Abstract
Introduction
One of the most compelling causes of perinatal mortality and morbidity is intrauterine growth restriction (IUGR). IUGR is linked with numerous health challenges that last lifelong, including neurodevelopmental impairment and a high incidence of brain dysfunction. There is mounting evidence that places the glutamatergic system at the center of the neurobiology and treatment of neurological diseases. Therefore, this study investigated the effects of postnatal glutathione intervention on the spatial memory and the expressions of vesicular glutamate transporter 1 (VGLUT1) in the hippocampus and the cerebellar cortex of Nω-nitro-L-arginine methyl (L-NAME)-induced rat model of IUGR.
Materials and Method
Twelve adult female rats were divided into Control and L-NAME groups; each containing 6 female rats. The control group received a single daily dose of normal saline while the L-NAME group was administered 50 mg/kg L-NAME daily from gestational day 9 until parturition. Offspring of the control rats were given free access to feeds while offspring from the L-NAME group were assigned into 3 groups: G1: given free access to feeds; G2 and G3 were administered 1.5 mg/kg body weight of glutathione from postnatal day (PND) 4–9 and PND 25–31 respectively. At the end of the intervention, Y-maze was conducted, and the rats euthanized on PND 35. The brain sections were processed, and immunofluorescence staining was performed using the Vectafluor Excel R.T.U Antibody kit.
Results
IUGR caused a significant 31.1% decrease in spontaneous alternation percentage (SAP), while early treatment with glutathione at PND 4–9 significantly (p < 0.01) increased SAP, while late treatment at PND 25–9 significantly decreased SAP compared to IUGR group. Furthermore, IUGR caused significant (p < 0.001) downregulation in corrected total cell fluorescence (CTCF) of VGLUT1 in both the hippocampus and cerebellar cortex. While treatment with glutathione caused upregulation in CTCF of VGLUT1 in the hippocampus and the cerebellar cortex.
Conclusion
Our results showed that early intervention with glutathione has significant therapeutic potential via upregulation of VGLUT1 expression in both hippocampus and cerebellar cortex, which positively correlated with enhanced spatial memory in IUGR rat model.
Keywords: IUGR, VGLUT1, Hippocampus, Cerebellar cortex, L-NAME, Glutathione
Highlights
-
•
IUGR is associated with health challenges; including neurodevelopmental impairment and a high incidence of brain dysfunction.
-
•
The role of postnatal glutathione intervention on neurodevelopment was evaluated in L-NAME induced rat model of IUGR.
-
•
IUGR downregulated the expression of VGLUT1 in both the hippocampus and cerebellar cortex and also impaired spatial memory.
-
•
Early intervention with GSH provides a promising remedial pathway to neuroprotection and symptomatic treatment in the IUGR rat model.
1. Introduction
Intrauterine (fetal) growth restriction (IUGR, FGR) is a complicated obstetrics challenge caused by a multiple set of maternal and fetal clinical pathologies. Common consequences of IUGR include neonatal mortality, stillbirth, poor neurological, and cardiovascular outcomes (Fleiss et al., 2019). The incidence of IUGR in high-income countries is between 3% and 9% of pregnancies, but dreadfully high, up to 30% of pregnancies in low-income countries (Fleiss et al., 2019). Globally, there are over 20 million Low birth weights (LBW) annually (UNICEF global database, 2012), with India, Pakistan, and Nigeria constituting about half of the LBW births (Black, 2015). The improved imaging techniques have increased our understanding of the clinical progression of IUGR and its poor neurodevelopmental outcomes (Fleiss et al., 2019).
Mounting research evidence has placed the glutamatergic system at the center of the neurobiology and treatment of diseases. This system is a powerful neuronal excitotoxin acclaimed to prompt accelerated or delayed neurotoxicity. Glutamatergic transmission is pivotal for regulating neuronal activity. The stored glutamate (Glu) is released from the synaptic vesicles by stimulation. The family of vesicular Glu transporters in mammals comprises three highly homologous proteins: VGLUT1–3 is responsible for the glutamatergic system’s homeostasis in the membrane of synaptic vesicles. VGLUT1 is a biochemical marker of glutamatergic neurons and glutamatergic synapses specifically (Orrego and Villanueva, 1993, Sanacora et al., 2008, Krystal et al., 1999, Du et al., 2020), and it is the most widely distributed in the cerebral and cerebellar cortices and hippocampus (Wojcik et al., 2004). Therefore, VGLUT1 holds the key to unlock new ideas and unveils the pathogenesis and preventive measures of many neurological diseases (Du et al., 2020).
