Abstract
Prolonged Covid‐19 is an emerging issue for patients with lymphoma or immune deficiency. We aimed to examine prolonged length of in‐hospital stay (LOS) due to Covid‐19 among patients with lymphoma and assess its determinants and outcomes. Adult patients with lymphoma admitted for Covid‐19 to 16 French hospitals in March and April, 2020 were included. Length of in‐hospital stay was analyzed as a competitor vs death. The study included 111 patients. The median age was 65 years (range, 19–92). Ninety‐four patients (85%) had B‐cell non‐Hodgkin lymphoma. Within the 12 months prior to hospitalization for Covid‐19, 79 patients (71%) were treated for their lymphoma. Among them, 63 (57%) received an anti‐CD20 therapy. Fourteen patients (12%) had relapsed/refractory disease. The median LOS was 14 days (range, 1–235). After a median follow‐up of 191 days (3–260), the 6‐month overall survival was 69%. In multivariable analyses, recent administration of anti‐CD20 therapy was associated with prolonged LOS (subdistribution hazard ratio 2.26, 95% confidence interval 1.42–3.6, p < 0.001) and higher risk of death (hazard ratio 2.17, 95% confidence interval 1.04–4.52, p = 0.039). An age ≥ 70 years and relapsed/refractory lymphoma were also associated with prolonged LOS and decreased overall survival. In conclusion, an age ≥ 70 years, a relapsed/refractory lymphoma and recent administration of anti‐CD20 therapy are risk factors for prolonged LOS and death for lymphoma patients hospitalized for Covid‐19. These findings may contribute to guide the management of lymphoma during the pandemic, support evaluating specific therapeutic approaches, and raise questions on the efficacy and timing of vaccination of this particular population.
1. INTRODUCTION
Patients with hematological malignancies have a higher risk of death from coronavirus disease 2019 (Covid‐19) than the general population. 1 , 2 Patients with lymphoma have an immune‐deficiency due to the biological features of lymphoma per se (hypogammaglobulinemia, neutropenia, lymphopenia) and its treatments, leading to an increased incidence and severity of infections. 3 , 4 High in‐hospital mortality related to severe Covid‐19 among patients with lymphoma has been reported in several countries, with 30‐day overall survivals (OS) ranging from 625 to 81%. 2 , 5 , 6 , 7 , 8 , 9 , 10 The risk of early death for patients with severe Covid‐19 and lymphoma increases with age and relapsed/refractory lymphoma disease. 5
The median length of in‐hospital stay (LOS) for Covid‐19 is reported to be 5 to 20 days depending on the requirement of intensive care. 11 , 12 With longer hindsight, the persistence of SARS‐CoV‐2 appears to be an emerging clinical issue for immunocompromised patients: the persistence of SARS‐CoV‐2 replication for at least 2 months was recently reported in a series of 20 immunocompromised patients treated for hematological malignancies. 13 Hueso et al. also reported a series of 17 patients with profound B‐cell depletion, prolonged Covid‐19 symptoms, negative SARS‐CoV‐2 serology, and positive SARS‐CoV‐2 polymerase chain reaction (PCR) in blood. 14 Moreover, among lymphoma treatments, anti‐CD20 monoclonal antibodies, such as rituximab and obinutuzumab, induce rapid B‐cell depletion, which may alter the generation of antibody responses to new pathogens, 15 , 16 possibly impacting the clinical course of Covid‐19. Indeed, at least nine case studies have reported that patients with B‐lineage hematological malignancies or auto‐immune diseases treated with B‐cell depletion experienced prolonged course of Covid‐19 with persistence of SARS‐CoV‐2 replication. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25
The incidence, risk factors, and outcomes of prolonged forms of Covid‐19 for patients with lymphoma are still only poorly assessed. In a previously published retrospective multicentric cohort, we reported a prolonged LOS of more than 30 days for 22% of patients with lymphoma and Covid‐19. 5 By increasing the follow‐up of this population from 1 to 6 months and following the inclusion of additional lymphoma patients from more centers in France, we aimed to examine prolonged LOS due to Covid‐19, and assess its determinants and outcomes.
2. METHODS
2.1. Setting
This retrospective multicentric study was conducted in 16 French hospitals. Briefly, adult patients with a past or current diagnosis of lymphoma, who were admitted for Covid‐19 in March and April, 2020, were identified in each hospital, as previously described. 5 Each local investigator confirmed Covid‐19 based on a positive PCR test result for SARS‐CoV‐2 from nasopharyngeal or oropharyngeal swabs, or on typical clinical history associated with chest computed tomography (CT) showing Covid‐19 lesions. Patients with any lymphoma subtype could be enrolled in the study, except those with lymphoblastic and lymphocytic lymphomas, which were investigated in other studies. Patients or their relatives (for those who were sedated) were informed according to French law on biomedical research. The ethics committee for research of the Université Paris‐Saclay approved this study (CER‐Paris‐Saclay‐2020‐045) and it was conducted in accordance with the Declaration of Helsinki. Information on the study is available at clinicaltrials.gov (NCT 04386512).
2.2. Data sources, assessment of variables, and definitions
Data were extracted from the patients' medical charts in each hospital by the local investigator. 5 Briefly, for each patient, data were obtained on their demographics, comorbidities, lymphoma history, including detailed chemotherapy and anti‐CD20 monoclonal antibody use (date of first and last administration), and lymphoma status at admission for Covid‐19 and at last follow‐up. Relapsed/refractory lymphoma was defined as progressive disease after more than two lines of treatment or progressive disease in palliative care due to comorbidities, regardless of the number of lines of treatment. Covid‐19‐related data included symptoms, laboratory tests, including complete blood counts, total immunoglobulin levels, SARS‐CoV‐2 RNA detection by PCR from nasal swabs, blood or bronchoalveolar lavage, and SARS‐CoV‐2 serology, imaging results, specific medications, oxygenation supply, and the modality of hospital discharge. Prolonged LOS due to Covid‐19 was defined as persisting or recurring Covid‐19 symptoms requiring a total LOS of more than 30 days.
