To the Editor:
Vaccination programs against SARS‐CoV‐2 are in full progress across the globe. The BNT162b2 mRNA (Pfizer) and the AZD1222 adenovirus vector (AstraZeneca‐AZ) vaccines account for the majority of vaccinations following the encouraging clinical trial results. 1 , 2 Age, gender and comorbidities, such as hematologic malignancies, may have an impact on antibody response. 3 , 4 , 5
The aim of this study was to compare the kinetics of SARS‐CoV‐2 neutralizing antibodies (NAbs) between the two vaccines at days 22 and 50 post‐vaccination. The possible influence of gender and body mass index (BMI) on the level of NAbs was also investigated.
The main inclusion criteria for participation in this study were: Age over 18 years, ability to sign the informed consent form, and eligibility for vaccination according to the national program for COVID‐19. Major exclusion criteria included the presence of immunosuppressive therapy, active malignant disease, and end‐stage renal disease. According to the National Immunization Program, only individuals aged 60–64 years had access to the AZ vaccine during the study period. For Pfizer, access to the vaccine was available to all ages 18 years and older. However, in this study, for comparability purposes, only subjects aged between 57 and 67 years, who received the Pfizer vaccine, were included to have comparable ages, that is, +3 and −3 from the age limits of the AZ vaccine. All individuals participated in a prospective study (NCT04743388) regarding the efficacy of vaccination for the prevention of COVID‐19 in Greece.
Data of the subjects were kept confidential in accordance with the General Data Protection Regulation (GDPR) rules (Regulation 2016/679 of European Parliament 2016). All names were kept confidential and immediately after collection, names were deleted and randomly replaced with a unique number. The study was approved by the respective Ethical Committee of Alexandra Hospital, in accordance with the Declaration of Helsinki and International Conference for harmonization for good clinical practice. All participants gave their written consent before participating in the study.
NAbs against SARS‐CoV‐2 were measured using a FDA‐approved methodology. The cPass™ SARS‐CoV2 NAbs Detection Kit (GenScript, Piscataway, NJ, USA) was used, which allows indirect detection of potential SARS‐CoV‐2 NAbs in blood by testing antibody‐mediated inhibition of SARS‐CoV‐2 RBD binding to the human host receptor angiotensin converting enzyme 2. The time points for blood collection and serum isolation were day 1 (D1, before the first Pfizer or AZ shot), D22 (before the second shot, only for Pfizer vaccine; second shot of AZ vaccine was scheduled for 12 weeks after the first shot), and D50 (4 weeks after the second Pfizer shot, 7 weeks after the first AZ shot). After venipuncture, serum was separated within 4 h of blood collection and stored at −80°C until the day of measurement. Stored samples from different time points of the same donor were measured in parallel assays.
After data collection, statistical analysis was performed in the Python programming language (v. 3.9.2). The analysis included descriptive statistics and statistical comparisons between groups. Prior to statistical comparisons, a normality test of the data distributions was performed using the Shapiro–Wilk criterion. In the case of normally distributed data, parametric comparison methods were used, while if all or one of the compared groups deviated from normality, non‐parametric methods were used. In all cases in this analysis, either one or all of the groups deviated from the normal distribution, so two‐group and multiple‐group analyses were performed using the nonparametric Mann–Whitney U test and Kruskal‐Wallis H test, respectively. Because comparisons were between different groups of subjects, namely men versus women or Pfizer vs. AZ vaccinated subjects, independent group comparisons were performed. For comparisons of nominal variables (e.g., occurrence of an adverse event with vaccine type), chi‐square analysis was used. In all cases, the significance level was set at 5% and a result was considered significant if the estimated p‐value (p) was below the significance level.
Figure 1 shows the percent inhibition of NAbs on days 1, 22, and 50 in the two groups of the study, vaccinated with either the Pfizer or the AZ vaccines. On the first day of observation (D1), that is, immediately before vaccination, eight subjects (10.3%) had inhibition above the threshold of 30% in the case of Pfizer's vaccine, while only one subject (1.4%) in the AZ group had inhibition above 30%. The median age of participants in both the Pfizer (N = 78) and AZ (N = 73) groups was 61 years (mean 61.8 and 61.5 years, respectively). Statistical comparison showed no significant differences between the two groups in terms of age at baseline (p = 0.897). In addition, there was no difference (p = 0.095) in NAbs titers at day 1, a result that allows later comparisons at days 22 and 50.
FIGURE 1.
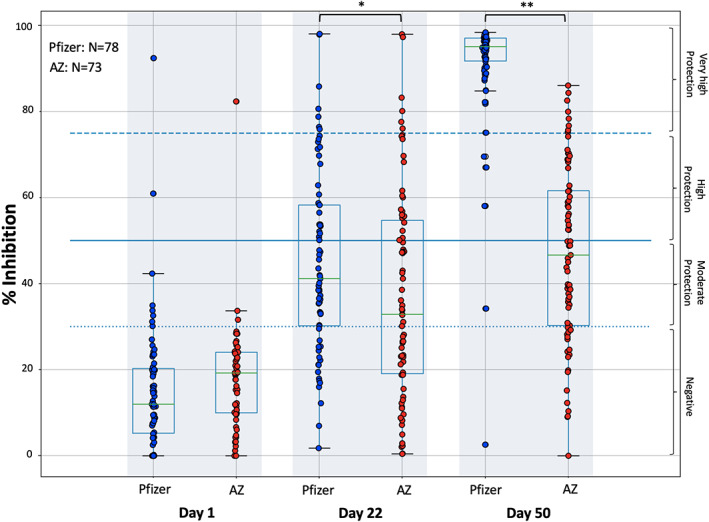
Inhibition (%) of SARS‐CoV‐2 binding to the human host receptor angiotensin converting enzyme‐2 after vaccination with the BNT162b2 mRNA vaccine (Pfizer) and the AZD1222 vaccine (AstraZeneca, AZ). NAbs were measured on day 1 (vaccination date), day 22, and day 50. Asterisks indicate statistically significant differences between the compared groups: *p‐value = 0.031, **p‐value <0.001. The boxplot borders refer to the quartiles of the distribution, while the overlaid dots represent the individual values of NAbs inhibition
At D22, a steep increase in NAbs was observed for both vaccines. The median inhibition values were 43.6% and 35.0% for the Pfizer and AZ groups, respectively. This difference was found to be statistically significant (p = 0.031), indicating a slightly higher percentage inhibition of SARS‐CoV‐2 by the Pfizer vaccine compared to the AZ vaccine. Overall, for the Pfizer group, seven (8.9%) and seventeen (21.8%) subjects had NAbs titers greater than 75% and less than 30%, respectively. For the AZ group, the number of subjects who had inhibition less than 30% was 32 (43.8%), while five of them (6.8%) had inhibition greater than 75%.