Accumulating evidence shows the significant role of the cerebellum in spatial navigation. This role has already been established at the hippocampal navigation level using behavioral, electrophysiological, and anatomical analyses in human and animal models (Rochefort et al., 2013a, Rochefort et al., 2013b).
The imbalance between oxidants and antioxidants is at the heart of oxidative stress. The import of oxidative stress state lies in the consequential oxidative cellular damage and associated sequelae that commences with cellular dysfunction and, ultimately, cell death (Ng et al., 2008).
The oxidative stress mechanisms provide viable treatment targets that might apply to multiple disorders, with dominant cellular antioxidant glutathione (GSH) stands out as a promising candidate (Ng et al., 2008). The need to understand IUGR pathological mechanisms is crucial for tailoring therapies to prevent or treat IUGR, and in particular, to reduce brain injury. Therefore, this study investigated the effects of postnatal glutathione intervention on the spatial memory and the expressions of VGLUT1 in the hippocampus and the cerebellar cortex of Nω-nitro-L-arginine methyl (L-NAME)-induced rat model of IUGR. This model was chosen because, unlike many other models of hypertension, it produced a dose-dependent hypertension in both non-pregnant and pregnant rats, which is maintained throughout pregnancy. This sustained hypertension leads to placental vascular abnormalities resulting in impaired maternal-fetal exchanges and consequently IUGR.
2. Materials and methods
2.1. Animals
The Research and Ethical Committee of the Olabisi Onabanjo University, Ago-Iwoye, approved (OOU/IREC/2019/45) all animal experiments performed in the present study. Twelve adult female Sprague-Dawley rats with comparable weights (230–260 g) and 6 male rats (320–360 g) were used in this experiment. This animal model was chosen as it combines the advantage of the short reproductive cycle (22 days) of the rat with the ease of maintenance. Animals were allowed free access to food and water in standard laboratory conditions throughout the entire experiment. Female rats were paired with male rats in the ratio of 2:1 and mated overnight for a maximum of 4 days; female rats were examined the next morning. Mating was confirmed by the visualization of spermatozoa in a vaginal smear and labeled as day 0 of pregnancy.
2.2. Intervention
Following confirmation of pregnancy, rats were randomly assigned two groups: group 1 served as a control group (no treatment or placebo). Group 2 received daily L-NAME at a dose of 50 mg/kg/d by gavage, beginning on day 9 of pregnancy and ending on day 19. The control group were given saline solution alone. Both groups were administered once daily until parturition.
2.3. Monitoring
Blood pressure was measured using a tail-cuff BP monitor (MRBP, IITC Life Sciences Inc., USA); the baseline systolic blood pressures were obtained on gestation day 8. Blood pressure measurements were repeated on and at gestational day (GD) 14 and 19. After the measurement of blood pressure, rats were placed singly in metabolic cages for a 24-h urine sample. Proteinuria measured using Labtex, LabMaxPlenno, Lagoa-Santa Brazil on GD 8, 14, and 19.
The rats in the control and LNAME were allowed to litter; after delivery, litters confirmed with IUGR from the LNAME mothers were recruited for the study. Litters from the control rats were given food and water ad libitum. The IUGR litters were randomly assigned into 3 groups; G1 served as the IUGR control litters; G2 litters were administered glutathione from postnatal day (PND) 4–9; G3 litters were administered glutathione from PND 25–31. Glutathione.
(GSH) was administered as (1.5 g /kg/ day i.p). GSH is a safe dietary supplement, its lethal dose 50 (LD50) in rodents is more than 5 g/kg (Weschawalit et al., 2017).
All litters were weighed daily during the period of administration to monitor the effect of glutathione on body weight. At the end of the intervention, the adolescent rats were subjected to neurobehavioral tests and subsequently euthanized, and brains dissected and fixed in a phosphate buffer formalin.
