Abstract
Background
COVID‐19 can cause severe disease with need of treatment in the intensive care unit (ICU) for several weeks. Increased knowledge is needed about the long‐term consequences.
Methods
This is a single‐center prospective follow‐up study of COVID‐19 patients admitted to the ICU for respiratory organ support between March and July 2020. Patients with invasive ventilation were compared with those with high‐flow nasal oxygen (HFNO) or non‐invasive ventilation (NIV) regarding functional outcome and health‐related qualify of life. The mean follow‐up time was 5 months after ICU discharge and included clinical history, three well‐validated questionnaires about health‐related quality of life and psychological health, pulmonary function test, 6‐minute walk test (6MWT) and work ability. Data were analyzed with multivariable general linear and logistic regression models with 95% confidence intervals.
Results
Among 248 ICU patients, 200 patients survived. Of these, 113 patients came for follow‐up. Seventy patients (62%) had received invasive ventilation. Most patients reported impaired health‐related quality of life. Approximately one‐third suffered from post‐traumatic stress, anxiety and depression. Twenty‐six percent had reduced total lung capacity, 34% had reduced 6MWT and 50% worked fulltime. The outcomes were similar regardless of ventilatory support, but invasive ventilation was associated with more bodily pain (MSD −19, 95% CI: −32 to −5) and <80% total lung capacity (OR 4.1, 95% CI: 1.3‐16.5).
Conclusion
Among survivors of COVID‐19 who required respiratory organ support, outcomes 5 months after discharge from ICU were largely similar among those requiring invasive compared to non‐invasive ventilation.
Keywords: COVID‐19, intensive care unit, long‐term effects
Editorial Comment.
In this study, survivors of COVID‐19 requiring ICU care were followed up following discharge with a battery of standardized questionnaires about health‐related quality of life and psychological health, in addition to physical status assessment. Months after discharge, many suffered from psychological sequelae of their acute illness, half had not returned to full‐time work, and a high percentage of them had reduced total lung capacity and 6‐minute walking capacity. This study underscores the high burden of chronic psychological and physical illness associated with the most severe form of viral pneumonias, such as the one resulting from COVID‐19.
1. INTRODUCTION
COVID‐19 is a disease with a varying disease spectrum, in terms of both disease severity and organs affected. The disease is caused by Severe Acute Respiratory Syndrome coronavirus 2 (SARS‐CoV‐2). 1 Approximately 81% who become infected with COVID‐19 get mild symptoms, whereas 14% get severe illness, that is, with severe dyspnea and hypoxia and 5% get critical illness with respiratory failure, shock or multi‐organ dysfunction. 2 Typical findings on computer tomography (CT)‐scans are bilateral ground glass changes which in some patients rapidly progress and result in acute respiratory distress syndrome (ARDS). 3 There is a significant pre‐existing comorbidity in the critically ill patients with COVID‐19 treated in the intensive care unit (ICU) such as hypertension, diabetes, cardiovascular disease, chronic lung disease and obesity. 4 , 5 There is no difference in the gender distribution in the number of sick patients, 1 but there is a predominance of men of those who become seriously ill. 6 Venous thromboembolic complications are common in patients with COVID‐19, which is why thrombosis prophylaxis is recommended in hospitalized patients, 7 and high‐dose prophylaxis is preferable in critical ill patients. 8 There are also reports of neurological complications in patients who have been severely ill due to COVID‐19 infection. 9 To optimize the recovery after severe COVID‐19 infection, increased knowledge is needed regarding the trajectory of recovery after a hospital stay and possible long‐term effects in affected patients. By incorporating patients’ perspectives of the illness and its treatments, through the use of patient‐reported measures, a better understanding of the outcome can be gained. One commonly used patient‐reported outcome measure is health‐related quality of life which refers to the potential impact of a disease on a person`s daily life and the activities that the person wishes to do. 10 Previous studies have indicated health‐related quality of life reductions in ICU survivors with a prolonged ICU stay (>5 days), up to one year after ICU discharge, compared with an age‐ and sex‐matched population. 11 However, the knowledge about recovery in patients with COVID‐19 after ICU treatment is limited. Therefore, the purpose of this prospective cohort study was to evaluate long‐term effects of COVID‐19 in critically ill patients treated in ICU and whether invasive ventilation was associated with worse health‐related quality of life, physical and psychological outcomes. Furthermore, the purpose was to evaluate whether there were differences in lung capacity and function level at follow‐up.
