Abstract
Background: Recruitment maneuvers (RMs) have heterogeneous effects on lung aeration and have adverse side effects. We aimed to identify morphological, anatomical, and functional imaging characteristics that might be used to predict the RMs on lung aeration in invasively ventilated patients.
Methods: We performed a systemic review. Studies included invasively ventilated patients who received an RM and in whom re-aeration was examined with chest computed tomography (CT), electrical impedance tomography (EIT), and lung ultrasound (LUS) were included.
Results: Twenty studies were identified. Different types of RMs were applied. The amount of re-aerated lung tissue after an RM was highly variable between patients in all studies, irrespective of the used imaging technique and the type of patients (ARDS or non-ARDS). Imaging findings suggesting a non-focal morphology (i.e., radiologic findings consistent with attenuations with diffuse or patchy loss of aeration) were associated with higher likelihood of recruitment and lower chance of overdistention than a focal morphology (i.e., radiological findings suggestive of lobar or segmental loss of aeration). This was independent of the used imaging technique but only observed in patients with ARDS. In patients without ARDS, the results were inconclusive.
Conclusions: ARDS patients with imaging findings suggestive of non-focal morphology show most re-aeration of previously consolidated lung tissue after RMs. The role of imaging techniques in predicting the effect of RMs on re-aeration in patients without ARDS remains uncertain.
Keywords: electrical impedance tomography, computed tomography, lung ultrasound, overdistention, recruitment maneuvers, ARDS
Introduction
A lung recruitment maneuver (RM) is a dynamic and transient increase in transpulmonary pressure aiming at (re-)opening collapsed lung parts and increasing end-expiratory lung volume (Lapinsky and Mehta, 2005). In theory, opening of collapsed or “non-aerated” lung areas decreases shunt, improving both oxygenation and removal of CO2 (Hedley-Whyte et al., 1964; Neumann et al., 1999). Furthermore, atelectatic areas might cause stress on, or deformation of, aerated regions, resulting in additional injury of lung parenchyma (Gattinoni et al., 2012). Accordingly, decreasing atelectatic areas with RM could protect the lungs, a strategy often referred to as the “open lung concept” (Hes, 2015).
The value of RMs without the use of any imaging monitoring is disputed, as, so far, clinical studies have failed to show benefit with regard to patient-centered outcomes—and even suggest harm (Cavalcanti et al., 2017). The absence of net benefit might be explained by the heterogeneity and unpredictable effects of RMs on lung aeration (Sahetya and Brower, 2017; Mancebo et al., 2019). The pressure threshold that should be overpassed during RMs to open atelectatic lung units is multifactorial and cannot be calculated precisely (Sahetya and Brower, 2017; Gattinoni et al., 2017). Furthermore, any increase in airways pressure will result in higher pressures in all lung parts, also those that are “open,” and these areas might be harmed by overdistention (Gattinoni et al., 2019). Thus, the benefit of RMs needs to be balanced between re-aeration and overdistention.
Changes in lung morphology indicative of re-aeration or overdistention can be estimated using lung imaging (Gattinoni et al., 2020). Various imaging techniques like chest computed tomography (CT), electrical impedance tomography (EIT), and lung ultrasound (LUS) have been suggested to be useful to evaluate lung morphology and function in an individual patient (Godet et al., 2015). We performed a systematic review to describe imaging-based methods to assess re-aeration after RMs in patients receiving invasive ventilation at the intensive care unit or the operating room. In this review, we focus on the variability of imaging-based method definitions and the clinical utility of baseline imaging characteristics.
Methods
This protocol was designed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Liberati et al., 2009). The study protocol has been registered on PROSPERO (CRD42020188056).
Eligibility Criteria
The PICO used to define eligibility criteria are the following: (1) P (population): invasive mechanical ventilation either in the intensive care unit (ICU) or the operating room (OR) with or without ARDS, (2) I (intervention): recruitment maneuver of any sort, (3) C (comparison): LUS and/or EIT and/or CT was used to evaluate re-aeration of previously consolidated lung tissue, (4) O: baseline image characteristics were reported and evaluated for their predictive value of recruitment.
Only original studies written in English were included, whereas animal studies, case reports, comments, letters, and studies that enrolled pediatric patients were not included.
Information Sources and Search
We searched EMBASE using PubMed on December 15, 2020 using the following key words: ((“diagnostic imaging” [Subheading] OR (“diagnostic” [All Fields] AND “imaging” [All Fields]) OR “diagnostic imaging” [All Fields] OR “ultrasound” [All Fields] OR “ultrasonography” [MeSH Terms] OR “ultrasonography” [All Fields] OR “ultrasound” [All Fields] OR “ultrasonics” [MeSH Terms] OR “ultrasonics” [All Fields]) OR (“ct” [All Fields]) OR “computed tomography” [All Fields]) OR ((“IEEE Int Conf Electro Inf Technol” [Journal] OR “eit” [All Fields]) OR “(electrical impedance tomography” [All Fields])) AND ((“positive-pressure respiration” [MeSH Terms] OR (“positive-pressure” [All Fields] AND “respiration” [All Fields]) OR “positive-pressure respiration” [All Fields] OR “peep” [All Fields]) AND Recruitment [All Fields]).
Study Selection
The identified studies were assessed for inclusion criteria based on title and then on abstract. For all selected papers, the full text was read and discussed between two authors (CP and LB). Studies that fulfilled the inclusion criteria were included in this review.
Data Collection
For each included study, we collected data related to patient characteristics and whether they referred to ARDS patients or not. The type of recruitment maneuver that was used was categorized as (a) sustained inflation, (b) sigh, (c) pressure-control ventilation, and (d) variable ventilation (Rocco et al., 2010). We recorded the criteria that were used to define a “responder” to recruitment and the baseline characteristics to identify factors that differentiate between “responders” and “non-responders.” For those studies including patients with ARDS, we documented whether authors classified patients as having “focal” (i.e., radiological attenuations with lobar or segmental distributions) or “non-focal” (i.e., radiological attenuation with diffuse or patchy distribution) abnormal lung morphology.
