Abstract
Many patients who have suffered from acute COVID infections have long-lasting symptoms affecting several organs including the brain. This long COVID status can include “brain fog” and cognitive deficits that can disturb activities of daily living and can delay complete recovery. Here, we report two cases of neurological long COVID with abnormal FDG PET findings marked by hypometabolic regions of the cingulate cortex.
Keywords: Long COVID, Cognitive involvement, Cingulate cortex
Introduction
The COVID pandemic has reached tens of millions of people around the world and has resulted in the deaths of several millions people [4]. Long-term complications are now described as the long COVID and affect many organs such as the heart, brain, kidney, pancreas and digestive system [8]. The post-acute COVID syndrome or long COVID syndrome [11] has been described at debated. Clinical signs can include fatigue, dyspnea, myalgia, diffuse pain, headaches, anxiety/depression and cognitive impairments (brain fog) [5]. In some cases additional explorations are normal. Long COVID can be seen not only in patients who have been hospitalized in an intensive care unit but also in patients who have had less serious forms that did not require hospital admissions.
The neurological complications of COVID include damage to the central nervous system (CNS) and the peripheral nervous system encompassing stokes, encephalitis, myelitis, myositis, Guillain Barré syndromes, and cognitive impairments. The pathophysiology of the disease is complex, incorporating direct viral neuronal damage, neuroinflammation, rupture of the blood brain barrier, microvasculitis and hypoxia [2, 5]. In the etiology of neurocognitive disorders occurring at a distance from the acute episode of COVID, involvement of the cingulate cortex has been little described, and we report here the cases of two patients who developed neurocognitive disorders after their acute COVID infectious episode with positive PCR. These patients had no risk factors such as obesity, diabetes or hypertension.
Patients and methods
Patient 1: a 45-year-old man in good health with no pathological history suffers from COVID in March 2020. After a week of progress with cough, fever, he was admitted to hospital due to dyspnea and hypoxia and put on oxygen therapy for 7 days. Blood C reactive protein (CRP) levels were increased during the acute phase. Then, he returns home and gradually develops cognitive disorders combining memory problems, slowness of ideation, general fatigue, anxiety, and depression without anosmia. Serotonin reuptake inhibitor therapy was introduced. In October 2020, the disorders persisted, and the neurological examination and the brain MRI was normal. Hospital anxiety and depression scale (HADS) was normal for depression and slightly increased for anxiety. Extensive neuropsychological tests revealed mildly affected episodic and visuo-spatial memory and a deficit of executive functions. EEG showed theta waves in the left temporal region and the FDG PET scan revealed abnormal hypometabolic regions (Fig. 1).
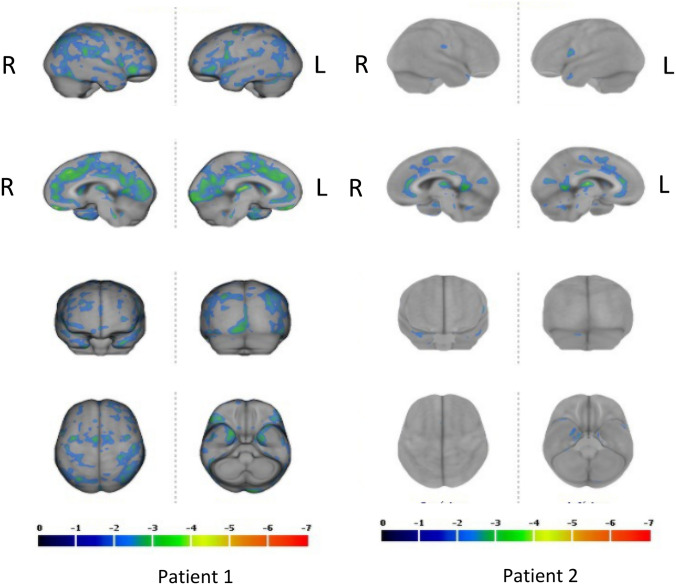
Fig. 1.
The cerebral Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) of patients 1 and 2. In patient 1, the posterior cingulate cortex and the precuneus are the most hypometabolic regions. In patient 2, anterior and posterior cingulate cortex are involved (more than 2SD)
Patient 2: a 43-year-old woman with no clinical history suffers from COVID in May 2020 with cough, fever, and fatigue for 7 days without anosmia. Blood CRP levels were increased during the acute phase. The signs gradually disappeared but in August 2020 general fatigue, walking and memory problems and speech deficit occurred. In November, brain and spinal cord MRIs were normal as well as the electromyogram. In January 2021, HADS scale was normal for anxiety and depression and extensive neuropsychological tests revealed impaired episodic memory and executive functions. The FDG PET scan shows abnormal hypometabolic areas (Fig. 1).
Results
The results are depicted in Figs. 1 and 2. Both MRI were found normal (Fig. 2). In the first patient (left panels), statistically significant hypometabolic regions (more than SD; as compared to normal control individuals) were localized in the anterior and posterior cingulate cortex as well as in the precuneus. Other cortical regions were less severely affected. In the second patients (right panels), hypometabolic regions were located in the anterior cingulate cortex. The posterior cingulate cortex was less severely affected (Fig. 1).
