Abstract
The uterus is the organ for embryo implantation and fetal development. Most current models of the uterus are centred around capturing its function during later stages of pregnancy to increase the survival in pre-term births. However, in vitro models focusing on the uterine tissue itself would allow modelling of pathologies including endometriosis and uterine cancers, and open new avenues to investigate embryo implantation and human development. Motivated by these key questions, we discuss how stem cell-based uteri may be engineered from constituent cell parts, either as advanced self-organising cultures, or by controlled assembly through microfluidic and print-based technologies.
Subject terms: Developmental biology, Embryogenesis
Bergmann et al. discuss the construction of synthetic uteri to model the earliest stages of human embryogenesis and associated pathologies. They highlight the constituent components from which a synthetic uterus may be engineered, propose a modular approach to assembling synthetic uteri and discuss how these technologies can shed light on implantation failure and uterine pathologies.
Introduction
The primary function of the uterus is to provide a suitable environment for the embryo to implant and gestate to full term. Recent single-cell transcriptional atlases of the uterus during the menstrual cycle1,2 and of the maternal–foetal interface during the first trimester of pregnancy in human3,4, provide comprehensive roadmaps to drive forward the development of stem cell-based models. The goal of such stem cell-based uterus models is to engineer a defined, flexible, and scalable system to address fundamental questions of reproductive biology. Major topics include mechanisms and pathologies of embryo implantation, embryogenesis, crosstalk between the developing embryo and the mother, and pathologies of the female reproductive tract. The first trimester of pregnancy is a particularly dynamic and critical stage when the embryo organises the body plan before the foetal growth phase in the second and third trimesters. Although complex in vitro models capable of complete recapitulation of the structure and function of the uterus may be necessary for a clinical setting to increase survival chances of premature fetuses5, such models would lack the scalability and experimental flexibility required for drug discovery and genetic screening.
In this review, we discuss the construction of stem cell-based uterus models to illuminate the earliest stages of human embryogenesis and associated pathologies. We outline the developmental origin of the uterus and consider its anatomy, function, and diseases. Building on recent breakthroughs in organoid culture, we highlight the constituent components from which a stem cell-based uterus may be engineered and propose a modular approach to assembling uterine models. We discuss approaches based on self-organisation as well as controlled assembly, either through microfluidic or print-based methodologies and conclude how these technologies can be used to tackle questions about implantation failure and key pathologies of the uterus.
Developmental origin of the uterus
In mammals, the uterus originates from the intermediate mesoderm which is positioned between the paraxial and lateral plate mesoderm after gastrulation6 (Fig. 1, week 3). At Carnegie stage (CS)10, the embryo folds to form the intraembryonic coeloms which are lined by lateral plate mesoderm and intermediate mesoderm, wherein the inner lining of the coeloms is the coelomic epithelium7 (Fig. 1, week 5). A subset of intermediate mesoderm cells undergoes mesenchymal-to-epithelial transition to form the nephric duct along the body. This transition requires Pax2 and Pax8, which induce expression of Lhx1 (Lim1)8, an important transcription factor of the urogenital system in both mouse and human9,10. The nephric ducts are required for the development of adult kidneys, the ureter, and the genital tract. The initially central portion of the nephric duct is known as the Wolffian (mesonephric) ducts.
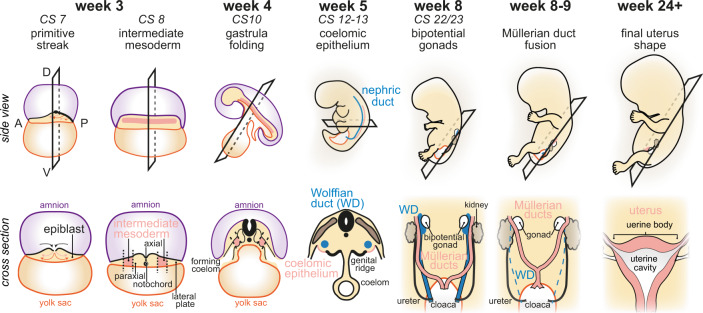
Fig. 1. Developmental origin of the uterus.
The development of the human uterus is shown in situ (top panel) and cross-sections (bottom panel). Gastrulation and primitive streak formation occur in Carnegie stage (CS)7 embryonic disc 3 weeks post-fertilisation, leading to the formation of intermediate mesoderm at CS 8. At CS10, the embryo has folded and established the intraembryonic coeloms. The nephric ducts develop from the mesodermal coelomic epithelium between CS12 and CS13. The central parts constitute the Wolffian ducts (WD). Concomitantly, the Müllerian ducts invaginate from the coelomic epithelium adjacent to the nephric duct and elongate towards the cloaca. By CS22–23, the bipotential gonads are fully established in proximity to the kidneys. Both are connected to the WDs and Müllerian ducts, ultimately leading to the cloaca. In female fetuses of around week 8–9, the WDs degenerate, and the Müllerian ducts fuse to form the uterine body and the upper vaginal tract. The uterus acquires its final shape at around 24 weeks post-fertilisation.
Morphogenetic rearrangements at CS12–16 lead to the insertion of the Wolffian ducts into the cloaca, the precursor of the bladder11,12. Intermediate mesoderm‑derived coelomic epithelial cells invaginate to form the Müllerian (paramesonephric) ducts at CS14–17, which elongate caudally along the Wolffian ducts at CS18–23. In mouse, the Müllerian and Wolffian ducts express Lhx1 and Wnt signalling is required for Wolffian duct elongation13,14. Histological studies of human embryos suggest that this morphogenetic process is conserved11,15. At CS23, Wolffian and Müllerian ducts together form the bipotential genital tract16. In males, the Müllerian ducts degenerate and the Wolffian ducts persist to form male reproductive organs. In females, it is the Müllerian ducts that develop into the female reproductive tract while the Wolffian ducts degenerate6. In either case, sex determination is controlled by gene expression from the X and Y chromosomes17,18.
Development of the uterus at week 8–9 commences with the fusion of the Müllerian ducts, which will undergo morphogenesis to form the uterus, fallopian tubes, cervix, and upper vaginal tract. Müllerian duct fusion in humans results in one central uterine cavity, in contrast to rodents, where Müllerian duct fusion is less extensive to allow the formation of two separated uterine horns11,16. At week 16, the developing human uterus starts to form glands in the endometrium. Endometrial glands gradually increase complexity and develop branches within the stroma until birth and will continue to develop postnatally until puberty11. This contrasts with mouse development where glands are formed exclusively after birth19,20. Nevertheless, both human and mouse endometrial gland formation requires WNT signalling21,22. Prenatal uterine development concludes with the formation of myometrium at week 22 and the uterus assumes its adult shape23. The final steps of human uterus development occur during puberty when the uterus further matures under the influence of sex steroid stimulation and initiates the menstrual cycle.
Anatomy and function of the uterus
The uterus is located within the pelvic area, between the bladder and the rectum. In humans, it is on average about 7 cm long and weighs around 60 g in the non-pregnant state (non-gravid), extending to up to five-fold in size during pregnancy (gravid). The uterine body contains a triangular uterine cavity with the isthmus leading to the cervix, which connects to the vaginal opening (Fig. 2a). The uterus is supported by uterine ligaments which, together with the ovaries and fallopian tubes, form the appendages of the uterus. Externally, the uterus consists of thick, smooth muscle bundles, the myometrium, and is covered by serous tissue termed perimetrium (Fig. 2b).
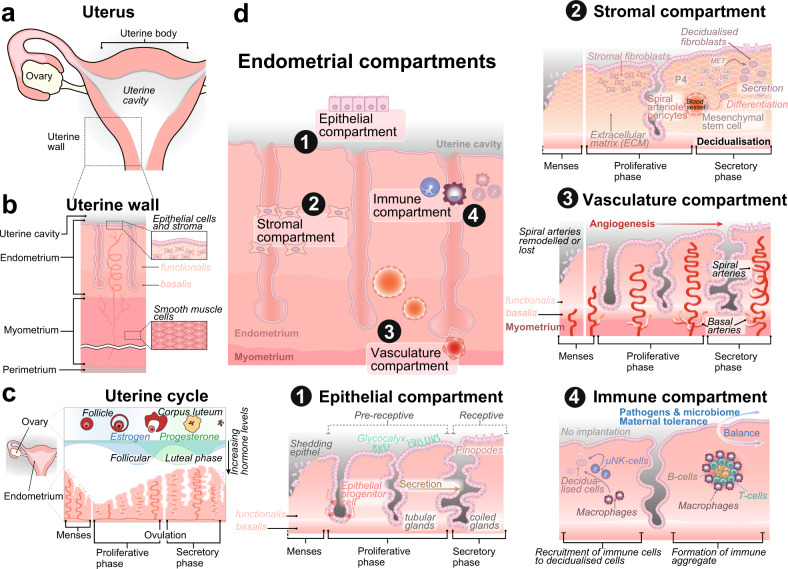
Fig. 2. Anatomical structure of the uterus.