Bias Assessment
The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) was used for the assessment of the methodologic quality of selected studies (Whiting, 2011). The four recommended domains (i.e., patient selection, index test, reference standard, and flow/timing) were assessed for low, high, or unclear risk of bias. As for the reference standard domain, CT was considered the “gold standard” for assessing lung re-aeration. Given the insufficient evidence to classify LUS or EIT as adequate reference tests to assess lung aeration, we considered the risk of bias to be high. Concerns regarding applicability for the first three domains were also assessed and scored as low, high, or unclear.
Synthesis of Results
The following data were combined into a table: patient group that was studied, number of patients, type of recruitment and maximal airway pressure reached, assessment of re-aeration of lung tissue, and criteria to define “responder.” The main findings of the study regarding heterogeneity in re-aerated lung tissue and differences between “responders” and “non-responders” were also shown. We further synthesized the current evidence for heterogeneity and prediction of recruitment response in an overview table, stratified per imaging method that was used. Finally, we linked the morphological characteristics derived from different imaging techniques of responders and non-responders in an overview figure.
Results
Included Studies
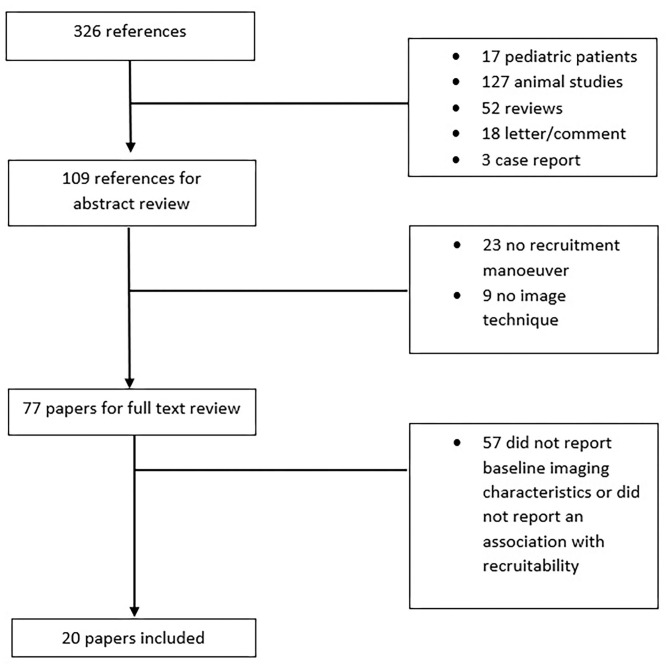
The described search resulted in 326 articles of which 249 were excluded based on the title and abstract review. Twenty out of the remaining 77 studies were included in this review based on full text review (Figure 1) and are summarized in Table 1. Seventeen studies included deeply sedated patients, while sedation level was not mentioned in the other three studies. All patients in the included studies were in supine position during RM. The majority of the included studies enrolled ARDS patients exclusively (14 studies, 70%). Three studies (15%) included a mixed population of intensive care unit patients, and in three studies (15%), patients undergoing elective operation were included. Three studies had the primary goal of quantification of potential for lung recruitment (Gattinoni et al., 2006; Camporota et al., 2019) or recruitment prediction (Constantin et al., 2010). Regarding lung imaging techniques, most of the studies (10 studies, 50%) assessed chest CT scan, followed by LUS (five studies, 25%) and EIT (five studies, 25%). Notably, chest CT was only used in studies that included patients with ARDS.
Figure 1.
Flow diagram of the study selection.
Table 1.
Studies included in this review.
| References | Patients | N | RM | Pmax | Imaging modality | Recruitment definition method | Outcome |
|---|---|---|---|---|---|---|---|
| He et al., 2020 | ICU (deeply sedated) | 30 | PC | NG | EIT | Ratio overdistended to recruited pixels | RM resulted in a high variability of the changes in the ration of overdistended to recruited pixels measured with EIT. No differences in the EELI and GI between responders and not responders to RM |
| Généreux et al., 2020 | OR (deeply sedated) | 45 | SI | 30 cm H2O | LUS | 12 areas derived LUS score | RM did not result in a significant improvement in LUS score |
| Karsten et al., 2019 | ICU (NM) | 15 | Sigh | 40 cm H2O | EIT | Local compliance (ODCL index) | RM resulted in the complete disappearance of collapsed units (ODCLindex) in all studied patients, but there was a high variation of the overdistention extension (19 ± 17%). After RM, the proportion of collapsed units was highly variable (0–50%), independent of the selected PEEP (5–13 cm H2O) |
| Zhao et al., 2019 | ARDS (deeply sedated) | 3 | Sigh | 35 cm H2O | EIT | Increase in ventilation in dependent areas | Those with ventilation distribution predominantly in the most dependent regions are likely non-responders to RM |
| Camporota et al., 2019 | ARDS (sedation level not mentioned) | 47 | SI | 45 cm H2O | CT | Proportion of re-aerated lung tissue compared with the total lung weight | RM resulted in a variable change in aerated lung tissue with a mean of 24.3% (−2–76). All patients were on ECMO and had a very high percentage of non-aerated lung tissue. Non-recruitable tissue varied between 50 and 80% of total lung weight |
| Eichler et al., 2018 | OR (deeply sedated) | 37 | Sigh | 40 cm H2O | EIT | EELI slope | A downward course of EELI may indicate the need for RM (EELI30sec/EELI0sec <1). This pattern of EELI inversed after RM and PEEP increase |
| Tang et al., 2017 | ARDS (deeply sedated) | 40 | PC | 35 cm H2O | LUS | Regasification score | RM resulted in significant changes in aeration in the anterior and lateral areas, but not in the posterior areas |
| Longo et al., 2017 | OR (deeply sedated) | 40 | Sigh | 35 cm H2O | LUS | Resolution of atelectasis | RM resolved atelectasis in all but 2/20 (10%) of the patients. The RM effect was assessed with TOE |
