To the Editor:
The emergent need to combat the coronavirus disease 2019 (COVID‐19) pandemic led to the development of several highly effective vaccines at unprecedented speeds, using highly advanced technology and scientific research. The initial trials demonstrated the safety of these vaccines, and the risk of serious adverse effects remains significantly low even after the vaccination of more than 1.5 billion people worldwide to date. The Ad26.COV2.S (Johnson & Johnson/Janssen) vaccine, a recombinant replication‐incompetent adenovirus type 26 vector COVID‐19 vaccine, was issued Emergency Use Authorization by the US Food and Drug Administration (FDA) on February 27, 2021, and has been administered to more than 10 million individuals as of June 1st, 2021.
In March 2021, a rare, life‐threatening syndrome was first described by the European Medicines Agency as thrombotic thrombocytopenic syndrome (TTS) following ChAdOx1 nCoV‐19 vaccination (AstraZeneca), a recombinant replication‐deficient chimpanzee adenovirus vector. 1 The syndrome, now recognized as vaccine‐induced thrombotic thrombocytopenia (VITT), results in pathologic anti‐platelet factor 4 (PF4) antibodies leading to thrombocytopenia and thrombosis in the absence of heparin exposure, a mechanism similar to “autoimmune” heparin‐induced thrombocytopenia (HIT). 2 , 3
On April 13th, 2021, six cases of VITT were reported in the United States following vaccination by the Ad26.COV2.S vaccine, all of whom were women who developed cerebral venous sinus thrombosis (CVST). 4 This led to an 11‐day pause in the administration of the vaccine, during which a thorough investigation led to its resumption by the Centers for Disease Control (CDC) and FDA, but revised to include a warning about this rare side effect. By April 27th, 2021, the number of VITT cases following Ad26.COV2.S vaccination reported in the US had risen to 15, all of whom were women of ages 20 to 50 years, and included 12 incidences of CVST. 5 , 6 To date, the only report of suspected VITT in a male Ad26.COV2.S vaccine recipient in the medical literature comes from the initial Johnson & Johnson phase 3 trials. One of the male trial participants who developed CVST, thrombocytopenia, and positive anti‐PF4 antibodies is suspected to have had VITT, but additional details have not been provided. 7 We hereby report the case of a man with confirmed VITT following Ad26.COV2.S vaccination, resulting in acute deep venous thrombosis (DVT) and bilateral pulmonary emboli (PE), and a post‐discharge course complicated by refractory thrombocytopenia.
A 48‐year‐old male with a history of asthma presented to a community emergency room in Salt Lake County, Utah, with bilateral lower extremity pain on April 26th, 2021, 19 days after receiving the Ad26.COV2.S vaccine on April 7th. The pain initially started in his toes a week prior (11 days post‐vaccination), and then progressed to his thighs bilaterally. He was healthy and exercised regularly, was not on any medications, and had never smoked. He flew out of town and back on March 30th and April 4th, respectively, with the longest continuous flight lasting around 4 h. He also flew again on April 22nd and April 25th on one‐hour long flights. His last known platelet count in 2019 was 177 × 109/L. In the emergency room, he had normal vital signs and oxygen saturation, and was found to have a platelet count of 74 × 109/L, fibrinogen of 254 mg/dL, D‐dimer of 15 109 ng/mL FEU, and an activated partial thromboplastin time (aPTT) of 31.8 s. Detailed laboratory work‐up and reference ranges are listed in Table 1. Venous duplex ultrasound of the lower extremities revealed non‐occlusive DVT in the bilateral popliteal veins extending to the gastrocnemius veins, an occlusive DVT of the left posterior tibial vein, as well as occlusive superficial venous thrombosis in the bilateral saphenous veins. He was discharged on rivaroxaban 15 mg twice‐daily with two‐day follow‐up with his primary care physician. He had no known exposure to heparin before the onset of symptoms and no exposure during his emergency room visit.
TABLE 1.
