Abstract
The term „nutritional cognitive neuroscience” was recently established to define a research field focusing on the impact of nutrition on cognition and brain health across the life span. In this overview, we summarize the robust evidence on the role of carotenoids as micronutrients with different biological properties in persons with cognitive (pre)frailty. As neurodegenerative processes during aging occur in a continuum from brain aging to dementia, we propose the name „nutritional cognitive neuroscience of aging“ to define research on the role of nutrition and micronutrients in cognitive frailty. Further studies are warranted which integrate carotenoid interventions in multidomain, personalized lifestyle strategies.
Keywords: Alzheimer's disease, Carotenoids, Cognitive frailty, Dementia, Frailty, Micronutrients
Highlights
-
•
Cognitive integrity is an essential element of healthy and active ageing.
-
•
Oxidative distress is strongly linked to neurodegeneration.
-
•
Consumption and levels of carotenoids are linked to cognitive frailty.
-
•
There is conflict of evidence for intervention trials with carotenoids in dementia.
-
•
Future studies with carotenoids should be within personalized and multidomain strategies.
1. Introduction
The term „Nutritional cognitive neuroscience” was recently established to define a rapidly expanding interdisciplinary field of research that seeks to understand nutrition's impact on cognition and brain health across the life span [1]. Indeed, nutrition, in its broad range of aspects from specific nutrients to whole diets, has been shown to affect brain structure and function. Therefore, nutritional cognitive neuroscience includes both pathophysiological investigations and nutritional intervention strategies in brain ageing and disease. The field has recently gained attention mainly due to two aspects, both related to the current demographic explosion with an impressive increase of the aging population. The first is the growing need to maintain cognitive integrity for healthy and active aging, in wellbeing and robustness [2,3]. The second is the rapidly increasing prevalence of and attention to age-related cognitive decline and dementias including mild cognitive impairment (MCI) [4] and Alzheimer disease (AD) [2,3]. Cognitive decline with and without dementia causes major public health concerns based on the fact that dementia is not curable. This is due to late diagnosis and a multifaceted pathophysiology, called multifactoriality. As multifactoriality increases with increasing age, the latter is the main risk factor for cognitive impairment and the large majority of dementia patients are old and very old, attention is shifted towards need of early diagnosis of cognitive changes and to slowing down their progression. Age-related cognitive decline including subjective cognitive impairment (SCI) [5] and MCI are object of a large body of investigations prompted at identifying the best possible preventive strategies. While the challenged search for effective anti-dementia drugs is ongoing, studies on the role of vascular- and lifestyle-related preventive strategies show that vascular risk control and lifestyle improvement are indeed able to slow down the progression of cognitive impairment [3,[6], [7], [8], [9]]. Among lifestyle interventions, cognitive training programs, physical exercise interventions and dietary strategies have gained a great deal of attention recently [7,9,10].
Several of these studies have been based upon the evidence that oxidative stress, a critical pathophysiological mechanism in aging [2,[11], [12], [13], [14]] as well as in the onset and progression of cognitive impairment [[15], [16], [17], [18], [19]], can be substantially influenced by physical activity and nutrition. Indeed, several biomarkers of oxidative stress and indicators of antioxidant micronutrient defense against free radicals have been shown to be associated with cognitive impairment with and without dementia [[15], [16], [17], [18], [19]]. Although the results of these studies are interesting, the interactions between the different components of lifestyle across the course of cognitive impairment have been not clearly identified yet.
The aim of this overview is to summarize existing knowledge in a field typically falling within the nutritional cognitive neuroscience, collected by the authors over several years of experience and concerning the analysis of a specific group of nutrient biomarker patterns, those related to carotenoids, coupled with modern metrics of cognitive performance and indices of brain health in advanced age. By integrating cutting-edge techniques from nutritional science and cognitive neuroscience, the newly established field of nutritional cognitive neuroscience of aging can be informed by both long-lasting research and advanced targeted understanding on the multifaceted relationship between nutrition and brain aging.