| Eronia et al., 2017 | ICU (deeply sedated) | 16 | SI | 40 cm H2O | EIT | EELI slope | A downward course of end-expiratory lung impedance may indicate the need for RM (10 min delta EELI >10%). This pattern of EELI inversed after RM and PEEP increase |
| Chiumello et al., 2016 | ARDS (sedation level not mentioned) | 22 | Sigh | NG | CT | Proportion of re-aerated lung tissue compared with the total lung weight | Responders to RM (increase in tissue >-100 HU) had higher amount of non-inflated tissue at PEEP 5 cmH2O (r2 = 0.44). This relation disappears when responders are defined by increase in tissue >-500 HU (r2 = 0.002) |
| *Caironi et al., 2015 | ARDS (deeply sedated) | 14 | PC | 45 cm H2O | CT | Proportion of re-aerated lung tissue compared with the total lung weight | Responders to RM had higher total lung weights. RM results in a highly variable recruitment of non-aerated lung tissue. This is independent of the severity of disease and baseline PEEP |
| de Matos et al., 2012 | ARDS (deeply sedated) | 51 | PC | 60 cm H2O | CT | Sectional lung weight re-aerated | RM resulted in variable aeration of previously non-aerated lung tissue: 45% (25–53). Responders to RM did not have a higher initial amount of non-aerated tissue (PEEP 10 cmH2O; r2 = 0.03) |
| Rode et al., 2012 | ARDS (deeply sedated) | 17 | Sigh | 30 cm H2O | LUS | Crater-like consolidations' borders leveling and abutting pleural line | RM resolved most (92%) of crater–like subpleural consolidations visible during ZEEP |
| Bouhemad et al., 2011 | ARDS (deeply sedated) | 40 | SI | 40 cm H2O | LUS | Increase lung re-aeration score | RM was unlikely to affect consolidations in posterior and caudal regions. RM responders were more likely to have non-focal rather than focal lung morphology |
| Constantin et al., 2010 | ARDS (deeply sedated) | 19 | SI | 40 cm H2O | CT | Proportion of re-aerated lung volume compared with the total lung volume | RM responders were more likely to have non-focal than focal lung morphology at ZEEP. Hyperinflation during RM is predicted by the lung volume between −800 and −900 HU in ZEEP (r2 = 0.77) |
| *Caironi et al., 2010 | ARDS (deeply sedated) | 68 | PC | 45 cm H2O | CT | Proportion of re-aerated lung tissue compared with the total lung weight | RM responders had more opening and closing lung tissue at PEEP 5 cm H2O. RM responders had a homogeneous cephalo-caudal distribution of non-aerated areas, while non-responders had a linear cephalo-caudal increase in non-aerated areas |
| Gattinoni et al., 2006 | ARDS (sedation level not mentioned) | 68 | PC | 45 cm H2O | CT | Proportion of re-aerated lung tissue compared with the total lung weight | RM had a variable effect on opening of lung tissue (median 9% range −10–60%). RM response was predicted by recruitment of lung tissue after increase in PEEP from 5 to 15 cm H2O (r2 = 0.72). RM response was predicted by the amount of non-aerated tissue at PEEP 5 cm H2O |
| Borges et al., 2006 | ARDS (deeply sedated) | 26 | PC | 60 cm H2O | CT | Proportion of re-aerated lung tissue compared with the total lung weight and proportion of re-aerated lung volume compared with the total lung volume | RM shows different responses with variation in lung opening pressures. RM at 40 cmH2O resulted in response in <50%, while this increased to 93% at 60 cm H2O |
| *Nieszkowska et al., 2004 | ARDS (sedation level not mentioned) | 32 | Sigh | NG | CT | Volume increase in non-aerated or poorly aerated areas | RM responders more frequently had non-focal morphology rather than focal (lobar) morphology (recruited volume: 572 ± 25 vs. 249 ± 159 ml). RM did not result in overinflation in patients with a diffuse morphology |
| Vieira et al., 1999 | ARDS (sedation level not mentioned) | 14 | Sigh | 45 cm H2O | CT | Total lung volume increases | RM responders more frequently had a non-focal morphology. RM responders more frequently had a biphasic lung density histogram with a peak at −700 to −900 HU >50 ml at ZEEP is related to a higher amount of overinflation with RM |
OR, operating room; N, number of enrolled patients; Pmax, maximum pressure used for recruitment maneuver; RM, lung recruitment maneuver; SI, sustained inflation; PC, pressure control; LUS, lung ultrasound; EIT, electrical impedance tomography; CT, computed tomography; ODCL, overdistention collapse index; PEEP, positive end-expiratory pressure; ZEEP, zero end-expiratory pressure; EELI, end expiratory lung impedance; LIL, left inferior lobe; TOE, transesophageal echocardiography; HU, Hounsfield units; COPD, chronic obstructive pulmonary disease.
Retrospective study.
Quality characteristics of the included studies, in relation to the aim of this systematic review, are presented in Supplementary Table 1. In two studies, there was a high concern regarding applicability of population selection. These two studies included a highly selective population, i.e., patients after cardiac surgery (Longo et al., 2017) or patients who underwent tracheostomy (Eichler et al., 2018).