Selected laboratory values
| Test name (units as applicable) | Result | Reference interval |
|---|---|---|
| CBC (initial presentation) | ||
| WBC (K/μL) | 5.8 | 3.2–10.6 |
| RBC (M/ μL) | 5.07 | 3.98–5.98 |
| Hemoglobin (g/dL) | 15.0 | 12.5–18.0 |
| Hematocrit (%) | 42.2 | 36.9–52.1 |
| MCV (fL) | 83.2 | 80.6–97.6 |
| MCHC (g/dL) | 35.5 | 33.4–35.3 |
| Platelets (K/μL) | 74 | 140–440 |
| Hemostasis Tests (initial presentation) | ||
| D‐dimer (ng/mL FEU) | 15 109 | 0–499 |
| PT (s) | 12.7 | 10–13 |
| aPTT (s) | 31.8 | 25.1–36.5 |
| Fibrinogen (mg/dL) | 254 | 150–430 |
| Thrombophilia Tests a | ||
| Prothrombin G20210A mutation | Not detected | Not detected |
| Factor V Leiden mutation | Not detected | Not detected |
| Lupus anticoagulant interpretation | Not detected | Not detected |
| Anticardiolipin IgG (GPL) | 5 | 0–14 |
| Anticardiolipin IgM (MPL) | 6 | 0–12 |
| β2‐glycoprotein 1 IgG (SGU) | 0 | 0–20 |
| β2‐glycoprotein 1 IgM (SMU) | 5 | 0–20 |
| SARS‐CoV‐2 Test | ||
| PCR | Not detected (nasopharyngeal swab specimen) | Not detected |
| VITT Tests (prior to IVIG therapy) | ||
| Anti‐PF4 antibody (OD) | 3.323 | ≤0.399 |
| Serotonin release assay | Positive |
Negative |
| P‐selectin expression assay | Positive |
Negative |
Protein C activity (clot‐based), protein S activity, and antithrombin activity not appropriate at the time of acute thrombosis and during rivaroxaban treatment.
The next day, he complained of pleuritic chest pain and presented to the emergency room at the University of Utah Hospital. A computerized tomography (CT) scan of the chest revealed multiple acute bilateral pulmonary emboli in the segmental and more proximal arteries. A hypercoagulable work‐up did not reveal any abnormalities (Table 1). A peripheral smear showed thrombocytopenia without schistocytes. The hematology service was consulted, and a presumptive diagnosis of VITT was made. The patient was immediately treated with 1 g/kg of intravenous immunoglobulins (IVIG) for 2 days, 1 mg/kg of prednisone, and switched from rivaroxaban to an intravenous argatroban infusion drip, during which he achieved therapeutic aPTT levels between 44 and 50 s. Magnetic resonance venography (MRV) and angiography (MRA) of the brain, performed due to symptoms of mild headaches, were unremarkable. His anti‐PF4 enzyme‐linked immunosorbent assay (ELISA) (LIFECODES PF4 IgG, Immucor, Norcross, GA), performed on a sample collected prior to initiating IVIG therapy, demonstrated a strongly positive result of 3.323 optical density (OD) units (reference interval < 0.399), consistent with a diagnosis of VITT. Serotonin release assay was positive. The patient's leg pain and chest pain resolved and his platelet count normalized to 150 × 109/L on the third day of hospitalization. His platelet count increased to 159 × 109/L on the fourth day, and he was discharged home with close follow‐up. Due to the prothrombotic nature of his syndrome, apixaban 10 mg twice daily was used instead of rivaroxaban to ensure more consistent drug levels, and the prednisone was tapered off. Over the subsequent month, the patient maintained persistently strong positive anti‐PF4 results (Figure 1), however, the SRA was no longer positive. Within a week post‐discharge, he developed recurrent thrombocytopenia, with a platelet count that down trended to 107 × 109/L (Figure 1). His thrombocytopenia appeared to somewhat correlate with prednisone dose adjustments, suggesting an autoimmune‐driven process, although it did not respond to repeat IVIG administration. Due to the normalized D‐Dimer levels and negative SRA, it was assumed that there was no further ongoing platelet activation.