2. Deregulation of redox balance and neurodegeneration
Nutrition and antioxidant micronutrient function in cognitive neuroscience emerged largely from the evidence of the role of oxidative distress and eustress in brain aging, neurodegeneration and age-related cognitive decline [17,20]. While the latter is acknowledged, conflicting results from interventional studies are due essentially to the multifactoriality and heterogeneity of the aging process including brain aging, which hinders the adequate disentaglement of one mechanism from other „pillars“ [21]. A paradigm shift necessary to the understanding of nutritional cognitive neuroscience consists of the well known, but largely neglected, fact that the main risk factor for age-related diseases including dementia is age and that the pathophysiology of aging with its multiple age-related changes strikingly resembles the characteristics of the aging brain, through a continuum from normal cognitive function to dementia [22,23]. In particular, the pathophysiology of age-related cognitive impairment and dementia is multifactorial, extremely complex and spans from traditional amyloid-β- and τ-hypotheses to genetic factors, to a lifelong exposure to the disequilibrium between the multitude of vascular and lifestyle protective and risk factors known [23]. In other words, main age-related neurodegenerative characteristics including structural, cellular, molecular, biochemical, vascular changes reflect intrinsic and extrinsic features of the aging process itself and are associated to cognition correlates [3,23]. Across the biomolecular to phenotypical to clinical manifestations, all age-related changes explain memory loss, impairment of other cognitive domains and neurological and neuropsychiatric systems. For the same reason, motoric alterations, posture, balance and gait disorders, sleep disturbances, sensory decrements, personality changes and mood disorders are part of the dementia features.
Within this complexity, the central nervous system (CNS), as a tissue that is highly dependent on O2, is particularly sensitive to changes in O2 levels and to the elevated production of derivatives of molecular oxygen (which are otherwise normally formed as an attribute of aerobic life), reactive oxygen species (ROS). The elevated formation of different ROS is termed ‘oxidative distress’, leads to molecular damage and is the pathologic counterpart of ‚oxidative eustress‘ - the equilibrium between ROS at physiological levels and their central role in redox signalling via different post-translational modifications [20].
Deregulation of redox balance as oxidative distress is strongly linked to neurodegeneration, including major long-term degenerative diseases such as AD, Parkinson disease, Huntington disease and amyotrophic lateral sclerosis [24]. Insults to the CNS, such as accumulation of protein aggregates associated with neurodegenerative diseases, cause oxidant generation — involving both neurons and microglia, the main phagocytes in the brain that serve as neuron-supporting cells. Generally, this response is meant to be protective by clearing debris and supporting neuronal survival. However, in some cases microglia become overactivated and overproduce reactive oxygen species (ROS) and reactive nitrogen species, thereby leading to neuroinflammation and impeding neuronal and oligodendroglial survival [25]. Furthermore, ischaemia-associated factors cause neuronal autotoxicity and breakdown of the blood–brain barrier [8,26,27]. The pleiotropy of ROS effects include several mechanisms investigated by transcriptomic, metabolomic, biochemical, immunohistochemical and behavioural analyses; there is also evidence that oxidants are mediators of psychological stress responses and that chronic psychological stress exposure promotes oxidative damage of nucleic acids and lipids, which could contribute to stress-induced ageing and mediate psychobiological resilience to oxidative damage in case of mild stress (reviewed in Ref. [20]).