Recruitment Methodology and Identification of “Responders”
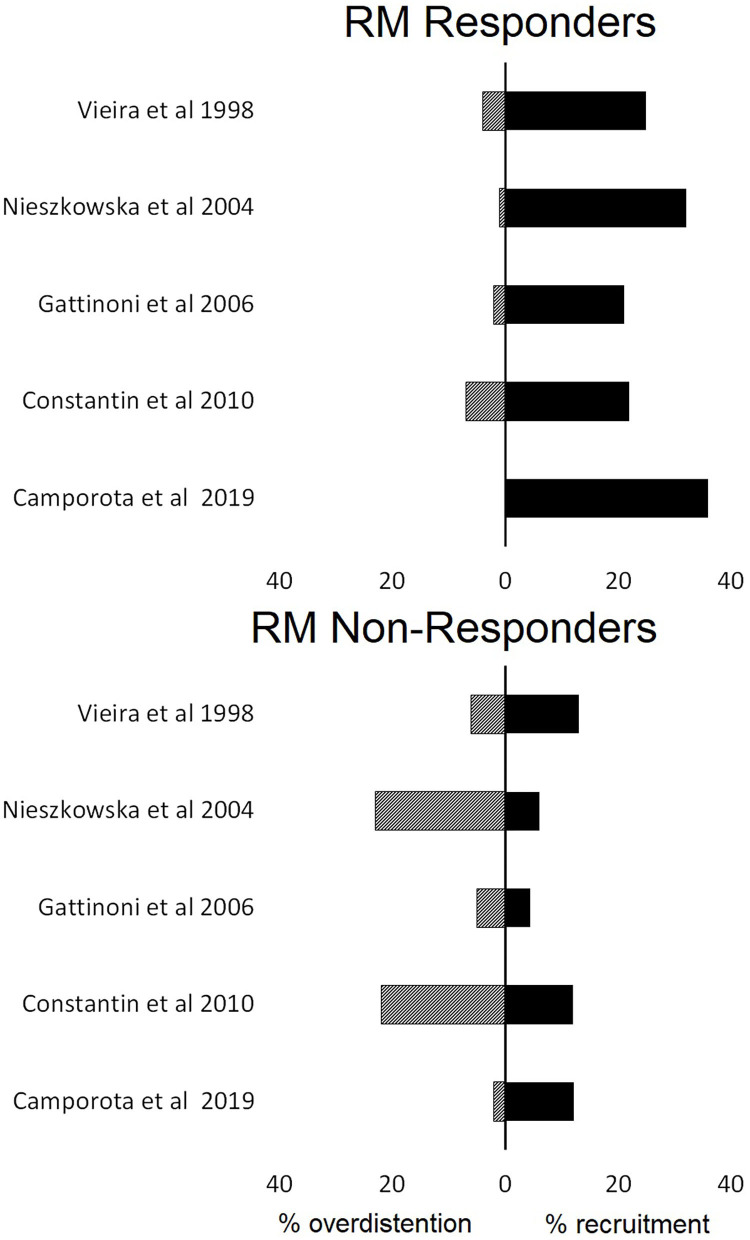
In eight studies (42%), a sigh, in six studies (31%), a pressure-control method, and in five studies (26%), a sustained inflation were used for the RM (Table 1). Applied maximum airway pressure varied widely, between 30 and 60 cm H2O. Classification of responders depended on the imaging technique used (Table 2). None of the studies defined the criteria to identify “responders” beforehand. Patients were classified post-hoc as “responders” and “non-responders” based on the median value of the study population in studies that quantified re-aeration by CT imaging. Recruitment “responders” generally had an increase in aeration of non-aerated lung tissue of more than 20% (Figure 2).
Table 2.
Findings related to the assessment of recruitment after recruitment maneuver application.
| Imaging modality | Definition of “recruitment” | Base-line PEEP | Maximum applied pressure (mean and range) |
|---|---|---|---|
| LUS | Decrease four points in LUS score (Généreux et al., 2020) | ZEEP (Bouhemad et al., 2011; Rode et al., 2012; Tang et al., 2017; Généreux et al., 2020), 6 cm H2O (Longo et al., 2017) |
34 cm H2O [30–40] |
| Maximum increase in regasification score (Tang et al., 2017) | |||
| Disappearance of atelectasis or B-lines (Bouhemad et al., 2011; Rode et al., 2012; Longo et al., 2017) | |||
| EIT | Any decrease in ODCLindex (Karsten et al., 2019) | ZEEP (Karsten et al., 2019; He et al., 2020), 5–8 cm H2O (Zhao et al., 2019), PEEP/FiO2 table PEEP(Eronia et al., 2017), 8 cm H2O (Eichler et al., 2018) |
39 cm H2O [35–40] |
| Reverse in EELI ratio from <1 to >1 (Eronia et al., 2017; Longo et al., 2017; Zhao et al., 2019) | |||
| Changes in the pixel ratio of overdistention to recruitment >15% (He et al., 2020) | |||
| CT | Decrease in non-aerated weight of lung (>-100 HU) (Borges et al., 2006; Gattinoni et al., 2006; Caironi et al., 2010, 2015; de Matos et al., 2012; Chiumello et al., 2016; Camporota et al., 2019) | ZEEP (Vieira et al., 1999; Nieszkowska et al., 2004; Constantin et al., 2010), 5 cm H2O (Gattinoni et al., 2006; Constantin et al., 2010; Caironi et al., 2015; Chiumello et al., 2016; Camporota et al., 2019), 10 cm H2O (de Matos et al., 2012), 5–10 cm H2O (Borges et al., 2006) |
48 cm H2O [40–60] |
| Decrease in non-aerated and poorly aerated weight of lung (>-500 HU; Chiumello et al., 2016) | |||
| Increase in the volume of gas penetrating in non-aerated areas (>-500 HU; Borges et al., 2006) | |||
| Increase in the volume of gas penetrating in non-aerated and poorly aerated areas (>-500 HU; Vieira et al., 1999; Nieszkowska et al., 2004; Constantin et al., 2010) |
PEEP, positive end-expiratory pressure; ZEEP, zero end-expiratory pressure; LUS, lung ultrasound; EIT, electrical impedance tomography; CT, computed tomography; EELI, end expiratory lung impedance; HU, Hounsfield units; ODCL, overdistention collapse index.
Figure 2.
The proportions of lung recruitment and lung overdistention in patients who were characterized responders or not responders to lung recruitment maneuvers (RM) based on computed tomography findings.
Heterogeneity in Re-aeration and Prediction of Positive Response to RM
Re-aeration after RM varied widely between studies, independent of the used image technique (Table 3). Unsurprisingly, most CT imaging studies showed that around 50% of patients are “non-responders” to recruitment because the median value was used as the cutoff value (Borges et al., 2006; Gattinoni et al., 2006; Caironi et al., 2015; Vieira et al., 1999; Camporota et al., 2019). Studies that used other imaging techniques did not mention the proportion of “non-responders,” though recruitment was described as “highly variable” (Karsten et al., 2019; Généreux et al., 2020).
Table 3.