2.3. Statistical analysis
Continuous variables are given as their medians and ranges and categorical variables as their frequencies and percentages. Qualitative variables were compared using Fisher's exact test. The OS was evaluated using Kaplan–Meier estimates from the time of hospitalization for Covid‐19 (or the time of the first SARS‐CoV‐2 PCR when symptoms of Covid‐19 occurred after admission for a cause other than Covid‐19) to the last follow‐up or date of death from any cause. Note, LOS was defined as the time between first admission and final discharge for Covid‐19 symptoms and analyzed as a competitor versus death. Living patients not discharged from the hospital at the time of the last follow up were censored at that time. The cumulative incidence of discharge from the hospital was estimated using the Kalbfleisch and Prentice method. 26 Factors associated with LOS were evaluated using a Fine and Gray proportional subdistribution hazard model. 27 Covariates considered in this analysis were gender, age (≥ 70 years vs below), body mass index (≥ 30 kg/m2 versus below), smoking status, presence of comorbidities (overall or hypertension only), main lymphoma subtypes (Hodgkin lymphoma, B‐cell non‐Hodgkin lymphoma [NHL], or T‐cell NHL), lymphopenia (< 1 G/L) hypogammaglobulinemia (< 4 g/L), recent (within 1 year) administration of any treatment for lymphoma or bendamustine, or anti‐CD20 monoclonal antibody, time between diagnosis of lymphoma and hospitalization for Covid‐19 (< 2 years vs ≥2 years), and lymphoma status (relapsed/refractory vs others). Multivariable analysis was performed from the subset of independent variables having univariate significance <0.1 and no missing data after a stepwise procedure of variable selection based on the Akaike information criterion. The impact of these selected variables on OS was also analyzed by a multivariable analysis using a Cox proportional hazard model. Statistical tests were two‐tailed and p values <0.05 were considered to denote statistical significance. Statistical analysis was performed using the R language and environment for statistical computing version 4.03 (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
3. RESULTS
3.1. Characteristics of the study population
The characteristics of the 111 included patients are summarized in Table 1 and Figure S1. Briefly, their median age was 65 years and 63% were male. Ninety‐four patients had B‐cell NHL (85%) and half (50%) were diagnosed with lymphoma more than 2 years before Covid‐19. The median LOS was 14 days (range, 1–235). After a median follow‐up of 191 days (range, 3–260), the 6‐month OS was 69% (95% Confidence Interval [CI] 60%–78%) (Figure S2). Twenty‐four patients died within 30 days after admission for Covid‐19. Among the 87 living patients 30 days after Covid‐19 diagnosis, 55 had been definitively discharged from the hospital, 31 were still hospitalized for Covid‐19 symptoms, and one was later re‐hospitalized for Covid‐19 symptoms recurrence. These 32 patients were considered as having prolonged LOS due to Covid‐19 (Figure S1).
TABLE 1.
Baseline characteristics of patients with lymphoma and Covid‐19 according to their clinical evolution
| Total population | Died within 30 days | Prolonged LOS for Covid‐19 > 30 days | Survived >30 days with LOS for Covid‐19 ≤ 30 days | |
|---|---|---|---|---|
| (n = 111) | (n = 24) | (n = 32) | (n = 55) | |
| Demographic characteristics | ||||
| Age, years | ||||
| Median (range) | 65 (19–92) | 76 (53–92) | 64 (43–87) | 64 (19–87) |
| ≥70, n (%) | 42 (38) | 17 (71) | 10 (31) | 15 (27) |
| Male gender, n (%) | 70 (63) | 16 (67) | 20 (63) | 34 (62) |
| Body mass index (kg/m2) | ||||
| Median (range) | 23.6 (15.9–41.6) | 24.1 (16.7–38.6) | 23.2 (16–41.5) | 24.5 (15.9–41.6) |
| ≥30, n (%) | 16 (14) | 3 (13) | 5 (16) | 8 (15) |
| Data missing, n (%) | 3 (3) | 2 (8) | 1 (3) | 0 (0) |
| Smoking status, n (%) | ||||
| Never smoked | 54 (49) | 10 (42) | 17 (53) | 27 (49) |
| Former smoker | 31 (28) | 9 (37) | 9 (28) | 13 (24) |
| Current smoker | 5 (4) | 1 (4) | 1 (3) | 3 (5) |
| Unknown | 21 (19) | 4 (17) | 5 (16) | 12 (22) |
| Comorbidities | ||||
| Comorbidity ≥1, n (%) | 75 (68) | 21 (88) | 22 (69) | 32 (58) |
| Hypertension | 45 (41) | 15 (63) | 10 (31) | 20 (36) |
| Diabetes | 22 (20) | 8 (33) | 5 (16) | 9 (16) |
| Chronic lung disease a | 10 (9) | 3 (13) | 3 (9) | 4 (7) |
| Cancer | 14 (13) | 6 (25) | 2 (6) | 6 (11) |
| HIV infection | 2 (2) | 0 (0) | 1 (3) | 1 (2) |
| Lymphoma characteristics | ||||
| Histological subtypes, n (%) | ||||
| Hodgkin lymphoma | 9 (8) | 1 (4) | 1 c (3) | 7 (13) |
| Diffuse large B‐cell lymphoma | 42 (38) | 15 (63) | 10 (31) | 17 (31) |
| Follicular lymphoma | 22 (20) | 0 (0) | 12 (38) | 10 (18) |
| Marginal zone lymphoma | 14 (13) | 2 (8) | 3 (9) | 9 (17) |
| Mantle cell lymphoma | 10 (9) | 3 (13) | 4 (13) | 3 (5) |
| Other B‐cell lymphoma | 6 (5) | 1 (4) | 2 (6) | 3 (5) |
| T‐cell lymphoma | 8 (7) | 2 (8) | 0 (0) | 6 (11) |
| Number of previous lymphoma treatment lines, n (%) | ||||
