Abstract
Objective
Patients with chronic rheumatic diseases (CRDs), such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), require special attention during the COVID‐19 pandemic as they are considered at risk of severe infections. Our objective was to assess the seroprevalence of SARS–CoV‐2 in patients with SLE and RA and to assess patient behavior, disease‐related symptoms, and mental health.
Methods
More than 900 participants were included: 405 patients with RA or SLE (CRD patients) and 513 blood donors. All participants had blood SARS–CoV‐2 total antibodies measured (sensitivity 96.7%, specificity 99.5%) and answered a questionnaire concerning behavior, anxiety, and symptoms of depression (Patient Health Questionnaire 9). The CRD patients were further asked about physical activity, adherence to medication, and disease‐related symptoms.
Results
CRD patients had a significantly lower seroprevalence of SARS–CoV‐2 antibodies (n = 1 of 365, 0.3%) compared to blood donors (n = 10 of 513, 1.9%; P = 0.03). Almost 60% of patients were unable to exercise as usual, and increased pain and disease activity was experienced by 34% and 24% of patients, respectively. Almost 10% of patients reduced or discontinued their immunosuppressive treatments at their own initiative. Symptoms of moderate depression were present in 19% of patients compared to 6.8% of blood donors (P < 0.001).
Conclusion
Low seroprevalence in patients with CRDs indicates successful mitigation of exposure to SARS–CoV‐2. However, this mitigation appears to occur at the expense of physical activity, experience of increased pain, disease activity, and symptoms of depression. There is a need for care providers to be aware of these negative side effects and for further studies to investigate the possible long‐term consequences.
INTRODUCTION
The COVID‐19 pandemic has complicated the management of systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) (throughout this work referred to collectively as chronic rheumatic diseases [CRDs]). Patients with CRDs are immunocompromised and generally vulnerable to infection (1). The fear of COVID‐19 and the shielding strategy undertaken by many CRD patients introduced new challenges in the management of the patients. Although recommendations have been developed to manage patients with CRDs by, i.e., the European Alliance of Associations for Rheumatology (EULAR) (2), strong evidence is still lacking to guide treatment decisions.
SIGNIFICANCE & INNOVATIONS.
Blood donors were significantly more likely to become infected with SARS–CoV‐2 compared to patients with chronic rheumatic disease (CRD) as evaluated by specific antibodies against SARS–CoV‐2 in blood.
During the pandemic, CRD patients were unable to exercise as usual and experienced increased pain and disease activity.
Approximately 10% of patients with CRD reduced or discontinued prescribed immunosuppressive treatment without their physician's recommendation.
The hypothesis of being at a higher risk of experiencing severe COVID‐19 when treated with immunosuppressants is not definitive (3). Some reports indicate that risk of a severe outcome of COVID‐19 is similar in most patients with CRDs to people without CRDs (3), while other reports have indicated the opposite (4).
Denmark has been a low‐incidence country, with a seroprevalence in June 2020 of 1.9% (5). One explanation for the low seroprevalence was a nationally mandated lock‐down during March to June 2020. The immediate need for information and lack thereof, particularly about patients’ risk of COVID‐19, triggered anxiety and isolation for many patients. Thus, the question remains whether the consequences of the lock‐down, e.g., isolation, depression, anxiety, lack of exercise, and reduced accessibility of rheumatology consult, overshadowed the benefits, considering the low prevalence of COVID‐19 in Denmark.
This study aimed to assess the seroprevalence of blood SARS–CoV‐2 total antibodies in patients with CRDs and blood donors during the first wave of the pandemic. We further evaluated patient behavior regarding medication, exercise, pain, and experienced disease activity during the pandemic. Finally, we investigated the differences in anxiety and depression in patients with CRDs compared with blood donors.