a The cavity of the human uterine body connects to the ovaries by the fallopian tubes. b The uterine wall consists of the endometrium, the smooth muscular myometrial layer, and the outermost perimetrium. The endometrial epithelium forms the border to the cavity (luminal epithelium) and deep-reaching endometrial glands (glandular epithelium) which are embedded in the stroma. Only the endometrial functionalis layer is shed during menstruation at the beginning of the human uterine cycle (c), while the non-hormone-responsive basalis layer remains intact. Oestrogen produced by the developing follicle in the ovaries induces the growth and proliferation of endometrial tissue during the proliferative (follicular) phase. Ovulation and release of the egg from the ovaries occur mid-cycle, which leads to increased progesterone levels during the secretory (luteal) phase. Degradation of the corpus luteum and decreasing hormone levels induce menses and re-start the uterine cycle if no embryo implants into the uterine wall. d The endometrium can be functionally distinguished into four compartments, which change throughout the uterine cycle. The luminal epithelium of the epithelial compartment (1) is shed during menses and rebuilt by differentiating epithelial progenitor cells migrating from uterine glands. The luminal epithelium becomes pre-receptive but avoids pre-mature attachment of an embryo by the formation of a highly charged glycocalyx layer. Uterine glands grow in size and increase secretion towards the secretory phase while changing shape to become coiled. The luminal epithelium grows apical cellular protrusions (pinopodes), which increases receptivity and allows embryo attachment and implantation. Decidualisation occurs during the secretory phase in the stromal compartment (2) and assists in the preparation of the endometrium for implantation. Mesenchymal stem cells surrounding spiral arteriole (specialised uterine blood vessels) pericytes differentiate to become secretory. Concomitantly, stromal fibroblasts undergo mesenchymal-to-epithelial transition (MET) under the influence of progesterone (P4) and convert to decidualised, secretory fibroblasts. Spiral arteries in the vascular compartment (3) branch from basal arteries, which supply the endometrial basalis layer during menses, when arteries of the functionalis layer are extensively remodelled or lost. Angiogenesis drives the formation of spiral arteries towards the secretory phase to increase the blood supply of the upper endometrium in preparation for embryo implantation. The uterine immune compartment (4) is a highly flexible system. Immune aggregates consisting of B-cells, T-cells and macrophages form and reside in the upper parts of the functional layer, maintaining the balance between defence against pathogens and external threats and sustaining the protective microbiome barrier necessary for uterine health. During pregnancy, the immune compartment provides maternal tolerance to an embryo which induces attachment to the uterine wall. If no successful implantation occurs, immune cells such as uterine natural killer (uNK) cells and macrophages are recruited to remove decidualised cells.
The endometrium is the inner mucosal layer, which surrounds the uterine cavity and is comprised of stroma and uterine glands. The endometrium changes cyclically in terms of function and appearance during the menstrual cycle (Fig. 2c). Apes, Old World monkeys, and some New World monkeys undergo a menstrual cycle characterised by external bleeding due to shedding of the outermost layer (menses)24. Most other mammalian species experience an oestrous cycle, in which the uterus undergoes remodelling throughout the cycle without shedding25. During human menstruation, only the endometrial functionalis layer facing the uterine cavity is shed while the basalis layer situated towards the myometrium remains unaffected by hormonal changes (Fig. 2b)26–28. The human menstrual cycle lasts on average 28 days and is orchestrated by steroidal hormones, including oestrogen and progesterone (Fig. 2c). Following menses, oestrogen is secreted by the ovaries, stimulating endometrial proliferation, which rebuilds the shed surface layer in the proliferative, or follicular, phase. The egg is released around day 14 of the cycle during ovulation. Progesterone secreted in the ovaries from the remnants of the follicle which contained the released egg, the corpus luteum, stimulates the thickening of the uterine lining and initiates the secretory, or luteal, phase. The progesterone signals from the corpus luteum induce stromal cells to undergo a transformation, termed decidualisation, in preparation for embryo implantation. In case of successful fertilisation, foetal trophoblast and maternal corpus luteum cooperatively sustain hormone levels to maintain the pregnancy, otherwise, with progesterone levels declining, the outermost endometrial functionalis layer is lost as a result of menstruation26–28.
The functionalis and basalis layers of the endometrium are composed of four tissue compartments (Fig. 2d):
Epithelial compartment: The luminal epithelium establishes the boundary to the uterine cavity and is remodelled during the menstrual cycle to become receptive for the implanting embryo. Uterine glands consist of connected glandular epithelium, which extends towards the myometrial border and cyclically change shape from tubular, in the proliferative phase, to coiled, in the secretory phase. The luminal epithelium is lost during menstruation, and the stroma is exposed to the uterine cavity. This leads to the transformation of glandular epithelial cells, which migrate towards the cavity and rebuild the luminal epithelium26.
Stromal compartment: The endometrial stroma is a connective tissue consisting of fibroblasts and extracellular matrix. In the mid-secretory phase, fibroblast-like stromal cells differentiate into rounded epithelioid-like cells in response to progesterone, a process termed decidualisation. Human decidualisation begins when stromal cells surrounding spiral arterioles in the upper two-thirds of the endometrium enlarge 6 days after ovulation in preparation for a potential pregnancy.
Vasculature compartment: Basal arteries branching from the uterine radial arteries supply the basalis layer and produce a capillary bed. This supports the functionalis layer viability during menstruation29, when its spiral arteries are extensively remodelled and partially lost. After menstruation, spiral arteries are rebuilt by angiogenesis. Progesterone levels control the blood flow, which increases in the secretory phase, and decreases towards the onset of menstruation. In the absence of progesterone, the blood supply to the spiral arteries stops, causing the stroma to become necrotic and ultimately leads to the onset of menstruation.
Immune compartment: Accounting for an estimated 10–15% of the stromal compartment, the endometrial immune compartment provides protection against pathogens and balances the commensal microbiome30,31. Importantly, it adopts an immunosuppressive state during embryo implantation and acquires maternal tolerance in reaction to hormonal changes32. The composition of the immune compartment changes significantly during the menstrual cycle33, including a substantial increase in uterine natural killer cells following ovulation, which represent the dominant uterine leucocytes during pregnancy.
Pregnancy is established when a competent blastocyst implants into the receptive endometrium. Embryo implantation begins with adplantation and apposition of the blastocyst at the site of implantation, allowing trophoblast cells to attach (Fig. 3a). Multinucleated syncytiotrophoblast penetrates the luminal epithelium of the endometrium, crosses the basement membrane, and invades the stromal compartment. In human and Great Apes, the conceptus burrows into the endometrium, resulting in an interstitial implantation, while in Old World and New World monkeys, the embryo remains within the uterine cavity and undergoes superficial implantation34,35 (Fig. 3b). After the initial stages of embryo implantation, human cytotrophoblast and differentiated extravillous trophoblast further invade and induce remodelling of the spiral arteries and maternal tissues. Decidualisation plays an important role in mediating the invasiveness of the trophoblast cells and subsequent placentation via the secretion of chemoattractants. Whilst in humans, decidualisation occurs naturally during the menstrual cycle and develops further following implantation, in Old World and New World monkeys decidualisation is initiated post-implantation and may be less extensive, or proceed more gradually34,35. In Old World monkeys, following initial attachment, but prior to the invasion, proliferation and hypertrophy of the epithelium occur at the periphery of the invading syncytiotrophoblast. The endometrial epithelium adjacent to the implantation site remodels its glandular appearance and undergoes a transformation into epithelial plaques35,36. Epithelial plaque formation does not occur in humans or Great Apes but has been observed in New World monkeys, including the marmoset34,37. The mechanisms controlling the different degrees of trophoblast invasion in monkeys and apes remain an intriguing open question and are highly relevant for pathologies affecting placental invasion.
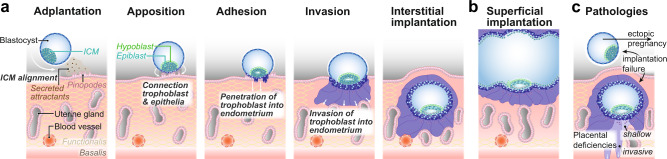
Fig. 3. Embryo development within the uterus and uterine pathologies.
a The stages leading to human interstitial embryo implantation and marmoset superficial implantation (b) are shown from left to right. The blastocyst arrives in the uterine cavity and hatches from the surrounding zone pellucida by day 5 post-fertilisation. It then moves along the uterine epithelium until it reaches a receptive environment provided by pinopodes before the inner cell mass (ICM) aligns towards the uterine epithelium (adplantation). The outer extraembryonal trophoblast cells of the blastocyst connect to the uterine cells (apposition) and penetrate through the luminal epithelium (adhesion). Concomitantly, the ICM differentiates into extraembryonal hypoblast and embryonal epiblast, which induce lumenogenesis. Trophoblast comprises of several layers of proliferative cytotrophblast (dark blue) and terminally differentiated, multinucleated syncytiotrophoblast (purple) which continue invasion into the endometrium towards nutrition-providing uterine glands and blood vessels, by degrading the extracellular matrix of the decidualised stroma. The human embryo is fully embedded in maternal endometrium following implantation. In contrast, the embryo is not fully engulfed by endometrium in superficial implantation (b) but stays within the uterine cavity. Common pathologies (c) associated with early stages of pregnancy are the failure of an embryo to implant into the uterine cavity wall, or ectopic pregnancy, when the embryo does not reach the uterine cavity for implantation, and instead implants mostly within the uterine tube. d Abnormal invasiveness causes placental deficiencies leading to placenta accreta spectrum disorders, caused by excessive trophoblast invasion, or pre-eclampsia, as a result of insufficient trophoblast invasion.
Pathologies of the gravid and non-gravid uterus
Human embryo implantation is an inherently inefficient process. Only around 25% of blastocysts implant into the uterus, representing a major hurdle to natural conception38–40 and rate-limiting step in assisted reproductive technologies41,42. In 2% of pregnancies, the embryo implants outside of the endometrium, often into the fallopian tubes, resulting in an ectopic pregnancy43. This can be life-threatening for the mother if left untreated and requires termination of the pregnancy. In about 2–8% of pregnancies, the trophoblast fails to invade the uterus properly, leading to pre-eclampsia44,45, which is characterised by high maternal blood pressure. Pre-eclampsia can result in seizures and, consequently is one of the leading causes of maternal morbidity and mortality worldwide with severe risks for both mother and the unborn child46. In 1 in 533 births47, excessive trophoblast invasion into deep maternal uterine tissues impairs separation of the placenta from the uterine wall following delivery48 (Fig. 3c). These placenta accreta spectrum disorders cause major obstetric maternal haemorrhage and sometimes require an emergency hysterectomy (removal of the uterus. Placenta accreta spectrum disorders remain a major cause of maternal death, with a mortality rate of up to 7%49. Antenatal diagnosis is limited to ultrasound methods and MRI, resulting in up to two-thirds of cases remaining undiagnosed until delivery. These pathologies are responsible for only a fraction of all pregnancy loss, and it is estimated that up to 1 in 4 pregnancies will result in a miscarriage40–42. Advanced in vitro models of the uterus should provide a strong basis for investigating the mechanisms of maternal–embryo interactions, and subsequent pregnancy loss.