Observed recruitment maneuver re-aeration effect and findings related to potential for lung re-aeration after recruitment maneuver according to the imaging module and the presence or not ARDS.
| ARDS | Non-ARDS | |
|---|---|---|
| Observed lung re-aeration with imaging analysis | ||
| LUS | 8% of evaluated consolidations did not respond to RM (Rode et al., 2012) | No change of LUS score after RM (Généreux et al., 2020) |
| 27% of patients had a re-aeration score ≥8 and an increase in lung volume more than 600 ml after RM (Bouhemad et al., 2011) | 10% of patients do not respond to RM (Longo et al., 2017) | |
| EIT | Extremely high variability in changes of the ratio between overdistention and collapsed ration (He et al., 2020) | Variable* compromise between the extension of lung collapse and overdistention after RM (Karsten et al., 2019) |
| CT | High variability* of potential recruitment tissue (Caironi et al., 2015) | |
| Potential recruitable tissue: 45% (range 5–75%; de Matos et al., 2012) | ||
| Potential recruitable tissue: 9% (range −10–60%; Gattinoni et al., 2006) | ||
| Potential recruitable tissue: 24.3% (range −2–76; Camporota et al., 2019) | ||
| High variability of opening lung pressures (Caironi et al., 2015) | ||
| Findings that predicted more lung re-aeration | ||
| LUS | Anterior located consolidations (Bouhemad et al., 2011; Tang et al., 2017) | |
| Crater-like sub-pleural consolidations (Rode et al., 2012) | ||
| EIT | Predominant ventilation in non-dependent areas (Zhao et al., 2019) | Decreasing pattern of EELI (delta EELI >10% or EELI index <1; Eronia et al., 2017; Eichler et al., 2018) |
| CT | Not aerated tissue (>-100 HU) >25–30% of total lung tissue (Gattinoni et al., 2006; Chiumello et al., 2016) | |
| Non-focal lung morphology (Nieszkowska et al., 2004; Constantin et al., 2010) | ||
| Homogeneous cephalo-caudal distribution of 40–50% non-aeration area (Caironi et al., 2010) | ||
| Opening and closing lung tissue (141 ± 81 g; Caironi et al., 2010) | ||
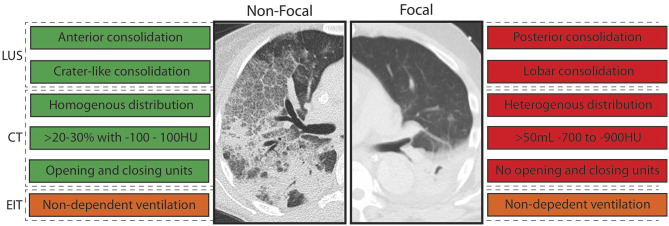
Imaging findings related to the amount of re-aerated lung tissue in patients with ARDS were the extent of lost aeration before RM, the distribution of non-aerated areas (craniocaudal and anteroposterior distribution), the morphology of non-aerated areas (e.g., crater-like consolidation), and functional lung characteristics related to tidal recruitment (tidal opening/closing tissue; Table 3). Findings that are more likely to resemble a diffuse or patchy loss of aeration (i.e., non-focal morphology) were suggestive of an increased likelihood of positive response to RMs (Figure 3). This was independent on the image technique employed.
Figure 3.
Imaging abnormalities that predicted response to recruitment maneuvers (RM) stratified per morphology. LUS, lung ultrasound; EIT, electrical impedance tomography; CT, computed tomography; HU, Houndsfield units; green, imaging abnormality in line with responder to RM; red, imaging abnormality in line with non-responder to RM; orange, imaging abnormality in line with responder with high uncertainty. Text boxes on the left: consistent with non-focal morphology. Text boxes on the right: consistent with focal morphology.
Only one study addressed the prediction of response to RM in patients in the operating room. A decreasing pattern of end-expiratory lung impedance (EELI) evaluated with EIT was found to be related to the amount of re-aerated lung tissue (Eichler et al., 2018; Table 3).
Overdistention
Overdistention was assessed in studies that used CT or EIT only, as LUS cannot be used for this purpose. Studies employing CT imaging showed the average percentage of overdistended lung volume to vary between 0 and 20% (Figure 2). EIT studies revealed the average overdistention secondary to RMs across patients to range between 5 and 30% (Karsten et al., 2019). Nevertheless, local overdistention in non-dependent areas may exceed 60% of that area (Eronia et al., 2017). “Non-responders” identified by CT had a higher increase in hyperinflated lung tissue compared with “responders” (Figure 2).
Discussion
The results of this systematic review can be summarized as follows: (a) data that quantify the potential for lung recruitment based on imaging are limited, (b) the definition of positive response to RMs was highly variable, and (c) patients with imaging characteristics suggestive for a non-focal morphology of ARDS seemed to show more re-aeration at RMs with moderate inspiratory pressures.
The included studies used a wide range of maximum airway pressures to recruit lung tissue. Most collapsed areas can be opened, but frequently only at very high airway pressures (Cressoni et al., 2017). Borges et al. found opening pressures of 60 cmH2O in patients with ARDS to be common, with coexistence of areas opening at lower and higher pressures in the majority of patients (Borges et al., 2006). In clinical practice, maximum airway pressure is often selected based on the hemodynamic fragility of the patient rather than the expected pressure needed for lung recruitment (Santos et al., 2015). This might explain why CT compared with LUS and EIT studies revealed higher recruitment pressures as transfer for CT imaging requires more hemodynamically stable patients (Constantin et al., 2019). Recent RCTs suggest airway pressures above 50 cm H2O to be associated with serious adverse events, even when the patient is exposed to it for a short period of time (Cavalcanti et al., 2017; Hodgson et al., 2019). As the different components that attribute to the compliance of the respiratory system (compliance of the lung and chest wall as well as intra-abdominal pressure) cannot be easily separated in clinical practice (Umbrello and Chiumello, 2018), assessing the RMs' effect with imaging techniques is important in clinical practice. Rather than defining the pressure at which the lung can be opened, it is more important to determine whether recruitment can be achieved with moderate airway pressures. In other words, when comparing patients with a similar expected risk of side effects due to a transient increase in inspiratory pressures, a patient who responds to the RM with reaeration of previously collapsed lung tissue may still benefit, but a patient without this response may not.