SUBJECTS AND METHODS
Subjects
SLE and RA outpatients at the Department of Rheumatology, Aarhus University Hospital, were included in the study. Patients were identified through hospital records. Inclusion criteria for RA patients were treatment with either a biologic or small‐molecule disease‐modifying antirheumatic drug (DMARD), and fulfillment of either the 1987 American College of Rheumatology (ACR) or 2010 ACR/EULAR Classification Criteria (6, 7). Inclusion criteria for SLE patients were fulfillment of the 1982 updated ACR criteria for SLE (8). Comorbidity was assessed using the Charlson Comorbidity Index. Disease‐specific morbidity for SLE patients was evaluated by the Systemic Lupus International Collaborating Clinics (SLICC) score. The 2 patient groups were selected because they represent some of our potential “high risk” patients concerning COVID‐19. Danish blood donors, included in the Danish Blood Donor Study, who answered a specific COVID‐19–related questionnaire, were included in the present study as controls. All subjects were from the same geographical area.
Questionnaires
After providing informed consent, CRD patients completed an electronic questionnaire concerning their mental and physical health, exercise, and behavior (the questionnaire was answered between May 25 and June 7) (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24716). Two patient research partners and 2 patient advisors from the Danish Rheumatism Association assisted in the creation of the questions. Symptoms of depression were assessed using the Patient Health Questionnaire 9 (PHQ‐9) (9), and symptoms of anxiety were evaluated using a national anxiety symptom questionnaire. Disease characteristics and Charlson Comorbidity Index data were obtained from the electronic health record. The blood donors’ questionnaire corresponded to that completed by the patients with CRDs, except for specific questions regarding rheumatic diseases. CRD patients who were positive for SARS–CoV‐2 antibodies or who previously tested positive with a polymerase chain reaction (PCR) test were interviewed about their symptoms of COVID‐19, disease duration, and severity.
Blood samples and SARS–CoV‐2 antibody testing
Blood samples from both CRD patients and blood donors were collected between June 8 and June 19 and analyzed at the Department of Clinical Microbiology at Aarhus University Hospital. Serum was tested for antibodies against SARS–CoV‐2 using a SARS–CoV‐2 total antibody enzyme‐linked immunosorbent assay (Wantai Biological Pharmacy Enterprise Co.) according to the manufacturer's instructions. The assay detects total antibodies in serum binding the SARS–CoV‐2 spike protein receptor‐binding domain.
Results were based on a single test result. The sample absorbance (A) value was divided by a cutoff (CO) value for the enzyme‐linked immunosorbent assay plate based on an average absorbance value for 3 negative kit controls. A/CO values: <0.9 (negative), 0.9–1.1 (inconclusive), and >1.1 (positive). Performance characteristics of the assay have been determined in a Danish validation study (sensitivity 96.7%, specificity 99.5%) (10). No cross‐reactivity was observed.
Statistical analysis
All values reported are medians with interquartile ranges (IQRs) unless otherwise stated. The statistical significance of differences was assessed using the Mann‐Whitney nonparametric test for continuous variables and Pearson's chi‐square test for categorical variables. Multivariate logistic regression with depression and behavioral changes as dependent variables and CRD, age, and sex as independent variables was performed (see Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24716).
Ethics
The Central Denmark Region Committee on Health Research Ethics was consulted concerning the present study (Ref. nr. 1‐10‐72‐1‐20). The project was approved by the Danish Data Protection Agency (1‐16‐02‐211‐20). The Danish Blood Donor Study was approved by the Danish Research Ethics Committees (M‐2009‐0237, SJ‐740) and the Danish Data Protection Agency (P‐2019‐99).
RESULTS
Subjects included
Through the hospital registries, we identified 455 RA patients who fulfilled the ACR 1987 classification criteria and were undergoing active treatment with either a biologic or small‐molecule DMARD. RA patients were contacted consecutively from a random list with the goal of including 200 RA patients. A total of 229 RA patients were contacted, and 199 (87%) consented to participation. Similarly, 215 SLE patients were identified and contacted, and 206 (96%) agreed to participate in the study. Thus, a total of 405 CRD patients were included, and 387 (95.6%) completed the questionnaire. During the same period of June 2020, 513 blood donors were included (Table 1).