FIGURE 1.
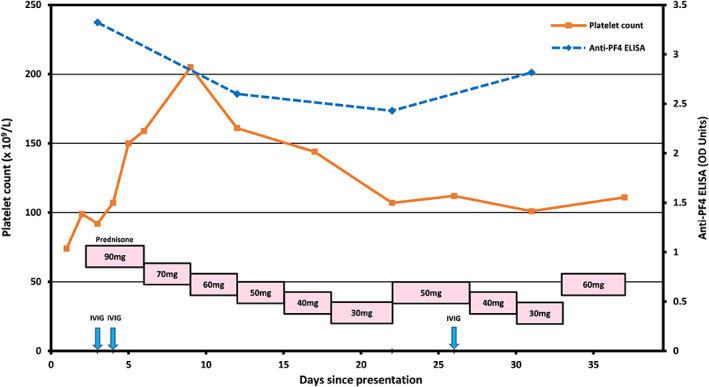
Platelet count and anti‐PF4 ELISA trends and therapeutic interventions since the time of initial presentation
To the best of our knowledge, this is the first published case in the medical literature describing a confirmed VITT diagnosis affecting a male Ad26.COV2.S vaccine recipient since the vaccine's authorization. This is also the first case describing the post‐discharge course. At the time of this report, the most recent update by the CDC describes a total of six men in the US affected by TTS (our patient included), with a 78% female predominance. 8 The most recent data from the UK also shows a female predominance in VITT following ChAdOx1 nCoV‐19 vaccination. 9 Furthermore, the majority of cases of VITT described to date appear to occur in unusual locations, namely CVST in 19 out of the recently reported 28 cases of VITT in the US, as well as a majority of the cases reported in Europe following ChAdOx1 nCoV‐19 vaccine. 10 As opposed to the previously reported cases of CVST and other unusual sites of thromboses, our patient developed extensive DVT and PE. Whether female sex is associated with increased likelihood of atypical thrombus location is unclear due to small numbers. The patient had no prior risk factors for thrombosis, other than two recent four‐hour long flights. His initial symptom of leg pain highlights the need for early awareness and recognition of VITT in recipients of the Ad26.COV2.S vaccine, and initiation of work‐up as guided by the International Society on Thrombosis and Hemostasis. The extreme rarity of this disorder in the US, and in men in particular, together with the patient's presenting clinical symptoms and recent travels may have led to VITT being underrecognized at first. Additionally, the initial presentation of leg pain related to DVT, as opposed to headaches or neurologic changes related to CVST, has been less commonly seen in VITT. 2 , 3 , 10
The strong positive results of IgG isotype anti‐PF4 ELISA in this case are similar to other reported cases of VITT. The anti‐PF4 antibody assay used in our laboratory detects antibodies reactive with PF4 when complexed with a polyanionic compound (polyvinyl sulfate). This assay has been reported to show strong positive results in patients with VITT, in contrast to other anti‐PF4 antibody assays, which may show weaker positive results or may even be negative. 11 In particular, rapid HIT immunoassays on automated coagulation analyzers, or in point of care formats, have shown negative results in most cases to date. Despite the reported sensitivity of ELISA assays, early immunoassay comparison studies of VITT samples have not identified any immunoassay successful in identifying all subjects. Functional testing in our laboratory was also performed with a serotonin release assay using both low and high heparin concentrations, with a positive result. Functional HIT assays using reagent platelets have shown variable results in the VITT patients reported to date, and may appear either negative or positive when performed in the presence of low and high heparin concentrations. 2 , 12 , 13 Buffer‐only SRA testing performed at an outside laboratory on residual pre‐treatment serum did not show definitive platelet reactivity, which was unexpected given the positive result with traditional SRA in the presence of heparin. P‐selectin expression assay (PEA) on the same pre‐treatment serum sample was positive, in keeping with reports that functional HIT assays with added exogenous PF4 are more sensitive for detection of VITT antibodies. 2 , 12 Repeat PEA and SRA testing performed a month later were both negative, suggesting no further ongoing platelet activation at the time of refractory thrombocytopenia.
