Abstract
It is thought that excessive production of reactive oxygen species (ROS) can be a causal component in many diseases, some of which have an inflammatory component. This led to an oversimplification whereby ROS are seen as inflammatory and antioxidants anti-inflammatory. This paper aims at reviewing some of the literature on thiols in host defense. The review will first summarize the mechanisms by which we survive infections by pathogens. Then we will consider how the redox field evolved from the concept of oxidative stress to that of redox regulation and how it intersects the field of innate immunity. A third section will analyze how an oversimplified oxidative stress theory of disease led to a hypothesis on the role of ROS and glutathione (GSH) in immunity, respectively as pro- and anti-inflammatory mediators. Finally, we will discuss some recent research and how to think out of the box of that oversimplification and link the role of thiols in redox regulation to the mechanisms by which we survive an infection outlined in the first section.
Keywords: Glutathione, Innate immunity, Nrf2, NF-kB, HIF-1α, Inflammation
Graphical abstract
1. Surviving pathogens: resistance versus tolerance
To describe this concept, we need to take a step back and consider the whole picture rather than focusing on one the aspects of response to pathogens (typically, immunity and inflammation). Without mentioning the mechanical barriers (e.g. skin) or the mucociliary escalator, we deal with an infection by two very different mechanisms: resistance and tolerance [[1], [2], [3]].
Resistance, or pathogen control, consists in killing the pathogen or inhibiting its growth. This is the field of immunity, of which innate immunity and the inflammatory response are one arm which is activated early before adaptive immunity develops a more sophisticated and specific arsenal of antibody-producing B-lymphocytes-derived plasma cells and cytotoxic T cells [4]. Resistance is also mediated by phagocytes producing bactericidal ROS and activating interferon (IFN) pathways that result in inhibition of viral proliferation.
Tolerance, or damage control, on the other hand, has not been systematically studied but is well known in plant physiology [5], and consists of various mechanisms that limit the damage that pathogens causes to the host, for instance by the neutralization of toxins released by the infection. Tolerance was described for respiratory infections; for instance, tissue protection mechanisms that can protect from infection without affecting the pathogen load have been described in mice infected with Legionella pneumophila, influenza or both [6,7]. Another example is how the host copes with malaria, not only by controlling the number of parasites but also by inducing heme oxygenase (HMOX1) that prevents the toxic effect of hemolysis-derived hemoglobin [8].
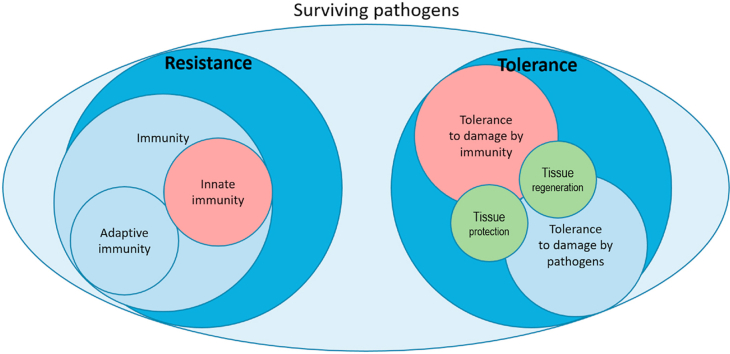
It is important to note, however, that if resistance and tolerance are completely different responses, their effector cells may be, in some cases, the same, and innate immunity was suggested to contribute to tissue repair and therefore tolerance [9,10]. A further overlap between the two concepts is that tolerance can include mechanisms that protect not only against the damage caused by the pathogen but also against host-mediated toxicity, such as excessive inflammation [3]. Tolerance can be mediated by stress responses and tissue repair mechanisms [11]. For instance, in an infection by Gram-negative bacteria, exposure to bacterial lipopolysaccharide (LPS, endotoxin) can induce a form of tachyphylaxis known as “endotoxin tolerance” [12,13]. This process switches off the inflammatory response and therefore further inflammation-induced tissue damage [12,13]. Fig. 1 provides a scheme of the main mechanisms of resistance and tolerance.
Fig. 1.
Resistance and tolerance as the two mechanisms to survive infections.
2. Innate immunity and inflammation
Innate immunity is triggered by pattern recognition receptors, such as the Toll-like receptors (TLRs) upon binding to pathogen-associated molecular patterns. In the case of Gram-negative bacteria, LPS binds TLR4 activating a signaling cascade that leads to the activation of NFkB and transcription of inflammatory genes [14,15]. The products of these genes, such as inflammatory cytokines or inducible cyclooxygenase generating arachidonic acid metabolites, then augment vascular permeability and, through the action of chemokines, recruit inflammatory cells, like neutrophils and macrophages, to the site of infection. Neutrophils in particular, but also macrophages, produce reactive oxygen species (ROS) as part of their bactericidal armamentarium during the NADPH oxidase (NOX)-mediated “oxidative burst” [16], a mechanism of pathogen resistance also present in plants [17].
In the 1980s, it became clear that most of the innate immune mechanisms of resistance are mediated by proinflammatory mediators called cytokines, such as interleukins (IL)-1, IL -6 and tumor necrosis factor (TNF). One important aspect of innate immunity is that the same mechanisms that are essential in limiting infection can also induce tissue damage to the host through the inflammatory response. This is known as the “cytokine theory of disease” that led to the development of some of the most effective biologicals currently approved for use for chronic inflammatory diseases, such as anti-TNF and anti-IL-6 antibodies [18,19].
3. Oxidative stress, antioxidants and immunity: the development of the concept of redox regulation
The popular view of the oxidative stress (OS) theory of disease is based on the idea that excessive production of reactive oxygen species (ROS) or insufficient antioxidant systems to cope with it are a causal component of many disease conditions [20]. The idea that free radicals can cause disease was first made explicit in a 1956 paper by Harman who put forward the “free radical theory of ageing” [21]. We have adopted the definition of “OS theory of disease” elsewhere [20], by analogy with other theories of disease that imply specific causal agents, like germs in the germ theory of disease [22] and inflammatory cytokines in the cytokine theory of disease [19]. However, by no means this implies that these theories are equal in terms of strength. In fact, while the germ theory of disease gained strength by the development of antibiotics and the cytokine theory by the approval of anti-cytokine antibodies (particularly anti-TNF and anti-IL-6), small antioxidants, acting to scavenge ROS, have not been approved yet for any indication. While, obviously, a theory can be perfectly valid even if it doesn't lead to any drug discovery, if should be noted that most studies hypothesizing a pathogenic role for OS, including the 1956 study by Harman [21], have more or less explicitly predicted that antioxidant treatments would be effective.
One of the possible reasons why the administration of antioxidants acting as ROS scavengers has not been successful is that ROS are not just toxic byproducts in the metabolism but also participate in the regulation of various signaling pathways, which defines as redox regulation [23]. Interestingly, this concept was first described when studying the role of ROS in infection and immunity. In 1991, a pioneering work by the laboratory of Patrick Baeuerle showed that hydrogen peroxide activates the transcription factor NFkB, an effect antagonized by the antioxidant N-acetylcysteine (NAC) [24]. In the same year, the group of Anthony Fauci reported that NAC and GSH inhibit the transcriptional activity of the HIV promoter when this is activated with the inflammatory cytokine TNF [25]. Those two studies indicated that ROS and low-molecular weight thiols can affect the activation of the transcription factors regulating the expression of inflammatory genes as well as the action of inflammatory cytokines.