Pathologies of the non-pregnant uterus include conditions relating to menstruation (dysmenorrhoea, menorrhagia), musculature (uterine prolapse), and genetic malformations (uterine septum, bicornuate uterus). There are around 9400 cases of endometrial cancers each year in the UK, with a 92% 5-year survival rate when diagnosed at the earliest stage compared to 15% when diagnosed at stage four50. The most common treatment for endometrial cancers is hysterectomy, potentially combined with radiotherapy or chemotherapy. Hysterectomy may also be necessary for other forms of cancers, including ovarian and cervical cancers, and gynaecological sarcoma. Endometriosis is a chronic condition affecting 10% of the female population51. It occurs when cells of the endometrium grow outside of the uterus52, most commonly in the ovaries and fallopian tubes. These cells remain hormone-responsive during the menstrual cycle, resulting in inflammation of the surrounding regions and eventual formation of scar tissues. Although there are strong heritability53,54, the risk factors and mechanisms remain unclear. There is currently no cure and treatment is limited to surgery, hormone treatment, and pain relief. Stem cell-based disease models have the potential to increase our mechanistic understanding of these pathologies, and thus advance clinical and regenerative treatments.
Building blocks for a stem cell-based uterus
The assembly of a stem cell-based uterus requires developmentally authentic cell lines as constituent parts (Fig. 4). Previous in vitro models of endometrium rely on cancer-derived cell lines55 which are likely to be compromised due to their carcinogenic origin. Consequently, we suggest that the construction of stem cell-based uteri should be based on either pluripotent stem cells (PSCs) or patient-specific primary cultures, depending on the research objective.
Fig. 4. The cellular building blocks of a stem-cell-based uterus.
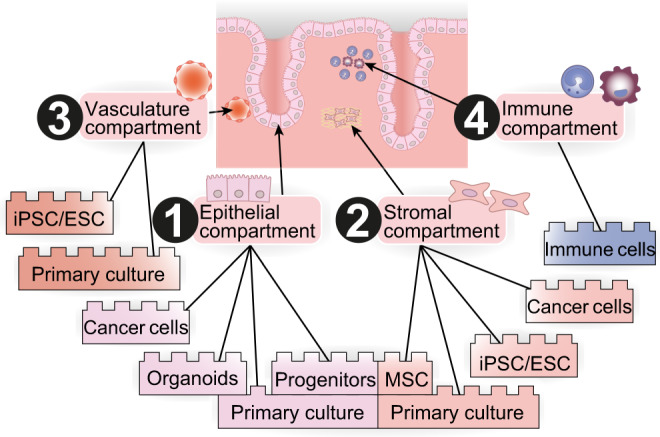
Appropriate cellular models of the epithelial compartment of the uterus (1) can be established as organoids and progenitor cell cultures, which can be derived from primary patient cells. The stromal compartment (2) can be founded by induced pluripotent stem cells (iPSC) or embryonal stem cells (ESC) cultures, or mesenchymal stem cells (MSC) derived from primary cell culture. The vasculature compartment (3) can be derived from iPSCs, ESCs or directly from primary cells, and immune cells of the endometrium build the immune compartment (4).
The differentiation of PSCs into the endometrial epithelium and stromal-like cells presents a promising avenue (Fig. 4). The first differentiation attempts were based on co-culture of PSCs with primary endometrial cultures56,57. However, these conditions were poorly defined, and the resulting cells were not fully characterised. Miyazaki et al. addressed this problem by differentiating human-induced PSCs (iPSCs) into endometrial stromal fibroblast-like cells in a 14-day embryoid-body-based approach in defined conditions58. The protocol closely follows in vivo endometrium development: iPSCs progress from primitive streak through intermediate mesoderm, coelomic epithelium and Müllerian duct-like stages. The first part of the differentiation is based on a protocol used to generate kidney progenitor cells59. Intermediate mesoderm-like cells express markers including LHX1 and PAX2. The fact that the intermediate mesoderm-like cells derived by this protocol can be used to make both kidney and endometrial-like cells, lends credibility to the developmental authenticity of the system. BMP inhibition prevents the acquisition of renal identity, while WNT activation is required for Müllerian duct and uterine gland development in vivo13,14,60,61. The resulting endometrial stromal fibroblast-like cells were hormone-responsive and resembled primary endometrial stromal cells as determined by RNA sequencing (RNA-seq). This protocol provides an encouraging starting point for iPSC-derived models of human endometrium.
Primary cultures of human adult tissue represent an alternative approach for building a stem cell-based uterus. Endometrium biopsy-derived epithelial and stromal cultures can be maintained in vitro and respond to hormonal stimuli59,62,63. Purified endometrial cell cultures have been used to understand physiological and pathological processes, including trophoblast invasion64. However, endometrium-derived primary culture offers limited potential for use in in vitro models since they cannot be propagated in vitro for many passages and tend to differentiate62,63. Recent advances in organoid culture demonstrate that when epithelial cells from endometrial primary cultures are dissociated, separated from the stromal compartment, and seeded into a 3D-extracellular matrix scaffold, they are capable self-organisation into hollow spheres formed by a single layer of columnar epithelium65–69. These spheres are called endometrial organoids as they recapitulate several physiological characteristics of the endometrium, and RNA-seq shows organoids share many similarities with in vivo endometrial glands66,70. In contrast to two-dimensional primary cultures, endometrial organoids can be readily propagated, stay genetically and phenotypically stable, and retain the capacity to respond to hormonal stimuli65,66,70,71. Strikingly, when exposed to sex hormones, such as progesterone or oestrogen, organoids develop characteristics of early pregnancy and are able to recapitulate the menstrual cycle65,66,70. Single-cell profiling identified several sub-populations in endometrial organoids, including proliferating, secretory, ciliated, and putative stem cell populations70. A subset of cells harbours the potential to generate either secretory or ciliated epithelial cells, which is consistent with the proposition of a progenitor population in vivo66. Overall, endometrial organoids represent the first step towards 3D-modelling of the endometrium and offer a sustainable source of endometrial epithelial cells71–74.
Assembly approaches
Due to the broad range of topics relating to uterine pathologies and maternal–embryo interactions, a single endometrial culture system is unlikely to address all biological questions of interest. Instead, a modular approach is required, with stem cell-based uteri variously assembled from the minimal components necessary to answer key questions74,75. Such modularity is advantageous due to its flexibility, whilst the use of minimal component sets avoids unnecessary confounding factors that may obscure interpretation and confers a degree of scalability. Here, we focus on three key approaches: (i) self-organising cultures to model early implantation; (ii) controlled assembly approaches to model trophoblast invasion, placentation, and embryo maintenance, or pathologies of the uterus; (iii) scaffold-based approaches that may be used to model pathologies of the uterus, with potential clinical implications.
Self-organising co-cultures
The co-culture of embryos alongside endometrial epithelial monolayers has long been suggested as a model for implantation and to gauge mechanisms of apposition and competence of blastocysts for maternal–embryo interactions76–84. Similarly, the culture of embryos with endometrial stromal cells provides opportunities for investigating aspects of trophoblast invasion at the earliest stages82,85–89. Multi-layered co-cultures combining epithelial and stromal cells offer a more complete model of early implantation90–92 but are limited by the availability of uterine tissue and embryo samples. Whilst issues with the availability of maternal tissues may be mitigated by appropriate community resources, such as biobanks, embryos are likely to remain the key bottleneck to these applications.
Endometrial organoids65,66,70,93 represent a suitable advancement over monolayer cultures, allowing for more complete modelling of endometrial tissues including the characteristic glandular structure. Since changes in endometrial vasculature represent a phenotypic readout of implantation, the incorporation of vasculature within organoid models would provide an important improvement (Fig. 5a). Blood vasculature has previously been induced in co-cultures of PSC-derived endothelial cells with pericytes94,95 or the introduction of mesodermal progenitor cells into organoid cultures96,97 (reviewed in ref. 98). It remains to be seen if such protocols can be adapted to allow survival and self-organisation of vascularised endometrial organoids.
