Our patient, a 24‐year‐old previously healthy male, presented with 10‐day history of severe abdominal pain, nausea, vomiting, and decreased oral intake. He had been seen at urgent care 2 days earlier for similar symptoms, treated for presumed gastritis with belladonna alkaloids, phenobarbital, antacid, and viscous lidocaine, and had moderate improvement. He received the Ad26.COV2.S vaccine 11 days prior to onset of symptoms.
He had diffuse abdominal tenderness and mild thrombocytopenia (66 × 103/μl), leukocytosis (11.4 × 103/μl), elevated D‐dimer (>5250 ng/ml) with normal hemoglobin 13.8 g/dl. That evening, he had worsening abdominal pain, maroon‐colored emesis and drop in hemoglobin to 7.9 g/dl. He was transferred to the intensive care unit. Testing for SARS‐CoV‐2 was negative. Contrast enhanced computerized tomography (CT) showed extensive occlusive thrombosis of the portal, superior mesenteric (SMV), and splenic veins with severe bowel wall thickening concerning for venous ischemia. Brain magnetic resonance venography was negative for cerebral venous thrombosis. He was treated with heparin for 20 h and then bivalirudin due to concern for vaccine‐induced thrombotic thrombocytopenia (VITT). He had esophagogastroduodenoscopy (EGD) on hospital Day 3 for hematemesis showing impressive congestive gastropathy, but no evidence of Mallory‐Weiss tear, gastritis, ulcer or carcinoma. His platelet count ranged from 66 × 103/μl to 82 × 103/μl and he continued bivalirudin. Repeat abdominal imaging on Day 4 showed worsening of his extensive portal, SMV and splenic vein thrombosis, worsening small bowel thickening, and splenic hypoenhancement suggesting infarction (Figure 1).
FIGURE 1.
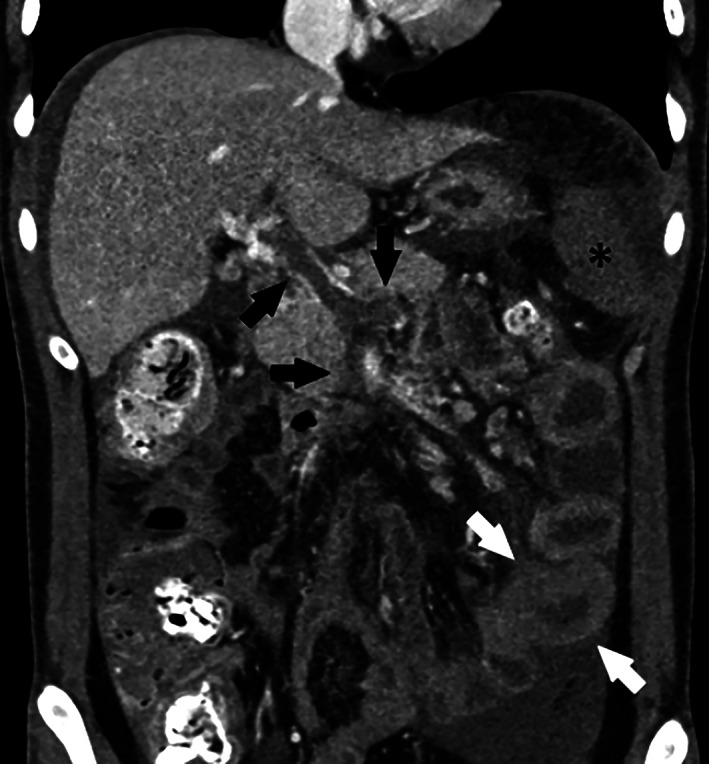
Pre‐TIPS coronal image from CT performed on Day 4 of hospitalization showing portal, SMV and splenic vein thrombosis (black arrows), bowel wall thickening (white arrows), and splenic hypoenhancement (asterisk) worse compared to the CT on admission
He was transferred to our institution for evaluation by interventional radiology. We administered intravenous immunoglobulin (IVIG) 1 g/kg on Days 5 and 6. After multidisciplinary discussion, we proceeded with transjugular intrahepatic portosystemic shunt (TIPS) 1 and thrombectomy to reduce the risk of further bowel ischemia and long‐term complications of portal hypertension. 2
We used intravascular ultrasound guidance with intracardiac echocardiography (ICE‐Boston Scientific) to gain access to the thrombosed portal venous system. We advanced, with some difficulty, a wire through the thrombosed portal vein and SMV into the patent distal SMV tributaries and deployed a 10 mm diameter TIPS stent (Gore Viatorr). We suctioned clot from the portal vein and SMV with an Inari FlowTriever 16 (Inari Medical, Irvine CA) and used the AngioJet (Boston Scientific) thrombectomy device with 3 milligrams of tissue plasminogen activator to clear the splenic vein.
The following morning, he had complete resolution of abdominal pain. Post‐procedure contrast enhanced computerized tomography demonstrated patency of the TIPS, SMV, and splenic veins, normal enhancement of the spleen and reduced small bowel wall thickening (Figure 2). The patient was evaluated by surgery as one loop of bowel had persistent wall thickening; but was managed non‐operatively. His diet was advanced without any further pain.
FIGURE 2.