| 0 | 12 (11) | 1 (4) | 2 (6) | 9 (16) |
| 1 | 65 (59) | 16 (67) | 17 (53) | 32 (58) |
| 2 | 16 (14) | 3 (12) | 5 (16) | 8 (15) |
| ≥3 | 18 (16) | 4 (17) | 8 (25) | 6 (11) |
| Lymphoma treatment, n (%) | ||||
| Any lymphoma therapy b | 79 (71) | 18 (75) | 26 (81) | 35 (64) |
| Anti‐CD20 monoclonal antibody b | 63 (57) | 15 (62) | 26 (81) | 22 (40) |
| As part of induction immuno‐chemotherapy | 50 (45) | 13 (54) | 18 (56) | 19 (35) |
| As maintenance following induction | 13 (12) | 2 (8) | 8 (25) | 3 (5) |
| Bendamustine b | 10 (9) | 5 (21) | 3 (9) | 2 (4) |
| Autologous stem cell transplant | 21 (19) | 5 (21) | 8 (25) | 8 (15) |
| Allogeneic stem cell transplant | 4 (4) | 1 (4) | 1 (3) | 2 (4) |
| CAR T‐cell | 5 (5) | 1 (4) | 2 (6) | 2 (4) |
| Lymphoma status at Covid‐19 diagnosis, n (%) | ||||
| Complete remission | 52 (47) | 8 (33) | 19 (59) | 25 (46) |
| Partial remission | 3 (3) | 0 (0) | 2 (6) | 1 (2) |
| Ongoing therapy <3 lines | 30 (27) | 10 (42) | 5 (16) | 15 (27) |
| Watch and wait | 12 (11) | 1 (4) | 1 (3) | 10 (18) |
| Relapsed/refractory | 14 (12) | 5 (21) | 5 (16) | 4 (7) |
| Time between diagnosis of lymphoma and hospitalization for Covid‐19 (months), median (range) | 24 (0–285) | 15 (1–246) | 35 (3–285) | 13 (0–201) |
Abbreviations: CAR, chimeric antigen receptor; HIV, human immunodeficiency virus, LOS, length of in‐hospital stay.
Chronic lung disease was defined as chronic obstructive pulmonary disease, asthma, or chronic bronchitis.
Treatment administered within the previous 12 months before hospitalization for Covid‐19.
Nodular lymphocyte‐predominant Hodgkin lymphoma.
3.2. Description and clinical evolution of patients with prolonged LOS for Covid‐19
The proportion of patients who had a prolonged LOS for Covid‐19 symptoms (> 30 days) was 29%. Their median LOS was 58 days (range, 31–235). As shown in Table 1, they had a median age of 64 years (range, 43–87) and 63% were male. Twenty‐two patients (69%) had at least one significant comorbidity. Noteworthy, three patients had an ongoing autoimmune disease (rheumatoid polyarthritis, Crohn's disease and ankylosing spondylitis) and another was being treated for human immunodeficiency virus infection. All patients had B‐cell NHL, except one, who had nodular lymphocyte‐predominant Hodgkin lymphoma, versus 42/55 (76%) among patients alive 30 days after Covid‐19 diagnosis who did not experience prolonged LOS for Covid‐19 (p = 0.01). Within the 12 months prior to hospitalization for Covid‐19, 26 patients (81%) were treated for their lymphoma. Among them, all patients received an anti‐CD20 monoclonal antibody, either administered as induction in association with chemotherapy for 18 (56%) (including the patient with nodular lymphocyte‐predominant Hodgkin lymphoma) or as maintenance following induction chemo‐immunotherapy for eight (25%). By contrast, only 22 of 55 patients (40%) alive 30 days after Covid‐19 diagnosis who did not experience prolonged LOS for Covid‐19 recently received an anti‐CD20 monoclonal antibody (p < 0.001). During the follow‐up after Covid‐19 diagnosis, seven patients received treatment for their lymphoma (Bruton's tyrosine kinase inhibitors for two patients and anti‐CD20 immunochemotherapy for five). One of them had a recurrent form of Covid‐19 after receiving anti‐CD20 immunochemotherapy for relapsing follicular lymphoma, 4 months after the initial Covid‐19 diagnosis.
The detailed clinical and biological characteristics at admission to the hospital of patients with prolonged LOS for Covid‐19 are detailed in Table S1. Of note, three patients had a prolonged LOS due both to long‐lasting Covid‐19 symptoms and evolution of the lymphoma (patients 20, 30 and 31 in Table S1). Fever and respiratory symptoms were common at admission, but seven patients (21%) initially had no fever. Four patients (12%) with fever had no respiratory symptoms. All 32 patients had chest CT showing bilateral ground‐glass opacities that were consistent with the diagnosis of Covid‐19. 28 Five patients (15%) did not require oxygen support, 11 (30%) required low‐dose supplemental oxygen, two (12%) non‐invasive ventilation or high‐flow oxygen, and 14 (42%) invasive mechanical ventilation. Corticosteroids were given to eight patients, all of whom recovered, most (n = 7) after receiving other treatments. Convalescent plasma was administered to nine patients, of whom eight recovered from their symptoms, and one died of prolonged Covid‐19 complicated by multiple organ failure 6 days after receiving half of the scheduled dose of convalescent plasma. Other treatments are detailed in Table S1.
Overall, 19 patients (58%) with prolonged LOS for Covid‐19 were admitted to the intensive care unit (ICU): 16 patients were admitted during the first 30 days of hospitalization for Covid‐19 and the other three at days 31, 34, and 42. Nine patients with prolonged LOS for Covid‐19 died (27%): six deaths (18%) were related to Covid‐19, two (6%) to multiple causes, including Covid‐19 and lymphoma progression, and one to lymphoma progression (Patient 32, Table S1).