On one hand, this connection between inflammatory cytokines and GSH prompted many studies on the protective effect of thiol antioxidants in various models of cytokine-mediated disease [[26], [27], [28]]. These studies also showed an inhibitory effect of thiol antioxidants on TNF production in vivo [29]. As a result, many researchers in the field worked on the assumption that ROS are pro-inflammatory mediators and GSH (as well as other thiols) is an anti-inflammatory molecule. The idea was that inflammation is associated with increased ROS production that, in turn, would augment NFkB activation and cytokine production, thus exacerbating inflammation. However, despite the fact that most preclinical studies showed a protective effect of NAC, the level of evidence required for drug approval has never been reached in adequately powered randomized clinical trials. Clinical trials with NAC in acute respiratory distress syndrome (ARDS) never showed a level of evidence for efficacy sufficient to recommend it as a treatment (reviewed in Ref. [30]).
In his discussion on mechanistic reasoning and clinical trials, Howick described how lack of efficacy could be explained by the fact that the effects of a compound on patients are a combination of its action on the desired pharmacological target and other, often less well defined, mechanisms [31]. The lack of success of thiols and other ROS scavengers might be due the fact that ROS are not only pathogenic mediators but also essential in the regulation of signaling molecules. Likewise, GSH may not be only a ROS scavenger but also a regulator of signaling molecules that can modulate the function of several redox-regulated proteins via disulfide formation, including glutathionylation [23,32]. Interestingly, many pathways and molecules associated with innate immunity are targets of glutathionylation [33]. These protein targets are at different levels of the inflammatory cascade, and include proteins interacting with Toll-like receptors, such as MyD88-adapter-like (MAL) [34], the p50 subunit of NF-kB [35] and the signal transducer and activator of transcription 3 (STAT3) [36]. Downstream of the inflammatory pathway, glutathionylation also occur at a specific cysteine of interleukin 1 beta (IL-1β), affecting its biological activity [37]. Of note, susceptibility of free protein cysteines to oxidation, including glutathionylation, is an important factor that determines whether a specific protein is susceptible to regulation by GSH/GSSG or not. It should also be noted that, in redox regulation, GSH acts in concert with hydrogen peroxide that can directly catalyse protein glutathionylation or do so by changing the GSH/GSSG ratio, with a possible catalytic role of redox enzymes [38].
Over twenty years ago, Joe McCord acknowledged that ROS are not “as we once thought, just a toxic but unavoidable byproduct of oxygen metabolism” (this sentence was referring to superoxide radicals) [39], and others noted that ROS-producing NADPH oxidases (NOX) evolved soon after the appearance of oxygen in the atmosphere [40]. The concept of redox regulation assigns a central role to hydrogen peroxide as a signaling molecule [23,32,41].
If ROS are essential signalling molecules in innate immunity, then the question arises as to the role of GSH. Is GSH acting only as a ROS scavenger, detoxifying hydrogen peroxide through the action of GSH peroxidase, or also regulating signaling pathways where redox-sensitive proteins are implicated?
4. Glutathione in inflammatory cells: a signaling molecule or a ROS scavenger?
Most of the studies on the anti-inflammatory role of GSH have been obtained using NAC as a precursor, or a mimic, of GSH. Consistently, most studies reported that NAC, in vivo or in vitro, inhibited the LPS-induced production of inflammatory cytokines, particularly TNF (see, for instance Refs. [29,[42], [43], [44], [45], [46], [47]], although one study using the macrophage cell line J774 observed an inhibition of TNF production by NAC only at 1.5% oxygen, where ROS production is higher than under atmospheric oxygen concentrations (21%) [42].
The reports on the effects of NAC in models of TNF-mediated inflammation are not consistent in their conclusions. While some studies showed that administration of NAC protects from LPS-induced lethality and pulmonary damage [27,28,48], others reported that the effect is dose-dependent, and that high NAC doses can increase LPS mortality [49]. There is, however, a consensus for an inhibitory effect on the production of inflammatory cytokines in vitro and in vivo, although there are reports, for instance, of NAC increasing LPS-induced IL-1β production in vitro [50], with the possibility that the effect of NAC may be concentration-dependent and observed only at the highest doses [46,47].
Induction of inflammation by LPS does not have a consistent effect of GSH levels in all experimental models. While some studies reported a decrease [[51], [52], [53]] and others an increase [46,54], most studies reported a lack of effect [[55], [56], [57], [58], [59], [60], [61], [62], [63]].
It is important to note, however, that NAC cannot be considered a specific tool to study the role of endogenous GSH. While it is a precursor of GSH synthesis, it is also a ROS scavenger itself and a potent reducing agent [64], and the mechanism of this is debated [65].
Other tools used to investigate the role of GSH are buthionine-SR-sulfoximine (BSO), that inhibits GSH synthesis from cysteine [66], or diethyl maleate (DEM), an electrophile that reacts with GSH by direct conjugation thus depleting it [67,68]. However, DEM is less specific [68,69]. Diethyl maleate can also be contaminated with dimethylfumarate [69], which is an Nrf2 inducer [70]. Thus, only BSO should be considered a reasonably specific tool to investigate the role of endogenous GSH. Table 1 summarizes the results of several studies investigating the effect of BSO on LPS-induced inflammatory cytokines. It can be seen that there is no consensus, so that the results from each study cannot be extrapolated outside the specific experimental conditions of cell culture, LPS concentrations, length of treatments and others. Thus, one cannot generalize the assumption that endogenous GSH is a negative regulator of the inflammatory response, and this may explain the lack of translational success, in terms of drugs approved so far, of the use of GSH and its precursors as therapeutic agents in inflammatory disease.
Table 1.
Effect of BSO in models of LPS-induced inflammatory cytokines.
| Model | Result | Ref | Year |
|---|---|---|---|
| vivo mice | TNF ↑ | [29] | 1992 |
| vitro U373 astrocytoma | IL-8 ↑ | [118] | 1997 |
| vivo mice | TNF ≈ | [119] | 1999 |
| vitro alveolar macrophages | TNF, IL-8 ↑ | [120] | 1999 |
| vivo mice | TNF ↓ | [121] | 1999 |
| vitro THP1 monocytic cells | IL-12 ↓ | [122] | 2001 |
| vitro epitelial cells | TNF ↑ | [123] a | 2002 |
| vitro epitelial cells | TNF ↑ | [124] a | 2002 |
| vitro epitelial cells | TNF, IL-1β, IL-6 ↑ | [125] a | 2002 |
| vitro epitelial cells | TNF, IL-1β, IL-6 ↑ | [126] a | 2002 |
| vitro epitelial cells | TNF, IL-1β, IL-6 ↑ | [127] a | 2002 |
| vitro U937 monocytic cells | TNF ↑ | [128] | 2005 |
| vitro THP1 monocytic cells | TNF ≈ | [129] | 2008 |
| vitro epithelial cells | TNF ↑ | [130] | 2008 |
| ex vivo mouse peritoneal macrophages | IL-6 ↓ | [131] | 2009 |
| vitro epithelial cells | TNF ↑ | [132] a | 2011 |
| vitro dendritic cells | IL-12, IL-27 ↓ | [133] | 2011 |
| vitro RAW264 macrophages | IL-1β ↓ | [134] | 2017 |
| vitro RAW264 macrophages | TNF ≈, IL-1β ↓ | [61] | 2017 |
| vivo rat | IL-1β, IL-6, fever ↓ | [135] | 2017 |
| vitro RAW264 macrophages | TNF ≈ | [136] | 2018 |
| vitro RAW264 macrophages | IL-1β, IL-6 ≈ | [62] | 2018 |
| vitro RAW264 macrophages | TNF ↓ | [137] | 2021 |
↑, increase; ↓ decrease; ≈ no effect.