2.4. Drugs and reagents
Procurement of L-NAME was from Sigma, St Louis, MO, USA. Ketamine, xylazine, and temgesic were obtained from Sigma (ST. Louis MO, USA). Vectafluor Excel R.T.U Antibody kit DyLight 488 Anti-Mouse IgG (DK-2488) was purchased from Vector Laboratories (USA), Anti-VGLUT1 (ab77822)was purchased from AbCam (AbCam, Cambridge, MA).
2.5. Euthanization and sample preparation
Rats were anesthetized with a ketamine (125 mg/Kg body weight) −xylazine (5 mg/Kg body weight). The brain tissues were transcardially perfused and fixed with phosphate-buffered saline (PBS) followed by 10% Neutral buffered formalin (NBF) (Andrade‐Valença et al., 2008). The excised brain tissues were then post-fixed in 10% NBF until further analysis.
2.6. Immunofluorescence
Coronal section, starting from antero-posterior (AP) coordinate = −2.52 mm to −3.48 mm posterior to the Bregma for CA3 region of the hippocampus and AP coordinate = −6.00 mm to −7.50 mm posterior to the Bregma for the cerebellum were excised (Paxinos and Watson, 2006).
Excised brain tissues fixed in 10% NBF were histologically processed using the automated tissue processor (Leica TP1020) to ensure adequate dehydration, clearing, and infiltration and subsequently embedded in paraffin wax. Coronal Section (3 μm thick) of the paraffin blocked brain samples were cut using a rotary microtome Leica RM2145 (Leica, Germany), placed on the water bath (Leica HI1210) set at 37 °C to produce sections free of folds. The sections were transferred to pre-coated slides and gently blotted to avoid the formation of wrinkles, and the slides were finally heated on the hot plate (Leica HI1220) set at 54 °C to ensure proper adherence of tissue sections to the slide, hence hindering possible fall-off during subsequent treatments. Sections were deparaffinized and rehydrated through xylene and descending ethanol series and immersed into preheated Vector antigen unmasking solution (H-3300), incubated for 20 min at 97 °C, and allowed to cool at room temperature. Sections were then washed in 0.01 M phosphate-buffered saline (PBS) for 5 min. Protein blocking was performed by incubating sections for 20 min with 2.5% Normal horse serum, with the excess serum from sections being tipped off. Incubation with primary antibodies; mouse Anti-VGLUT1 overnight, diluted at 1:100. Sections were washed for 5 min in PBS and incubated for 15 min with an amplifier antibody. The sections were re-washed for 5 min in PBS and incubated for 30 min with VectaFluor reagent. Sections were then washed for 5 min twice in PBS and mounted in VectaShield mounting media containing 4′, 6-diamidino-2-phenylindole (DAPI), and then allowed to cure at room temperature. Negative controls were processed likewise, but the incubation with primary antibodies was omitted.
2.7. Photomicrography and image quantification
Sections were viewed and images captured with an Axioscope A1 microscope (Carl Zeiss, Germany) around the CA3 region of the hippocampus. Photomicrographs were analyzed using custom-written scripts for Fiji/ImageJ (NIH, Bethesda, MD, USA). The corrected total cell fluorescence (CTCF) was calculated using the formula: CTCF = Integrated Density – (Area of selected cell x Mean fluorescence of background readings) (Hammond, 2014).
2.8. Statistical analysis
Data were analyzed using Student t-test or one-way ANOVA followed by Tukey’s posthoc test, where applicable using the GraphPad Prism version 7.0 (GraphPad Inc, USA) statistical software package. Results are expressed as mean ±SEM, and p < 0.05 was considered significant.
3. Results
3.1. L-NAME-induced hypertension and proteinuria in pregnant mice
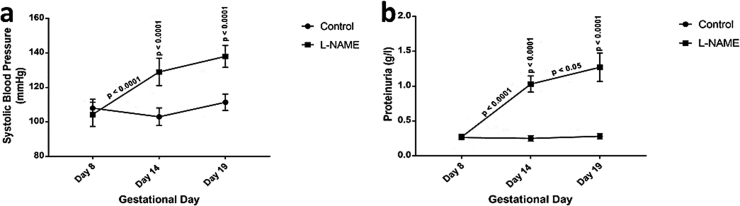
The mean SBP measured by tail-cuff methods and analyzed by a two-way-ANOVA showed significant interaction [F (2, 24) = 66.17 p < 0.0001] between treatments and gestational days. Significant day effect [F (2, 24) = 68/82 p < 0.0001] was also observed between the gestational days. In addition, significant treatment effect [F (1, 24) = 68.82 p < 0.0001] was noted between the LNAME and the control. Tukey’s posthoc test showed significantly (p < 0.0001) higher SBP for the IUGR rats at GD 14 (129 ± 5.4 mm Hg) and GD 19 (138 ± 4.3 mm Hg) compared to their respective controls (109 ± 3.8 mm Hg and 111.4 ± 2.9 mm Hg) (See Fig. 1a).