3. Carotenoids and cognitive frailty
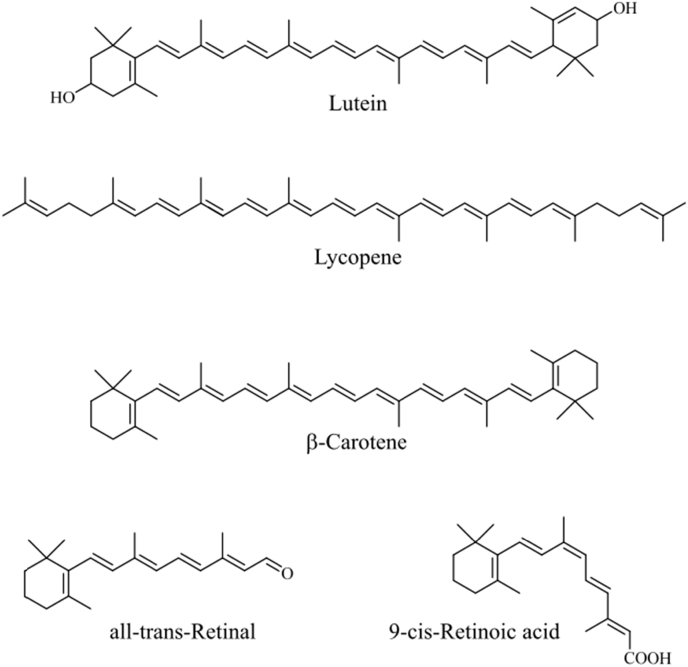
Tissue carotenoids are robust biomarkers of dietary exposure, occurring as natural lipophilic yellow-orange pigments present in various organisms, such as plants, animals, and microorganisms. The orange color of carrots and the red color of tomatoes are due to their carotenoid components. Plant, algae, and fungi produce >600 different types of carotenoids, while animals obtain carotenoids from food since they cannot synthesize them. About 40 different carotenoids are found in human organisms with α- and β-carotene, β-cryptoxanthin, lycopene, lutein, zeaxanthin the most prominent (Fig. 1). In addition to dietary supply, carotenoid bioavailability, metabolism and distribution depends on individual host factors including diseases, lifestyle, sex, age, and genetic makeup [28]. Together these factors determine an individual carotenoid pattern in the human blood and tissues and influence the generation of carotenoid metabolites and their distribution.
Fig. 1.
Chemical structures of selected carotenoids and retinoids.
Consumption and levels of circulating carotenoids have been linked to health effects in context with several age-related diseases as well as to cognitive and physical performance [29]. While several mechanisms of action have to be disclosed yet, the biochemistry of carotenoids is important to understand their beneficial properties.
Biological properties of carotenoids are closely related to their unique structure which also determines their physicochemical characteristics. Carotenoids usually consist of 8 isoprenoid and thus contain 40 carbon atoms. Most of them are composed of a central chain with conjugated double bonds substituted with various cyclic and acyclic substituents [30]. The polyene chain is responsible for the color of the compounds and determines their antioxidant activity. Double bonds of carotenoids may occur in the cis/trans (E/Z) isomeric form. However, of the numerous possible isomers the all-trans configuration, selected mono and poly-cis isomers are preferentially formed. Carotenoids which are composed only of carbon and hydrogen atoms are very lipophilic and tend to accumulate in lipophilic surroundings like membranes or lipoproteins. Xanthophylls, a subgroup of carotenoids which carry at least one oxygen atom are somewhat less lipophilic and the functional residue has impact on antioxidant activity, biochemical effects and spatial orientation. In membranes xanthophylls like lutein can basically take two different orientations. When the OH-groups are located at opposite hydrophilic surfaces they span the double layer [31]. When both OH-groups are fixed at the same hydrophilic surface the orientation of the xanthophyll is perpendicular to hydrophobic core. Carotenoids also tend to aggregate in membranes. Orientation and aggregation of carotenoids in biological membranes has impact on membrane fluidity, stability, permeability, and its biological function as a signaling platform; e.g. formation of lipid rafts. It has been suggested that specific xanthophyll-membrane interactions are relevant for proper function of neural membranes which might explain the selective occurrence of lutein and zeaxanthin in brain and retina [32].