Table 1.
Demographic characteristics of the study participants*
| SLE | RA | Blood donors | |
|---|---|---|---|
| Patients included, no. | 206 | 199 | 513 |
| Female sex at birth | 188 (91.3) | 142 (71.4) | 252 (49.1) |
| Age, median (IQR) years | 49.0 (38.3–60.1) | 62.5 (51.6–70.5) | 47 (33–57) |
| BMI, median (IQR) kg/m2 | 24.2 (21.8–27.8) | 25.8 (22.5–28.9) | 25.5 (23.1–28.4) |
| Disease duration, median (IQR) years | 11.9 (6.0–24.0) | 14.0 (8.0–21.0) | – |
| Charlson Comorbidity Index, median (IQR) | 2 (1–3) | 3 (2–4) | – |
| Smoking | |||
| Active | 21 (11) | 26 (14) | – |
| Previous | 75 (38) | 95 (50) | – |
| Never | 100 (51) | 70 (37) | – |
| Hypertension | 53 (25.7) | 46 (23.4) | – |
| Caucasian | 198 (96.1) | 198 (99.5) | – |
| Rheumatoid arthritis | |||
| Anti‐CCP positivity | – | 146 of 196 (74.5) | – |
| IgM RF positivity | – | 137 (69) | – |
| Erosive disease on radiograph | – | 158 (79) | – |
| Systemic lupus erythematosus | |||
| ACR classification criteria | |||
| Malar rash | 129 (63) | – | – |
| Discoid rash | 133 (6) | – | – |
| Photosensitivity | 105 (51) | – | – |
| Oral ulcers | 61 (30) | – | – |
| Nonerosive arthritis | 172 (84) | – | – |
| Pleuritis or pericarditis | 61 (30) | – | – |
| Renal disorder | 60 (29) | – | – |
| Neurologic disorder | 14 (79) | – | – |
| Hematologic disorder | 151 (73) | – | – |
| Immunologic disorder, | 188 (91) | – | – |
| Positive antinuclear antibody | 204 (99) | – | – |
| SLICC score, median (IQR) | 1 (0–2) | – | – |
Values are the number (%) unless indicated otherwise. ACR = American College of Rheumatology; Anti‐CCP = anti–cyclic citrullinated peptide; BMI = body mass index; IQR = interquartile range; RA = rheumatoid arthritis; RF = rheumatoid factor; SLE = systemic lupus erythematosus; SLICC = Systemic Lupus International Collaborating Clinics.
The RA patients were significantly older than the SLE patients and blood donors. The RA patients had a median disease duration of 14 years, 146 (74.5%) of 196 were anti–citrullinated protein antibody–positive, and 158 (79%) had erosive disease. The majority of the RA patients were treated with a tumor necrosis factor inhibitor (n = 108, 54%), and 99 (50%) of all received concomitant methotrexate (see Supplementary Table 3, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24716).
SLE patients had an average age of 49 years and a mean disease duration of 12 years. Most of the SLE patients were antinuclear antibody positive (n = 204, 99%) and were women (n = 188, 91%), and 60 (29%) with nephritis, with a median SLICC damage score of 1. They were primarily being treated with hydroxychloroquine (n = 151, 73%), prednisolone (n = 82, 40%), or azathioprine (n = 44, 21%) (see Supplementary Table 3, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24716). RA patients had a significantly higher Charlson Comorbidity Index of 3 compared to 2 for the SLE patients (P > 0.001). The CRD cohort had a combined median age of 57 years, and the blood donors, a median age of 47 years (P > 0.001). The CRD patients had a body mass index of 25.0 kg/m2, which was no different than the blood donors of 25.5 kg/m2 (P = 0.06).