2. METHODS
2.1. Study design and patients
This prospective cohort study included survivors of severe COVID‐19 infection who had been admitted to either of the two ICUs (ICU A, and ICU B) at Södersjukhuset, Stockholm, Sweden during the period from 25 March to 13 August 2020. All critically ill patients with a positive Polymerase Chain Reaction for COVID‐19 and treated for respiratory failure with invasive ventilation, high‐flow treatment with oxygen (HFNO) or non‐invasive ventilation (NIV) in the ICU were eligible for inclusion, see flowchart Figure 1. All patients received standard treatment during this period with oxygen, thromboprophylaxis and antibiotics in almost all cases. In the beginning of the pandemic, all patients received chloroquine phosphate for a short period of time. At the end of the inclusion period, patients at the ICU received corticosteroids. Study approval was granted from the Swedish Ethical Review Authority (Dnr 2020‐03760) and written informed consent was obtained from all included patients.
FIGURE 1.
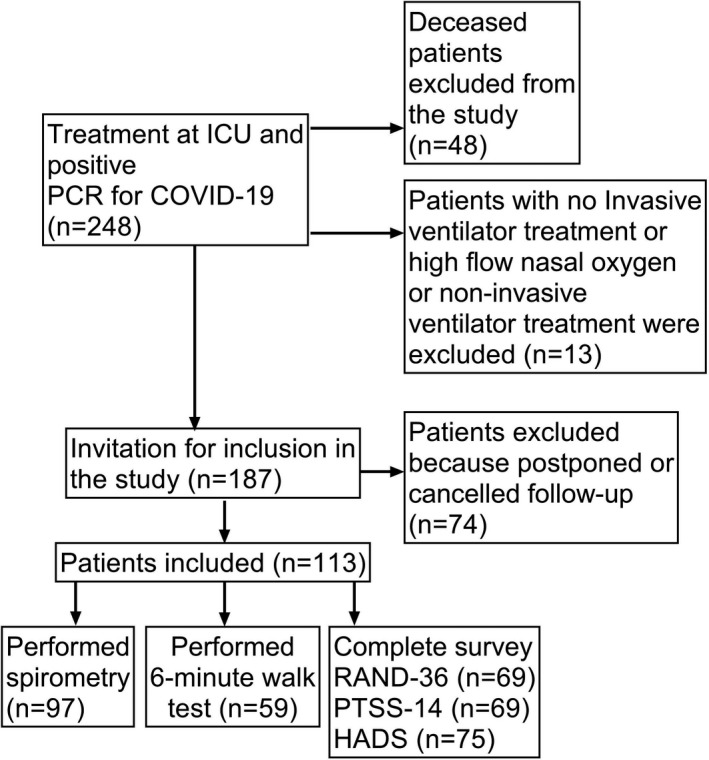
Flowchart of study inclusion
2.2. Data collection
Surviving patients were invited for a follow‐up visit at the hospital between 2 and 7 months after ICU‐discharge for examination and screening for symptoms related to COVID‐19. Together with the invitation for the follow‐up, a questionnaire kit assessing health‐related quality of life and psychological well‐being was sent to the patients. Each ICU had their own follow‐up clinic. At follow‐up, all patients met a physician for medical assessment. At clinic A, all patients met a nurse for review of their ICU stay and a physiotherapist for assessment of physical capacity with 6‐minute walk test (6MWT). Due to limited resources, few patients visiting ICU follow‐up B underwent 6MWT and not everyone met a nurse and a physiotherapist. Self‐reported data on current and former work ability were also collected at the appointment. Patients were invited for pulmonary function testing on a separate occasion.