3.3. Laboratory findings and virological monitoring of patients with prolonged LOS for Covid‐19
The most common laboratory findings at admission were elevated C‐reactive protein (> 5 mg/L) for 29/32 evaluated patients (91%), profound hypogammaglobulinemia (< 4 g/L) for 8/25 (32%), and lymphopenia (< 1 G/L) for 21/30 (70%). Twenty‐seven patients had positive nasal swab PCR tests at diagnosis, whereas it was negative for five patients, although they all had typical clinical symptoms of Covid‐19. In those PCR negative patients, the diagnosis of SARS‐CoV‐2 pneumonia was established by an infectiologist expert based on clinical background and chest CT. 28 Covid‐19 was later confirmed for three of these five patients by SARS‐CoV‐2 positive blood PCR and for one of these five patients by positive PCR in bronchoalveolar lavage.
Twenty‐six of the 32 patients with prolonged LOS had multiple PCR tests during the follow‐up and six had only a single SARS‐CoV‐2 PCR test performed. Among the patients with repeated testing, SARS‐CoV‐2 PCR from nasal swabs or bronchoalveolar lavage was positive up to 143 days after initial diagnosis. The SARS‐CoV‐2 RNA was positive in blood for 10 of 13 evaluated patients and remained positive for a median of 35 days (range, 4–123) after the diagnosis of Covid‐19 (of note, seven of the positive cases were in ICU). Also, SARS‐CoV‐2 IgG‐IgM serology was assessed for 19 patients and remained negative for 13 patients (up to 172 days after the diagnosis of Covid‐19), whereas only two patients reached positive serology (four other patients became antibody positive shortly after receiving convalescent plasma administration). The clinical and virological evolution of six representative patients with prolonged LOS for Covid‐19 is illustrated in Figure 1.
FIGURE 1.
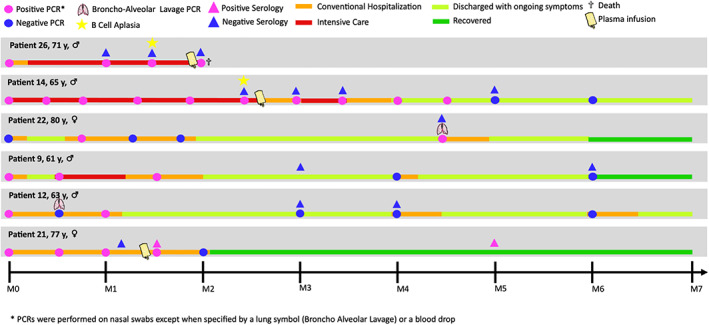
Clinical and biological evolution of six illustrative patients with prolonged LOS for Covid‐19. These six patients had repeated SARS‐CoV‐2 PCR testing as per institutional policies to document clearance of infection. SARS‐CoV‐2 PCR from nasal swabs or bronchoalveolar lavage remained positive for more than 1 month and up to 143 days after initial diagnosis. SARS‐CoV‐2 IgG‐IgM serology remained negative for all six patients, except one who had a transient positive serology after receiving convalescent plasma therapy. The lymphoma subtype, history of treatments and biological characteristics are detailed in the Table S1. LOS: length of in‐hospital stay [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Risk factors for prolonged LOS and death of Covid‐19
Univariate and multivariable analyses of the factors associated with LOS are shown in Table 2 and Figure 2. An age ≥ 70, hypertension, the presence of comorbidity, NHL subtype, relapsed/refractory lymphoma, recent use of bendamustine, and recent anti‐CD20 treatment were all associated with a longer LOS in univariate analysis. Recent anti‐CD20 treatment administered as induction with chemotherapy was associated with increased LOS (subdistribution hazard ratio [sHR] 1.79, 95% CI 1.1–2.91, p = 0.019, vs no anti‐CD20 within 12 months) whereas recent anti‐CD20 treatment as maintenance following induction chemo‐immunotherapy tended to be associated with increased LOS (sHR 1.87, 95% CI 0.9–3.89, p = 0.095) (data not shown). In the multivariable analysis, an age ≥ 70 years (2.34, 95% CI 1.32–4.17, p = 0.004), relapsed/refractory lymphoma (sHR 3.12, 95% CI 1.13–8.61, p = 0.028), and recent administration of anti‐CD20 monoclonal antibody (sHR 2.26, 95% CI 1.42–3.60, p < 0.001) were significantly associated with increased LOS. These variables were also found to be associated with an increased risk of death in a multivariable analysis (Table 2): age ≥ 70 years (HR 4.08, 95% CI 1.94–8.57, p < 0.001), relapsed/refractory lymphoma (HR 3.34, 95% CI 1.58–7.06, p = 0.002) and recent administration of anti‐CD20 monoclonal antibody (HR 2.17, 95% CI 1.04–4.52, p = 0.039). Of note, there was a trend toward an association between the presence of comorbidities and both increased LOS (sHR 1.50, 95% CI 0.91–2.48, p = 0.109) and risk of death (HR 2.50, 95% CI 0.95–6.57, p = 0.064) (Figure 2). Figure S3 summarizes the clinical evolution of the patients who recently received or not an anti‐CD20 monoclonal antibody.
TABLE 2.