SARS–CoV‐2 antibody and PCR test
All the blood donors (n = 513) and 365 of the CRD patients (90.1%) had SARS–CoV‐2 antibodies measured (Figure 1). Significantly more blood donors (n = 10, 1.9%) than patients (n = 1, 0.3%) tested positive for SARS–CoV‐2 antibodies (P = 0.03). A subsequent interview revealed that the antibody‐positive CRD patient had a subclinical, asymptomatic infection. Fifty‐one CRD patients (13.2%) reported a total of 60 PCR tests performed for SARS–CoV‐2 RNA prior to inclusion. All tests were negative.
Figure 1.
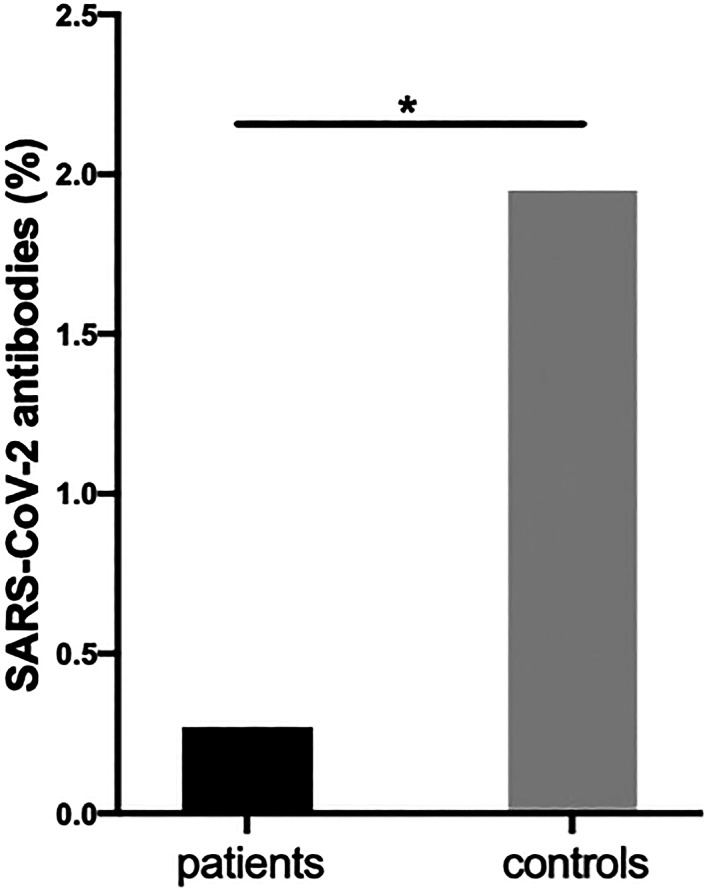
Presence of SARS–CoV‐2 antibodies in chronic rheumatic disease patients (1 of 365, 0.3%) and blood donors (10 of 513, 1.9%). * P = 0.03.
Behavioral changes
More CRD patients reported a change in behavior compared to blood donors (Table 2). Whereas both CRD patients and blood donors adjusted their behavior with regard to handwashing (P = 0.90) and sneezing in the elbow (P = 0.70), CRD patients were more likely to avoid public transportation, avoid large gatherings, and stay home compared to blood donors (all P ≤ 0.001) (Table 2). Logistic regression adjusting for age and sex did not change this conclusion (see Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24716).
Table 2.