Our case demonstrates that VITT following Ad26.COV2.S vaccination occurs in men, with variability in presenting symptoms and thrombus locations that may lead to underrecognition of the syndrome. It also highlights the importance of continued monitoring and follow‐up for this disorder. In our case, the patient developed refractory thrombocytopenia which we believe is immune‐mediated, but not associated with further platelet activation. Refractory thrombocytopenia may pose a bleeding risk for patients on therapeutic anticoagulation, and guidelines on the optimal management of this phenomenon are urgently needed. Importantly, we emphasize the need for early recognition of VITT syndrome by physicians, as underrecognition may lead to increased morbidity and mortality, whereas early recognition may permit rapid treatment and improvement of symptoms, as seen in our case.
CONFLICT OF INTEREST
MYL has received advisory board honoraria from Sanofi Genzyme, Argenx, Dova Pharmaceuticals, Hema Biologics. MYA, KAM, KJS report no conflict of interest.
AUTHOR CONTRIBUTIONS
Mouhamed Yazan Abou‐Ismail, Karen A. Moser, Kristi J. Smock, Ming Y. Lim all contributed to the writing of the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. COVID‐19 vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets. European Medicines Agency. https://www.ema.europa.eu/en/news/covid‐19‐vaccine‐astrazeneca‐benefits‐still‐outweigh‐risks‐despite‐possible‐link‐rare‐blood‐clots. Accessed May 2021.
- 2. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384:2092‐2101. 10.1056/nejmoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cines DB, Bussel JB. SARS‐CoV‐2 vaccine–induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254‐2256. 10.1056/nejme2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cases of cerebral venous sinus thrombosis with thrombocytopenia after receipt of the Johnson & Johnson COVID‐19 vaccine. Centers for Disease Control and Prevention. https://emergency.cdc.gov/han/2021/han00442.asp. Accessed May 1, 2021.
- 5. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID‐19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients–United States, April 2021. Centers for Disease Control and Prevention. https://www.cdc.gov/mmwr/volumes/70/wr/mm7017e4.htm. 2021. Accessed May 1, 2021. [DOI] [PMC free article] [PubMed]
- 6. See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination. JAMA. 2021. 10.1001/jama.2021.7517. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sadoff J, Davis K, Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination—response from the manufacturer. N Engl J Med. 2021;384(20):1965–1966. 10.1056/nejmc2106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID‐19 vaccination. 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf. Updated May 12, 2021. Accessed May 31, 2021.
- 9. Coronavirus vaccine ‐ weekly summary of Yellow Card reporting. Medicines & Healthcare Products Regulatory Agency. https://www.gov.uk/government/publications/coronavirus‐covid‐19‐vaccine‐adverse‐reactions/coronavirus‐vaccine‐summary‐of‐yellow‐card‐reporting. Accessed May 21, 2021.
- 10. Makris M, Pavord S, Lester W, Scully M, Hunt B. Vaccine‐induced immune thrombocytopenia and thrombosis (VITT). Res Pract Thromb Haemost. 2021;5(5):e12529. 10.1002/rth2.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Platton S, Bartlett A, MacCallum P, et al. Evaluation of laboratory assays for anti‐platelet factor 4 antibodies after ChAdOx1 nCOV‐19 vaccination. J Thromb Haemost. 2021. 10.1111/jth.15362. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2202‐2211. 10.1056/nejmoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine‐related thrombosis following AstraZeneca COVID‐19 vaccination: guidance statement from the GTH. Hämostaseologie. 2021. 10.1055/a-1469-7481. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