Data on clinical details were prospectively recorded and included sex, age, body mass index (BMI), smoking habits, comorbidities, ICU length of stay and Simplified Acute Physiological Score III (SAPS 3), which is a scoring system used to predict mortality risk in the ICU. A higher score at ICU admission indicates a higher mortality risk. 12
2.3. Outcomes
2.3.1. Health‐related quality of life (RAND‐36
Health‐related quality of life was assessed with the 36‐item questionnaire RAND‐36 Item Health Survey (RAND Corp) which includes eight domains: Physical functioning (10 items), role physical (4 items), bodily pain (2 items), general health (5 items), vitality (4 items), social functioning (2 items), role emotional (3 items) and emotional well‐being (5 items). 13 Questionnaire responses were linearly transformed into scores between 0 and 100 (according to the manual), 14 where a higher score represents a better perceived health‐related quality of life. 13 , 15 Normative data for RAND‐36 in the general population are available from a random sample of adults in south of Sweden. In 1999, the Short form‐36 (identical to RAND‐36) questionnaire was mailed to 10.000 individuals aged between 20 and 74 years and complete data were collected from 6093 people (61%). 16 These data can be obtained as mean values for men and women in different age groups. In our study, each patient was individually matched on these mean values of the corresponding age and sex groups. 14
2.3.2. Post‐traumatic stress symptom scale ‐14 (PTSS‐14)
Post‐traumatic stress symptoms were assessed with the questionnaire PTSS‐14 validated for ICU survivors with 14 questions screening for ongoing stress symptoms), each scored from 1 (never) to 7 (always) rendering a total score between 14 and 98 with a higher score indicating more problems. Score above 45 is commonly used as a cut‐off for clinically significant symptoms of post‐traumatic stress. 17
2.3.3. Hospital anxiety and depression scale (HADS)
The questionnaire consists of 14 items, assessing symptoms of anxiety and symptoms of depression in two separate subscales 18 and is well validated for critically ill patients. 19 Each item generates a score between 0 and 3 points. A subscale score of ≥8 indicates clinical symptoms of anxiety or depression. 20
2.3.4. Pulmonary function testing
Total lung capacity (TLC) examined with whole body plethysmography and diffusion capacity for carbon monoxide (DLCO) (uncorrected value) were measured according to standard methods. 21 , 22 Outcomes were expressed according to reference values (Hedenström). 23 , 24 TLC ≥80% of predicted and DLCO ≥80% were used as cut‐offs, in line with previous studies of COVID‐19. 25 , 26
2.3.5. Six‐minute walk test
The 6MWT measures the distance that a patient can quickly walk on a hard‐flat surface at six minutes and was performed with pulse oximetry according to standard methods. 27 Predicted values were calculated with regard to age, sex and BMI and the observed values were compared to the predicted values. 28
2.3.6. Work ability
Self‐reported data on work ability at the time of follow‐up were assessed for patients <65 years who had been working fulltime before onset of COVID‐19.
2.4. Statistical analysis
Descriptive statistics were presented as counts (n), proportions (%), means with standard deviation (SD) and medians with interquartile range (IQR) according to type and distribution of data. Potential differences between groups were analyzed by Fisher´s Exact Test, Student's t‐test and Mann‐Whitney U‐test, where appropriate. Two questionnaires (HADS and PTSS‐14) had missing items and these were handled with mean imputation. 29
Multivariable general linear regression models were used to estimate mean score differences (MSD) with 95% confidence intervals (CI) between health‐related quality of life in patients divided into two groups: HFNO or/and NIV and invasive ventilation. Wilcoxon Signed Ranks Test was used to compare health‐related quality of life with age‐ and sex‐matched data. 14 To investigate associations, logistic regression was used and presented as odds ratios (OR) with 95% CI. The multivariable models were adjusted for age (<50, 50 to <65 and ≥65), sex (men/women), BMI (<25, 25 to 30 and >30), comorbidities (diabetes, hypertension and chronic lung disease). Smoking habits were defined as never/ever smokers. Potential non‐response biases among patients who came for follow‐up were analyzed regarding patient characteristic variables of responders and non‐responders.
Statistical analyses were performed with IBM SPSS Statistics software 25.0 (IBM Corp., Armonk, NY, USA) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) and Graph Pad Prism 8 (GraphPad Software Inc, San Diego, CA, USA). A two‐sided P‐value <.05 was regarded as statistically significant.
3. RESULTS
3.1. Patients
Between March and August 2020, a total of 248 patients with COVID‐19 were treated at the two ICUs. Out of these, 200 (81%) patients survived. Thirteen patients were excluded because they had been admitted to the ICU for other reasons than respiratory failure, leaving 187 eligible for follow‐up. Out of these, 113/187 (60%) came for follow‐up. Among the 74 patients who did not come for follow‐up, several had a scheduled follow‐up appointment in another hospital to which they had been moved to due to lack of beds in the ICU. For the others, the reason for declining follow‐up was unknown. With regard to those who came for follow‐up, there were no statistically significant differences in patient characteristics between responders (who completed the questionnaires and performed pulmonary function test and 6MWT) and non‐responders, except for lower participation rate on pulmonary function test for patients with chronic lung disease (File S1).