Univariate and multivariable analyses of the determinants of length of in hospital stay and overall survival
| Length of in‐hospital stay | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable analysis | Univariate analysis | Multivariable analysis b | |||||
| sHR (95% CI) | p value | sHR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Gender (male vs female) | 1.11 (0.70–1.76) | 0.665 | — | — | 1.47 (0.70–3.07) | 0.307 | — | — |
| Age ≥ 70 years | 2.49 (1.47–4.21) | 0.001 | 2.34 (1.32–4.17) | 0.004 | 4.73 (2.30–9.75) | <0.001 | 4.08 (1.94–8.57) | <0.001 |
| Comorbidities (≥1 vs 0) | 1.98 (1.24–3.14) | 0.004 | 1.50 (0.91–2.48) | 0.109 | 3.42 (1.32–8.85) | 0.011 | 2.50 (0.95–6.57) | 0.064 |
| Hypertension | 1.64 (1.01–2.66) | 0.044 | — | — | 2.34 (1.19–4.62) | 0.014 | — | — |
| Obesity (BMI ≥ 30 kg/m2) | 1.01 (0.97–1.06) | 0.546 | — | — | 1.02 (0.96–1.08) | 0.579 | — | — |
| Smoking status (vs never smoker) | ||||||||
| Former smoker | 1.45 (0.81–2.60) | 0.215 | — | — | 1.77 (0.85–3.66) | 0.126 | — | — |
| Current smoker | 0.76 (0.27–2.14) | 0.608 | — | — | 0.68 (0.09–5.15) | 0.709 | — | — |
| Histological subtype (vs B‐cell NHL) | ||||||||
| T‐cell lymphoma | 0.65 (0.28–1.51) | 0.318 | — | — | 0.71 (0.17–2.96) | 0.636 | — | — |
| Hodgkin lymphoma | 0.43 (0.20–0.90) | 0.024 | — | — | 0.28 (0.04–2.07) | 0.214 | — | — |
| Time from lymphoma diagnosis to admission for Covid‐19 (>12 months) | 1.00 (1.00–1.01) | 0.480 | — | — | 1.00 (1.00–1.01) | 0.455 | — | — |
| Lymphopenia (<1 G/L) c | 1.55 (0.96–2.51) | 0.071 | — | — | 2.67 (1.02–6.97) | 0.044 | — | — |
| Hypogammaglobulinemia (<4 g/L) d | 1.45 (0.67–3.13) | 0.339 | — | — | 1.30 (0.42–4.03) | 0.649 | — | — |
| Lymphoma treatment | ||||||||
| Anti‐CD20 monoclonal antibody a | 1.83 (1.16–2.89) | 0.009 | 2.26 (1.42–3.60) | <0.001 | 1.60 (0.78–3.29) | 0.198 | 2.17 (1.04–4.52) | 0.039 |
| Bendamustine a | 3.37 (1.06–10.73) | 0.039 | — | — | 3.26 (1.42–7.52) | 0.006 | — | — |
| Any lymphoma therapy a | 1.45 (0.89–2.37) | 0.140 | — | — | 1.27 (0.58–2.81) | 0.55 | — | — |
| Relapsed/refractory lymphoma | 3.64 (1.32–9.98) | 0.012 | 3.12 (1.13–8.61) | 0.028 | 3.43 (1.63–7.18) | 0.001 | 3.34 (1.58–7.06) | 0.002 |
Abbreviations: BMI, body mass index; CI, confidence interval; NHL, non‐Hodgkin lymphoma; sHR, sub‐distribution hazard ratio.
Treatment administrated within the previous 12 months before hospitalization for Covid‐19.
Performed with the subset of independent variables found associated with length of in‐hospital stay at the 0.1% level.
Missing data in six patients.
Missing data in 45 patients.
FIGURE 2.
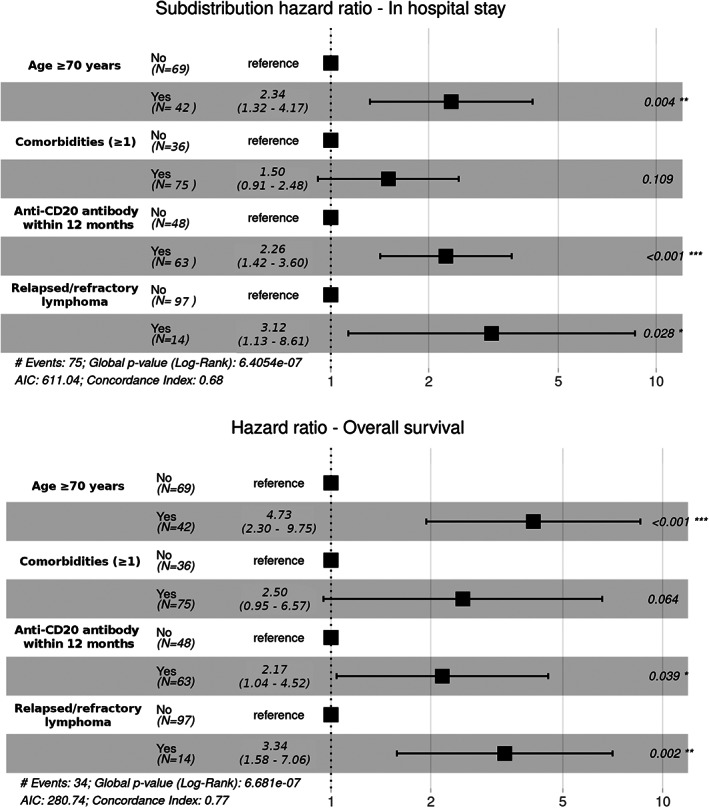
Forest plots for determinants of length of in‐hospital stay and overall survival. AIC: Akaike information criterion
4. DISCUSSION
Long‐lasting Covid‐19 symptoms are a major emerging concern for many individuals who have survived a SARS‐Cov‐2 infection. 29 , 30 , 31 Apart from mild‐to‐moderate long‐lasting symptoms reported for the general population, several reports described the persistence of SARS‐CoV‐2 replication with severe symptoms for immunocompromised patients, including those with lymphoma. 13 , 14 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 Here, we analyzed the determinants of LOS due to Covid‐19 symptoms in a multicentric retrospective cohort of patients with lymphoma. The major findings are the high incidence of prolonged LOS for Covid‐19 symptoms in this population (29% of patients required more than 30 days of hospitalization for Covid‐19 symptoms), and the association between longer LOS and recent administration of anti‐CD20 therapy, an age ≥ 70 years, and relapsed/refractory lymphoma status.
