on these previous reports, we set ORT categories with thresholds of 180, 240, and 300 min, and performed a sensitivity analysis in patients with an OPT of ≤ 6 h. Consequently, we focused on the early time window and observed consistent time–outcome relationships across all ages. However, we did not intend to determine the time limit for endovascular therapy. Our registry previously revealed that endovascular reperfusion therapy is effective, regardless of the onset-to-door time15.
Although a marginal association of early reperfusion with survival was observed in patients aged 70 to < 80 years, no associations were noted between ORT and mortality in the other age subgroups. In addition, no association between ORT and sICH was found in any age subgroup. Previous reports from the meta-analysis of the HERMES collaborators and a large practical database documented that both mortality and sICH were not associated with the onset-to-randomization time or OPT1,12, which is consistent with our current results. However, in patients aged ≥ 80 years, the odds ratios for mortality and sICH tended to be low. This may also be due to an age-related selection bias for endovascular therapy.
The clinical background characteristics in the present study were not different from those in previous reports. Male sex13,14, hypertension13, cardiogenic embolism14, and high NIHSS score14 have been reported to be associated with elderly patients undergoing endovascular thrombectomy. Early hospital arrival was reported to be associated with atrial fibrillation, a high NIHSS score, and ambulance use in ischemic stroke patients28. This may be because atrial fibrillation is associated with stroke severity29. In our cohort, the proportion of patients with ASPECTS ≥ 6 in patients aged < 70 years and the proportion of those with ICA or M1 segment occlusion in patients aged of 70 to < 80 years was different among the ORT categories, although there were no trends according to ORT.
Several limitations of the current study should be noted. First, this was an observational study; thus, the selection of endovascular therapy depended on the practicing physician. Moreover, patients with unsuccessful reperfusion were excluded from the analysis. Furthermore, 8% of patients were excluded from the analysis owing to missing data. These factors may have led to a potential selection bias, although we conducted sensitivity analyses. Second, although we systematically registered stroke patients with acute large-vessel occlusion over a 2-year period at 46 centers, the sample size was not large enough to fully evaluate the time–outcome effect in each age subgroup. Third, the imaging methods were not standardized and perfusion imaging was not analyzed in this study. We used two types of ASPECTS: ASPECTS on CT, an ASPECTS on DWI in patients with anterior circulation occlusion. Although we conducted a sensitivity analysis, this was not sufficient to adjust for the impact of imaging on the outcome. Therefore, a study with modern standardized imaging methods is needed to evaluate the impact of time to reperfusion. Fourth, the assessments of the mRS scores could be biased, as acute treatment was not completely masked although the assessments were performed by independent physicians. Fifth, the assessments of the mTICI scores could be biased. A previous report revealed a discrepancy in the assessment of the mTICI score between local operators and independent core laboratories30. This may have led to a selection bias.
Conclusions
Our large prospective registry of acute large-vessel occlusion revealed that early reperfusion was associated with better outcomes across all age subgroups. Achieving early successful reperfusion is important in patients of all ages, even in octogenarians.
Supplementary information
Acknowledgements
We thank all investigators involved in RESCUE (Recovery by Endovascular Salvage for Cerebral Ultra-Acute Embolism)-Japan Registry 2. This study was supported in part by the Japan Agency for Medical Research and Development; the Japanese Society for Neuroendovascular Therapy; the Ministry of Health, Labor and Welfare of Japan; Medtronic; Stryker; and Medicos Hirata. The funding sources did not participate in any part of the study, from study conception to article preparation.
Author contributions
Ke.T., S.Y., K.U. and M.T. designed and conceptualized the study. Ke.T. analyzed the data and drafted the manuscript for intellectual content. S.Y., K.U., H.Y., N.S. and T.M. had major roles in the acquisition of data. Ke.T., S.Y., K.U., H.Y., N.S., H.K., H.M., M.E., Y.O., KazuoK, KazumiK, Mak.S., N.T., Ka.T., E.F., Y.M., K.M., T.K., S.O., T.S., Man.S, M.T., T.N., H.N., and T.M. revised the manuscript for intellectual content.
Data availability
The data, analytic methods, and study materials will not be made available to other researchers for the purpose of reproducing the results or replicating the procedure.