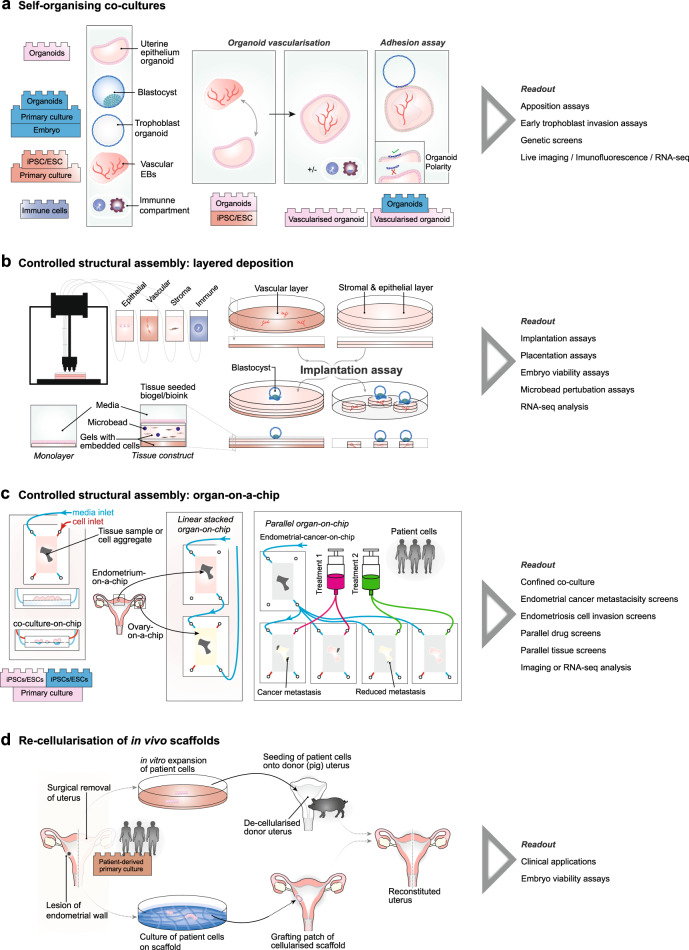
Fig. 5. Engineering of a stem cell-based uterus.
a Co-culture systems to model the uterus during embryo implantation. Advances include vascularisation of endometrial organoids and integration of immune components. The co-culture of vascularised organoids alongside embryo should allow apposition assays if the polarity of organoids can be inverted. b Apposition, adhesion and trophoblast invasion could be modelled in complex tissue constructs engineered by layered deposition of 3D cell-embedded gels using 3D printing. c Automatised culture can be obtained by using organ-on-a-chip technology, allowing high-throughput screening of patient-derived uterine cells for co-culture and cell-invasion assays, but also enable linear stacking of multiple components of the female reproductive tract on-a-chip, such as ovarian and fallopian tube-cell containing chips. d Pathologies associated with the human uterus, such as a medically required surgical removal of the uterus or a lesion of the endometrial wall following cancer treatment, could be targeted with stem cell-based uteri. Patient-derived primary cells could be expanded in vitro, and seeded onto a de-cellularised donor uterus in order to avoid immune reaction of the patient following re-grafting of the donor uterus. Patient primary cell culture on a scaffold allows to obtain a cellularised grafting patch which can be transplanted to reconstitute the patient’s uterus.
Human blastocyst or first-trimester placenta-derived trophoblast stem cells provide an exciting avenue to model the extraembryonic compartment99–102. Trophoblast stem cells are capable of self-renewal and differentiation into syncytiotrophoblast and trophoblastic vesicles103,104. The latter may provide suitable surrogate models for human blastocysts to overcome the ethical and practical limitations associated with human embryo research. Indeed, the culture of trophoblast spheroids alongside endometrial epithelial cells105–116 or stromal cells89,108,117–119 has already been demonstrated. A key step forward would therefore be the co-culture of vascularised endometrial organoids with trophoblast organoids (Fig. 5a). Hurdles to overcome include identification of suitable media that allow for survival and development of these divergent organoid systems, and issues arising from the reversal of the apicobasal polarity, with microvilli facing towards the organoid lumen, rather than outwards where contact with the embryo-surrogate would occur (Fig. 5a).
PSC-based approaches have also emerged as useful platforms for modelling embryonic and extraembryonic tissues, and may soon represent useful proxies for embryos. In mouse, co-culture of embryonic and extraembryonic cell types allows the formation of embryo-like structures reminiscent of pre-implantation blastocysts120,121 and post-implantation mouse embryos122,123 (reviewed in ref. 124). A combination of embryonic and extraembryonic tissues is likely to be required to faithfully recapitulate maternal–embryo interactions, rather than extraembryonic trophoblast tissues alone (Fig. 5a). Indeed, secreted ligands from the mouse inner cell mass (ICM) induce proliferation in adjacent polar trophectoderm125,126 and play essential roles in implantation. This might be of particular relevance for primate development, where the blastocyst implants with the ICM oriented towards the endometrium127,128. Continuing advances in the field will provide stem cell-based embryo models, which are bound to outperform models based on trophoblast organoids alone. Ultimately, the establishment of robust stem cell-based embryos in human and non-human primates will be an essential goal for the development of realistic and scalable models of early pregnancy.
Selective incorporation of components of the immune system129–131 represents an additional advantage of assembled stem cell-based uteri. The importance of immune cells in preventing diseases33,132 as well as their immunomodulatory role during pregnancy132–139 is well established, and the distribution of immune cells is highly dynamic within the menstrual cycles (reviewed in ref. 33). The inclusion of immune components into organoid models would be an asset to delineate mechanisms of immune tolerance, albeit at the expense of increased complexity. Careful consideration should be given as to which components of the immune system to include in order to retain tractability of the organoid approaches. The abundance of uterine natural killer cells within the uterus during pregnancy may present a natural starting point. Due to the plasticity of components of the immune system care must be taken to minimise undesired transdifferentiation of immune components in culture. Fluorescence labelling and live-cell imaging will allow tracking of immune cell movements and, combined with single-cell profiling endpoint analysis, will provide candidate regulators for immunomodulatory functions of the uterus.
Controlled structural assembly: layered deposition and organ-on-a-chip
Decidualisation and placentation represent protracted biological processes that extend over large spatial scales. Trophoblast invasion, which is an important part of placentation, has been quantified using invasion assays. Traditional approaches include the culture of trophoblast cells in Matrigel-coated trans-well inserts with their invasiveness being monitored. These techniques are limited in scope and incapable of capturing the complex interplay between trophoblast and maternal tissues. Full modelling of trophoblast invasion, placentation, and pathologies such as placenta accreta, requires tissue constructs more akin to the maternal interface.
Print-based technologies have been suggested for a variety of tissue constructions140,141 including muscle142 and neural tissues143. These approaches are based on the layered deposition of appropriate gel-embedded cells or bio-inks by 3D printing technologies144. The increased spatial scale of these tissue constructs over organoids may require the integration of functional vasculature for oxygen and nutrient supply. Blood vasculature has already been induced in Matrigel and hyaluronic acid (HA)-based hydrogels145,146 and the deposition of epithelial-cell-laden hydrogel channels within printed constructs effectively allows the formation of vasculature147–150. Another exciting approach entails sacrificial networks, which are cell matrices with embedded dissolvable filaments. Upon the breakdown of these filaments, the created space can be seeded with endothelial cells to generate defined vasculature151–155. In the future, sacrificial networks may be routinely combined with print-based approaches155,156 (reviewed in refs. 98,157). A limitation of explicit engineering of vasculature is the decreased complexity of branching compared to natural systems. Alternative approaches include seeding of vascularised cell aggregates96,97 onto a basal scaffolding on which subsequent layers of stromal and epithelial cell-laden gels or organoid-containing gels are deposited (Fig. 5b). To increase parallelisation for screening purposes, constructs could feasibly be engineered over micro-patterned plates158,159, or alternatively by the embedding of multiple vascularised endometrial organoids within gels that then provide the appropriate signals for invasion (Fig. 5b). A key advantage of print-based approaches is the flexible inclusion of localised signalling by incorporation of chemically laden microbeads within gels, e.g., angiogenic signals to promote vasculogenesis or angiogenesis, or candidate signals to modulate trophoblast invasion.
Functional models of the uterus in different primate systems could be applied to interrogate species-specific differences in the mode of invasion, specifically allowing comparative studies of superficial versus interstitial implantation. Optimisation of such models to allow long-term survival of stem cell-based embryos will provide a powerful asset for investigating the causes of first trimester miscarriages.
Microfluidic “organ-on-a-chip” concepts157,160–162 in which cell-aggregates or primary sample tissues from a specific organ are cultured within a microfluidic device represent an alternative platform for modelling of the uterus162 (Fig. 5c). In particular, endometrium-on-a-chip has been used to combine primary human stromal cells with epithelial cells to allow hormone-responsive differentiation of stromal cells into decidua163. An important advantage of custom microfluidic devices is the ability to co-culture different cells or cell aggregates at an interface whilst exerting precise control over the signalling environment. This is exemplified in the recent generation of embryonic disc-like and amnion-like cells in structures reminiscent of the early human post-implantation embryo164. Modifications of these microfluidic devices would allow for co-culture of layers of endometrial stromal and endometrial epithelial cells, alongside trophoblast cells, each with cell-specific signalling environments. This approach could be used to monitor trophoblast invasion into endometrium on-a-chip (Fig. 5c).
Modularity is one of the key strengths of microfluidic devices. This was recently shown in a model of the female reproductive tract by linear integration of chips containing tissues from fallopian tubes, the uterus, and cervix with shared perfusion between modules165. This system supported murine ovarian follicles to produce the human 28-day menstrual cycle and demonstrated the feasibility of modelling complex multi-cellular behaviour by integrating a system of simpler component parts. Extensions of this experimental setup may be of particular use in disease modelling e.g., to test hypotheses about the mechanisms of endometriosis52. The linear combination of a chip with endometrial tissues alongside chips containing other tissues with a shared circulating perfusion should provide a powerful assay to gauge retrograde menstruation under hormone varying environments using cells from patients with and without endometriosis. Complex parallel arrays may provide further flexibility, for example allowing a culture of endometrial cancer samples with downstream samples of disease-free endometrium and adjacent organ tissues and one-way connective perfusion, which could be used to investigate metastasis of cancer. Indeed, a single metastatic tumour sample could be connected to multiple identical downstream tissue samples, each with different drug treatment, thus allowing parallelised tissue-specific drug screening (Fig. 5c).
Re-cellularisation of in vivo-derived tissue scaffolds
A variety of uterine conditions require surgical interventions, including complications during pregnancy. In some cases, previous surgical procedures, including caesarean sections, may impact the ability of the uterus to support a foetus to full term or serve as an aggravating factor in other uterine conditions47–49. Stem cell-based models may provide a starting basis for regenerative approaches to address these issues.