Behavioral changes, mental health, pain, and disease activity*
| CRD | Blood donors | P | |
|---|---|---|---|
| Behavioral changes due to the risk of COVID‐19 | |||
| Washing hands more often | 91.6 | 91.4 | 0.92 |
| Coughing or sneezing in the elbow | 83.0 | 84.0 | 0.67 |
| Wearing face mask | 5.0 | 2.4 | 0.001 |
| Restricting the use of public transport | 47.4 | 32.9 | <0.001 |
| Avoiding places where many people are gathered | 80.7 | 70.0 | <0.001 |
| Staying at home | 51.6 | 35.3 | <0.001 |
| Symptoms of moderate depression, PHQ‐9 score of ≥10 | 19.01 | 6.75 | <0.001 |
| Effect of anxiety on daily function, anxiety score of >5 | 5.21 | 4.88 | 0.825 |
| COVID‐19 test | |||
| Have you been tested for COVID‐19? | 13.2 | – | – |
| Did you test positive? | 0 | – | – |
| Training, pain, and disease activity | |||
| Have you been able to exercise as usual? (yes) | 41.4 | – | – |
| Have you been less physically active due to COVID‐19? (yes) | 44.7 | – | – |
| Has the degree of physical activity increased the pain from your rheumatic disease? (yes) | 33.9 | – | – |
| Have you experienced increased disease activity during COVID‐19? (yes) | 23.5 | – | – |
| Have you started taking other medicines/supplements to reduce the risk of COVID‐19? (yes) | 8.0 | – | – |
| Have you on your own initiative reduced/discontinued your immunosuppressive treatment due to concerns about COVID‐19? (yes) | 9.3 | – | – |
Values are the percentage. CRD = chronic rheumatic diseases; PHQ‐9 = Patient Health Questionnaire 9.
Exercise, pain, and disease activity
More than half of the CRD patients (n = 227 of 387, 58.7%) were unable to exercise as usual and 45% (n = 173) reported being less physically active. CRD patients experienced increased pain (n = 131, 34%) and an increase in disease activity (n = 91, 23.5%). The pandemic also affected how CRD patients took their medication; 8.0% (n = 31) started taking other medications or supplements to reduce the risk of COVID‐19, and 9.3% (n = 36) reduced or discontinued their prescribed treatment at their own initiative.
Depression and anxiety
A significantly larger proportion of CRD patients (n = 73, 18.9%) had symptoms of moderate depression evaluated by a PHQ‐9 score of ≥10, compared to blood donors (n = 34; P < 0.001) (Table 2). SLE patients (n = 47 of 195, 24.1%) were more affected by moderate depression than RA patients (n = 26 of 189, 13.8%; P = 0.01). There was no difference in anxiety symptoms that affected daily function between CRD patients and blood donors (P = 0.825) (Table 2). Adjustment for age and sex using logistic regression did not change conclusions regarding depression or anxiety (see Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24716).
DISCUSSION
The study aimed to assess how the first wave of the global COVID‐19 pandemic affected patients with CRDs compared to blood donors in a well‐defined geographical region and with comparable exposure time. Assessed by SARS–CoV‐2 total antibodies in blood, we found that CRD patients had a significantly lower prevalence compared to blood donors. However, this lower prevalence appears to occur at the expense of decreased physical activity, experience of increased pain, self‐perceived disease activity, and symptoms of depression.
Even though Denmark has so far been a low‐incidence country (5), we were surprised by the low seroprevalence of SARS–CoV‐2 antibodies in the CRD patients. A person becomes SARS–CoV‐2 antibody–positive 1–2 weeks after the onset of COVID‐19. The assay used in the present study has a high diagnostic sensitivity and specificity, and no cross‐reactivity was observed in a large validation study that included samples from patients with autoimmune diseases (10). Antibodies against SARS–CoV‐2 were measured, as we also aimed to identify CRD patients and controls who had subclinical infection (11). Up to 18% of SARS–CoV‐2 infections are subclinical (12). The antibody response is potentially weaker in patients with CRDs, which could be due to the use of DMARDs or their underlying immune‐mediated condition. This response may lead to false‐negative results. However, in a severely immunocompromised patient population (chronic lymphatic leukemia), anti–SARS–CoV‐2 antibodies were still present in 67% of patients 3 months after clinical COVID‐19 (13). An alternative explanation for our observations is provided by the results of the questionnaire, as CRD patients were significantly more isolated compared to blood donors and thus less exposed to SARS–CoV‐2. Substantiating this interpretation, none of the patients (13.2%) who were tested for SARS–CoV‐2 (PCR test) prior to inclusion tested positive.