Fig. 1.
Mean Systolic blood pressure and proteinuria in pregnant mice.Each point represents mean ± S.E.M, with a significant difference at p < 0.05 (*) & p < 0.0001 (****).
The proteinuria level assessed by a dipstick method and analysed by a two way-ANOVA showed significantly interaction [F (2, 24) = 19.78 p < 0.0001] between treatments and gestational days. Significant day effect [F (2, 24) = 22.73 p < 0.0001] was also observed between the gestational days. In addition, significant treatment effect [F (1, 24) = 53.1 p < 0.0001] was noted between the LNAME and the control. Tukey’s post-hoc test showed significantly (p < 0.0001) higher for IUGR rats at GD 14 (1.03 ± 0.36 g/l) and GD 19 (1.27 ± 0.30 g/l) compared to their respective controls (0.25 ± 0.07 g/l and 0.28 ± 0.05 g/l) (See Fig. 1b).
3.2. Glutathione remediates IUGR associated spatial memory impairment
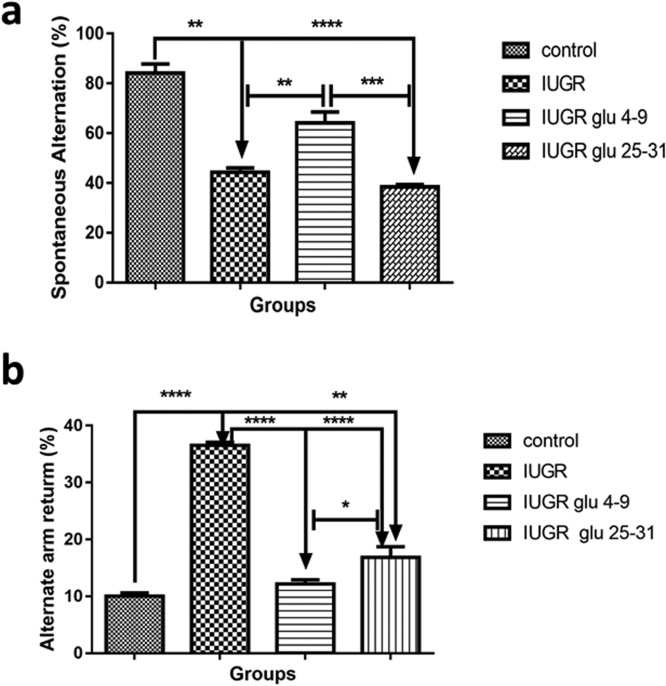
One-way ANOVA showed a significant [F (3, 20) = 16.91, p = 0.0005] difference between the groups means. While Tukey's multiple comparisons tests showed significant (p < 0.01) decrease in the spontaneous alternation percentage (SAP) in the IUGR (44.3. % loss) and IUGR+Glutathione PND25–31(38.4% loss) rats, when compared to the control rats. Early treatment (IUGR+Glutathione PND4–9)caused significant (p < 0.01) constituting about 50% increase in SAP when compared with the IUGR rats as shown in Fig. 2a.
Fig. 2.
Percentage spontaneous alternation and alternate arm return in adolescence IUGR mice.Each point represents mean ±S.E.M, with a significant difference at p < 0.05 (*), p < 0.01 (**), p < 0.001 (***)&p < 0.0001 (****).
Alternate arm return (AAR) using Y maze is use to test for memory impairment. The percentage of impairment is calculated as ({AAR/total number of entry-2} X100). We observed a significant increase in the percentage impairments at p < 0.0001 in the IUGR rats compared to the control. However, both early and late treatment with glutathione significantly (p < 0.0001) reduced the percentage impairments, see Fig. 2b.