Patient characteristics of the 113 patients who came for follow‐up are presented in Table 1. Seventy patients (62%) had received invasive ventilation and 43 (38%) HFNO) or NIV in the ICU. Patient characteristics were similar between the groups, except that there was a significantly higher proportion of men (58/70, 83%) among patients with invasive ventilation compared to HFNO or NIV (28/43, 65%, P = .041) and patients with invasive ventilation had a higher SAPS 3 median score [57 (IQR 52‐60) vs 53 (IQR 49‐58), P = .026] and a significant longer stay in the ICU with median 21 days (IQR 15‐30) in comparison to 4 days (IQR 3‐8) for the group with HFNO or NIV (P < .001) (Table 1). Median time of follow‐up was 5 months (IQR 4‐5) after ICU discharge with no differences in mean time between the groups.
TABLE 1.
Patient and clinical characteristics of included patients (n = 113)
|
HFNO or NIV n = 43 |
Invasive ventilator treatment n = 70 |
|
|---|---|---|
| Male n (%) | 28 (65) | 58 (83)* |
| Age, years, mean (SD) | 58 (14) | 58 (12) |
| BMI, Kg/m2, median (IQR) | 28 (26‐30) | 28 (26‐30) |
| Hypertension n (%) | 16 (37) | 33 (47) |
| Diabetes n (%) | 7 (16) | 14 (20) |
| Cardiovascular disease n (%) | 5 (12) | 7 (10) |
| Chronic lung disease n (%) | 11 (26) | 11 (16) |
| Ever smoker n (%) | 16 (37) | 28 (40) |
| SAPS 3 (ICU admission), median (IQR) | 53 (49‐58) | 57 (52‐60)* |
| Length of ICU stay (days), median (IQR) | 4 (3‐8) | 21 (15‐30)* |
Abbreviations: BMI, body mass index; HFNO, high‐flow nasal oxygen; ICU, Intensive care unit; IQR, interquartile range; NIV, non‐invasive ventilation;SAPS, Simplified Acute Physiology Score; SD, Standard deviation.
Statistically significant value (P < .05).
3.2. Health‐related quality of life
Five months after ICU discharge, patients reported impaired health‐related quality of life in all domains, compared with an age‐ and sex‐matched reference population, (Table 2, Figure 2). There were no differences in health‐related quality of life between patients with HFNO or NIV and invasive ventilation. After adjustment for confounders, the results remained similar between groups except for more bodily pain in patients who had received invasive ventilation (MSD −19, 95% CI: −32 to −5) (Table 3).
TABLE 2.
Health‐related qualify of life (RAND‐36) in COVID‐19 patients with respiratory distress and ventilation support compared with a reference population, presented as mean scores with 95% confidence intervals
| HRQL domains | All patients | HFNO or NIV | Invasive ventilation support | Reference population |
|---|---|---|---|---|
| Mean scores (95% CI) | Mean scores (95% CI) | Mean scores (95% CI) | Age and sex matched data in mean scores (95% CI) | |
| Total number | 69 | 28 | 41 | |
| Physical function | 62 (55‐68) | 61 (50‐71) | 62 (54‐70) | 81 (79‐83) |
| Role physical | 29 (20‐39) | 30 (15‐45) | 29 (16‐41) | 75 (72‐78) |
| Bodily pain | 59 (51‐67) | 67 (55‐79) | 54 (44‐63) | 71 (70‐72) |
| General health | 49 (45‐54) | 48 (39‐57) | 51 (45‐56) | 70 (69‐72) |
| Vitality | 48 (42‐54) | 44 (32‐56) | 50 (44‐57) | 68 (67‐69 |
| Social function | 63 (57‐70) | 64 (53‐74) | 61 (54‐69) | 88 (87‐88) |
| Role emotional | 58 (48‐69) | 59 (42‐76) | 58 (44‐71) | 82 (81‐84) |
| Mental health | 67 (62‐72) | 66 (57‐75) | 68 (62‐74) | 81 (81‐82) |
Abbreviations: CI, confidence intervals; HFNO, high‐flow nasal oxygen; HRQL, Health‐related quality of life; NIV, non‐invasive ventilation.