A prolonged evolution of Covid‐19 requiring a longer LOS has been reported in several case studies on immune‐compromised patients. 13 , 14 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 25 In the present study, the median LOS was 14 days for the whole cohort and 20 days (range, 2–235) for patients who received recent anti‐CD20 treatment, whereas in the general population the reported Covid‐19 median LOS was 5 days worldwide (outside of China) 11 and, in France 11 days for all patients hospitalized between March 1 and June 15, 2020. The proportion of patients requiring more than 30 days of hospitalization for Covid‐19 was 29% in this study whereas it was 8% in the general population in France. 12 Most patients with prolonged LOS for Covid‐19 had B‐cell NHL (31/32) and were recently treated for their lymphoma (26/32). All of them received a B‐cell depletion therapy, either combined with chemotherapy (n = 18) or alone as maintenance (n = 8). The only patient with prolonged LOS for Covid‐19 without B‐cell NHL had a nodular lymphocyte‐predominant Hodgkin lymphoma recently treated with anti‐CD20 based chemotherapy. These observations suggest an association between B‐cell depletion and the risk of prolonged LOS for Covid‐19.
The analysis of the risk factors associated with prolonged LOS for Covid‐19 in competition with death confirmed that one of the main risk factors for prolonged hospitalization for Covid‐19 symptoms was the administration of anti‐CD20 monoclonal antibody within the previous 12 months (sHR: 2.26). This finding was supported by associations of similar magnitude observed between LOS and anti‐CD20 monoclonal antibody, given either combined with chemotherapy (sHR: 1.79) or as maintenance treatment (sHR: 1.87), although the latter did not reach statistical significance (p = 0.095). In accordance with these findings, a recent case series reported 17 consecutive patients with profound B‐cell depletion and prolonged Covid‐19 symptoms, among whom 15 previously received an anti‐CD20 monoclonal antibody. 14 Fourteen of these patients were treated for lymphoma, one for Waldenström macroglobulinemia, one for multiple sclerosis and one was diagnosed with common variable immune deficiency. All patients had positive SARS‐CoV‐2 RNA in blood, and none had developed a neutralizing antibody response. Patients with B‐cell NHL were also shown to have lower rates of seroconversion and antibody levels compared to other hematological patients. 32 Consistently with these findings, in the present study, 10 of 13 evaluated patients had positive SARS‐CoV‐2 RNA in blood lasting up to 123 days after Covid‐19 diagnosis. The persistence of positive SARS‐CoV‐2 PCR in nasal swabs or in bronchoalveolar lavage was also observed up to 143 days after initial admission. Moreover, only two patients (out of 19 with available results) with prolonged LOS for Covid‐19 developed a positive serology against SARS‐CoV‐2. 33 A lower rate of seroconversion and a longer shedding of the virus (61 days vs 33 days) had also been reported in patients treated with anti‐CD20 monoclonal antibodies in a large study assessing the seroconversion after Covid‐19 in patients with cancer. 34
Further supporting the specific impact of B‐cell depletion on the course of Covid‐19, cases of severe Covid‐19 have also been reported for patients treated with anti‐CD20 therapy for various auto‐immune diseases, including fatal cases for two patients with rheumatoid arthritis. 35 , 36 , 37 , 38 In the present study, recent anti‐CD20 therapy also had a detrimental impact on long‐term OS (HR: 2.17) regardless of age and relapsed/refractory status. Overall, these findings raise questions about the management of patients with lymphoma during the Covid‐19 pandemic, for whom there are currently no consensus guidelines. Although avoiding or delaying the administration of anti‐CD20 monoclonal antibodies might be discussed in certain circumstances in order to prevent prolonged Covid‐19, further studies are necessary before recommendation can be made to change clinical practice. The decision concerning the use of anti‐CD20 therapy should not preclude the patient from receiving the most efficacious treatment strategy and requires consideration of the disease characteristics and the patient's history.
Vaccination against SARS‐CoV‐2 was shown to prevent Covid‐19 in the general population. 39 , 40 We can hypothesize that vaccination of patients, their proxies and hospital workers might therefore benefit directly to patients, who receive or are planned to receive an anti‐CD20 treatment. However, several studies have shown a blunted response to vaccination in patients with lymphoma or autoimmune disease treated with rituximab. 41 , 42 The B‐cell depletion at the time of vaccination may strongly impact the generation of B‐cell memory and the levels of neutralizing high‐affinity antibodies. As recently reported in patients with chronic lymphocytic leukemia, none of the patients exposed to anti‐CD20 antibodies within the 12 months prior to vaccination had an antibody‐mediated response to BNT162b2 mRNA Covid‐19 vaccine. 43 It may also hamper the formation of functional memory CD8+ T‐cells 44 and the maintenance of follicular helper CD4+ T‐cells responses. 45 Therefore, whenever possible, SARS‐CoV‐2 vaccination might be considered before the onset of anti‐CD20 monoclonal antibody therapy although therapeutic B‐cell depletion may also alter the efficacy of already established antiviral memory T‐cell responses. 44 Likely, forthcoming studies will determine the optimal SARS‐CoV‐2 vaccination schedule in lymphoma patients.
Apart from B‐cell depletion, other factors were found to contribute to LOS for Covid‐19. In the present study, an age ≥ 70 years (sHR: 2.34) and a relapsed/refractory lymphoma status (sHR: 3.12) were both associated with longer LOS. Moreover, although recent overall administration of lymphoma treatment per se was not associated with a prolonged LOS, recent use of bendamustine was associated with longer LOS in univariate analysis (sHR: 3.37). However, among the 10 patients who had received bendamustine, eight had also received anti‐CD20 therapy, six had a relapsed/refractory lymphoma and only three patients (including two with relapsed/refractory lymphoma) were hospitalized for more than 30 days. Further studies are needed to explore the impact of bendamustine on the evolution of Covid‐19. Cases of prolonged shedding of viable SARS‐CoV‐2 for at least 2 months have also been described for patients undergoing hematopoietic cell transplantation or receiving chimeric antigen receptor T‐cell therapy for various hematological malignancies. 13 In the present study, prolonged Covid‐19 was also observed for patients who had previously undergone hematopoietic cell transplantation (autologous for eight and allogeneic for one) or received cellular therapy (n = 2).