Fig. 2.
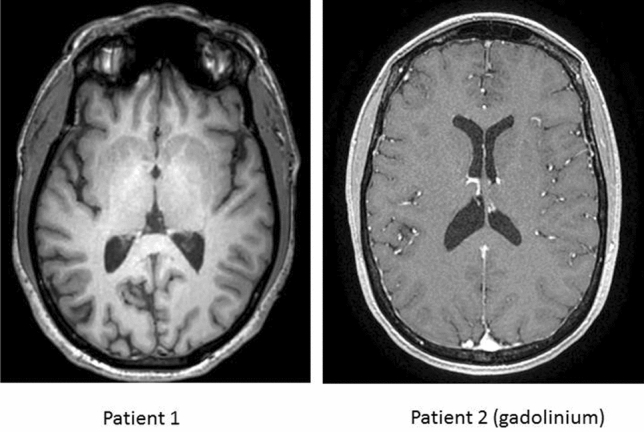
Brain MRI of patient 1 (left panel and patient 2 (right panel). Both MRI were found normal
Discussion
Both patients complained of brain fog and cognitive symptoms confirmed by neuropsychological tests and which could be related at least to the dysfunction of the cingulate cortex as revealed cerebral FDG PET scans. It is interesting to note that MRI were considered in the normal range in both cases. The cingulate cortex is involved in many cognitive functions. Previous experimental and clinical studies have shown that the anterior and posterior cingulate cortex are implicated in emotions, memory, depression, and decision of action [7, 7]. The anterior cingulate cortex receives inputs from the orbitofrontal cortex in the outcome rewards. The posterior cingulate cortex has outcomes towards the hippocampus. These deficient brain connections could explain the cognitive signs observed in these patients and characterized by episodic memory deficits and abnormal executive and attentional functions. Hypometabolisms of the cingulate cortex have been observed in several neurological and psychiatric diseases including mild cognitive impairment due to Alzheimer’s disease, severe depression and internet gaming disorder [1, 3, 6, 10]. Cortical hypometabolisms were clearly more pronounced in the first patient who experienced a more severe COVID evolution. Patient 1 was admitted for several days and received oxygen supplementation, whereas patient 2 has been hill at home for 2 weeks. Hypometabolic PET regions are more widespread in patient 1 affecting the cingulate, the precuneus and some other cortical regions, whereas only the cingulate cortex was affected in patient 2.The nature of the brain lesions linked to cognitive signs and abnormal PET results are unknown in these two cases. Whereas probable hypoxia was present in the first case, it was absent in the second patient. Delayed neuroinflammation could also be a possible cause to explain longstanding brain dysfunctions. In the future, in COVID patients developing neurocognitive disorders, it seems important to evaluate brain metabolisms with PET scans at least to verify the integrity of the cingulate cortex (and other regions) and to implement appropriate cognitive remediation in these patients.
Declarations
Conflicts of interest
The authors declare no conflict of interests regarding this work.
References
- 1.Boeve BF. Mild cognitive impairment associated with underlying Alzheimer's disease versus Lewy body disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S41–44. doi: 10.1016/S1353-8020(11)70015-3. [DOI] [PubMed] [Google Scholar]
- 2.Boldrini M, Canoll PD, Klein RS. How COVID-19 affects the brain. JAMA Psychiat. 2021 doi: 10.1001/jamapsychiatry.2021.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutinho AMN, Porto FHG, Zampieri PF, Otaduy MC, Perroco TR, Oliveira MO, Nunes RF, Pinheiro TL, Bottino CMC, Leite CC, Buchpiguel CA. Analysis of the posterior cingulate cortex with [18F]FDG-PET and Naa/mI in mild cognitive impairment and Alzheimer's disease: correlations and differences between the two methods. Dement Neuropsychol. 2015;9:385–393. doi: 10.1590/1980-57642015DN94000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SK, Jeong H, Im JJ, Lee SH, Chung YA. PET hypometabolism of the prefrontal-cingulate cortices in internet gaming disorder. Front Psychiatry. 2020;11:566518. doi: 10.3389/fpsyt.2020.566518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lichenstein SD, Verstynen T, Forbes EE. Adolescent brain development and depression: a case for the importance of connectivity of the anterior cingulate cortex. Neurosci Biobehav Rev. 2016;70:271–287. doi: 10.1016/j.neubiorev.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021 doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolls ET. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct. 2019;224:3001–3018. doi: 10.1007/s00429-019-01945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su L, Cai Y, Xu Y, Dutt A, Shi S, Bramon E. Cerebral metabolism in major depressive disorder: a voxel-based meta-analysis of positron emission tomography studies. BMC Psychiatry. 2014;14:321. doi: 10.1186/s12888-014-0321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taribagil P, Creer D, Tahir H. 'Long COVID' syndrome. BMJ Case Rep. 2021;14:e241485. doi: 10.1136/bcr-2020-241485. [DOI] [PMC free article] [PubMed] [Google Scholar]