This review also revealed several challenges associated with the quantification of lung re-aeration with image technics: there is poor agreement between imaging techniques, and there is no universal definition of recruitment response. Chiumello et al. found poor agreement between CT and LUS with respect to assessment of re-aeration, which is not unexpected since LUS is a semiquantitative method assessing only the subpleural areas (Chiumello et al., 2018). Furthermore, the role of LUS in assessing overdistention is currently unknown (Bouhemad et al., 2015). Pleural line displacement identified with LUS, as well the number of A-lines are relevant indexes that are currently being studied (Martins and Nogué, 2020; Tonelotto et al., 2020). EIT quantifies collapsed lung units based on local changes in compliance (Costa et al., 2009). However, compliance might be more related to the improvement or deterioration of already ventilated lung units than the real recruitment of atelectatic lung units (Chiumello et al., 2016). Even though CT is considered the gold standard in detecting lung recruitment, defining the degree of re-aeration remains challenging. Potentially recruitable lung tissue, determined by CT, is mainly expressed as percentage of total lung volume since absolute values depend on lung dimensions. However, expressing recruitment as percentage implies mathematical coupling with the total atelectatic volume, which is at least debatable (de Matos et al., 2012). Gattinoni et al. introduced the terms “high” and “low” recruitment responders based on the median percentage of potentially recruitable lung tissue determined by CT (Gattinoni et al., 2006). Worth mentioning, different median percentages of potentially recruitable tissue were reported in later studies (Camporota et al., 2019; de Matos et al., 2012), probably due to heterogeneity in inclusion characteristics and application of various maximum airway pressures. Given that recruitment is a continuous spectrum that depends on applied airway pressure and several imaging characteristics, speaking about “responders” from “non-responders” is a false dichotomization.
We set out to determine the role of imaging techniques in predicting the lung response to RM. The main strength of this review is the systematic and integrative approach. We excluded studies that based assessment of recruitment on mechanical or oxygenation variables as those can be influenced by factors other than recruitment of lung tissue, which is also known as the recruitment paradox (Amato and De Santis Santiago, 2016). We also acknowledge several limitations. First, we had to perform secondary analyses of many included studies as they were not intended to quantify potential for lung re-aeration, limiting statistical comparisons between groups. Second, we did not directly compare imaging techniques. Each method has intrinsic limitations, such as visualization of the subpleural region only for LUS and the need for patient transport for CT, which justify preferential use of one technique over another in specific situations. Of note, the definition and method of recruitment varied between studies even when the same image technique was used, which made direct comparisons impossible. Third, given the undefined role of LUS and EIT in the assessment of recruitment, a significant number of trials had an unclear risk of bias.
All features predictive of increased lung re-aeration after RM are consistent with a non-focal morphology of ARDS. Patients with focal ARDS lack, by definition, ventral consolidations not limited to the subpleural space and show a heterogeneous distribution of consolidation with less opening and closing, which renders them very unlikely to be recruitable. In line with this notion, patients with non-focal morphology were typically recruitable, while patients with focal morphology were not (Puybasset et al., 2000; Constantin et al., 2010). Notably, atelectasis is usually located in the dorsal lung areas in patients without lung injury requiring invasive mechanical ventilation (Longo et al., 2017; Pereira et al., 2018) implying a “focal” morphology. This may explain the lack of RM efficiency to increase lung aeration in invasively ventilated patients in the operating room (Généreux et al., 2020). Although the results of this review are not conclusive for patients without ARDS, it stresses the need for further research into lung morphology and its relation to lung re-aeration with robust imaging technics in these patients.
By integrating data from multiple studies to morphological classifications, we present a framework used to better design and interpret future studies. We have to acknowledge that this classification is imperfect, as one EIT study that only included three patients suggested that predominant ventilation in the non-dependent areas predicted recruitment, while this is not a feature that is consistent with non-focal morphology of ARDS. The relation between re-aeration and improvement in ventilation perfusion mismatch and heart function was not evaluated in this review (Karbing et al., 2020). Furthermore, in this review, we investigated the imaging techniques' role in predicting RM effects in deeply sedated patients without considering the optimal level of PEEP that would be required after recruitment to keep the lung open. Rather than a final classification, we suggest that the morphological classification is a good starting point to further improve from, with the addition of other predictors. Furthermore, more attention should be drawn to the quantification of overdistention rather than measurement of re-aeration alone. Balancing the assessment of negative and positive effects may improve our understanding as to what patients may or may not benefit from RMs.
Conclusions
We conclude that defining positive response to RMs using imaging techniques is challenging and not yet well-elucidated. Variations in RM method, population selection, as well as different imaging techniques should be taken into consideration in future studies. Given the adverse events associated with high maximum airway pressures, only the lungs of specific patients can be re-aerated with moderate maximum airway pressures. Lung ultrasound and CT characteristics consistent with non-focal morphology of ARDS are predictive of more re-aeration in response to recruitment maneuver. The morphological characteristics related to successful response to RMs in patients without ARDS have not been studied to date.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