These different publications report results from the same experiment and should not be considered replications of published data.
5. Could NFkB be important in resistance and Nrf2 in tolerance, and what are the other players?
The activation of NFkB by LPS is acknowledged as a key pathway in innate immunity, the early-onset mechanism of resistance through pathogen control [71,72]. In fact, NFkB target genes (such as cytokines, cyclooxygenase, nitric oxide synthase) induce the classical inflammatory response in terms of recruitment of neutrophils and macrophages, activation of their killing mechanisms and antigen presentation that also contribute to the activation of adaptive immunity.
On the other hand, activation of Nrf2 is often associated with an anti-inflammatory response [73], part of which mediated by carbon monoxide produced by HMOX1 [74]. The anti-inflammatory action of carbon monoxide has been observed in lipopolysaccharide-induced models of septic shock [75] as well as in tissue-specific models of inflammatory disease [76,77].
Activation of Nrf2 also results in the inhibition of the production of inflammatory cytokines [78,79]. The main role of Nrf2, however, is adapting the organism to survive against oxidants and other electrophiles [80,81] including various xenobiotics [82]. The activation of Nrf2 by LPS has been reported in different models [61,83,84], and several papers demonstrated the key role of Nrf2-induced HMOX1 not only in the tolerance to malaria mentioned above but also in protecting from LPS lethality or polymicrobial sepsis [[84], [85], [86]]. When considering HMOX1 as a Nrf2 target gene in the context of inflammation, however, it is important to note that HMOX1 is not solely controlled by Nrf2 [74], and its induction by LPS or other Toll-like receptor agonists has a different pattern compared to other Nrf2 target genes [87,88]. Finally, Nrf2 is negatively regulated by Glycogen Synthase Kinase-3β (GSK3beta) [80,89,90] and this may contribute to the diminished LPS-induced cytokine production observed with GSK3beta inhibitors [91].
In addition to NFkB and Nrf2, hypoxia-inducible factor 1α (HIF-1α) should be considered. This transcription factor is important in adapting to low oxygen concentrations leading to physiological responses that promote oxygen delivery through the induction of erythropoietin (EPO) and vascular endothelial growth factor (VEGF), as well as cellular responses leading to a switch to glycolysis [92,93]. In particular, EPO has been shown to act as a tissue-protective cytokine that limits damage by inflammatory mediators and promotes tissue repair [94,95]. In the context of this review, EPO administration protects from lethality and organ damage in animal models of sepsis [96,97] and improves survival of mice with cerebral malaria without affecting parasitemia [98].
Therefore, the HIF-1α pathway could also play a role in tolerance, by protecting the host from the damage induced by the pathogen and/or by the immune response to it. Although the main role of HIF-1α is oxygen sensing, rather than ROS sensing as in the case of Nrf2, several reports indicate that it is redox regulated, and for this reason we discuss it here. In particular, ROS can stabilize HIF-1α by inactivating the prolyl hydroxylase responsible for its destabilization [99] as well as by increasing its mRNA levels via NFkB [100]. ROS can also stimulate the transcription of several HIF-1α target genes [101]. Conversely, antioxidant molecules have the opposite effect, with NAC and Vitamin C reducing HIF-1α levels [102] and ebselen and pyrrolidine dithiocarbamate inhibiting ROS-mediated activation of the transcription of EPO [101].
Whether HIF-1α has a pro- or anti-inflammatory role is debated. However, while in the context of cancer HIF-1α has been considered pro-inflammatory [103], in the context of innate immunity many data suggest the opposite. In fact, administration of chemical HIF-1α stabilizers inhibits NFkB activation and inflammatory cytokine production [[104], [105], [106], [107]], and protects from TNF-induced tissue damage [108,109] as well as Clostridium difficile-induced intestinal injury [110].
Finally, although not discussed in the present review, it should be mentioned that the three transcription factors I discussed are not independent from each other. It is important to highlight that Nrf2 and NFkB are not separate and independent. Studies have shown that Nrf2 can inhibit NFkB activation [86,111]. Signaling via HIF-1α is promoted by Nrf2, as shown by experiments where Nrf2 or its inhibitor were knocked out [[112], [113], [114]].
Stabilization of HIF-1α by the prolyl hydroxylase inhibitor dimethyloxalylglycine inhibits the production of inflammatory cytokines in models of inflammation and endotoxic/septic shock [107,109,115] although it should be noted that in specific microenvironments, such as in cancer, a pro-inflammatory role of HIF-1α has been described [116,117].
6. Conclusions
From the literature reviewed here, one could come to the general conclusion that NFkB has a key role in resistance while Nrf2 and HIF-1a are more important in tolerance and that, in general, most antioxidants have an inhibitory effect, and ROS a stimulating one, on these transcription factors. In this context, GSH does not inhibit the expression of all the genes associated with innate immunity but rather facilitates the induction via TLR4 of a subset of genes associated with the antiviral response while those for most inflammatory cytokines are independent of GSH, at least in the mouse macrophage experimental model discussed in this review.
We might therefore conclude that, while a schematic view of NFkB mediating resistance and Nrf2/HIF-1α mediating tolerance may hold, GSH-mediated redox regulation is not a master switch in the system but rather a signaling mechanism that fine tunes those systems by adding an additional control to regulated subset of genes [61].
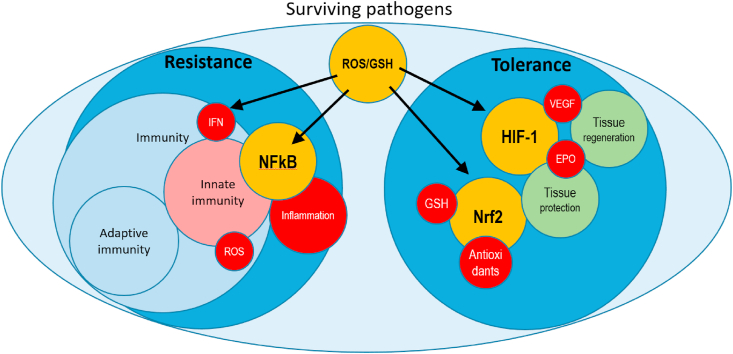
Fig. 2 is an attempt to describe the different roles of transcription factors and of the effectors of resistance and tolerance, as well as indicating where redox regulation can act, either through ROS or GSH/GSSG, or both. It should be noted that the role of ROS as signaling molecules vs. toxic mediators depends on their intracellular concentrations [23].