Competing interests
Dr. Todo reports lecturer fees from Medtronic and Stryker. Dr. Yoshimura reports research grants from Medico’s Hirata, Medtronic, and Termo and lecturer fees from Medtronic, Kaneka, and Stryker. Dr. Yamagami reports research grants from Bristol-Myers Squibb; lecturer fees from Stryker, Terumo, Medtronic, Medico’s Hirata, Johnson and Johnson, Bayer, Daiichi-Sankyo, Bristol-Myers Squibb, Boehringer Ingelheim, Takeda, and Otsuka Pharmaceutical; and membership in the advisory boards of Daiichi-Sankyo and Biomedical Solutions. Dr. Sakai reports a research grant from Termo, lecturer fees from Jimro, Johnson & Johnson, Medico’s Hirata, Medtronic, and Stryker; and membership in the advisory boards of Jimro and Medtronic. Dr. Nakamura reports lecturer fees from Johnson & Johnson, Medtronic, and Stryker. Dr. Kimura reports lecturer fees from Medtronic. Dr. Matsumaru discloses lecturer fees from Medtronic, Stryker, Terumo, Johnson & Johnson, Kaneka, and Jimro. Dr. Minematsu reports lecturer fees from Stryker and membership in the advisory board of Medico’s Hirata. The other authors report no conflicts related to the subject matter of the article.
Footnotes
The original online version of this Article was revised: The original version of this Article contained errors in Figure 3, where the x-axis label for the graph showing “Patients (%) with a good outcome” was obstructed by an image element.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Change history
9/8/2021
A Correction to this paper has been published: 10.1038/s41598-021-97242-2
Contributor Information
Kenichi Todo, Email: ktodo@neurol.med.osaka-u.ac.jp.
RESCUE-Japan Registry 2 Investigators:
Kazunori Toyoda, Masataka Takeuchi, Masafumi Morimoto, Toshiyuki Onda, Masunari Shibata, Shinichi Yoshimura, Nobuyuki Sakai, Takahiro Ohta, Keisuke Imai, Ryo Itabashi, Masayuki Ezura, Taro Yamashita, Norihito Fukawa, Naoto Kimura, Ryosuke Doijiri, Hajime Ohta, Yukiko Enomoto, Chisaku Kanbayashi, Ikuya Yamaura, Hideyuki Ishihara, Yuki Kamiya, Makoto Hayase, Kouhei Nii, Junya Kobayashi, Hiroaki Yasuda, Ryushi Kondo, Daisuke Yamamoto, Manabu Sakaguchi, Junichiro Satomi, Yoshiki Yagita, Akira Handa, Atsushi Shindo, Nagayasu Hiyama, Naoki Toma, Tomoyuki Tsumoto, Kazumi Kimura, Wataro Tsuruta, Keigo Matsumoto, Yoshihiro Kiura, Takaaki Yamazaki, Taketo Hatano, Yoshihisa Matsumoto, Takao Kojima, Norio Ikeda, Shigeyuki Sakamoto, Hiroyuki Ohnishi, Koichi Haraguchi, and Naoyuki Uchiyama
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92100-7.
References
- 1.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, HERMES collaborators et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 2.Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- 3.Mocco J, Zaidat OO, von Kummer R, Yoo AJ, Gupta R, Lopes D, THERAPY Trial Investigators et al. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016;47:2331–2338. doi: 10.1161/STROKEAHA.116.013372. [DOI] [PubMed] [Google Scholar]
- 4.Muir KW, Ford GA, Messow CM, Ford I, Murray A, Clifton A, PISTE Investigators et al. Endovascular therapy for acute ischaemic stroke: the pragmatic ischaemic stroke thrombectomy evaluation (PISTE) randomised, controlled trial. J. Neurol. Neurosurg. Psychiatry. 2017;88:38–44. doi: 10.1136/jnnp-2016-314117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, DAWN Trial Investigators et al. Thrombectomy 6 to 24 h after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, DEFUSE 3 Investigators et al. Thrombectomy for stroke at 6 to 16 h with selection by perfusion imaging. N. Engl. J. Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mueller-Kronast NH, Zaidat OO, Froehler MT, Jahan R, Aziz-Sultan MA, Klucznik RP, STRATIS Investigators et al. Systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke: primary results of the STRATIS registry. Stroke. 2017;48:2760–2768. doi: 10.1161/STROKEAHA.117.016456. [DOI] [PubMed] [Google Scholar]
- 8.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, HERMES Collaborators et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316:1279–1288. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 9.Sheth SA, Jahan R, Gralla J, Pereira VM, Nogueira RG, Levy EI, SWIFT-STAR Trialists et al. Time to endovascular reperfusion and degree of disability in acute stroke. Ann. Neurol. 2015;78:584–593. doi: 10.1002/ana.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahan R, Saver JL, Schwamm LH, Fonarow GC, Liang L, Matsouaka RA, et al. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA. 2019;322:252–263. doi: 10.1001/jama.2019.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonarow GC, Reeves MJ, Zhao X, Olson DM, Smith EE, Saver JL, et al. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation. 2010;121:879–891. doi: 10.1161/CIRCULATIONAHA.109.892497. [DOI] [PubMed] [Google Scholar]