CP performed the literature search, drafted the manuscript, and approved the submitted version of the manuscript. MRS, LH, NH, MH, and FP revised the manuscript for critical content and approved the submitted version of the manuscript. MJS and LB conceived the study, revised the manuscript for critical content, and approved the submitted version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.666941/full#supplementary-material
References
- Amato M. B. P., De Santis Santiago R. R. (2016). The recruitability paradox. Am. J. Respir. Crit. Care Med. 193, 1192–1194. 10.1164/rccm.201601-0178ED [DOI] [PubMed] [Google Scholar]
- Borges J. B., Okamoto V. N., Matos G. F., Caramez M. P., Arantes P. R., Barros F., et al. (2006). Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 174, 268–278. 10.1164/rccm.200506-976OC [DOI] [PubMed] [Google Scholar]
- Bouhemad B., Brisson H., Le-Guen M., Arbelot C., Lu Q., Rouby J. J. (2011). Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am. J. Respir. Crit. Care Med. 183, 341–347. 10.1164/rccm.201003-0369OC [DOI] [PubMed] [Google Scholar]
- Bouhemad B., Mongodi S., Via G., Rouquette I. (2015). Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology 122, 437–447. 10.1097/ALN.0000000000000558 [DOI] [PubMed] [Google Scholar]
- Caironi P., Carlesso E., Cressoni M., Chiumello D., Moerer O., Chiurazzi C., et al. (2015). Lung recruitability is better estimated according to the Berlin definition of acute respiratory distress syndrome at standard 5 cm H2O rather than higher positive end-expiratory pressure: a retrospective cohort study. Crit. Care Med. 43, 781–790. 10.1097/CCM.0000000000000770 [DOI] [PubMed] [Google Scholar]
- Caironi P., Cressoni M., Chiumello D., Ranieri M., Quintel M., Russo S. G., et al. (2010). Lung opening and closing during ventilation of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 181, 578–586. 10.1164/rccm.200905-0787OC [DOI] [PubMed] [Google Scholar]
- Camporota L., Caricola E. V., Bartolomeo N., Di Mussi R., Wyncoll D. L. A., Meadows C. I. S., et al. (2019). Lung recruitability in severe acute respiratory distress syndrome requiring extracorporeal membrane oxygenation. Crit. Care Med. 47,1177–1183. 10.1097/CCM.0000000000003837 [DOI] [PubMed] [Google Scholar]
- Cavalcanti A. B., Suzumura É. A., Laranjeira L. N., Paisani D. M., Damiani L. P., Guimarães H. P., et al. (2017). Effect of lung recruitment and titrated Positive End-Expiratory Pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome - a randomized clinical trial. JAMA 318, 1335–1345. 10.1001/jama.2017.14171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Del Sorbo L., Grieco D. L., Junhasavasdikul D., Rittayamai N., Soliman I., et al. (2019). Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 201, 178–187. 10.1164/rccm.201902-0334OC [DOI] [PubMed] [Google Scholar]
- Chiumello D., Marino A., Brioni M., Cigada I., Menga F., Colombo A., et al. (2016). Lung recruitment assessed by respiratory mechanics and computed tomography in patients with acute respiratory distress syndrome what is the relationship? Am. J. Respir. Crit. Care Med. 193, 1254–1263. 10.1164/rccm.201507-1413OC [DOI] [PubMed] [Google Scholar]
- Chiumello D., Mongodi S., Algieri I., Vergani G. L., Orlando A., Via G. (2018). Assessment of lung aeration and recruitment by ct scan and ultrasound in acute respiratory distress syndrome patients*. Crit. Care Med. 46, 1761–1768. 10.1097/CCM.0000000000003340 [DOI] [PubMed] [Google Scholar]
- Constantin J. M., Grasso S., Chanques G., Aufort S., Futier E., Sebbane M. (2010). Lung morphology predicts response to recruitment maneuver in patients with acute respiratory distress syndrome. Crit. Care Med. 38, 1108–1117. 10.1097/CCM.0b013e3181d451ec [DOI] [PubMed] [Google Scholar]
- Constantin J. M., Jabaudon M., Lefrant J. Y., Jaber S., Quenot J. P., Langeron O., et al. (2019). Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 7, 870–880. 10.1016/S2213-2600(19)30138-9 [DOI] [PubMed] [Google Scholar]
- Costa E. L., Borges J. B., Melo A., Suarez-Sipmann F., Toufen C., Jr, Bohm S. H., et al. (2009). Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intens. Care Med. 35, 1132–1137. 10.1007/s00134-009-1447-y [DOI] [PubMed] [Google Scholar]
- Cressoni M., Chiumello D., Algieri I., Brioni M., Chiurazzi C., Colombo A., et al. (2017). Opening pressures and atelectrauma in acute respiratory distress syndrome. Intens. Care Med. 43, 603–611. 10.1007/s00134-017-4754-8 [DOI] [PubMed] [Google Scholar]
- de Matos G. F., Stanzani F., Passos R. H., Fontana M. F., Albaladejo R., Caserta R. E. (2012). How large is the lung recruitability in early acute respiratory distress syndrome: a prospective case series of patients monitored by computed tomography. Crit. Care 16:R4. 10.1186/cc10602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler L., Mueller J., Grensemann J., Frerichs I., Zöllner C., Kluge S. (2018). Lung aeration and ventilation after percutaneous tracheotomy measured by electrical impedance tomography in non-hypoxemic critically ill patients: a prospective observational study. Ann. Intens. Care 8:110. 10.1186/s13613-018-0454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eronia N., Mauri T., Maffezzini E., Gatti S., Bronco A., Alban L., et al. (2017). Bedside selection of positive end-expiratory pressure by electrical impedance tomography in hypoxemic patients: a feasibility study. Ann. Intens. Care 7:76. 10.1186/s13613-017-0299-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Caironi P., Cressoni M., Chiumello D., Ranieri V. M., Quintel M., et al. (2006). Lung recruitment in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 354, 1775–1786. 10.1056/NEJMoa052052 [DOI] [PubMed] [Google Scholar]
- Gattinoni L., Carlesso E., Caironi P. (2012). Stress and strain within the lung. Curr. Opin. Crit. Care 18, 42–47. 10.1097/MCC.0b013e32834f17d9 [DOI] [PubMed] [Google Scholar]
- Gattinoni L., Collino F., Maiolo G., Rapetti F., Romitti F., Tonetti T., et al. (2017). Positive end-expiratory pressure: How to set it at the individual level. Ann. Transl. Med. 5:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Collino F., Maiolo G., Rapetti F., Romitti F., Tonetti T., et al. (2017). Positive end-expiratory pressure: how to set it at the individual level. Ann. Transl. Med. 5:288. 10.21037/atm.2017.06.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Marini J. J., Quintel M. (2020). Recruiting the acutely injured lung: how and why? Am. J. Respir. Crit. Care Med. 201, 130–132. 10.1164/rccm.201910-2005ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- Généreux V., Chassé M., Girard F., Massicotte N., Chartrand-Lefebvre C., Girard M. (2020). Effects of positive end-expiratory pressure/recruitment manoeuvres compared with zero end-expiratory pressure on atelectasis during open gynaecological surgery as assessed by ultrasonography: a randomised controlled trial. Br. J. Anaesth. 124, 101–109. 10.1016/j.bja.2019.09.040 [DOI] [PubMed] [Google Scholar]