Fig. 2.
Regulators (yellow) and effectors (red) of resistance and tolerance. Left: innate immunity controls pathogens by ROS production by phagocytes (mainly antibacterial) and upregulation of the IFN pathway (mainly antiviral) as well as activation of the inflammatory response through activation of NFkB. Right: tolerance includes tissue protection by Nrf2-mediated upregulation of antioxidant systems and HIF-1-mediated induction of tissue-protective cytokines (e.g. EPO) as well as promotion of tissue repair through induction of growth factors (e.g. VEGF). Please note the dual role of ROS and GSH both as effectors and regulators. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The fact that GSH regulates the response to pathogens by a more complex mechanism that just acting as a ROS scavenger can explain why, in some instances, thiol antioxidants were not effective in clinical trials despite a very convincing mechanistic theory. It may not be surprising that administration of these reducing agents could in fact interfere with key signaling pathways, including those outlined in this review.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Schneider D.S., Ayres J.S. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 2008;8(11):889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Read A.F., Graham A.L., Raberg L. Animal defenses against infectious agents: is damage control more important than pathogen control. PLoS Biol. 2008;6(12):e4. doi: 10.1371/journal.pbio.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense strategy. Science. 2012;335(6071):936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy K., Weaver C. Garland science; 2016. Janeway's Immunobiology. [Google Scholar]
- 5.Caldwell R.M., Schafer J.F., Compton L.E., Patterson F.L. Tolerance to cereal leaf rusts. Science. 1958;128(3326):714–715. doi: 10.1126/science.128.3326.714. [DOI] [PubMed] [Google Scholar]
- 6.Jamieson A.M., Pasman L., Yu S., Gamradt P., Homer R.J., Decker T., Medzhitov R. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science. 2013;340(6137):1230–1234. doi: 10.1126/science.1233632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillai P.S., Molony R.D., Martinod K., Dong H., Pang I.K., Tal M.C., Solis A.G., Bielecki P., Mohanty S., Trentalange M., Homer R.J., Flavell R.A., Wagner D.D., Montgomery R.R., Shaw A.C., Staeheli P., Iwasaki A. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science. 2016;352(6284):463–466. doi: 10.1126/science.aaf3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos S., Carlos A.R., Sundaram B., Jeney V., Ribeiro A., Gozzelino R., Bank C., Gjini E., Braza F., Martins R., Ademolue T.W., Blankenhaus B., Gouveia Z., Faisca P., Trujillo D., Cardoso S., Rebelo S., Del Barrio L., Zarjou A., Bolisetty S., Agarwal A., Soares M.P. Renal control of disease tolerance to malaria. Proc. Natl. Acad. Sci. U. S. A. 2019;116(12):5681–5686. doi: 10.1073/pnas.1822024116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasaki A., Pillai P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014;14(5):315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luan H.H., Wang A., Hilliard B.K., Carvalho F., Rosen C.E., Ahasic A.M., Herzog E.L., Kang I., Pisani M.A., Yu S., Zhang C., Ring A.M., Young L.H., Medzhitov R. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell. 2019;178(5):1231–1244. doi: 10.1016/j.cell.2019.07.033. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soares M.P., Teixeira L., Moita L.F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 2017;17(2):83–96. doi: 10.1038/nri.2016.136. [DOI] [PubMed] [Google Scholar]
- 12.Biswas S.K., Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30(10):475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Cavaillon J.M., Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit. Care. 2006;10(5):233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akira S., Takeda K., Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2(8):675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R., Janeway C., Jr. Innate immunity. N. Engl. J. Med. 2000;343(5):338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 16.Babior B.M. Phagocytes and oxidative stress. Am. J. Med. 2000;109(1):33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 17.Lamb C., Dixon R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Biol. 1997;48(1):251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 18.Clark I.A., Rockett K.A. The cytokine theory of human cerebral malaria. Parasitol. Today. 1994;10(10):410–412. doi: 10.1016/0169-4758(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 19.Tracey K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghezzi P., Jaquet V., Marcucci F., Schmidt H.H. The oxidative stress theory of disease: levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017;174:1784–1796. doi: 10.1111/bph.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 22.Smith K.A. Louis Pasteur, the father of immunology? Front. Immunol. 2012;3:68. doi: 10.3389/fimmu.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 24.Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalebic T., Kinter A., Poli G., Anderson M.E., Meister A., Fauci A.S. Suppression of human immunodeficiency virus expression in chronically infected monocytic cells by glutathione, glutathione ester, and N-acetylcysteine. Proc. Natl. Acad. Sci. U. S. A. 1991;88(3):986–990. doi: 10.1073/pnas.88.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villa P., Ghezzi P. Effect of N-acetyl-L-cysteine on sepsis in mice. Eur. J. Pharmacol. 1995;292(3–4):341–344. doi: 10.1016/0926-6917(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 27.Bernard G.R., Lucht W.D., Niedermeyer M.E., Snapper J.R., Ogletree M.L., Brigham K.L. Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J. Clin. Invest. 1984;73(6):1772–1784. doi: 10.1172/JCI111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davreux C.J., Soric I., Nathens A.B., Watson R.W., McGilvray I.D., Suntres Z.E., Shek P.N., Rotstein O.D. N-acetyl cysteine attenuates acute lung injury in the rat. Shock. 1997;8(6):432–438. [PubMed] [Google Scholar]
- 29.Peristeris P., Clark B.D., Gatti S., Faggioni R., Mantovani A., Mengozzi M., Orencole S.F., Sironi M., Ghezzi P. N-acetylcysteine and glutathione as inhibitors of tumor necrosis factor production, Cell. Immunol. 1992;140(2):390–399. doi: 10.1016/0008-8749(92)90205-4. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre R.C., Jr., Pulido E.J., Bensard D.D., Shames B.D., Abraham E. Thirty years of clinical trials in acute respiratory distress syndrome. Crit. Care Med. 2000;28(9):3314–3331. doi: 10.1097/00003246-200009000-00034. [DOI] [PubMed] [Google Scholar]