FIGURE 2.
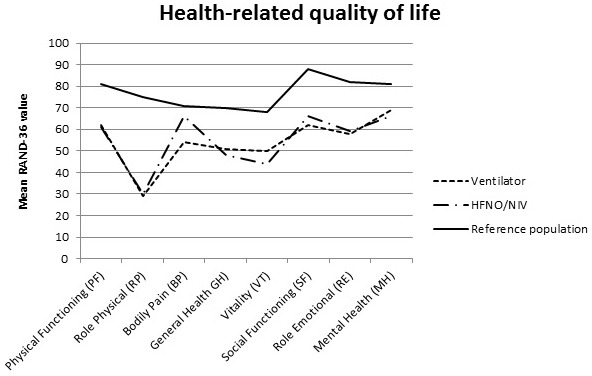
Health‐related quality of life for ICU survivors with COVID‐19 with invasive ventilator treatment, high‐flow nasal oxygen (HFNO)/non‐invasive ventilator (NIV) treatment and for an age‐specific normative population
TABLE 3.
Health‐related quality of life (RAND‐36) comparisons between COVID‐19 patients with invasive ventilation and HFNO or NIV (reference), with adjustment for age, sex, BMI and comorbidities presented as mean score differences with 95% confidence intervals
| HRQL domains | Univariable analysis | Multivariable analysis |
|---|---|---|
| MSD (95% CI) | MSD (95% CI) | |
| Physical function | 2 (−11 to 15) | −3 (−16 to 10) |
| Role physical | −2 (−21 to 18) | ‐5 (−26 to 1) |
| Bodily pain | −14 (−28 to 1) | −19 ( −32 to −5)* |
| General health | 3 (−7 to 13) | 0 (−10 to 11) |
| Vitality | 6 (−6 to 19) | 4 (−9 to 17) |
| Social function | −3 (−15 to 10) | −5 (−18 to 8) |
| Role emotional | −2 (−23 to 20) | −5 (−28 to 17) |
| Mental health | 2 (−8 to 13) | 0 (−11 to 11) |
Abbreviations: CI, confidence intervals; HFNO, high‐flow nasal oxygen; HRQL, Health‐related quality of life; MSD, mean score differences; NIV, non‐invasive ventilation.
Statistically significant value (P < .05).
3.3. Symptoms of post‐traumatic stress, anxiety and depression
Symptoms of post‐traumatic stress was found in 24/69 patients (35%). For anxiety and depression, 25/75 (33%) and 27/75 (36%) patients reported clinically relevant problems. Compared to patients with HFNO or NIV, those with invasive ventilation did not seem to have a statistically significant increased risk of post‐traumatic stress (OR 1.8, 95% CI: 0.6 to 6.1), anxiety (OR 1.7, 95% CI: 0.6 to 5.3) or depression (OR: 1.1, 95% CI: 0.4 to 3.1) (Table 4).
TABLE 4.
Associations of invasive ventilation on total lung capacity, symptoms of post‐traumatic stress, anxiety and depression compared with HFNO or NIV (reference) in COVID‐19 patients with adjustment for age, sex, BMI, and comorbidities, presented as odds ratios with 95% confidence intervals
| Outcomes | Univariable analysis | Multivariable analysis |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Total lung capacity <80% | 4.2 (1.4‐15.5)* | 4.1 (1.3‐16.5)* |
| PTSS‐14 > 45 | 1.3 (0.5‐3.8) | 1.8 (0.6‐6.1) |
| HADS anxiety ≥8 | 1.7 (0.6‐4.8) | 1.7 (0.6‐5.3) |
| HADS depression ≥8 | 1.0 (0.4‐2.5) | 1.1 (0.4‐3.1) |
Abbreviations: CI, confidence intervals; HADS, Hospital anxiety and depression scale; HFNO, high‐flow nasal oxygen; NIV, non‐invasive ventilation; OR, odds ratio; PTSS‐14, Post‐traumatic stress symptom scale ‐14; TLC, total lung capacity.
Statistically significant value (P < .05).