Among the attempts to treat prolonged Covid‐19, corticosteroids administration and/or convalescent plasma led to favorable outcomes in our study, but the small number of patients treated precludes drawing definitive conclusions. In particular, administration of Covid‐19 convalescent plasma was shown to lead to rapid clinical improvement for eight of nine patients, in line with the results reported by Hueso et al. 14 A larger study comparing convalescent plasma to other treatment approaches is warranted to evaluate the short‐term and long‐term benefits of this innovative strategy for lymphoma patients experiencing prolonged Covid‐19. The benefit of an earlier administration of convalescent plasma therapy for B‐cell depleted patients remains also to be determined. 46
Limitations of the study include its retrospective nature and the selection of hospitalized patients, excluding patients with mild symptoms. Other limitations were the lack of systematic monitoring of SARS‐CoV‐2 PCR and the anti‐SARS‐CoV‐2 immune responses, and the heterogeneity of treatments received for Covid‐19. In conclusion, a high proportion of patients with B‐cell lymphoma have a prolonged LOS for Covid‐19. Administration of anti‐CD20 therapy within the previous 12 months before Covid‐19 is one of the main risk factors for longer LOS and death from Covid‐19. The risk of prolonged LOS for Covid‐19 was also higher for patients ≥70 years of age and with relapsed/refractory disease. Our findings may contribute to the elaboration of guidelines for the management of lymphoma patients during the Covid‐19 pandemic. Although convalescent plasma may be a promising approach to treat B‐cell depleted patients with prolonged Covid‐19, further studies are needed to compare its efficacy to other strategies. As B‐cell depletion may also affect the generation of primary and memory B‐cell and T‐cell responses, unresolved issues are whether these patients are protected against re‐infection after SARS‐CoV‐2 infection or gain effective immunological memory after SARS‐CoV‐2 vaccination.
CONFLICT OF INTEREST
The authors certify that there is no conflict of interest with any organization concerning the material presented in this manuscript.
Rémy Duléry reports personal fees from Takeda, Novartis, and Biotest and non‐financial support from Gilead outside the submitted work. Roberta Di Blasi reports personal fees from Gilead and Novartis outside the submitted work. Serge Bologna reports personal fees from Janssen and Roche outside the submitted work. Guillaume Cartron reports personal fees from Roche, Celgene, Sanofi, Gilead, Janssen, and Abbvie outside the submitted work. Karine Lacombe reports personal fees and non‐financial support from Gilead, MSD, Abbvie, ViiV Healthcare, and Janssen outside the submitted work. Caroline Besson reports research funding from Roche and non‐financial support from Takeda and Roche outside the submitted work.
AUTHOR CONTRIBUTIONS
Sylvain Lamure and Rémy Duléry designed the data collection, collected and analyzed the data, and wrote the article. Marc Delord analyzed the data, performed statistical analysis, and commented on the manuscript. Roberta Di Blasi, Adrien Chauchet, Thomas Hueso, Bénédicte Deau‐Fischer, Bernard Drenou, Carole Soussain, Pierre Feugier, Cédric Rossi, Nicolas Noël, Sylvain Choquet, Serge Bologna, Bertrand Joly, Laure Philippe, Milena Kohn, Sandra Malak, Guillemette Fouquet, Etienne Daguindau, and Yassine Taoufik performed the data collection and some of the data interpretation and reviewed the manuscript. Catherine Thiéblemont, Guillaume Cartron, and Karine Lacombe reviewed the study design and the article. Caroline Besson designed the study, contributed to the analysis, and wrote the article.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
We thank all the clinicians and patients in the participating centers for their contributions to this multicenter study. We are grateful to the Centre Hospitalier de Versailles, in particular Philippe Rousselot and Laure Morisset for promoting the research and for supporting the editing. We thank France Lymphome Espoir for reviewing the study material, LYSA for discussions on the design of this study, and Sophie Rigaudeau and Cécile Lauréana for their contribution to the collection of the cases.
Duléry R, Lamure S, Delord M, et al. Prolonged in‐hospital stay and higher mortality after Covid‐19 among patients with non‐Hodgkin lymphoma treated with B‐cell depleting immunotherapy. Am J Hematol. 2021;96:934–944. 10.1002/ajh.26209
Rémy Duléry and Sylvain Lamure contributed equally and are co‐first authors.
Marc Delord and Roberta Di Blasi contributed equally and are co‐third authors.
DATA AVAILABILITY STATEMENT
The dataset supporting this article is available upon demand to the corresponding author and to the promoter (Centre Hospitalier de Versailles).
REFERENCES
- 1. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID‐19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737‐e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Averbuch D, Orasch C, Cordonnier C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European conference on infections in leukemia. Haematologica. 2013;98:1826‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maschmeyer G, De Greef J, Mellinghoff SC, et al. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European conference on infections in leukemia (ECIL). Leukemia. 2019;33:844‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lamure S, Duléry R, Di Blasi R, et al. Determinants of outcome in Covid‐19 hospitalized patients with lymphoma: a retrospective multicentric cohort study. EClinicalMedicine. 2020;27:100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glenthøj A, Jakobsen LH, Sengeløv H, et al. SARS‐CoV‐2 infection among patients with haematological disorders: severity and one‐month outcome in 66 Danish patients in a nationwide cohort study. Eur J Haematol. 2020;106:72‐81. 10.1111/ejh.13519. [DOI] [PubMed] [Google Scholar]
- 7. Wood WA, Neuberg DS, Thompson JC, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a report from the ASH research collaborative data hub. Blood Adv. 2020;4:5966‐5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rüthrich MM, Giessen‐Jung C, Borgmann S, et al. COVID‐19 in cancer patients: clinical characteristics and outcome‐an analysis of the LEOSS registry. Ann Hematol. 2021;100(2):383‐393. 10.1007/s00277-020-04328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. García‐Suárez J, de la Cruz J, Cedillo Á, et al. Impact of hematologic malignancy and type of cancer therapy on COVID‐19 severity and mortality: lessons from a large population‐based registry study. J Hematol Oncol. 2020;13:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee LYW, Cazier J‐B, Starkey T, et al. COVID‐19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rees EM, Nightingale ES, Jafari Y, et al. COVID‐19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Golberg E. Parcours hospitalier des patients atteints de la Covid‐19 lors de la première vague de l'épidémie. 2020: 39.