- 31.Howick J., Glasziou P., Aronson J.K. Problems with using mechanisms to solve the problem of extrapolation. Theor. Med. Bioeth. 2013;34(4):275–291. doi: 10.1007/s11017-013-9266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Autreaux B., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 33.Mullen L., Mengozzi M., Hanschmann E.M., Alberts B., Ghezzi P. How the redox state regulates immunity. Free Radic. Biol. Med. 2020;157:3–14. doi: 10.1016/j.freeradbiomed.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Hughes M.M., Lavrencic P., Coll R.C., Ve T., Ryan D.G., Williams N.C., Menon D., Mansell A., Board P.G., Mobli M., Kobe B., O'Neill L.A.J. Solution structure of the TLR adaptor MAL/TIRAP reveals an intact BB loop and supports MAL Cys91 glutathionylation for signaling. Proc. Natl. Acad. Sci. U. S. A. 2017;114(32):E6480–E6489. doi: 10.1073/pnas.1701868114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pineda-Molina E., Klatt P., Vazquez J., Marina A., Garcia de Lacoba M., Perez-Sala D., Lamas S. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40(47):14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 36.Xie Y., Kole S., Precht P., Pazin M.J., Bernier M. S-glutathionylation impairs signal transducer and activator of transcription 3 activation and signaling. Endocrinology. 2009;150(3):1122–1131. doi: 10.1210/en.2008-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X., Liu P., Zhang C., Chiewchengchol D., Zhao F., Yu H., Li J., Kambara H., Luo K.Y., Venkataraman A., Zhou Z., Zhou W., Zhu H., Zhao L., Sakai J., Chen Y., Ho Y.S., Bajrami B., Xu B., Silberstein L.E., Cheng T., Xu Y., Ke Y., Luo H.R. Positive regulation of interleukin-1beta bioactivity by physiological ROS-mediated cysteine S-glutathionylation. Cell Rep. 2017;20(1):224–235. doi: 10.1016/j.celrep.2017.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stocker S., Van Laer K., Mijuskovic A., Dick T.P. The conundrum of hydrogen peroxide signaling and the emerging role of peroxiredoxins as redox relay hubs. Antioxidants Redox Signal. 2018;28(7):558–573. doi: 10.1089/ars.2017.7162. [DOI] [PubMed] [Google Scholar]
- 39.McCord J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000;108(8):652–659. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 40.Thannickal V.J. Oxygen in the evolution of complex life and the price we pay. Am. J. Respir. Cell Mol. Biol. 2009;40(5):507–510. doi: 10.1165/rcmb.2008-0360PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandel N.S., Trzyna W.C., McClintock D.S., Schumacker P.T. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J. Immunol. 2000;165(2):1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y., Li L., Hang Q., Fang Y., Dong X., Cao P., Yin Z., Luo L. gamma-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019;20:157–166. doi: 10.1016/j.redox.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mo M., Li S., Dong Z., Li C., Sun Y., Li A., Zhao Z. S-allylmercaptocysteine ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammation and oxidative stress via nuclear factor kappa B and Keap1/Nrf2 pathways. Int. Immunopharm. 2020;81:106273. doi: 10.1016/j.intimp.2020.106273. [DOI] [PubMed] [Google Scholar]
- 45.Berrino E., Carradori S., Angeli A., Carta F., Supuran C.T., Guglielmi P., Coletti C., Paciotti R., Schweikl H., Maestrelli F., Cerbai E., Gallorini M. Dual carbonic anhydrase IX/XII inhibitors and carbon monoxide releasing molecules modulate LPS-mediated inflammation in mouse macrophages. Antioxidants. 2021;10(1) doi: 10.3390/antiox10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song M., Kellum J.A., Kaldas H., Fink M.P. Evidence that glutathione depletion is a mechanism responsible for the anti-inflammatory effects of ethyl pyruvate in cultured lipopolysaccharide-stimulated RAW 264.7 cells. J. Pharmacol. Exp. Therapeut. 2004;308(1):307–316. doi: 10.1124/jpet.103.056622. [DOI] [PubMed] [Google Scholar]
- 47.Palacio J.R., Markert U.R., Martinez P. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm. Res. 2011;60(7):695–704. doi: 10.1007/s00011-011-0323-8. [DOI] [PubMed] [Google Scholar]
- 48.Gatti S., Faggioni R., Echtenacher B., Ghezzi P. Role of tumour necrosis factor and reactive oxygen intermediates in lipopolysaccharide-induced pulmonary oedema and lethality. Clin. Exp. Immunol. 1993;91(3):456–461. doi: 10.1111/j.1365-2249.1993.tb05924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sprong R.C., Winkelhuyzen-Janssen A.M., Aarsman C.J., van Oirschot J.F., van der Bruggen T., van Asbeck B.S. Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am. J. Respir. Crit. Care Med. 1998;157(4 Pt 1):1283–1293. doi: 10.1164/ajrccm.157.4.9508063. [DOI] [PubMed] [Google Scholar]
- 50.Parmentier M., Hirani N., Rahman I., Donaldson K., MacNee W., Antonicelli F. Regulation of lipopolysaccharide-mediated interleukin-1beta release by N-acetylcysteine in THP-1 cells. Eur. Respir. J. 2000;16(5):933–939. doi: 10.1183/09031936.00.16593300. [DOI] [PubMed] [Google Scholar]
- 51.Arribas B., Rodriguez-Cabezas M.E., Comalada M., Bailon E., Camuesco D., Olivares M., Xaus J., Zarzuelo A., Galvez J. Evaluation of the preventative effects exerted by Lactobacillus fermentum in an experimental model of septic shock induced in mice. Br. J. Nutr. 2009;101(1):51–58. doi: 10.1017/S0007114508986876. [DOI] [PubMed] [Google Scholar]
- 52.Payabvash S., Ghahremani M.H., Goliaei A., Mandegary A., Shafaroodi H., Amanlou M., Dehpour A.R. Nitric oxide modulates glutathione synthesis during endotoxemia. Free Radic. Biol. Med. 2006;41(12):1817–1828. doi: 10.1016/j.freeradbiomed.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Zhang C., Walker L.M., Mayeux P.R. Role of nitric oxide in lipopolysaccharide-induced oxidant stress in the rat kidney. Biochem. Pharmacol. 2000;59(2):203–209. doi: 10.1016/s0006-2952(99)00324-x. [DOI] [PubMed] [Google Scholar]
- 54.Fay M., Jampy-Fay M., Akarid K., Gougerot-Pocidalo M.A. Protective effect of LPS and poly A:U against immune oxidative injury: role of thiols released by activated macrophages. Free Radic. Biol. Med. 1995;18(4):649–654. doi: 10.1016/0891-5849(94)00173-h. [DOI] [PubMed] [Google Scholar]
- 55.Minamiyama Y., Takemura S., Koyama K., Yu H., Miyamoto M., Inoue M. Dynamic aspects of glutathione and nitric oxide metabolism in endotoxemic rats. Am. J. Physiol. 1996;271(4 Pt 1):G575–G581. doi: 10.1152/ajpgi.1996.271.4.G575. [DOI] [PubMed] [Google Scholar]
- 56.Nathens A.B., Marshall J.C., Watson R.W., Dackiw A.P., Rotstein O.D. Diethylmaleate attenuates endotoxin-induced lung injury. Surgery. 1996;120(2):360–366. doi: 10.1016/s0039-6060(96)80310-2. [DOI] [PubMed] [Google Scholar]
- 57.Romao P.R., Fonseca S.G., Hothersall J.S., Noronha-Dutra A.A., Ferreira S.H., Cunha F.Q. Glutathione protects macrophages and Leishmania major against nitric oxide-mediated cytotoxicity. Parasitology. 1999;118(Pt 6):559–566. doi: 10.1017/s0031182099004278. [DOI] [PubMed] [Google Scholar]