3.4. Pulmonary function testing
Ninety‐seven patients out of 113 (86%) performed pulmonary function testing (ie, measurement of TLC and DLCO) at mean time 6 months after discharge from ICU. The majority of patients had a normal pulmonary function, but 25/97 (26%) had TLC <80% of predicted and 34/96 (35%) DLCO <80%. Those with HFNO or NIV had a better TLC at follow‐up with median 91%, IQR 84%‐98%, compared with those who had invasive ventilation (median 85%, IQR 73%‐90%) (P = .0013). Patients with invasive ventilation were more likely to have reduced lung volumes (ie, TLC) (OR 4.1 95% CI: 1.3‐16.5) (Table 4). No statistically significant differences were seen in diffusion capacity between patients with HFNO or NIV and invasive ventilation (median 93%, IQR 74%‐111% vs median 86%, IQR 73%‐98%).
3.5. Six‐minute walk test
All patients from ICU A and three patients from ICU B performed 6MWT. In total, fifty‐nine patients (59/113) (52%) performed 6MWT. Out of these 20/59 (34%) had a reduced walking capacity assessed as <80% of predicted. For the whole group (n = 59), the median walking distance was 88% of predicted (IQR 74%‐99%). The median saturation measured with pulse oximetry pre‐test was 98% (IQR 96%‐98%) and 97% (IQR 95%‐98%) directly after the test. No statistically significant differences were seen between the groups.
3.6. Work ability
Among those younger than 65 years of age (n = 74) 46 (62%) worked fulltime before onset of COVID‐19. Five months after ICU discharge, 23/46 (50%) had returned to full time work. There were no significant differences between those with HFNO or NIV and invasive ventilation [9/19 (47%) vs 14/27 (52%)].
4. DISCUSSION
To date, few studies have reported long‐term outcomes in severely ill patients with COVID‐19 and there is a great need of this knowledge to be able to offer adequate help to the survivors. This study indicates that health‐related quality of life was reduced 5 months after ICU discharge. Psychological distress was present in approximately one‐third of the patients. Reduced lung volumes (ie, TLC) was seen in 26% of the patients and 35% had impaired diffusion capacity. A reduced walking distance at 6MWT was seen in 34%. Half of the survivors previously working fulltime had returned to fulltime work. Most outcomes were similar regardless of type of ventilation support, except that those with invasive ventilation reported more pain and more commonly had a reduced pulmonary function.
In previous studies, the health‐related quality of life has shown to be significantly impaired after a severe COVID‐19 infection. A report including 19 ICU survivors with acute respiratory distress syndrome (ARDS) following COVID‐19, found quality‐of‐life impairments for all patients and approximately half of them complained about psychological distress at 3‐month follow‐up. 30 Similar results were reported from another 3‐month follow‐up study of 22 ARDS survivors with COVID‐19. 31 In a cohort study including 1733 patients with COVID‐19, of which 122 had needed HFNO, NIV or invasive ventilation, it was found that the more severely ill patients suffered from fatigue or muscle weakness and psychological problems 6 months after symptom onset. 26 These results are similar to what was found in this cohort.
The low health‐related quality‐of‐life scores found in our cohort are comparable to previous reported results of acute respiratory distress syndrome (ARDS) survivors, 32 except for role physical and role emotional which were lower for the COVID‐19 survivors. Another meta‐analysis of 1104 ICU survivors found that 22% of the patients experienced post‐traumatic distress syndrome. 33 The prevalence rate varied depending on the time of assessment and were at the highest at the time of discharge and shortly thereafter. Compared to this result, the prevalence of psychological distress in COVID‐19 patients was higher for post‐traumatic stress.
In our cohort, the majority had a normal lung function at the time‐point of follow‐up with small differences with regard to days in the ICU. In a 3‐month follow‐up study of 124 patients with COVID‐19, almost all of the critical ill patients (n = 20) had abnormalities on CT (17 investigated), but despite this, a relatively good lung function. 34 In our study, no significant differences were seen on 6MWT or the ability to return to work with regard to number of ICU days. Therefore, the recovery process seems not to be depending on severity of disease at such great extent as could be expected.
Patients with COVID‐19 seem to have impaired health in the months after ICU discharge, irrespective of ICU length of stay or type of ventilation support. In a Swedish study, comorbidities such as type 2 diabetes, hypertension and obesity were all risk factors for a severe cause of COVID‐19 requiring invasive ventilation. 35 These comorbidities, common in our cohort, may affect not only the severity of disease but also the ability to recover.