3.3. Glutathione enhances relative brain weights in IUGR rats
A significant decrease (p < 0.01) accounting for 20.6% and 22.3% loss in the relative brain weights was observed in the IUGR and IUGR+Glutathione PND25–31 groups, respectively, when compared to the control. While a significant increase (p < 0.05) in the relative brain weights was noted in the IUGR+Glutathione PND4–9 group when compared to the IUGR group, see Fig. 3.
Fig. 3.
Percentage spontaneous alternation and alternate arm return in adolescence IUGR mice.Each point represents mean ±S.E.M, with a significant difference at p < 0.05 (*) & p < 0.01 (**).
3.4. Early treatment with GSH mitigates the IUGR induced downregulated expressions of VGLUT1 in
-
(a)
the hippocampus
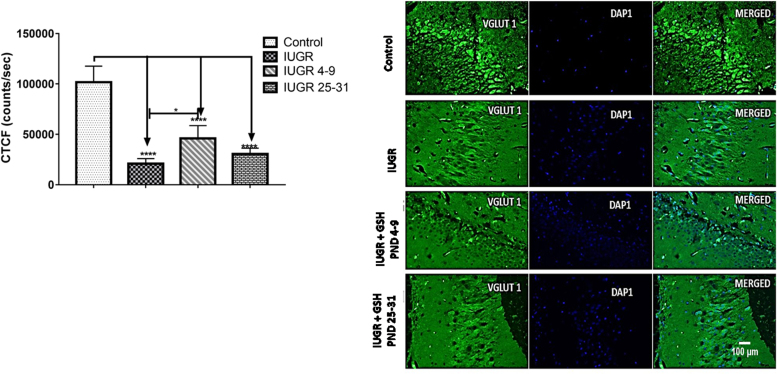
One-way ANOVA showed a significant [F (3, 20) = 68.20, p < 0.0001] difference between the groups means. While Tukey's multiple comparisons tests showed significant (p < 0.0001) downregulation in corrected total cell fluorescence (CTCF) of positive VGLUT1 cells expression in the hippocampus of the IUGR (65.5% loss), IUGR+ Glutathione PND4–9 (37.7% loss), and IUGR+Glutathione PND25–31(53.8% loss), rats when compared to the control rats. Significant (p = 0.0101) constituting about 37.3% upregulation in CTCF positive VGLUT1 cells expression in the hippocampus of IUGR+ Glutathione PND4–9 was noted when compared with the IUGR rats as shown in Fig. 4.
-
(a)
the cerebellar cortex.
Fig. 4.
Representative images showing CA3 region labeled by vesicular glutamate transporter 1 (VGLUT1) immunofluorescence in the hippocampus (scale bar: 100 µm). Mean hippocampal VGLUT1 corrected total Cell fluorescence. Each bar represents mean ±S.E.M, with a significant difference at p < 0.05 (*) & p < 0.0001 (****).
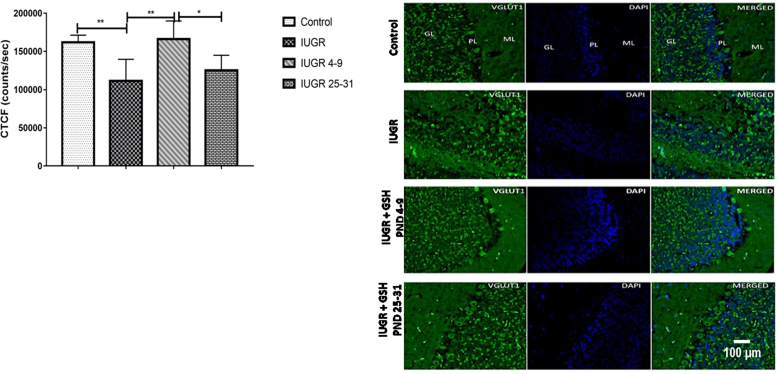
One-way ANOVA showed a significant [F (3, 19) = 8.831, p = 0.0007] difference between the groups means. While Tukey's multiple comparisons tests showed significant (p < 0.006) downregulation in corrected total cell fluorescence (CTCF) of positive VGLUT1 cells expression in the cerebellum of the IUGR rats by about 36.9% when compared with the control; Significant (p = 0.0019) downregulation accounting for about 28.0% loss of CTCF in IUGR+Glutathione PND25–31 when compared with Glutathione PND4–9. We also noted significant upregulation in CTCF positive VGLUT1 cell expression constituting about 28.1% increase CTFC in the hippocampus of IUGR + Glutathione PND4–9 when compared with the IUGR rats, as shown in Fig. 5.