- 58.Venketaraman V., Dayaram Y.K., Talaue M.T., Connell N.D. Glutathione and nitrosoglutathione in macrophage defense against Mycobacterium tuberculosis. Infect. Immun. 2005;73(3):1886–1889. doi: 10.1128/IAI.73.3.1886-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong X., Thimmulappa R., Kombairaju P., Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J. Immunol. 2010;185(1):569–577. doi: 10.4049/jimmunol.0902315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balabanli B., Balaban T. Investigation into the effects of boron on liver tissue protein carbonyl, MDA, and glutathione levels in endotoxemia. Biol. Trace Elem. Res. 2015;167(2):259–263. doi: 10.1007/s12011-015-0301-z. [DOI] [PubMed] [Google Scholar]
- 61.Diotallevi M., Checconi P., Palamara A.T., Celestino I., Coppo L., Holmgren A., Abbas K., Peyrot F., Mengozzi M., Ghezzi P. Glutathione fine-tunes the innate immune response toward antiviral pathways in a macrophage cell line independently of its antioxidant properties. Front. Immunol. 2017;8:1239. doi: 10.3389/fimmu.2017.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tocmo R., Parkin K. S-Alk(en)ylmercaptocysteine suppresses LPS-induced pro-inflammatory responses in murine macrophages through inhibition of NF-kappaB pathway and modulation of thiol redox status. Free Radic. Biol. Med. 2018;129:548–558. doi: 10.1016/j.freeradbiomed.2018.10.424. [DOI] [PubMed] [Google Scholar]
- 63.Staples S., Wall S.B., Li R., Tipple T.E. Selenium-independent antioxidant and anti-inflammatory effects of thioredoxin reductase inhibition in alveolar macrophages. Life Sci. 2020;259:118285. doi: 10.1016/j.lfs.2020.118285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samuni Y., Goldstein S., Dean O.M., Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta. 2013;1830(8):4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 65.Ezerina D., Takano Y., Hanaoka K., Urano Y., Dick T.P. N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem. Biol. 2018;25(4):447–459 e4. doi: 10.1016/j.chembiol.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griffith O.W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J. Biol. Chem. 1979;254(16):7558–7560. [PubMed] [Google Scholar]
- 67.Bannai S. Induction of cystine and glutamate transport activity in human fibroblasts by diethyl maleate and other electrophilic agents. J. Biol. Chem. 1984;259(4):2435–2440. [PubMed] [Google Scholar]
- 68.Anderson M.E. Glutathione: an overview of biosynthesis and modulation. Chem. Biol. Interact. 1998;111–112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 69.Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol. Ther. 1991;51(2):155–194. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- 70.Linker R.A., Lee D.H., Ryan S., van Dam A.M., Conrad R., Bista P., Zeng W., Hronowsky X., Buko A., Chollate S., Ellrichmann G., Bruck W., Dawson K., Goelz S., Wiese S., Scannevin R.H., Lukashev M., Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(Pt 3):678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 71.Ghosh S., May M.J., Kopp E.B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 72.Lemaitre B., Nicolas E., Michaut L., Reichhart J.M., Hoffmann J.A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 73.Dinkova-Kostova A.T., Liby K.T., Stephenson K.K., Holtzclaw W.D., Gao X., Suh N., Williams C., Risingsong R., Honda T., Gribble G.W., Sporn M.B., Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. U. S. A. 2005;102(12):4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ryter S.W., Alam J., Choi A.M. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 2006;86(2):583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 75.Motterlini R., Nikam A., Manin S., Ollivier A., Wilson J.L., Djouadi S., Muchova L., Martens T., Rivard M., Foresti R. HYCO-3, a dual CO-releaser/Nrf2 activator, reduces tissue inflammation in mice challenged with lipopolysaccharide. Redox Biol. 2019;20:334–348. doi: 10.1016/j.redox.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Ali Z., Ollivier A., Manin S., Rivard M., Motterlini R., Foresti R. Therapeutic effects of CO-releaser/Nrf2 activator hybrids (HYCOs) in the treatment of skin wound, psoriasis and multiple sclerosis. Redox Biol. 2020;34:101521. doi: 10.1016/j.redox.2020.101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Babu D., Motterlini R., Lefebvre R.A. CO and CO-releasing molecules (CO-RMs) in acute gastrointestinal inflammation. Br. J. Pharmacol. 2015;172(6):1557–1573. doi: 10.1111/bph.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heiss E., Herhaus C., Klimo K., Bartsch H., Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001;276(34):32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 79.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tebay L.E., Robertson H., Durant S.T., Vitale S.R., Penning T.M., Dinkova-Kostova A.T., Hayes J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015;88(Pt B):108–146. doi: 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayes J.D., McLellan L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999;31(4):273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 82.Xu C., Li C.Y., Kong A.N. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm. Res. (Seoul) 2005;28(3):249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 83.Vijayan V., Baumgart-Vogt E., Naidu S., Qian G., Immenschuh S. Bruton's tyrosine kinase is required for TLR-dependent heme oxygenase-1 gene activation via Nrf2 in macrophages. J. Immunol. 2011;187(2):817–827. doi: 10.4049/jimmunol.1003631. [DOI] [PubMed] [Google Scholar]
- 84.Cuadrado A., Martin-Moldes Z., Ye J., Lastres-Becker I. Transcription factors NRF2 and NF-kappaB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014;289(22):15244–15258. doi: 10.1074/jbc.M113.540633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thimmulappa R.K., Scollick C., Traore K., Yates M., Trush M.A., Liby K.T., Sporn M.B., Yamamoto M., Kensler T.W., Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem. Biophys. Res. Commun. 2006;351(4):883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thimmulappa R.K., Lee H., Rangasamy T., Reddy S.P., Yamamoto M., Kensler T.W., Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 2006;116(4):984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ingram S., Mengozzi M., Sacre S., Mullen L., Ghezzi P. Differential induction of nuclear factor-like 2 signature genes with toll-like receptor stimulation. Free Radic. Biol. Med. 2019;135:245–250. doi: 10.1016/j.freeradbiomed.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 88.Kim K.H., Lyu J.H., Koo S.T., Oh S.-R., Lee H.-K., Ahn K.-S., Sadikot R.T., Joo M. MyD88 is a mediator for the activation of Nrf2. Biochem. Biophys. Res. Commun. 2011;404(1):46–51. doi: 10.1016/j.bbrc.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 89.Salazar M., Rojo A.I., Velasco D., de Sagarra R.M., Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281(21):14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 90.Hayes J.D., Chowdhry S., Dinkova-Kostova A.T., Sutherland C. Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of beta-TrCP and GSK-3. Biochem. Soc. Trans. 2015;43(4):611–620. doi: 10.1042/BST20150011. [DOI] [PubMed] [Google Scholar]