- Godet T., Constantin J. M., Jaber S., Futier E. (2015). How to monitor a recruitment maneuver at the bedside. Curr. Opin. Crit. Care 21, 253–258. 10.1097/MCC.0000000000000195 [DOI] [PubMed] [Google Scholar]
- He H., Chi Y., Long Y., Yuan S., Frerichs I., Möller K., et al. (2020). Influence of overdistension/recruitment induced by high positive end-expiratory pressure on ventilation-perfusion matching assessed by electrical impedance tomography with saline bolus. Crit. Care. 29:586. 10.1186/s13054-020-03301-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley-Whyte J., Laver M. B., Bendixen H. H. (1964). Effect of changes in tidal ventilation on physiologic shunting. Am. J. Physiol. 206, 891–897. 10.1152/ajplegacy.1964.206.4.891 [DOI] [PubMed] [Google Scholar]
- Hes D. R. (2015). Recruitment maneuvers and PEEP titration. Respir. Care 60, 1688–1704. 10.4187/respcare.04409 [DOI] [PubMed] [Google Scholar]
- Hodgson C. L., Cooper D. J., Arabi Y., King V., Bersten A., Bihari S., et al. (2019). Maximal recruitment open lung ventilation in acute respiratory distress syndrome (PHARLAP): a phase II. Multicenter randomized controlled clinical trial. Am. J. Respir. Crit. Care Med. 200, 1363–1372. 10.1164/rccm.201901-0109OC [DOI] [PubMed] [Google Scholar]
- Karbing D. S., Panigada M., Bottino N., Spinelli E., Protti A., Rees S. E., et al. (2020). Changes in shunt, ventilation/perfusion mismatch, and lung aeration with PEEP in patients with ARDS: a prospective single-arm interventional study. Crit. Care 24, 1–13. 10.1186/s13054-020-2834-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten J., Voigt N., Gillmann H.-J., Stueber T. (2019). Determination of optimal positive end-expiratory pressure based on respiratory compliance and electrical impedance tomography: a pilot clinical comparative trial. Biomed. Eng. Biomed. Tech. 64, 135–145. 10.1515/bmt-2017-0103 [DOI] [PubMed] [Google Scholar]
- Lapinsky S. E., Mehta S. (2005). Bench-to-bedside review: recruitment and recruiting maneuvers. Crit Care 9, 60–65. 10.1186/cc2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 21:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo S., Siri J., Acosta C., Palencia A., Echegaray A., Chiotti I., et al. (2017). Lung recruitment improves right ventricular performance after cardiopulmonary bypass: a randomised controlled trial. Eur. J. Anaesthesiol. 34, 66–74. 10.1097/EJA.0000000000000559 [DOI] [PubMed] [Google Scholar]
- Mancebo J., Mercat A., Brochard L. (2019). Maximal lung recruitment in ARDS: a nail in the coffin. Am. J. Respir. Crit. Care Med. 200, 1331–1333. 10.1164/rccm.201908-1615ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins S. R., Nogué R. (2020). Vertical displacement of pleura: a new method for bronchospasm evaluation? Ultrasound J. 12, 10–13. 10.1186/s13089-020-00184-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P., Rothen H. U., Berglund J. E., Valtysson J., Magnusson A., Hedenstierna G. (1999). Positive end-expiratory pressure prevents atelectasis during general anaesthesia even in the presence of a high inspired oxygen concentration. Acta Anaesthesiol. Scand. 43, 295–301. 10.1034/j.1399-6576.1999.430309.x [DOI] [PubMed] [Google Scholar]
- Nieszkowska A., Lu Q., Vieira S., Elman M., Fetita C., Rouby J. J. (2004). Incidence and regional distribution of lung overinflation during mechanical ventilation with positive end-expiratory pressure. Crit. Care Med. 32, 1496–1503. 10.1097/01.CCM.0000130170.88512.07 [DOI] [PubMed] [Google Scholar]
- Pereira S. M., Tucci M. R., Morais C. C. A., Simões C. M., Tonelotto B. F. F., Pompeo M. S., et al. (2018). Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology 129, 1070–1081. 10.1097/ALN.0000000000002435 [DOI] [PubMed] [Google Scholar]
- Puybasset L., Gusman P., Muller J.-C., Cluzel P., Coriat P., Rouby J.-J., et al. (2000). Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure. Intens. Care Med. 26, 1215–1227. 10.1007/s001340051340 [DOI] [PubMed] [Google Scholar]
- Rocco P. R., Pelosi P., De Abreu M. G. (2010). Pros and cons of recruitment maneuvers in acute lung injury and acute respiratory distress syndrome. Expert Rev. Respir. Med. 4, 479–489. 10.1586/ers.10.43 [DOI] [PubMed] [Google Scholar]
- Rode B., Vučić M., Siranović M., Horvat A., Krolo H., Kelečić M., et al. (2012). Positive end-expiratory pressure lung recruitment: comparison between lower inflection point and ultrasound assessment. Wien Klin Wochenschr. 124, 842–847. 10.1007/s00508-012-0303-1 [DOI] [PubMed] [Google Scholar]
- Sahetya S. K., Brower R. G. (2017). Lung recruitment and titrated PEEP in moderate to severe ARDS. JAMA 318:1327. 10.1001/jama.2017.13695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R. S., Silva P. L., Pelosi P., Rocco P. R. (2015). Recruitment maneuvers in acute respiratory distress syndrome: the safe way is the best way. World J. Crit. Care Med. 4, 278–286 10.5492/wjccm.v4.i4.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K. Q., Yang S. L., Zhang B., Liu H. X., Ye D. Y., Zhang H. Z., et al. (2017). Ultrasonic monitoring in the assessment of pulmonary recruitment and the best positive end-expiratory pressure. Medicine 96:e8168. 10.1097/MD.0000000000008168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelotto B., Pereira S. M., Tucci M. R., Vaz D. F., Vieira J. E., Malbouisson L. M., et al. (2020). Intraoperative pulmonary hyperdistention estimated by transthoracic lung ultrasound: a pilot study. Anaesth. Crit. Care Pain Med. 39, 825–831. 10.1016/j.accpm.2020.09.009 [DOI] [PubMed] [Google Scholar]
- Umbrello M., Chiumello D. (2018). Interpretation of the transpulmonary pressure in the critically ill patient. Ann. Transl. Med. 6, 383–383. 10.21037/atm.2018.05.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira S. R., Puybasset L., Lu Q., Richecoeur J., Cluzel P., Coriat P., et al. (1999). A scanographic assessment of pulmonary morphology in acute lung injury: significance of the lower inflection point detected on the lung pressure- volume curve. Am. J. Respir. Crit. Care Med. 159, 1612–1623. 10.1164/ajrccm.159.5.9805112 [DOI] [PubMed] [Google Scholar]
- Whiting P. F. (2011). QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155:529. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Lee L. C., Chang M. Y., Frerichs I., Chang H. T., Gow C. H., et al. (2019). The incidence and interpretation of large differences in EIT-based measures for PEEP titration in ARDS patients. J. Clin. Monit. Comput. 10. 10.07/s10877-019-00396-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.