Fig. 5.
Representative images showing cortical layers labeled by vesicular glutamate transporter 1 (VGLUT1) immunofluorescence in the cerebellum (scale bar: 100 µm). Mean cerebellar VGLUT1 corrected total Cell fluorescence. Each bar represents mean ±S.E.M, with a significant difference at p < 0.05 (*) & p < 0.01 (**).
4. Discussion
The intrauterine fetal environment could provide perfect or adverse conditions for neurodevelopment (Shallie and Naicker, 2019). Our findings showed IUGR caused impaired spatial memory, reduced relative brain weight, and downregulated expression of VGLUT1 in the hippocampus and cerebellar cortex in adolescent rats. These results could be due to the poor neurodevelopmental outcomes associated with IUGR (Fleiss et al., 2019). There is a significant increase in neurodevelopmental deficits, such as cognitive and learning impairments, and cerebral palsy in infants born with IUGR (Fleiss et al., 2019). This study induced a symmetrical rat model of IUGR, which affected both the brain and the body weights, as shown by the significantly reduced relative brain weights in the IUGR group. This model does not spare the brain from the damaging effects of IUGR during brain development and is associated with worse neurological outcomes. Damaging stimuli during development, such as IUGR, affect the trophic actions of secreted factors such as serotonin, tryptophan, GABAergic synapse, and glutamate metabolism. All these alter brain development trajectories, leading to loss of cells, processes, and factors that are vital building blocks of the brain. Ultimately, this impairs microglia's normal function in promoting proliferation, pathfinding, myelination, and synaptogenesis (Fleiss et al., 2019, Pedroso et al., 2019).
To understand the impaired spatial memory noted in our study, we assessed GLU's role by examining the expression of VGLUT1. In healthy states, Glu plays a significant role in synaptic plasticity, learning, and memory, and it is vital in the pathophysiology of neurological diseases. The downregulated expression of VGLUT1 in the hippocampus and the cerebellum observed in this study could be due to the vital role VGLUT1 plays in the learning and memory, principally by affecting synaptic Glu transport and long-term potentiation (Cheng et al., 2011, Du et al., 2020). Moreover, it can affect the development of various neurological diseases (Hermann and Chopp, 2012, Castillo et al., 2003, Hossmann, 2006, Du et al., 2020). The hippocampus and the cerebellar network is implicated in the emergence of goal-directed actions from the Spatio-temporal organization of self-motion information (Babayan et al., 2017). In this network, the cerebellum supports learning of the sequence, taking into account contextual information from the hippocampus. In contrast, the hippocampus reciprocates by prompting previous events' memory to differentiate intersections in sequence-based navigation (Babayan et al., 2017, Rochefort et al., 2013a, Rochefort et al., 2013b, Rondi-Reig et al., 2014).
Currently, there are no effective medical interventions for IUGR, so we initiated an early and late postnatal intervention with an antioxidant. Antioxidant's choice was predicated based on a previous study that implicated oxidative stress in fetal programming as supported by epidemiological evidence of oxidant indices and low birth weight in preeclampsia (Roberts and Lain, 2002). Therefore, oxidative stress may be a connecting link between intrauterine insult and programming consequences after birth (Thompson and Al-Hasan, 2012). In selecting a suitable treatment that targets oxidative stress, the wide variability in antioxidant properties must be considered.
In this study, we selected GSH as a suitable target for IUGR induced oxidative stress. Our results indicated that early postnatal (PND 4–9) treatment with GSH was able to ameliorate the adverse effect of IUGR on spatial memory, relative brain, and on the expression of VGLUT1. PND 4–9 corresponds to the peak brain growth spur (Most active period of brain development). This period also signifies the most venerable period (Bockhorst et al., 2008, Semple et al., 2013), and invariably affect the normal development of the hippocampal and cerebellar structures with the attendant's impairments observed in this study. While late treatment (PND 25–31) corresponds to the period of structural maturation (Maximum volume of gray matter and cortical thickness), which represent the later and inactive period of brain development (Tsujimoto, 2008, Bansal and Zoeller, 2008, Semple et al., 2013). This late intervention, however, could not produce any significant remedial effect. Glutathione is mechanistically the most generic cellular antioxidant, which enhances its appeal as a treatment target in the absence of a definite understanding of more fundamental oxidative stress mechanisms (Ng et al., 2008). The generation of reactive oxygen species (ROS) is usually balanced by the cell's antioxidant defense mechanisms, which maintains its redox state and is vital in physiological regulation in both the embryo and fetus (Ng et al., 2008). Thus, antioxidants provide a promising remedial pathway to neuroprotection in symptomatic treatment and might be a promising pathway.