Partial reconstruction of the uterus has been achieved using tissue grafts166 or xenogeneic tissue grafts167. A recent study in rabbits restored the function of rabbit uteri following extensive excision of the endometrium using a biodegradable polymer scaffold seeded with autologous primary cells168 (Fig. 5d). This approach yielded increased implantation rates and sustained pregnancy to term, in contrast to unseeded scaffolds or no-scaffold controls. Whilst graft-based approaches may prove useful for the repair of the uterus following trauma, they cannot address severe pathologies where a hysterectomy may be required. Uterus transplantations are one possibility169–172 and have allowed successful livebirths173–175. However, transplantations are limited by the availability of organ donors. Alternatively, recellularisation approaches in which appropriate cell lineages are seeded onto decellularised scaffolds, have been developed in animal models for lungs176–180 (reviewed in ref. 181), heart182–184, liver185, oesophagus186, trachea187, bladder172,188, liver189, and vagina190. Decellularisation of rat and pig uteri has been shown to preserve vasculature191,192, and allow for preliminary recellularisation using neonatal rat uterine cells and rat mesenchymal stem cells192 or mixtures of stromal and epithelial stem cells from human endometrial origin191. Studies in sheep have systematically compared different decellularisation approaches of uteri for their ability to support recellularisation of sheep PSCs189. The possibility of using animal scaffolds presents an opportunity for clinical settings (Fig. 5d). However, notable issues associated with the removal of xenoantigens and risks of transmission of animal viruses remain193. Finally, the ability of donor uteri from deceased patients to sustain live births174 may open up the possibility of donor scaffolds. Together, these early studies suggest that repair or reconstruction of uteri using pathogen-free, patient-specific cells may represent a long-term treatment solution to some of the most severe uterine pathologies.
Outlook
Investigation of the earliest stages of implantation and maternal–embryo interactions with human embryos has long been limited by the 14-day rule194. Stem cell-based approaches to mimic the maternal environment synergise with recent advances in modelling embryogenesis124, and together will illuminate the critical stages of human periimplantation development. Modelling primate implantation with stem cell-based uteri is charged with biomedical potential and provides a unique opportunity for basic science. The development of multi-organoid systems and controlled structural assembly of uterus models should facilitate large-scale genetic and drug screening, enable cross-species comparative studies, and shed new light in the field of human embryogenesis that has previously been obscured. Stem cell-based uterus and embryo research will raise important ethical questions that will require careful consideration by the research community and beyond. Ultimately, advances in basic science go hand-in-hand with progress in clinical applications, and the next few years promise to see striking steps forward in the advancement of women’s reproductive health.
Supplementary information
Acknowledgements
We thank the members of the Boroviak lab, in particular Dylan Siriwardena, Erin Slatery, and Max Lycke, as well as Geraldine Jowett and Christos Kyprianou for fruitful discussions and helpful comments on the manuscript. This research is generously supported by the Wellcome Trust (WT RG89228), the Centre for Trophoblast Research, and the Isaac Newton Trust.
Author contributions
C.A.P., T.E.B., S.B., M.S. and C.M. contributed to conceptualisation and writing of the manuscript. S.B., M.S. and C.M. created figures with input from all authors.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer Reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christopher A. Penfold, Email: cap76@cam.ac.uk
Thorsten E. Boroviak, Email: teb45@cam.ac.uk
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02233-8.
References
- 1.Wang W, et al. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 2020;26:1644–1653. doi: 10.1038/s41591-020-1040-z. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Alonso, L. et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. bioRxiv10.1101/2021.01.02.425073 (2021). [DOI] [PMC free article] [PubMed]
- 3.Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature563, 347–353 (2018). [DOI] [PMC free article] [PubMed]
- 4.Liu, Y. et al. Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res. 28, 819–832 (2018). [DOI] [PMC free article] [PubMed]
- 5.Partridge, E. A. et al. An extra-uterine system to physiologically support the extreme premature lamb. Nat. Commun. 8, 1–16 (2017). [DOI] [PMC free article] [PubMed]
- 6.Kobayashi, A. & Behringer, R. R. Developmental genetics of the female reproductive tract in mammals. Nat. Rev. Genet.4, 969–980 (2003). [DOI] [PubMed]
- 7.Cochard, L. Netter’s atlas of human embryology. Igarss2014, (2014) 10.1007/s13398-014-0173-7.2.
- 8.Bouchard, M., Souabni, A., Mandler, M., Neubüser, A. & Busslinger, M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 16, 2958–2970 (2002). [DOI] [PMC free article] [PubMed]
- 9.Huang, C. C., Orvis, G. D., Kwan, K. M. & Behringer, R. R. Lhx1 is required in Müllerian duct epithelium for uterine development. Dev. Biol. 389, 124–136 (2014). [DOI] [PMC free article] [PubMed]
- 10.Zhang, W. et al. Identification and functional analysis of a novel LHX1 mutation associated with congenital absence of the uterus and vagina. Oncotarget8, 8785 (2017). [DOI] [PMC free article] [PubMed]
- 11.Robboy SJ, Kurita T, Baskin L, Cunha GR. New insights into human female reproductive tract development. Differentiation. 2017;97:9–22. doi: 10.1016/j.diff.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uetani N, Bouchard M. Plumbing in the embryo: developmental defects of the urinary tracts. Clin. Genet. 2009;75:307–317. doi: 10.1111/j.1399-0004.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- 13.Guioli S, Sekido R, Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Dev. Biol. 2007;302:389–398. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Orvis GD, Behringer RR. Cellular mechanisms of Müllerian duct formation in the mouse. Dev. Biol. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto R. Development of the human Müllerian duct in the sexually undifferentiated stage. Anat. Rec. - Part A. 2003;272:514–519. doi: 10.1002/ar.a.10061. [DOI] [PubMed] [Google Scholar]
- 16.Cunha GR, et al. Development of the human female reproductive tract. Differentiation. 2018;103:46–65. doi: 10.1016/j.diff.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.She ZY, Yang WX. Sry and SoxE genes: how they participate in mammalian sex determination and gonadal development? Semin. Cell Dev. Biol. 2017;63:13–22. doi: 10.1016/j.semcdb.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Stévant I, Nef S. Genetic control of gonadal sex determination and development. Trends Genet. 2019;35:346–358. doi: 10.1016/j.tig.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Cooke PS, Spencer TE, Bartol FF, Hayashi K. Uterine glands: development, function and experimental model systems. Mol. Hum. Reprod. 2013;19:547–558. doi: 10.1093/molehr/gat031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelleher AM, Demayo FJ, Spencer TE. Uterine glands: developmental biology and functional roles in pregnancy. Endocr. Rev. 2019;40:1424–1445. doi: 10.1210/er.2018-00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carta L, Sassoon D. Wnt7a is a suppressor of cell death in the female reproductive tract and is required for postnatal and estrogen-mediated growth. Biol. Reprod. 2004;71:444–454. doi: 10.1095/biolreprod.103.026534. [DOI] [PubMed] [Google Scholar]
- 22.Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131:2061–2072. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- 23.Fritsch H, Hoermann R, Bitsche M, Pechriggl E, Reich O. Development of epithelial and mesenchymal regionalization of the human fetal utero-vaginal anlagen. J. Anat. 2013;222:462–472. doi: 10.1111/joa.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catalini L, Fedder J. Characteristics of the endometrium in menstruating species: lessons learned from the animal kingdom. Biol. Reprod. 2020;102:1160–1169. doi: 10.1093/biolre/ioaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billhaq DH, Lee SH, Lee S. The potential function of endometrial-secreted factors for endometrium remodeling during the estrous cycle. Anim. Sci. J. 2020;91:13333. doi: 10.1111/asj.13333. [DOI] [PubMed] [Google Scholar]
- 26.Slayden, O. V. D. & Calhoun, A. The primate uterus: cyclic changes. In Encyclopedia of Reproduction (ed. Skinner M.) 312–319 (Elsevier, 2018).
- 27.Bartelmez GW. Histological studies of menstruating mucous membrane of human uterus. Contrib. Embryol. 1933;24:143–186. [Google Scholar]
- 28.Bartelmez GW. Cyclic changes in the endometrium of the rhesus monkey (Macaca mulatta) Contrib. Embryol. Carnegie Inst. 1951;34:99–146. [Google Scholar]
- 29.Chaudhry, R. & Chaudhry, K. Anatomy, Abdomen and Pelvis, Uterine Arteries (StatPearls, 2018). [PubMed]
- 30.Zhou JZ, Way SS, Chen K. Immunology of the uterine and vaginal mucosae. Trends Immunol. 2018;39:355–314. doi: 10.1016/j.it.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017;8:875. doi: 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderstraeten A, Tuyaerts S, Amant F. The immune system in the normal endometrium and implications for endometrial cancer development. J. Reprod. Immunol. 2015;109:7–16. doi: 10.1016/j.jri.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Lee SK, Kim CJ, Kim D-J, Kang J. Immune cells in the female reproductive tract. Immune Netw. 2015;15:16. doi: 10.4110/in.2015.15.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter AM, Enders AC, Pijnenborg R. The role of invasive trophoblast in implantation and placentation of primates. Philos. Trans. R. Soc. B. 2015;370:20140070. doi: 10.1098/rstb.2014.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross C, Boroviak TE. Origin and function of the yolk sac in primate embryogenesis. Nat. Commun. 2020;11:3760. doi: 10.1038/s41467-020-17575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enders AC, King BF. Early stages of trophoblastic invasion of the maternal vascular system during implantation in the macaque and baboon. Am. J. Anat. 1991;192:329–346. doi: 10.1002/aja.1001920403. [DOI] [PubMed] [Google Scholar]
- 37.Luckett WP. Comparative development and evolution of the placenta in primates. Contributions Primatol. 1974;3:142–234. [PubMed] [Google Scholar]
- 38.Leridon, H. Démographie des échecs de la reproduction. In Les Accidents Chromosomiques de la Reproduction (eds Boué, A. & Thibault, C.) 13–27 (Inserm, 1973).