This cohort received care at a general hospital in Stockholm area, including a heterogenic population and there is no reason to believe that this cohort would be any different from other ICU patients with COVID‐19. In countries where the healthcare organization is similar, these results could contribute with knowledge on how to assess and accommodate expected problems after severe COVID‐19 disease and respiratory distress.
A methodological strength of the study is the prospective design and the use of well‐validated outcome questionnaires and tests, which reduce the risk of information bias. Results were adjusted for known confounders. However, residual confounding cannot be completely eliminated. The study also holds a number of limitations. The lack of baseline data is a major limitation of the study. Pre‐ICU data on functional ability and quality of life are difficult to obtain as an ICU admission is difficult to foresee but may prevent identification of new‐onset problems. Approximately 40% of the patients declined follow‐up or cancelled their appointment and further all patients did not return complete questionnaires, which might induce a risk of selection bias. To speculate, it might have been that those who did not come for follow‐up did not have the strength to come. In that case, a potential underestimation of reported problems could be present. With regard to those who came for follow‐up, non‐response analysis showed few differences in patient characteristics between responders and non‐responders. Health‐related quality‐of‐life data from the Swedish reference population were obtained in 1999 and we cannot preclude that there have been changes in the reference data since then. However, we have no reason to believe that the changes are immense. Furthermore, despite that a higher number of survivors who had received invasive ventilation reported symptoms of post‐traumatic stress, anxiety and depression, no statistically significant differences between the groups were seen. However, the limited number of participants in the study induces a risk of type II error. Most patients who performed the 6MWT had been admitted to only one of the ICUs, but the ICUs have close collaboration with similar treatment strategies. Therefore, there is little reason to believe that the results cannot be extrapolated to all included patients. The outcome was mostly based on self‐reported data, which inevitably incorporate a risk of misclassification. Also, the time interval for follow‐up varied between 2 and 7 months which might have had impact on the results. However, mean time for follow‐up was similar between the comparison groups. Finally, a larger sample size would likely have improved the generalizability of the results.
In conclusion, health‐related quality of life was reduced 2‐7 months after ICU discharge and psychological distress was present in approximately one‐third of the patients and work ability had decreased. Most outcomes were similar regardless of whether the patient had been on invasive ventilation or not, except for bodily pain and pulmonary function, which were worse for patients with invasive ventilation who had a longer ICU stay.
CONFLICT OF INTERESTS
The authors declare that they have no competing interest.
AUTHOR CONTRIBUTIONS
AS co‐designed the study, characterized patients, summarized data, interpreted data and wrote the manuscript. AH co‐designed the study, characterized patients, summarized data and interpreted data. PL co‐designed the study, interpreted data and helped writing the manuscript. SFT and JS characterized patients, co‐designed the study and helped writing the manuscript. MR co‐designed the study and helped writing the manuscript. AG co‐designed the study and helped writing the manuscript. MAF performed most of the statistical analyses. MS co‐designed the study, interpreted data and helped writing the manuscript. EJA performed statistical analyses, interpreted data and helped writing the manuscript. PD designed the study, summarized data, interpreted data and helped writing manuscript. All authors read and approved the final manuscript.
Supporting information
File S1
ACKNOWLEDGEMENTS
The authors acknowledge all patients who participated in the study and caregivers involved in the follow‐up.
Schandl A, Hedman A, Lyngå P, et al. Long‐term consequences in critically ill COVID‐19 patients: A prospective cohort study. Acta Anaesthesiol Scand. 2021;65:1285–1292. 10.1111/aas.13939
Funding information
Departmental funding only.