In conclusion, IUGR remains a global health care issue. Our results showed that early intervention with GSH; an antioxidant provides a promising remedial pathway to neuroprotection and symptomatic treatment via the upregulation of VGLUT1 expression in both hippocampus and cerebellar cortex; which positively correlated with enhanced spatial memory in the IUGR rat model.
Compliance with ethical standards
All procedures performed in studies involving animals were in accordance with the institutional guidelines for the care and use of animals.
CRediT authorship contribution statement
PDS Acquired the financial support for the project leading to this publication. PDS and TN conceptualized the research ideas and formulated there search goals and aim. PDS and OFS designed the methodology, including the selection of the IUGR model. AIS and MKO conducted the research, specifically they performed the experiments including the neurobehavioral test. PDS and OFS performed the immunofluorescence technique. TN provided the study materials, reagents, and laboratory space for the analyses. PDS and AIS performed the statistical analysis. AIS and MKO prepared the initial draft. PDS, OFS and TN provided critical review and revision of the initial draft. All authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors report no declarations of interest.
Acknowledgement
The International Brain Research Organization-African Regional Committee (IBRO-ARC), 2017 and International Society funded this work for Neurochemistry/International Brain Research Organization (ISN/IBRO) Postdoctoral fellowship, 2017. We want to acknowledge the contributions of Denis Margolis and Dr. Olayemi K Ijomone.
References
- Andrade‐Valença L.P.A., Valença M.M., Velasco T.R., Carlotti Jr C.G., Assirati J.A., Galvis‐Alonso O.Y., Neder L., Cendes F., Leite J.P. Mesial temporal lobe epilepsy: clinical and neuropathologic findings of familial and sporadic forms. Epilepsia. 2008;49(6):1046–1054. doi: 10.1111/j.1528-1167.2008.01551.x. [DOI] [PubMed] [Google Scholar]
- Babayan B.M., Watilliaux A., Viejo G., Paradis A.L., Girard B., Rondi-Reig L. A hippocampo-cerebellar centred network for the learning and execution of sequence-based navigation. Sci. Rep. 2017;7(1):1–16. doi: 10.1038/s41598-017-18004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R., Zoeller R.T. Polychlorinated biphenyls (Aroclor 1254) do not uniformly produce agonist actions on thyroid hormone responses in the developing rat brain. Endocrinology. 2008;149(8):4001–4008. doi: 10.1210/en.2007-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R.E. Vol. 81. Karger Publishers; 2015. Global prevalence of small for gestational age births; pp. 1–7. (Low Birthweight Baby: Born Too Soon or Too Small). [DOI] [PubMed] [Google Scholar]
- Bockhorst K.H., Narayana P.A., Liu R., Ahobila‐Vijjula P., Ramu J., Kamel M., Wosik J., Bockhorst T., Hahn K., Hasan K.M., Perez‐Polo J.R. Early postnatal development of rat brain: in vivo diffusion tensor imaging. J. Neurosci. Res. 2008;86(7):1520–1528. doi: 10.1002/jnr.21607. [DOI] [PubMed] [Google Scholar]
- Castillo J., Alvarez-Sabin J., Davalos A., Diez-Tejedor E., Lizasoain I., Martinez-Vila E., Vivancos J., Zarranz J.J. Consensus review. Pharmacological neuroprotection in cerebral ischemia: is it still a therapeutic option? Neurologia. 2003;18(7):368–384. [PubMed] [Google Scholar]
- Cheng X.R., Yang Y., Zhou W.X., Zhang Y.X. Expression of VGLUTs contributes to degeneration and acquisition of learning and memory. Neurobiol. Learn. Mem. 2011;95(3):361–375. doi: 10.1016/j.nlm.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Du X., Li J., Li M., Yang X., Qi Z., Xu B., Liu W., Xu Z., Deng Y. Research progress on the role of type I vesicular glutamate transporter (VGLUT1) in nervous system diseases. Cell Biosci. 2020;10(1):1–10. doi: 10.1186/s13578-020-00393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss B., Wong F., Brownfoot F., Shearer I.K., Baud O., Walker D.W., Gressens P., Tolcos M. Knowledge gaps and emerging research areas in intrauterine growth restriction-associated brain injury. Front. Endocrinol. 2019;10:188. doi: 10.3389/fendo.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond Luke. The University of Queensland; Australia: 2014. Measuring cell fluorescence using ImageJ; p. 5d853e60.https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html Copyright 2014, Martin Fitzpatrick Revision. [Google Scholar]