The effects of physical isolation, inactivity, pain, disease activity, and mental health on the patient with CRD intertwine and are hard to untangle. Nearly 10% of the study population reduced or discontinued their immunosuppressive medication, which could possibly lead to disease flare and increased pain in some of the patients. EULAR has stated that patients with CRDs should keep taking their medication (2), but uncertainty about immunosuppressants and the risk of COVID‐19 has led to some patients ignoring expert recommendations.
The present study underlines the fact that the COVID‐19 pandemic facilitates an environment that endorses physical inactivity. Nearly 60% of CRD patients were not able to exercise as usual, resulting in almost 50% being less physically active, and a third experienced increased pain. Structured physical activity is an integral part of the treatment for patients with CRDs and is advocated by EULAR as an essential part of the standard of care (14). Inactivity in patients with chronic diseases is associated with poor physical and mental health and an increased risk in both all‐cause mortality and disease‐specific mortality (15).
A larger proportion of patients with CRD experienced symptoms of depression compared to blood donors, but depression is known to be significantly more prevalent among patients with CRDs than in the general population. Thus, we cannot conclude that symptoms of depression in the current study per se were related to physical inactivity and/or physical isolation during the pandemic. Furthermore, age could be a potential confounder of these results, as the CRD patients were significantly older than the controls. However, SLE patients, with the highest rate of depression, had an age comparable to our controls, and thus age is unlikely to be a confounding factor.
It is apparent that the global pandemic will be prevalent for a protracted period, and we should acknowledge the potential long‐term consequences of the current recommendations for patients with CRDs. Patients receiving immunosuppressants in regions of high incidence of SARS–Cov‐2 infection in Italy did not seem to have a higher risk of serious complications compared to the general population (16), and studies indicate that most patients with CRDs do not have a higher frequency of mortality and poor outcome compared to the general population (3, 17), although further studies are needed to clarify this aspect. Looking at the consequences of the self‐imposed isolation strategy that some patients with CRDs have chosen, a superior approach would possibly be to obey the recommendation targeted at the general population and avoid strict physical isolation.
The strengths of this study are that this is the first work to evaluate the number of SARS–CoV‐2–infected individuals in a cohort of patients with CRDs by measuring the seroprevalence of antibodies against SARS–CoV‐2. The study included ~80% of all SLE patients and >40% of all RA patients treated with either a biologic or small‐molecule DMARD in our clinic, representing a group of significantly immunosuppressed CRD patients. Further, the study included blood donors from the same region, included and sampled during the same 2‐week period as the CRD patients. Thus, the exposure time for patients and blood donors in this study is unbiased.
There are also limitations to the study. We wished to answer the question of whether having a CRD or receiving DMARD treatment would put CRD patients at a high or low risk of COVID‐19. However, due to shielding and thus nonexposure, the incidence of infected individuals in the CRD group was too low for these questions to be addressed. Low seroprevalence of SARS–CoV‐2 Ig in the CRD patients could be due to immunosuppressive treatment. We do not think this possibility is the case, as symptomatic CRD patients were tested with pharyngeal swap and PCR test, and none of the CRD patients in the study had tested positive prior to inclusion. The potential patients with asymptomatic disease and unmeasurable antibodies cannot be ruled out.
A social desirability bias cannot be excluded. Patients would likely answer questions in a manner that would be viewed favorably by their treating rheumatologist, i.e., to questions about stopping medication. However, for the seroprevalence, we do not think social desirability has influenced the result. Blood donors are not representative of the general population, and hence could represent a selection bias. However, the seroprevalence of SARS–CoV‐2 in the blood donors included in the study reflected the seroprevalence in the general population of June 2020 in Denmark (5) and would therefore not bias our results.