- 39.Racowsky C. High rates of embryonic loss, yet high incidence of multiple births in human art: Is this paradoxical? Theriogenology. 2002;57:87–96. doi: 10.1016/S0093-691X(01)00659-8. [DOI] [PubMed] [Google Scholar]
- 40.Macklon NS, Geraedts JPM, Fauser BCJM. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum. Reprod. Update. 2002;8:333–343. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- 41.Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- 42.Boomsma CM, et al. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum. Reprod. 2009;24:1427–1435. doi: 10.1093/humrep/dep011. [DOI] [PubMed] [Google Scholar]
- 43.Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum. Reprod. Update. 2014;20:250–261. doi: 10.1093/humupd/dmt047. [DOI] [PubMed] [Google Scholar]
- 44.Stanek J. Histological features of shallow placental implantation unify early-onset and late-onset preeclampsia. Pediatr. Dev. Pathol. 2019;22:112–122. doi: 10.1177/1093526618803759. [DOI] [PubMed] [Google Scholar]
- 45.Fisher SJ. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015;213:S115–S122. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Sayed AAF. Preeclampsia: a review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan. J. Obstet. Gynecol. 2017;56:593–598. doi: 10.1016/j.tjog.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am. J. Obstet. Gynecol. 2005;192:1458–1461. doi: 10.1016/j.ajog.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 48.Jauniaux E, Bunce C, Grønbeck L, Langhoff-Roos J. Prevalence and main outcomes of placenta accreta spectrum: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019;221:208–218. doi: 10.1016/j.ajog.2019.01.233. [DOI] [PubMed] [Google Scholar]
- 49.Silver RM, Barbour KD. Placenta accreta spectrum. Accreta, increta, and percreta. Obstet. Gynecol. Clin. North Am. 2015;42:381–402. doi: 10.1016/j.ogc.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Bannister, N. & Broggio, J. Cancer Survival by Stage at Diagnosis for England (Experimental Statistics). (Office for National Statistics, 2016).
- 51.Rogers PAW, et al. Priorities for endometriosis research: recommendations from an international consensus workshop. Reprod. Sci. 2009;16:335–346. doi: 10.1177/1933719108330568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019;15:666–682. doi: 10.1038/s41574-019-0245-z. [DOI] [PubMed] [Google Scholar]
- 53.Stefansson H, et al. Genetic factors contribute to the risk of developing endometriosis. Hum. Reprod. 2002;17:555–559. doi: 10.1093/humrep/17.3.555. [DOI] [PubMed] [Google Scholar]
- 54.Gallagher CS, et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat. Commun. 2019;10:4857. doi: 10.1038/s41467-019-12536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weimar CHE, Post Uiterweer ED, Teklenburg G, Heijnen CJ, Macklon NS. In-vitro model systems for the study of human embryo–endometrium interactions. Reprod. BioMed. Online. 2013;27:673–688. doi: 10.1016/j.rbmo.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Song T, et al. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng.—Part A. 2015;21:353–361. doi: 10.1089/ten.tea.2014.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye L, et al. Generation of human female reproductive tract epithelium from human embryonic stem cells. PLoS ONE. 2011;6:e21136. doi: 10.1371/journal.pone.0021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyazaki K, et al. Generation of progesterone-responsive endometrial stromal fibroblasts from human induced pluripotent stem cells: role of the WNT/CTNNB1 pathway. Stem Cell Rep. 2018;11:1136–1155. doi: 10.1016/j.stemcr.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Classen-Linke I, Kusche M, Knauthe R, Beier HM. Establishment of a human endometrial cell culture system and characterization of its polarized hormone responsive epithelial cells. Cell Tissue Res. 1996;287:171–185. doi: 10.1007/s004410050743. [DOI] [PubMed] [Google Scholar]
- 60.Deutscher E, H Hung-ChangYao. Essential roles of mesenchyme-derived beta-catenin in mouse Müllerian duct morphogenesis. Dev. Biol. 2007;307:227–236. doi: 10.1016/j.ydbio.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arango NA, et al. Conditional deletion of β-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev. Biol. 2005;288:276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 62.Li, D. et al. Development and characterization of a polarized human endometrial cell epithelia in an air-liquid interface state. Stem Cell Res. Ther. 9, 1–7 (2018). [DOI] [PMC free article] [PubMed]
- 63.Chen, J. & Roan, N. Isolation and culture of human endometrial epithelial cells and stromal fibroblasts. BIO-PROTOCOL5, (2015). [DOI] [PMC free article] [PubMed]
- 64.Mannelli C, Ietta F, Avanzati AM, Skarzynski D, Paulesu L. Biological tools to study the effects of environmental contaminants at the feto–maternal interface. Dose-Response. 2015;13:1559325815611902. doi: 10.1177/1559325815611902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boretto, M. et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development144, 1775–1786 (2017). [DOI] [PubMed]
- 66.Turco MY, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017;19:568–577. doi: 10.1038/ncb3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eritja N, et al. ERα-mediated repression of pro-inflammatory cytokine expression by glucocorticoids reveals a crucial role for TNFα and IL1α in lumen formation and maintenance. J. Cell Sci. 2012;125:1929–1944. doi: 10.1242/jcs.095067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eritja N, et al. Long-term estradiol exposure is a direct mitogen for insulin/EGF-primed endometrial cells and drives PTEN loss-induced hyperplasic growth. Am. J. Pathol. 2013;183:277–287. doi: 10.1016/j.ajpath.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 69.Eritja N, et al. A novel three-dimensional culture system of polarized epithelial cells to study endometrial carcinogenesis. Am. J. Pathol. 2010;176:2722–2731. doi: 10.2353/ajpath.2010.090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fitzgerald, H. C., Dhakal, P., Behura, S. K., Schust, D. J. & Spencer, T. E. Self-renewing endometrial epithelial organoids of the human uterus. Proc. Natl. Acad. Sci. USA116, 23132–23142 (2019). [DOI] [PMC free article] [PubMed]
- 71.Alzamil L, Nikolakopoulou K, Turco MY. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 2020;28:35–51. doi: 10.1038/s41418-020-0565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hibaoui Y, Feki A. Organoid models of human endometrial development and disease. Front. Cell Dev. Biol. 2020;8:84. doi: 10.3389/fcell.2020.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gu ZY, Jia SZ, Liu S, Leng JH. Endometrial organoids: a new model for the research of endometrial-related diseases. Biol. Reprod. 2020;103:918–926. doi: 10.1093/biolre/ioaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fitzgerald, H. C., Schust, D. J. & Spencer, T. E. In vitro models of the human endometrium: evolution and application for women’s health+. Biol. Reprod. 10.1093/biolre/ioaa183 (2020). [DOI] [PMC free article] [PubMed]
- 75.Cui Y, Zhao H, Wu S, Li X. Human female reproductive system organoids: applications in developmental biology, disease modelling, and drug discovery. Stem Cell Rev. Rep. 2020;16:1173–1184. doi: 10.1007/s12015-020-10039-0. [DOI] [PubMed] [Google Scholar]
- 76.Lindenberg S, Nielsen MH, Lenz S. In vitro studies of human blastocyst implantation, In Vitro Studies of Human Blastocyst Implantation. Ann. N. Y. Acad. Sci. 1985;442:368–374. doi: 10.1111/j.1749-6632.1985.tb37541.x. [DOI] [PubMed] [Google Scholar]
- 77.Simón C, et al. Coculture of human embryos with autologous human endometrial epithelial cells in patients with implantation failure. J. Clin. Endocrinol. Metab. 1999;84:2638–2646. doi: 10.1210/jcem.84.8.5873. [DOI] [PubMed] [Google Scholar]
- 78.Simón, C. et al. Embryonic Regulation of Integrins β 3,α 4, and α 1 in Human Endometrial Epithelial Cells in Vitro 1. J. Clin. Endocrinol. Metab. 82, 2607–2616 (1997). [DOI] [PubMed]
- 79.Meseguer M, et al. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol. Reprod. 2001;64:590–601. doi: 10.1095/biolreprod64.2.590. [DOI] [PubMed] [Google Scholar]
- 80.Caballero-Campo P, et al. Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation. Mol. Hum. Reprod. 2002;8:375–384. doi: 10.1093/molehr/8.4.375. [DOI] [PubMed] [Google Scholar]
- 81.Dominguez F, et al. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5, and CCR2B in the human endometrium and the human blastocyst. Mol. Hum. Reprod. 2003;9:189–198. doi: 10.1093/molehr/gag024. [DOI] [PubMed] [Google Scholar]
- 82.Tan Y, et al. A model for implantation: coculture of blastocysts and uterine endometrium in mice. Biol. Reprod. 2005;72:556–560. doi: 10.1095/biolreprod.104.032821. [DOI] [PubMed] [Google Scholar]
- 83.González RR, et al. Leptin and leptin receptor are expressed in the human endometrium and endometrial leptin secretion is regulated by the human blastocyst. J. Clin. Endocrinol. Metab. 2000;85:4883–4888. doi: 10.1210/jcem.85.12.7060. [DOI] [PubMed] [Google Scholar]
- 84.Ojosnegros S, Seriola A, Godeau AL, Veiga A. Embryo implantation in the laboratory: an update on current techniques. Hum. Reprod. Update. 2021;27:501–530. doi: 10.1093/humupd/dmaa054. [DOI] [PubMed] [Google Scholar]
- 85.Carver J, et al. An in-vitro model for stromal invasion during implantation of the human blastocyst. Hum. Reprod. 2003;18:283–290. doi: 10.1093/humrep/deg072. [DOI] [PubMed] [Google Scholar]
- 86.Grewal, S., Carver, J. G., Ridley, A. J. & Mardon, H. J. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc. Natl. Acad. Sci. USA105, 16189–16194 (2008). [DOI] [PMC free article] [PubMed]
- 87.Teklenburg G, Salker M, Heijnen C, Macklon NS, Brosens JJ. The molecular basis of recurrent pregnancy loss: Impaired natural embryo selection. Mol. Hum. Reprod. 2010;16:886–895. doi: 10.1093/molehr/gaq079. [DOI] [PubMed] [Google Scholar]