- Hermann D.M., Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11(4):369–380. doi: 10.1016/S1474-4422(12)70039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann K.A. Pathophysiology and therapy of experimental stroke. Cell. Mol. Neurobiol. 2006;26(7–8):1055–1081. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal J.H., D’Souza C.D., Petrakis I.L., Belger A., Berman R.M., Charney D.S., Abi-Saab W., Madonick S. NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv. Rev. Psychiatry. 1999;7(3):125–143. [PubMed] [Google Scholar]
- Ng F., Berk M., Dean O., Bush A.I. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008;11(6):851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Orrego F., Villanueva S. The chemical nature of the main central excitatory transmitter: a critical appraisal based upon release studies and synaptic vesicle localization. Neuroscience. 1993;56(3):539–555. doi: 10.1016/0306-4522(93)90355-j. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Elsevier; 2006. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. [Google Scholar]
- Pedroso A.P., Dornellas A.P., de Souza A.P., Pagotto J.F., Oyama L.M., Nascimento C.M., Klawitter J., Christians U., Tashima A.K., Ribeiro E.B. A proteomics–metabolomics approach indicates changes in hypothalamic glutamate–GABA metabolism of adult female rats submitted to intrauterine growth restriction. Eur. J. Nutr. 2019;58(8):3059–3068. doi: 10.1007/s00394-018-1851-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J.M., Lain K.Y. Recent insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23(5):359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- Rochefort C., Lefort J.M., Rondi-Reig L. The cerebellum: a new key structure in the navigation system. Front. Neural Circuits. 2013;7:35. doi: 10.3389/fncir.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C., Lefort J.M., Rondi-Reig L. The cerebellum: a new key structure in the navigation system. Front. Neural Circuits. 2013;7:35. doi: 10.3389/fncir.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondi-Reig L., Paradis A.L., Lefort J.M., Babayan B.M., Tobin C. How the cerebellum may monitor sensory information for spatial representation. Front. Syst. Neurosci. 2014;8:205. doi: 10.3389/fnsys.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G., Zarate C.A., Krystal J.H., Manji H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug. Discov. 2008;7(5):426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple B.D., Blomgren K., Gimlin K., Ferriero D.M., Noble-Haeusslein L.J. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallie P.D., Naicker T. The placenta as a window to the brain: a review on the role of placental markers in prenatal programming of neurodevelopment. Int. J. Dev. Neurosci. 2019;73:41–49. doi: 10.1016/j.ijdevneu.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Thompson L.P., Al-Hasan Y. Impact of oxidative stress in fetal programming. J. Pregnancy. 2012;2012:1–8. doi: 10.1155/2012/582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. Neuroscientist. 2008;14(4):345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- UNICEF global database 2012, from Multiple Indicator Cluster Surveys (MICS), Demographic Health Surveys (DHS), and other national surveys. Monitoring the situation of children and women. New York, UNICEF, 2013. http://www.childinfo.org/low_birthweight_status_trends.html. (Accessed 24 February 2014).
- Weschawalit S., Thongthip S., Phutrakool P., Asawanonda P. Glutathione and its antiaging and antimelanogenic effects. Clin. Cosmet. Investig. Dermatol. 2017;10:147–153. doi: 10.2147/CCID.S128339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik S.M., Rhee J., Herzog E., Sigler A., Jahn R., Takamori S., Brose N., Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc. Natl. Acad. Sci. 2004;101(18):7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]