At D50, the difference in inhibition between Pfizer's vaccine and AZ was even greater, reaching a median inhibition value of 95.6% in the case of Pfizer compared to the median estimate of 41.1% for AZ (p < 0.001). In this point we have to stress that the Pfizer group had received the second vaccine dose four weeks before (on D22), while the AZ group had received only the first vaccination dose (on D1). In the case of Pfizer's vaccine, half of the subjects developed inhibition greater than 95%, the majority of them (73 subjects or 93.6%) had NAbs inhibitory titers greater than 75%, while only two (2.6%) were classified as negative (less than 30%). In the AZ group, the number of subjects with inhibition greater than 75% was eight (10.9%), while eighteen subjects (24.6%) had NAbs inhibition less than 30%.
A similar analysis was also performed with respect to gender to identify possible gender differences in the development of percentage inhibition of SARS‐CoV‐2 between the two vaccines. It was found that both males and females in the Pfizer group developed inhibition levels significantly faster compared to AZ group (p‐values equal to 0.032 and 0.027 for males and females, respectively) from day 1 to 22, after only one vaccination with each vaccine, respectively.
The possible influence of BMI on NAbs titers was also investigated. In the Pfizer group, 33.7% had normal weight, 48.2% were overweight, and 18.1% were obese. In the AZ group, 28.4% of subjects had normal weight, 40.7% were overweight, and 30.9% were obese. No significant differences were observed between BMI groups and vaccine type (p‐values of Kruskal‐Wallis test were 0.174, 0.258 and 0.242 for D1, D22 and D50, respectively). Furthermore, when BMI was treated as a scale variable, the paired cross‐correlations between BMI and NAbs inhibition level yielded a correlation coefficient of no more than 0.125, indicating no association.
Regarding the safety profile, no adverse events occurred with the Pfizer vaccine in 44 subjects (56.4%), while 34 of them (43.6%) experienced at least one grade 1/2 adverse event after the first vaccination (i.e., fatigue, fever, chills, or myalgia) which lasted for up to 72 h. In the AZ group, 27 subjects (37.0%) experienced no adverse events after vaccination, while 46 subjects (63.0%) experienced at least one, grade 1/2, adverse event. No severe adverse event of grade 3 or more was observed with either vaccine. Chi‐square analysis revealed a statistically significant association between the type of vaccine and the occurrence of an adverse event. In particular, vaccination with AZ showed a positive association with the occurrence of adverse events (p = 0.018).
Despite the relatively small sample size, this study showed that NAbs inhibitory values reached moderate levels with Pfizer and AZ at three weeks after the first vaccination, with slightly higher values after vaccination with Pfizer. At day 50, that is, 4 weeks after the second vaccination with Pfizer, the inhibition levels for Pfizer's vaccine became very high, with half of them showing inhibition of more than 95%, while the inhibition levels of AZ remain almost at the moderate levels of D22, as these patients had not received the second vaccine dose yet. Although many countries have opted for a 12‐week interval between the two doses of AZ in order to increase the number of vaccinated people with at least one dose, 6 our results advocate for a shorter administration schedule, especially as more vaccines are becoming available. Furthermore, males and females treated with Pfizer vaccine achieved significantly faster viral inhibition at D22 than those treated with the AZ vaccine. BMI was found to have no significant effect on NAbs titers. Finally, it was found that the AZ vaccine was significantly associated with a higher incidence of mild adverse events compared to the Pfizer vaccine.
CONFLICT OF INTEREST
The authors declare no relevant conflict of interest.
ACKNOWLEDGMENTS
We thank Christine‐Ivy Liacos, PhD, Nefeli Mavrianou, PhD Ioanna Charitaki, NR, Nikoletta‐Aikaterini Kokkali, NR, Mrs Stamatia Skourti and Mr Theodoros Konstantakopoulos for administrative, technical, and/or material support. We also thank SYN‐ENOSIS (Greece), AEGEAS (Greece) and IEMBITHEK (Greece) for partially funding this study, as well as all of the study participants for donating their time and samples.
Evangelos Terpos and Ioannis P. Trougakos equal contribution as first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Terpos E, Trougakos IP, Apostolakou F, et al. Age‐dependent and gender‐dependent antibody responses against SARS‐CoV‐2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol. 2021. 10.1002/ajh.26185. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low Neutralizing Antibody Responses Against SARS‐CoV‐2 in Elderly Myeloma Patients After the First BNT162b2 Vaccine Dose. Blood. 2021. 10.1182/blood.2021011904. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021. 10.1182/blood.2021011568. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voysey M, Costa Clemens SA, Madhi SA, et al. Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