We had a high participation and response rate in the study, but we are aware that nonparticipants and nonresponders could influence our result. Still, the most common reason for CRD patients not to participate was fear of leaving home to have blood drawn. We would as follows expect the nonparticipation to cut the bias toward negative seroprevalence for the nonresponders.
Although our results suggest that isolation is associated with apparent protection against COVID‐19, the results also raise a concern regarding the possible consequences of isolation for patients with CRDs. The potential consequence of physical isolation is a risk of severe mental health issues, physical inactivity, self‐medication, increased pain, and increased disease activity. The long‐term consequences of our recommendations for patients with CRDs should be taken into account when tackling the continuing pandemic.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Ammitzbøll had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Ammitzbøll, Erikstrup, Thomsen, Hauge, Troldborg.
Acquisition of data
Ammitzbøll, Andersen, Vils, Mistegaard, Mikkelsen, Erikstrup, Thomsen, Troldborg.
Analysis and interpretation of data
Ammitzbøll, Hauge, Troldborg.
Supporting information
Supplementary Table 1 Supplementary Table
Supplementary Table 2 Regression analysis. Effect of age, sex and SLE or RA diagnosis on behavioral changes and depression
Supplementary Table 3 Immunosuppresice treatment of patients with SLE and RA
ACKNOWLEDGMENTS
We are thankful for the help with creation of the questionnaire by Lene Mandrup Thomsen, Nanna Bacci Hartz, Lene Lau, and Jeanette Andersen.
Supported by the Danish Rheumatism Association.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52:53–61. [DOI] [PubMed] [Google Scholar]
- 2. Landewé R, Machado P, Kroon F, Bijlsma H, Burmester G, Carmona L, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS‐CoV‐2. Ann Rheum Dis 2020;79:851–8. [DOI] [PubMed] [Google Scholar]
- 3. Gianfrancesco M, Hyrich KL, Al‐Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bachiller‐Corral J, Boteanu A, Garcia‐Villanueva MJ, de la Puente C, Revenga M, Diaz‐Miguel MC, et al. Risk of severe coronavirus infection (COVID‐19) in patients with inflammatory rheumatic diseases. J Rheumatol 2021;48:1098–102. [DOI] [PubMed] [Google Scholar]
- 5. Erikstrup C, Hother C, Pedersen O, Mølbak K, Skov R, Holm D, et al. Estimation of SARS‐CoV‐2 infection fatality rate by real‐time antibody screening of blood donors. Clin Infect Dis 2020;72:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 7. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 8. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 9. Kroenke K, Spitzer R, Williams J. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harritshoej LH, Gybel‐Brask M, Afzal S, Kamstrup PR, Joergensen CS, Thomsen MK, et al. Comparison of sixteen serological SARS‐CoV‐2 immunoassays in sixteen clinical laboratories. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2020. [Google Scholar]
- 11. Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis 2020;71:778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID‐19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 2020;25:2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roeker L, Knorr D, Pessin M, Ramanathan L, Thompson M, Leslie L, et al. Anti‐SARS‐CoV‐2 antibody response in patients with chronic lymphocytic leukemia. Leukemia 2020;34:3047–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rausch Osthoff AK, Niedermann K, Braun J, Adams J, Brodin N, Dagfinrud H, et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis 2018;77:1251–60. [DOI] [PubMed] [Google Scholar]
- 15. Booth F, Roberts C, Thyfault J, Ruegsegger G, Toedebusch R. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev 2017;97:1351–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl 2020;26:832–4. [DOI] [PubMed] [Google Scholar]
- 17. Serling‐Boyd N, D'Silva K, Hsu T, Wallwork R, Fu X, Gravallese E, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis 2021;80:660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Supplementary Table
Supplementary Table 2 Regression analysis. Effect of age, sex and SLE or RA diagnosis on behavioral changes and depression
Supplementary Table 3 Immunosuppresice treatment of patients with SLE and RA