REFERENCES
- 1. Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID‐19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 3. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kass DA, Duggal P, Cingolani O. Obesity could shift severe COVID‐19 disease to younger ages. Lancet. 2020;395(10236):1544‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peckham H, de Gruijter NM , Raine C, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID‐19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jonmarker S, Hollenberg J, Dahlberg M, et al. Dosing of thromboprophylaxis and mortality in critically ill COVID‐19 patients. Crit Care. 2020;24(1):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bowling A. Measuring health: a review of subjective health, well‐being and quality of life measurement scales. Maidenhead, UK: Open University Press; 2017. [Google Scholar]
- 11. Gerth AMJ, Hatch RA, Young JD, Watkinson PJ. Changes in health‐related quality of life after discharge from an intensive care unit: a systematic review. Anaesthesia. 2019;74(1):100‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3–From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hays RD, Sherbourne CD, Mazel RM. The RAND 36‐item health survey 1.0. Health Econ. 1993;2(3):217‐227. [DOI] [PubMed] [Google Scholar]
- 14. Sullivan M, Taft C. SF‐36 Hälsoenkät: Swedish manual and interpretation guide, 2nd edn. Gothenburg: Sahlgrenska University Hospital; 2002. [Google Scholar]
- 15. Orwelius L, Nilsson M, Nilsson E, et al. The Swedish RAND‐36 health survey ‐ reliability and responsiveness assessed in patient populations using Svensson's method for paired ordinal data. J Patient Rep Outcomes. 2017;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nordlund A, Ekberg K, Kristenson M. EQ‐5D in a general population survey–a description of the most commonly reported EQ‐5D health states using the SF‐36. Qual Life Res. 2005;14(4):1099‐1109. [DOI] [PubMed] [Google Scholar]
- 17. Twigg E, Humphris G, Jones C, Bramwell R, Griffiths RD. Use of a screening questionnaire for post‐traumatic stress disorder (PTSD) on a sample of UK ICU patients. Acta Anaesthesiol Scand. 2008;52(2):202‐208. [DOI] [PubMed] [Google Scholar]
- 18. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361‐370. [DOI] [PubMed] [Google Scholar]
- 19. Jutte JE, Needham DM, Pfoh ER, Bienvenu OJ. Psychometric evaluation of the Hospital Anxiety and Depression Scale 3 months after acute lung injury. J Crit Care. 2015;30(4):793‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brennan C, Worrall‐Davies A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta‐analysis of case‐finding ability. J Psychosom Res. 2010;69(4):371‐378. [DOI] [PubMed] [Google Scholar]
- 21. Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single‐breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49:1600016. [DOI] [PubMed] [Google Scholar]
- 22. Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511‐522. [DOI] [PubMed] [Google Scholar]
- 23. Hedenström H, Malmberg P, Agarwal K. Reference values for lung function tests in females. Regression equations with smoking variables. Bull Eur Physiopathol Respir. 1985;21(6):551‐557. [PubMed] [Google Scholar]
- 24. Hedenström H, Malmberg P, Fridriksson HV. Reference values for lung function tests in men: regression equations with smoking variables. Ups J Med Sci. 1986;91(3):299‐310. [DOI] [PubMed] [Google Scholar]
- 25. Han X, Fan Y, Alwalid O, et al. Six‐month follow‐up chest CT findings after severe COVID‐19 pneumonia. Radiology. 2021;299(1):E177‐E186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laboratories ACoPSfCPF . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166(1):111‐117. [DOI] [PubMed] [Google Scholar]
- 28. Enright PL, Sherrill DL. Reference equations for the six‐minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384‐1387. [DOI] [PubMed] [Google Scholar]
- 29. Bell ML, Fairclough DL, Fiero MH, Butow PN. Handling missing items in the Hospital Anxiety and Depression Scale (HADS): a simulation study. BMC Res Notes. 2016;9(1):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valent A, Dudoignon E, Ressaire Q, Dépret F, Plaud B. Three‐month quality of life in survivors of ARDS due to COVID‐19: a preliminary report from a French academic centre. Anaesth Crit Care Pain Med. 2020;39(6):740‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Truffaut L, Demey L, Bruyneel AV, et al. Post‐discharge critical COVID‐19 lung function related to severity of radiologic lung involvement at admission. Respir Res. 2021;22(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dowdy DW, Eid MP, Dennison CR, et al. Quality of life after acute respiratory distress syndrome: a meta‐analysis. Intensive Care Med. 2006;32(8):1115‐1124. [DOI] [PubMed] [Google Scholar]
- 33. Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van den Borst B , Peters JB, Brink M, et al. Comprehensive health assessment three months after recovery from acute COVID‐19. Clin Infect Dis. 2020. 10.1093/cid/ciaa1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Svensson P, Hofmann R, Häbel H, Jernberg T, Nordberg P. Association between cardiometabolic disease and severe COVID‐19: a nationwide case‐control study of patients requiring invasive mechanical ventilation. BMJ Open. 2021;11(2):e044486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1