- 88.Teklenburg G, et al. Cell lineage specific distribution of H3K27 trimethylation accumulation in an in vitro model for human implantation. PLoS ONE. 2012;7:e32701. doi: 10.1371/journal.pone.0032701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weimar CHE, et al. Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos. PLoS ONE. 2012;7:e41424. doi: 10.1371/journal.pone.0041424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park DW, et al. A well-defined in vitro three-dimensional culture of human endometrium and its applicability to endometrial cancer invasion. Cancer Lett. 2003;195:185–192. doi: 10.1016/S0304-3835(03)00131-9. [DOI] [PubMed] [Google Scholar]
- 91.Bentin-Ley U, et al. Isolation and culture of human endometrial cells in a three-dimensional culture system. J. Reprod. Fertil. 1994;101:327–332. doi: 10.1530/jrf.0.1010327. [DOI] [PubMed] [Google Scholar]
- 92.Petersen A, et al. The antiprogesterone Org 31710 inhibits human blastocyst-endometrial interactions in vitro. Fertil. Steril. 2005;83:1255–1263. doi: 10.1016/j.fertnstert.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 93.Abbas Y, et al. Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus. 2020;10:20190079. doi: 10.1098/rsfs.2019.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orlova VV, et al. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat. Protoc. 2014;9:1514–1531. doi: 10.1038/nprot.2014.102. [DOI] [PubMed] [Google Scholar]
- 95.Orlova VV, et al. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler. Thromb. Vasc. Biol. 2014;34:177–186. doi: 10.1161/ATVBAHA.113.302598. [DOI] [PubMed] [Google Scholar]
- 96.Markou M, et al. Tissue engineering using vascular organoids from human pluripotent stem cell derived mural cell phenotypes. Front. Bioeng. Biotechnol. 2020;8:278. doi: 10.3389/fbioe.2020.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wörsdörfer P, et al. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 2019;9:15663. doi: 10.1038/s41598-019-52204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grebenyuk S, Ranga A. Engineering organoid vascularization. Front. Bioeng. Biotechnol. 2019;7:39. doi: 10.3389/fbioe.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cinkornpumin JK, et al. Naive human embryonic stem cells can give rise to cells with a trophoblast-like transcriptome and methylome. Stem Cell Rep. 2020;15:198–213. doi: 10.1016/j.stemcr.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dong, C. et al. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. Elife9, 263–267 (2020). [DOI] [PMC free article] [PubMed]
- 101.Turco MY, et al. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature. 2018;564:263–267. doi: 10.1038/s41586-018-0753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haider S, et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 2018;11:537–551. doi: 10.1016/j.stemcr.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Okae H, et al. Derivation of human trophoblast stem cells. Cell Stem Cell. 2018;22:50–63.e6. doi: 10.1016/j.stem.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 104.Lee CQE, et al. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Rep. 2016;6:257–272. doi: 10.1016/j.stemcr.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galan A, et al. The human blastocyst regulates endometrial epithelial apoptosis in embryonic adhesion. Biol. Reprod. 2000;63:430–439. doi: 10.1093/biolreprod/63.2.430. [DOI] [PubMed] [Google Scholar]
- 106.Hohn HP, Linke M, Denker HW. Adhesion of trophoblast to uterine epithelium as related to the state of trophoblast differentiation: In vitro studies using cell lines. Mol. Reprod. Dev. 2000;57:135–145. doi: 10.1002/1098-2795(200010)57:2<135::AID-MRD4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 107.Ho H, Singh H, Aljofan M, Nie G. A high-throughput in vitro model of human embryo attachment. Fertil. Steril. 2012;97:974–978. doi: 10.1016/j.fertnstert.2012.01.116. [DOI] [PubMed] [Google Scholar]
- 108.Holmberg, J. C. et al. An in vitro model for the study of human implantation. Am. J. Reprod. Immunol. 67, 169–178 (2012). [DOI] [PMC free article] [PubMed]
- 109.Xiong T, et al. Administration of calcitonin promotes blastocyst implantation in mice by up-regulating integrin β3 expression in endometrial epithelial cells. Hum. Reprod. 2012;27:3540–3551. doi: 10.1093/humrep/des330. [DOI] [PubMed] [Google Scholar]
- 110.Kinnear S, Salamonsen LA, Francois M, Harley V, Evans J. Uterine SOX17: a key player in human endometrial receptivity and embryo implantation. Sci. Rep. 2019;9:15495. doi: 10.1038/s41598-019-51751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heneweer C, Adelmann HG, Hendric Kruse L, Denker HW, Thie M. Human uterine epithelial RL95-2 cells reorganize their cytoplasmic architecture with respect to Rho protein and F-actin in response to trophoblast binding. Cells Tissues Organs. 2003;175:1–8. doi: 10.1159/000073432. [DOI] [PubMed] [Google Scholar]
- 112.Heneweer C, Schmidt M, Denker HW, Thie M. Molecular mechanisms in uterine epithelium during trophoblast binding: the role of small GTPase RhoA in human uterine Ishikawa cells. J. Exp. Clin. Assist. Reprod. 2005;2:4. doi: 10.1186/1743-1050-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mo B, et al. ECC-1 cells: a well-differentiated steroid-responsive endometrial cell line with characteristics of luminal epithelium. Biol. Reprod. 2006;75:387–394. doi: 10.1095/biolreprod.106.051870. [DOI] [PubMed] [Google Scholar]
- 114.Uchida H, et al. Histone deacetylase inhibitor-induced glycodelin enhances the initial step of implantation. Hum. Reprod. 2007;22:2615–2622. doi: 10.1093/humrep/dem263. [DOI] [PubMed] [Google Scholar]
- 115.Aboussahoud W, Bruce C, Elliott S, Fazeli A. Activation of Toll-like receptor 5 decreases the attachment of human trophoblast cells to endometrial cells in vitro. Hum. Reprod. 2010;25:2217–2228. doi: 10.1093/humrep/deq185. [DOI] [PubMed] [Google Scholar]
- 116.Liu S, Yang X, Liu Y, Wang X, Yan Q. SLeX/L-selectin mediates adhesion in vitro implantation model. Mol. Cell. Biochem. 2011;350:185–192. doi: 10.1007/s11010-010-0697-x. [DOI] [PubMed] [Google Scholar]
- 117.Harun R, et al. Cytotrophoblast stem cell lines derived from human embryonic stem cells and their capacity to mimic invasive implantation events. Hum. Reprod. 2006;21:1349–1358. doi: 10.1093/humrep/del017. [DOI] [PubMed] [Google Scholar]
- 118.Gonzalez M, et al. Expansion of human trophoblastic spheroids is promoted by decidualized endometrial stromal cells and enhanced by heparin-binding epidermal growth factor-like growth factor and interleukin-1β. Mol. Hum. Reprod. 2011;17:421–433. doi: 10.1093/molehr/gar015. [DOI] [PubMed] [Google Scholar]
- 119.He D, et al. H19 regulates trophoblastic spheroid adhesion by competitively binding to let-7. Reproduction. 2019;157:423–430. doi: 10.1530/REP-18-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rivron NC, et al. Blastocyst-like structures generated solely from stem cells. Nature. 2018;557:106–111. doi: 10.1038/s41586-018-0051-0. [DOI] [PubMed] [Google Scholar]
- 121.Sozen B, et al. Self-organization of mouse stem cells into an extended potential blastoid. Dev. Cell. 2019;51:698–712.e8. doi: 10.1016/j.devcel.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harrison SE, Sozen B, Christodoulou N, Kyprianou C, Zernicka-Goetz M. Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science (80-.). 2017;356:eaal1810. doi: 10.1126/science.aal1810. [DOI] [PubMed] [Google Scholar]
- 123.Sozen B, et al. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol. 2018;20:1229–1229. doi: 10.1038/s41556-018-0187-z. [DOI] [PubMed] [Google Scholar]
- 124.Shahbazi MN, Siggia ED, Zernicka-Goetz M. Self-organization of stem cells into embryos: A window on early mammalian development. Science (80-.). 2019;364:948–951. doi: 10.1126/science.aax0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuan H, Corbi N, Basilico C, Dailey L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 1995;9:2635–2645. doi: 10.1101/gad.9.21.2635. [DOI] [PubMed] [Google Scholar]
- 126.Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/S0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 127.Sharma A, Kumar P. Understanding implantation window, a crucial phenomenon. J. Hum. Reprod. Sci. 2012;5:2–6. doi: 10.4103/0974-1208.97777. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 128.Vinatier, D. & Monnier, J. C. The fetal-maternal interface. Description of its elements from an immunologic perspective. J. Gynecol. Obstet. Biol. Reprod. (Paris). 19, 691–700 (1990). [PubMed]
- 129.Neal JT, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175:1972–1988.e16. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cattaneo CM, et al. Tumor organoid–T-cell coculture systems. Nat. Protoc. 2020;15:15–39. doi: 10.1038/s41596-019-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jowett GM, et al. ILC1 drive intestinal epithelial and matrix remodelling. Nat. Mater. 2020;20:250–259. doi: 10.1038/s41563-020-0783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sojka DK, Yang L, Yokoyama WM. Uterine natural killer cells. Front. Immunol. 2019;10:960. doi: 10.3389/fimmu.2019.00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zenclussen AC, Hämmerling GJ. Cellular regulation of the uterine microenvironment that enables embryo implantation. Front. Immunol. 2015;6:321. doi: 10.3389/fimmu.2015.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Meyer N, Zenclussen AC. Immune cells in the uterine remodeling: are they the target of endocrine disrupting chemicals? Front. Immunol. 2020;11:246. doi: 10.3389/fimmu.2020.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laird SM, et al. A review of immune cells and molecules in women with recurrent miscarriage. Hum. Reprod. Update. 2003;9:163–174. doi: 10.1093/humupd/dmg013. [DOI] [PubMed] [Google Scholar]
- 136.Schumacher A, Costa SD, Zenclussen AC. Endocrine factors modulating immune responses in pregnancy. Front. Immunol. 2014;5:196. doi: 10.3389/fimmu.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Geldenhuys J, Rossouw TM, Lombaard HA, Ehlers MM, Kock MM. Disruption in the regulation of immune responses in the placental subtype of preeclampsia. Front. Immunol. 2018;9:1659. doi: 10.3389/fimmu.2018.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fukui A, et al. Changes of NK cells in preeclampsia. Am. J. Reprod. Immunol. 2012;67:278–286. doi: 10.1111/j.1600-0897.2012.01120.x. [DOI] [PubMed] [Google Scholar]