- 12.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, American Heart Association Stroke Council et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 13.Son S, Kang DH, Hwang YH, Kim YS, Kim YW. Efficacy, safety, and clinical outcome of modern mechanical thrombectomy in elderly patients with acute ischemic stroke. Acta Neurochir. (Wien) 2017;159:1663–1669. doi: 10.1007/s00701-017-3269-y. [DOI] [PubMed] [Google Scholar]
- 14.Koizumi S, Ota T, Shigeta K, Amano T, Ueda M, Matsumaru Y, et al. Onset to reperfusion time was not important in mechanical thrombectomy for elderly patients: a retrospective multicenter study in Tama area, Tokyo. Cerebrovasc. Dis. 2018;46:89–96. doi: 10.1159/000492867. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura S, Sakai N, Uchida K, Yamagami H, Ezura M, Okada Y, et al. Endovascular therapy in ischemic stroke with acute large-vessel occlusion: recovery by endovascular salvage for cerebral ultra-acute embolism Japan registry 2. J. Am. Heart Assoc. 2018;7:e008796. doi: 10.1161/JAHA.118.008796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/S0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 17.Barber PA, Hill MD, Eliasziw M, Demchuk AM, Pexman JH, Hudon ME, ASPECTS Study Group et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76:1528–1533. doi: 10.1136/jnnp.2004.059261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tei H, Uchiyama S, Usui T, Ohara K. Posterior circulation ASPECTS on diffusion-weighted MRI can be a powerful marker for predicting functional outcome. J. Neurol. 2010;257:767–773. doi: 10.1007/s00415-009-5406-x. [DOI] [PubMed] [Google Scholar]
- 19.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.STR.19.5.604. [DOI] [PubMed] [Google Scholar]
- 20.Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994;25:2220–2226. doi: 10.1161/01.STR.25.11.2220. [DOI] [PubMed] [Google Scholar]
- 21.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, SITS-MOST investigators et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 23.Lansberg MG, Mlynash M, Hamilton S, Yeatts SD, Christensen S, Kemp S, DEFUSE 3 Investigators et al. Association of thrombectomy with stroke outcomes among patient subgroups: secondary analyses of the DEFUSE 3 randomized clinical trial. JAMA Neurol. 2019;76:447–453. doi: 10.1001/jamaneurol.2018.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tekle WG, Hassan AE, Jadhav AP, Haussen DC, Budzik RF, Bonafe A, DAWN Trial Investigators et al. Impact of periprocedural and technical factors and patient characteristics on revascularization and outcome in the DAWN trial. Stroke. 2020;51:247–253. doi: 10.1161/STROKEAHA.119.026437. [DOI] [PubMed] [Google Scholar]
- 25.Kaesmacher J, Chaloulos-Iakovidis P, Panos L, Mordasini P, Michel P, Hajdu SD, et al. Mechanical thrombectomy in ischemic stroke patients with alberta stroke program early computed tomography score 0–5. Stroke. 2019;50:880–888. doi: 10.1161/STROKEAHA.118.023465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo G, Mo D, Tong X, Liebeskind DS, Song L, Ma N, et al. Factors associated with 90-day outcomes of patients with acute posterior circulation stroke treated by mechanical thrombectomy. World Neurosurg. 2018;109:e318–e328. doi: 10.1016/j.wneu.2017.09.171. [DOI] [PubMed] [Google Scholar]
- 27.Package 'rms'. https://cran.r-project.org/web/packages/rms/rms.pdf. Accessed 27 May 2016.
- 28.Matsuo R, Yamaguchi Y, Matsushita T, Hata J, Kiyuna F, Fukuda K, Fukuoka Stroke Registry Investigators et al. Association between onset-to-door time and clinical outcomes after ischemic stroke. Stroke. 2017;48:3049–3056. doi: 10.1161/STROKEAHA.117.018132. [DOI] [PubMed] [Google Scholar]
- 29.Kimura K, Minematsu K, Yamaguchi T, Japan Multicenter Stroke Investigators’ Collaboration (J-MUSIC) Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry. 2005;76:679–683. doi: 10.1136/jnnp.2004.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang G, Treurniet KM, Jansen IGH, Emmer BJ, van den Berg R, Marquering HA, MR CLEAN Registry et al. Operator versus core lab adjudication of reperfusion after endovascular treatment of acute ischemic stroke. Stroke. 2018;49:2376–2382. doi: 10.1161/STROKEAHA.118.022031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, analytic methods, and study materials will not be made available to other researchers for the purpose of reproducing the results or replicating the procedure.