Post‐TIPS coronal image from CT performed on Day 1 after TIPS showing normal appearing portal, SMV and splenic veins (black arrows) flowing into a patent TIPS (white arrow). The spleen enhanced normally (asterisk) and the appearance of the bowel was improved
No other causes of thrombophilia were identified including Factor V Leiden, prothrombin gene G20210A, JAK2 V617F mutations, decreased protein C and S, antithrombin and ADAMTS13 activity or elevated homocysteine. Both free protein S (67%, reference range of 74–146) and factor VII activity (49, reference range 50%–129% were mildly decreased, likely from liver synthetic dysfunction. He had no evidence of a lupus anticoagulant or anticardiolipin, and beta‐2‐glycoprotein antibodies. Testing for heparin‐induced thrombocytopenia by latex immunoassay was negative twice (<0.600 U/ml, <1 negative) and his serotonin release assay was negative. However, he had evidence of anti‐PF4 IgG antibodies by ELISA (OD 2.47, >0.5 positive), confirming vaccine‐induced thrombotic thrombocytopenia.
On Day 12, bivalirudin was stopped and he started apixaban 5 mg every 12 h. His platelet count was 292 × 103/μl. On Day 13, he was discharged home.
We present the first male patient with extensive mesenteric thromboses in the setting of recent Ad26.COV2.S vaccination. He had negative HIT screen and positive heparin PF4 IgG antibodies. He was treated with bivalirudin and IVIG, and subsequently underwent TIPS and thrombectomy for persistent symptomatic thrombosis.
We did not identify any inherited disorders or suggestive family history. An extensive evaluation of acquired causes only identified PF4 IgG antibodies and a greatly elevated D‐dimer and C‐reactive protein. These findings and onset of symptoms after recent vaccination confirm the diagnosis of VITT.
Our decision to perform TIPS and mechanical thrombectomy rather than catheter directed thrombolysis was two‐fold. First, due to the degree of intrahepatic portal vein thrombosis, catheter directed thrombolysis alone could fail in the absence of adequate outflow with continued risk of progressive bowel ischemia. Second, the patient presented with hematemesis, so the risk of bleeding with thrombolysis was unacceptably high.
In addition to venous ischemia of the bowel, extensive splanchnic venous thrombosis can cause ascites and variceal bleeding. 2 If not successfully treated, chronically thrombosed veins present a formidable challenge for interventional 3 and surgical treatments. 4 We postulate that the IVIG and continuous non‐heparin anticoagulant decreased his risk of occlusion of his TIPS and recurrent thrombosis.
Reports of thrombotic events after Ad26.COV2.S vaccination are sparse. Muir, et al. described a 48‐year‐old Caucasian female who had thrombocytopenia with cerebral venous sinus and splanchnic thromboses after Ad26.COV2.S vaccination. She was treated with argatroban (as an alternative to anticoagulation with heparin) and IVIG. Her platelets recovered, though she was still critically ill at time of the report. 5 Clark et al. described a 40‐year‐old Caucasian female with thrombocytopenia, cerebral venous sinus thrombosis, and pulmonary embolism following Ad26.COV2.S vaccination. She was treated with bivalirudin and her platelets rapidly returned to normal. She was discharged on Day 6 with resolution of headache and no clinical sequelae of thromboses at the time of the report. 6
This case suggests that thrombectomy and TIPS can be safe and effective for severe VITT involving the splanchnic circulation. Additional studies are needed to establish the role of mechanical intervention in VITT, particularly in cases refractory to medical therapies.
This case also highlights that vaccine‐induced thrombotic thrombocytopenia with life‐threatening illness can also affect males. We are not aware of any male deaths from VITT, but further studies are needed to establish gender differences.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1. Supplementary Appendix
Dhoot R, Kansal A, Handran C, et al. Thrombocytopenia and splanchnic thrombosis after Ad26.COV2.S vaccination successfully treated with transjugular intrahepatic portosystemic shunting and thrombectomy. Am J Hematol. 2021;96(9):1180–1182. 10.1002/ajh.26258
REFERENCES
- 1. Valentin N, Korrapati P, Constantino J, Young A, Weisberg I. The role of transjugular intrahepatic portosystemic shunt in the management of portal vein thrombosis. Eur J Gastroenterol Hepatol. 2018;30(10):1187‐1193. 10.1097/MEG.0000000000001219. [DOI] [PubMed] [Google Scholar]
- 2. Wang JT, Zhao HY, Liu YL. Portal vein thrombosis. Hepatobiliary Pancreat Dis Int. 2005;4(4):515‐518. [PubMed] [Google Scholar]
- 3. Salem R, Vouche M, Baker T, et al. Pretransplant portal vein recanalization‐transjugular intrahepatic portosystemic shunt in patients with complete obliterative portal vein thrombosis. Transplantation. 2015;99(11):2347‐2355. 10.1097/TP.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 4. Ozer A, Aktas H, Yilmaz TU, et al. Liver transplant in patients with portal vein thrombosis: the experience of 55 patients. Exp Clin Transplant. 2019. 10.6002/ect.2018.0260. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5. Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384(20):1964‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clark RT, Johnson L, Billotti J, et al. Early outcomes of bivalirudin therapy for thrombotic thrombocytopenia and cerebral venous sinus thrombosis after Ad26.COV2.S vaccination: a case report. Ann Emerg Med. 2021. 10.1016/j.annemergmed.2021.04.035. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary Appendix