- 139.Moffett-King A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 140.Kang HW, et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 141.Tamay DG, et al. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 2019;7:164. doi: 10.3389/fbioe.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim JH, et al. 3D Bioprinted human skeletal muscle constructs for muscle function restoration. Sci. Rep. 2018;8:12307. doi: 10.1038/s41598-018-29968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim JH, et al. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 2020;11:1025. doi: 10.1038/s41467-020-14930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nahmias Y, Schwartz RE, Verfaillie CM, Odde DJ. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol. Bioeng. 2005;92:129–136. doi: 10.1002/bit.20585. [DOI] [PubMed] [Google Scholar]
- 145.Kusuma, S. et al. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl. Acad. Sci. USA110, 12601–12606 (2013). [DOI] [PMC free article] [PubMed]
- 146.Kusuma, S. & Gerecht, S. Derivation of endothelial cells and pericytes from human pluripotent stem cells. Methods Mol. Biol. 1307, 213-22 (2015). [DOI] [PubMed]
- 147.Xu C, Chai W, Huang Y, Markwald RR. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol. Bioeng. 2012;109:3152–3160. doi: 10.1002/bit.24591. [DOI] [PubMed] [Google Scholar]
- 148.Kinoshita K, Iwase M, Yamada M, Yajima Y, Seki M. Fabrication of multilayered vascular tissues using microfluidic agarose hydrogel platforms. Biotechnol. J. 2016;11:1415–1423. doi: 10.1002/biot.201600083. [DOI] [PubMed] [Google Scholar]
- 149.Roudsari LC, Jeffs SE, Witt AS, Gill BJ, West JL. A 3D poly(ethylene glycol)-based tumor angiogenesis model to study the influence of vascular cells on lung tumor cell behavior. Sci. Rep. 2016;6:32726. doi: 10.1038/srep32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang YS, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 152.Wu W, Deconinck A, Lewis JA. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011;23:H178–H183. doi: 10.1002/adma.201004625. [DOI] [PubMed] [Google Scholar]
- 153.Bertassoni LE, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–2211. doi: 10.1039/C4LC00030G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li S, Liu YY, Liu LJ, Hu QX. A versatile method for fabricating tissue engineering scaffolds with a three-dimensional channel for prevasculature networks. ACS Appl. Mater. Interfaces. 2016;8:25096–25103. doi: 10.1021/acsami.6b07725. [DOI] [PubMed] [Google Scholar]
- 155.Miller JS, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kolesky, D. B., Homan, K. A., Skylar-Scott, M. A. & Lewis, J. A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA113, (2016). [DOI] [PMC free article] [PubMed]
- 157.Liu Y, Gill E, Huang YYS. Microfluidic on-chip biomimicry for 3D cell culture: A fit-for-purpose investigation from the end user standpoint. Future Sci. OA. 2017;3:FSO173. doi: 10.4155/fsoa-2016-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Deglincerti A, et al. Self-organization of human embryonic stem cells on micropatterns. Nat. Protoc. 2016;11:2223–2232. doi: 10.1038/nprot.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods. 2014;11:847–854. doi: 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat. Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 161.Dittrich PS, Manz A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006;5:210–218. doi: 10.1038/nrd1985. [DOI] [PubMed] [Google Scholar]
- 162.Mancini V, Pensabene V. Organs-on-chip models of the female reproductive system. Bioengineering. 2019;6:103. doi: 10.3390/bioengineering6040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gnecco JS, et al. Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann. Biomed. Eng. 2017;45:1758–1769. doi: 10.1007/s10439-017-1797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zheng Y, et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature. 2019;573:421–425. doi: 10.1038/s41586-019-1535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xiao S, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017;8:14584. doi: 10.1038/ncomms14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ansari AH, Gould K, Turner RJ. Segmental uterine horn replacement in rabbit using umbilical vein. Obstet. Gynecol. 1982;60:733–737. [PubMed] [Google Scholar]
- 167.Taveau JW, et al. Regeneration of uterine horn using porcine small intestinal submucosa grafts in rabbits. J. Investig. Surg. 2004;17:81–92. doi: 10.1080/08941930490422456. [DOI] [PubMed] [Google Scholar]
- 168.Magalhaes RS, Williams JK, Yoo KW, Yoo JJ, Atala A. A tissue-engineered uterus supports live births in rabbits. Nat. Biotechnol. 2020;38:1280–1287. doi: 10.1038/s41587-020-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fageeh W, Raffa H, Jabbad H, Marzouki A. Transplantation of the human uterus. Int. J. Gynecol. Obstet. 2002;76:245–251. doi: 10.1016/S0020-7292(01)00597-5. [DOI] [PubMed] [Google Scholar]
- 170.Ozkan O, et al. Preliminary results of the first human uterus transplantation from a multiorgan donor. Fertil. Steril. 2013;99:470–476. doi: 10.1016/j.fertnstert.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 171.Brännström M, et al. First clinical uterus transplantation trial: a six-month report. Fertil. Steril. 2014;101:1228–1236. doi: 10.1016/j.fertnstert.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 172.Brännström M, et al. Uterus transplantation: a rapidly expanding field. Transplantation. 2018;102:569–577. doi: 10.1097/TP.0000000000002035. [DOI] [PubMed] [Google Scholar]
- 173.Brännström M, et al. One uterus bridging three generations: first live birth after mother-to-daughter uterus transplantation. Fertil. Steril. 2016;106:261–266. doi: 10.1016/j.fertnstert.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 174.Ejzenberg D, et al. Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Obstet. Gynecol. Surv. 2019;74:261–263. doi: 10.1097/01.ogx.0000557824.84633.0b. [DOI] [PubMed] [Google Scholar]
- 175.Testa G, et al. First live birth after uterus transplantation in the United States. Am. J. Transplant. 2018;18:1270–1274. doi: 10.1111/ajt.14737. [DOI] [PubMed] [Google Scholar]
- 176.Petersen TH, et al. Tissue-engineered lungs for in vivo implantation. Science (80-.). 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ott, H. C. et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 16, 927–933 (2010). [DOI] [PubMed]
- 178.Scarritt ME, et al. Re-endothelialization of rat lung scaffolds through passive, gravity-driven seeding of segment-specific pulmonary endothelial cells. J. Tissue Eng. Regen. Med. 2018;12:786. doi: 10.1002/term.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wallis JM, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng. - Part C. 2012;18:420–432. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nagao RJ, et al. Preservation of capillary-beds in rat lung tissue using optimized chemical decellularization. J. Mater. Chem. B. 2013;1:4801–4808. doi: 10.1039/c3tb20640h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Uriarte, J. J., Uhl, F. E., Rolandsson Enes, S. E., Pouliot, R. A. & Weiss, D. J. Lung bioengineering: advances and challenges in lung decellularization and recellularization. Curr. Opin. Organ Transplant. 23, 673–678 (2018). [DOI] [PMC free article] [PubMed]
- 182.Ott HC, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 183.VeDepo MC, Detamore MS, Hopkins RA, Converse GL. Recellularization of decellularized heart valves: progress toward the tissue-engineered heart valve. J. Tissue Eng. 2017;8:2041731417726327. doi: 10.1177/2041731417726327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Robertson MJ, Dries-Devlin JL, Kren SM, Burchfield JS, Taylor DA. Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS ONE. 2014;9:e90406. doi: 10.1371/journal.pone.0090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Uygun, B. E. et al. Decellularization and recellularization of whole Livers. J. Vis. Exp. 10.3791/2394 (2010). [DOI] [PMC free article] [PubMed]
- 186.Nieponice A, et al. Patch esophagoplasty: esophageal reconstruction using biologic scaffolds. Ann. Thorac. Surg. 2014;97:283–288. doi: 10.1016/j.athoracsur.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 187.Hung SH, Su CH, Lin SE, Tseng H. Preliminary experiences in trachea scaffold tissue engineering with segmental organ decellularization. Laryngoscope. 2016;126:2520–2527. doi: 10.1002/lary.25932. [DOI] [PubMed] [Google Scholar]
- 188.Yoo, J. J., Meng, J., Oberpenning, F. & Atala, A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology51, 221–225 (1998). [DOI] [PubMed]
- 189.Tiemann TT, et al. Towards uterus tissue engineering: a comparative study of sheep uterus decellularisation. Mol. Hum. Reprod. 2020;26:167–178. doi: 10.1093/molehr/gaaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Raya-Rivera AM, et al. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet. 2014;384:329–336. doi: 10.1016/S0140-6736(14)60542-0. [DOI] [PubMed] [Google Scholar]
- 191.Campo H, et al. De- and recellularization of the pig uterus: a bioengineering pilot study. Biol. Reprod. 2017;96:34–45. doi: 10.1095/biolre/bio143396. [DOI] [PubMed] [Google Scholar]
- 192.Miyazaki K, Maruyama T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials. 2014;35:8791–8800. doi: 10.1016/j.biomaterials.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 193.Scarritt ME, Pashos NC, Bunnell BA. A review of cellularization strategies for tissue engineering of whole organs. Front. Bioeng. Biotechnol. 2015;3:43. doi: 10.3389/fbioe.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Warnock, M. Report of the Committee of Inquiry Into Human Fertilisation and Embryology, Vol. 9314 (HM Stationery Office, 1984).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.