- 13. Aydillo T, Gonzalez‐Reiche AS, Aslam S, et al. Shedding of viable SARS‐CoV‐2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586‐2588. 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B‐cell‐depleted patients with protracted COVID‐19. Blood. 2020;136:2290‐2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gea‐Banacloche JC. Rituximab‐associated infections. Semin Hematol. 2010;47:187‐198. [DOI] [PubMed] [Google Scholar]
- 16. Tudesq J‐J, Cartron G, Rivière S, et al. Clinical and microbiological characteristics of the infections in patients treated with rituximab for autoimmune and/or malignant hematological disorders. Autoimmun Rev. 2018;17:115‐124. [DOI] [PubMed] [Google Scholar]
- 17. Helleberg M, Niemann CU, Moestrup KS, et al. Persistent COVID‐19 in an immunocompromised patient temporarily responsive to two courses of Remdesivir therapy. J Infect Dis. 2020;222:1103‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tepasse P‐R, Hafezi W, Lutz M, et al. Persisting SARS‐CoV‐2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. Br J Haematol. 2020;190:185‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baang JH, Smith C, Mirabelli C, et al. Prolonged SARS‐CoV‐2 replication in an immunocompromised patient. J Infect Dis. 2020;223:23‐27. 10.1093/infdis/jiaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS‐CoV‐2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183(7):1901‐1912.e9. 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore JL, Ganapathiraju PV, Kurtz CP, Wainscoat B. A 63‐year‐old woman with a history of non‐Hodgkin lymphoma with persistent SARS‐CoV‐2 infection who was seronegative and treated with convalescent plasma. Am J Case Rep. 2020;21:e927812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kos I, Balensiefer B, Roth S, et al. Prolonged course of COVID‐19‐associated pneumonia in a B‐cell depleted patient after rituximab. Front Oncol. 2020;10:1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yasuda H, Tsukune Y, Watanabe N, et al. Persistent COVID‐19 pneumonia and failure to develop anti‐SARS‐CoV‐2 antibodies during rituximab maintenance therapy for follicular lymphoma. Clin Lymphoma Myeloma Leuk. 2020;20:774‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karataş A, İnkaya AÇ, Demiroğlu H, et al. Prolonged viral shedding in a lymphoma patient with COVID‐19 infection receiving convalescent plasma. Transfus Apher Sci. 2020;59:102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Betrains A, Godinas L, Woei‐A‐Jin FJSH, et al. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol. 2021;192(6):1100‐1105. 10.1111/bjh.17266. [DOI] [PubMed] [Google Scholar]
- 26. Prentice RL, Kalbfleisch JD, Peterson AV, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541‐554. [PubMed] [Google Scholar]
- 27. Fine J, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496‐509. [Google Scholar]
- 28. Lei J, Li J, Li X, Qi X. CT imaging of the 2019 novel coronavirus (2019‐nCoV) pneumonia. Radiology. 2020;295:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malta M. My journey with COVID‐19. EClinicalMedicine. 2020;27:100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long‐COVID: analysis of COVID cases and their symptoms collected by the Covid symptoms study app. medRxiv. 2020;20214494. 10.1101/2020.10.19.20214494. [DOI] [Google Scholar]
- 31. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220‐232. 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cattaneo C. Longitudinal Serological Response to Sars‐COV‐2 in Patients Affected by Hematologic Diseases. ASH, 2020. https://ash.confex.com/ash/2020/webprogram/Paper141355.html. Accessed Jan 22, 2021.
- 33. Ni L, Ye F, Cheng M‐L, et al. Detection of SARS‐CoV‐2‐specific humoral and cellular immunity in COVID‐19 convalescent individuals. Immunity. 2020;52:971‐977.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thakkar A, Pradhan K, Jindal S, et al. Patterns of seroconversion for SARS‐CoV‐2 IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer. 2021;2:392‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schulze‐Koops H, Krueger K, Vallbracht I, Hasseli R, Skapenko A. Increased risk for severe COVID‐19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. 2021;80:e67. 10.1136/annrheumdis-2020-218075. [DOI] [PubMed] [Google Scholar]
- 36. Guilpain P, Le Bihan C, Foulongne V, et al. Rituximab for granulomatosis with polyangiitis in the pandemic of covid‐19: lessons from a case with severe pneumonia. Ann Rheum Dis. 2021;80:e10. [DOI] [PubMed] [Google Scholar]
- 37. Avouac J, Airó P, Carlier N, Matucci‐Cerinic M, Allanore Y. Severe COVID‐19‐associated pneumonia in 3 patients with systemic sclerosis treated with rituximab. Ann Rheum Dis. 2021;80:e37. 10.1136/annrheumdis-2020-217864. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38. Benucci M, Quartuccio L, Li Gobbi F, et al. Persistence of rT‐PCR‐SARS‐CoV‐2 infection and delayed serological response, as a possible effect of rituximab according to the hypothesis of Schulze‐Koops et al. Ann Rheum Dis. 2020. 10.1136/annrheumdis-2020-218590. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yri OE, Torfoss D, Hungnes O, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118:6769‐6771. [DOI] [PubMed] [Google Scholar]
- 42. Nazi I, Kelton JG, Larché M, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122:1946‐1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;blood.2021011568. 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Durali D, de Goër de Herve M‐G, Gasnault J, Taoufik Y. B cells and progressive multifocal leukoencephalopathy: search for the missing link. Front Immunol. 2015;6:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Libster R, Marc GP, Wappner D, et al. Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl J Med. 2021;384(7):610‐618. 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The dataset supporting this article is available upon demand to the corresponding author and to the promoter (Centre Hospitalier de Versailles).