- 91.Lv H., Liu Q., Wen Z., Feng H., Deng X., Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3beta-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Semenza G.L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology. 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 93.Semenza G.L. Hypoxia-inducible factor 1 and cardiovascular disease. Annu. Rev. Physiol. 2014;76:39–56. doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brines M.L., Ghezzi P., Keenan S., Agnello D., de Lanerolle N.C., Cerami C., Itri L.M., Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc. Natl. Acad. Sci. U. S. A. 2000;97(19):10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brines M., Cerami A. The receptor that tames the innate immune response. Mol. Med. 2012;18:486–496. doi: 10.2119/molmed.2011.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heitrich M., Garcia D.M., Stoyanoff T.R., Rodriguez J.P., Todaro J.S., Aguirre M.V. Erythropoietin attenuates renal and pulmonary injury in polymicrobial induced-sepsis through EPO-R, VEGF and VEGF-R2 modulation. Biomed. Pharmacother. 2016;82:606–613. doi: 10.1016/j.biopha.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 97.Coldewey S.M., Khan A.I., Kapoor A., Collino M., Rogazzo M., Brines M., Cerami A., Hall P., Sheaff M., Kieswich J.E., Yaqoob M.M., Patel N.S., Thiemermann C. Erythropoietin attenuates acute kidney dysfunction in murine experimental sepsis by activation of the beta-common receptor. Kidney Int. 2013;84(3):482–490. doi: 10.1038/ki.2013.118. [DOI] [PubMed] [Google Scholar]
- 98.Kaiser K., Texier A., Ferrandiz J., Buguet A., Meiller A., Latour C., Peyron F., Cespuglio R., Picot S. Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J. Infect. Dis. 2006;193(7):987–995. doi: 10.1086/500844. [DOI] [PubMed] [Google Scholar]
- 99.Lee H.Y., Lee T., Lee N., Yang E.G., Lee C., Lee J., Moon E.Y., Ha J., Park H. Src activates HIF-1alpha not through direct phosphorylation of HIF-1alpha specific prolyl-4 hydroxylase 2 but through activation of the NADPH oxidase/Rac pathway. Carcinogenesis. 2011;32(5):703–712. doi: 10.1093/carcin/bgr034. [DOI] [PubMed] [Google Scholar]
- 100.Bonello S., Zahringer C., BelAiba R.S., Djordjevic T., Hess J., Michiels C., Kietzmann T., Gorlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler. Thromb. Vasc. Biol. 2007;27(4):755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 101.Chandel N.S., Maltepe E., Goldwasser E., Mathieu C.E., Simon M.C., Schumacker P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. U. S. A. 1998;95(20):11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao P., Zhang H., Dinavahi R., Li F., Xiang Y., Raman V., Bhujwalla Z.M., Felsher D.W., Cheng L., Pevsner J., Lee L.A., Semenza G.L., Dang C.V. HIF-dependent antitumorigenic effect of antioxidants in vivo. Canc. Cell. 2007;12(3):230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corcoran S.E., O'Neill L.A. HIF1alpha and metabolic reprogramming in inflammation. J. Clin. Invest. 2016;126(10):3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shang L., Kang W., Li S., Ge S. Prolyl hydroxylase inhibitor DMOG suppressed inflammatory cytokine production in human gingival fibroblasts stimulated with Fusobacterium nucleatum. Clin. Oral Invest. 2019;23(7):3123–3132. doi: 10.1007/s00784-018-2733-2. [DOI] [PubMed] [Google Scholar]
- 105.Shang L., Wang T., Tong D., Kang W., Liang Q., Ge S. Prolyl hydroxylases positively regulated LPS-induced inflammation in human gingival fibroblasts via TLR4/MyD88-mediated AKT/NF-kappaB and MAPK pathways. Cell Prolif. 2018;51(6) doi: 10.1111/cpr.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scholz C.C., Cavadas M.A., Tambuwala M.M., Hams E., Rodriguez J., von Kriegsheim A., Cotter P., Bruning U., Fallon P.G., Cheong A., Cummins E.P., Taylor C.T. Regulation of IL-1beta-induced NF-kappaB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc. Natl. Acad. Sci. U. S. A. 2013;110(46):18490–18495. doi: 10.1073/pnas.1309718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hams E., Saunders S.P., Cummins E.P., O'Connor A., Tambuwala M.T., Gallagher W.M., Byrne A., Campos-Torres A., Moynagh P.M., Jobin C., Taylor C.T., Fallon P.G. The hydroxylase inhibitor dimethyloxallyl glycine attenuates endotoxic shock via alternative activation of macrophages and IL-10 production by B1 cells. Shock. 2011;36(3):295–302. doi: 10.1097/SHK.0b013e318225ad7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hindryckx P., De Vos M., Jacques P., Ferdinande L., Peeters H., Olievier K., Bogaert S., Brinkman B., Vandenabeele P., Elewaut D., Laukens D. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. J. Immunol. 2010;185(10):6306–6316. doi: 10.4049/jimmunol.1002541. [DOI] [PubMed] [Google Scholar]
- 109.Takeda K., Ichiki T., Narabayashi E., Inanaga K., Miyazaki R., Hashimoto T., Matsuura H., Ikeda J., Miyata T., Sunagawa K. Inhibition of prolyl hydroxylase domain-containing protein suppressed lipopolysaccharide-induced TNF-alpha expression. Arterioscler. Thromb. Vasc. Biol. 2009;29(12):2132–2137. doi: 10.1161/ATVBAHA.109.196071. [DOI] [PubMed] [Google Scholar]
- 110.Hirota S.A., Fines K., Ng J., Traboulsi D., Lee J., Ihara E., Li Y., Willmore W.G., Chung D., Scully M.M., Louie T., Medlicott S., Lejeune M., Chadee K., Armstrong G., Colgan S.P., Muruve D.A., MacDonald J.A., Beck P.L. Hypoxia-inducible factor signaling provides protection in Clostridium difficile-induced intestinal injury. Gastroenterology. 2010;139(1):259–269 e3. doi: 10.1053/j.gastro.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmed S.M., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 112.Kim T.H., Hur E.G., Kang S.J., Kim J.A., Thapa D., Lee Y.M., Ku S.K., Jung Y., Kwak M.K. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Canc. Res. 2011;71(6):2260–2275. doi: 10.1158/0008-5472.CAN-10-3007. [DOI] [PubMed] [Google Scholar]
- 113.Ke B., Shen X.D., Zhang Y., Ji H., Gao F., Yue S., Kamo N., Zhai Y., Yamamoto M., Busuttil R.W., Kupiec-Weglinski J.W. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J. Hepatol. 2013;59(6):1200–1207. doi: 10.1016/j.jhep.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ji X., Wang H., Zhu J., Zhu L., Pan H., Li W., Zhou Y., Cong Z., Yan F., Chen S. Knockdown of Nrf2 suppresses glioblastoma angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Int. J. Canc. 2014;135(3):574–584. doi: 10.1002/ijc.28699. [DOI] [PubMed] [Google Scholar]
- 115.Hirai K., Furusho H., Hirota K., Sasaki H. Activation of hypoxia-inducible factor 1 attenuates periapical inflammation and bone loss. Int. J. Oral Sci. 2018;10(2):12. doi: 10.1038/s41368-018-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taylor C.T., Colgan S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017;17(12):774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scholz C.C., Taylor C.T. Targeting the HIF pathway in inflammation and immunity. Curr. Opin. Pharmacol. 2013;13(4):646–653. doi: 10.1016/j.coph.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 118.Tanaka C., Kamata H., Takeshita H., Yagisawa H., Hirata H. Redox regulation of lipopolysaccharide (LPS)-induced interleukin-8 (IL-8) gene expression mediated by NF kappa B and AP-1 in human astrocytoma U373 cells. Biochem. Biophys. Res. Commun. 1997;232(2):568–573. doi: 10.1006/bbrc.1997.6264. [DOI] [PubMed] [Google Scholar]
- 119.Kang K.W., Pak Y.M., Kim N.D. Diethylmaleate and buthionine sulfoximine, glutathione-depleting agents, differentially inhibit expression of inducible nitric oxide synthase in endotoxemic mice. Nitric Oxide. 1999;3(3):265–271. doi: 10.1006/niox.1999.0233. [DOI] [PubMed] [Google Scholar]
- 120.Gosset P., Wallaert B., Tonnel A.B., Fourneau C. Thiol regulation of the production of TNF-alpha, IL-6 and IL-8 by human alveolar macrophages. Eur. Respir. J. 1999;14(1):98–105. doi: 10.1034/j.1399-3003.1999.14a17.x. [DOI] [PubMed] [Google Scholar]
- 121.Wang F., Wang L.Y., Wright D., Parmely M.J. Redox imbalance differentially inhibits lipopolysaccharide-induced macrophage activation in the mouse liver. Infect. Immun. 1999;67(10):5409–5416. doi: 10.1128/iai.67.10.5409-5416.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dobashi K., Aihara M., Araki T., Shimizu Y., Utsugi M., Iizuka K., Murata Y., Hamuro J., Nakazawa T., Mori M. Regulation of LPS induced IL-12 production by IFN-gamma and IL-4 through intracellular glutathione status in human alveolar macrophages. Clin. Exp. Immunol. 2001;124(2):290–296. doi: 10.1046/j.1365-2249.2001.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haddad J.J., Saade N.E., Safieh-Garabedian B. Redox regulation of TNF-alpha biosynthesis: augmentation by irreversible inhibition of gamma-glutamylcysteine synthetase and the involvement of an IkappaB-alpha/NF-kappaB-independent pathway in alveolar epithelial cells. Cell. Signal. 2002;14(3):211–218. doi: 10.1016/s0898-6568(01)00233-9. [DOI] [PubMed] [Google Scholar]
- 124.Haddad J.J. The involvement of L-gamma-glutamyl-L-cysteinyl-glycine (glutathione/GSH) in the mechanism of redox signaling mediating MAPK(p38)-dependent regulation of pro-inflammatory cytokine production. Biochem. Pharmacol. 2002;63(2):305–320. doi: 10.1016/s0006-2952(01)00870-x. [DOI] [PubMed] [Google Scholar]
- 125.Haddad J.J. Redox regulation of pro-inflammatory cytokines and IkappaB-alpha/NF-kappaB nuclear translocation and activation. Biochem. Biophys. Res. Commun. 2002;296(4):847–856. doi: 10.1016/s0006-291x(02)00947-6. [DOI] [PubMed] [Google Scholar]
- 126.Haddad J.J., Safieh-Garabedian B., Saade N.E., Lauterbach R. Inhibition of glutathione-related enzymes augments LPS-mediated cytokine biosynthesis: involvement of an IkappaB/NF-kappaB-sensitive pathway in the alveolar epithelium. Int. Immunopharm. 2002;2(11):1567–1583. doi: 10.1016/s1567-5769(02)00117-0. [DOI] [PubMed] [Google Scholar]
- 127.Haddad J.J., Land S.C. Redox signaling-mediated regulation of lipopolysaccharide-induced proinflammatory cytokine biosynthesis in alveolar epithelial cells. Antioxidants Redox Signal. 2002;4(1):179–193. doi: 10.1089/152308602753625942. [DOI] [PubMed] [Google Scholar]
- 128.Strasser E.M., Wessner B., Manhart N., Roth E. The relationship between the anti-inflammatory effects of curcumin and cellular glutathione content in myelomonocytic cells. Biochem. Pharmacol. 2005;70(4):552–559. doi: 10.1016/j.bcp.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 129.Cao J., Jiang L., Zhang X., Yao X., Geng C., Xue X., Zhong L. Boric acid inhibits LPS-induced TNF-alpha formation through a thiol-dependent mechanism in THP-1 cells. J. Trace Elem. Med. Biol. 2008;22(3):189–195. doi: 10.1016/j.jtemb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 130.Zhang F., Wang X., Wang W., Li N., Li J. Glutamine reduces TNF-alpha by enhancing glutathione synthesis in lipopolysaccharide-stimulated alveolar epithelial cells of rats. Inflammation. 2008;31(5):344–350. doi: 10.1007/s10753-008-9084-0. [DOI] [PubMed] [Google Scholar]
- 131.Pruett S.B., Cheng B., Fan R., Tan W., Sebastian T. Oxidative stress and sodium methyldithiocarbamate-induced modulation of the macrophage response to lipopolysaccharide in vivo. Toxicol. Sci. 2009;109(2):237–246. doi: 10.1093/toxsci/kfp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haddad J.J. A redox microenvironment is essential for MAPK-dependent secretion of pro-inflammatory cytokines: modulation by glutathione (GSH/GSSG) biosynthesis and equilibrium in the alveolar epithelium. Cell. Immunol. 2011;270(1):53–61. doi: 10.1016/j.cellimm.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 133.Kamide Y., Utsugi M., Dobashi K., Ono A., Ishizuka T., Hisada T., Koga Y., Uno K., Hamuro J., Mori M. Intracellular glutathione redox status in human dendritic cells regulates IL-27 production and T-cell polarization. Allergy. 2011;66(9):1183–1192. doi: 10.1111/j.1398-9995.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 134.Bolling A.K., Solhaug A., Morisbak E., Holme J.A., Samuelsen J.T. The dental monomer hydroxyethyl methacrylate (HEMA) counteracts lipopolysaccharide-induced IL-1beta release-Possible role of glutathione. Toxicol. Lett. 2017;270:25–33. doi: 10.1016/j.toxlet.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 135.Wrotek S., Domagalski K., Jedrzejewski T., Dec E., Kozak W. Buthionine sulfoximine, a glutathione depletor, attenuates endotoxic fever and reduces IL-1beta and IL-6 level in rats. Cytokine. 2017;90:31–37. doi: 10.1016/j.cyto.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 136.Fernandes A.M.M., Vilela P.G.F., Valera M.C., Bolay C., Hiller K.A., Schweikl H., Schmalz G. Effect of bleaching agent extracts on murine macrophages. Clin. Oral Invest. 2018;22(4):1771–1781. doi: 10.1007/s00784-017-2273-1. [DOI] [PubMed] [Google Scholar]
- 137.Schweikl H., Birke M., Gallorini M., Petzel C., Bolay C., Waha C., Hiller K.A., Buchalla W. HEMA-induced oxidative stress inhibits NF-kappaB nuclear translocation and TNF release from LTA- and LPS-stimulated immunocompetent cells. Dent. Mater. 2021;37(1):175–190. doi: 10.1016/j.dental.2020.10.029. [DOI] [PubMed] [Google Scholar]