3.1. Carotenoids as antioxidants
Experimental data have proven that carotenoids are efficient lipophilic antioxidants in vivo [33]. Upon exposure of polyunsaturated acids to free radicals like the hydroxyl or hydrogen radical, lipid peroxidation is induced and a number of secondary reactive oxygen species such as lipid hydroperoxides, cyclic peroxides or peroxyl radicals are generated. In further reaction sequences reactive aldehydes are formed. Lipid peroxidation and chemical reactions of peroxidation products impair membrane properties and interfere with the proper function of relevant biomolecules, mainly proteins. Carotenoids exhibit antioxidant properties that may interfere with lipid peroxidation. Although the basic chemistry of carotenoid radical interaction is not completely understood, radical scavenging usually involves the transfer of a hydrogen atom or electron, yielding a carotenoid radical or carotenoid radical ions, respectively.
Carotenoid radicals are thought to be stabilized via electron delocalisation over the entire system of π-bonds. In contrast to other antioxidants, carotenoids are destroyed in this process and cannot be regenerated like vitamin E. The contribution of carotenoid-dependent radical scavenging to entire in-vivo antioxidant network is still a matter of debate but also indirect effects of carotenoids linked to activation of antioxidant defense enzymes are likely important [34]. There is evidence that carotenoids act as prooxidants under specific conditions and then contribute to an oxidative load. Antioxidant versus prooxidant behaviour depends on the concentration of the carotenoid itself, oxygen tension and chemical surroundings. It has been postulated that either too low or too high concentrations of the compounds are unfavourable. Thus, benefits and risks have been related to the intake of carotenoids with both anti- and prooxidative properties [35] and optimal carotenoid levels are required for antioxidant activity in cells and tissues.
Carotenoids with an extended system of conjugated double bonds are very efficient quenchers of singlet molecular oxygen and excited triplet state molecules [33]. They are part of the light-harvesting complex in plants and among other tasks responsible for the prevention oxidative damage due to light induced formation of reactive oxygen species. Upon physical quenching of excited state compounds carotenoids remain intact and undergo several cycles before they are destroyed in chemical reactions. Singlet oxygen is also generated in the human organism especially in light exposed tissue when suitable sensitizers are present. However, also the light-independent chemical generation of singlet oxygen has been described [36].
Absorption of visible light in the range of about 450 nm is a typical for carotenoids with nine conjugated double bonds. The retinal xanthophylls lutein and zeaxanthin are selectively taken up and comprise the major macular pigments. Due to their absorption properties they are efficient filters for high-intensity, short-wavelength visible light which adds to their antioxidant function and contributes to the protection against light-induced oxidative stress [37]. Lutein and zeaxanthin are suggested to play a role in the prevention of age-related macular degeneration [37].
3.2. Vitamin A and carotenoid metabolites
Carotenoids which carry at least one beta ionone ring are precursors of vitamin A and considerably contribute to human vitamin A supply. Due to its structure, β-carotene is the only carotenoid which can be cleaved to yield two molecules of all-trans-retinal [38]. Cleavage of the central double bond is catalysed by the enzyme β-carotene-15, 15'-oxygenase (BCO1), which also takes α-carotene, lycopene and some apo-carotenals as substrates [39]. Recently, it has been confirmed that the enzymatic activity of β-carotene-oxygenase 2 (BCO2), which converts carotenoids into more polar metabolites, is conserved in humans. This enzyme is expressed at high levels in human retinas and plays a role in controlling carotenoid homeostasis. Its activity may help to explain why the apocarotenoid substrates e.g. xeaxanthin, achieve a steady state level after supplementation and it provides a mechanism for the catabolism of oxidized carotenoid metabolites through asymmetric cleavage by the generating β-ionone and β-apo-10′-carotenal ([40] JBC 295, 15553). BCO2 was also effective in protecting human cell lines from oxidative stress induced by carotenoids that have been associated with adverse health effects in animal models and their offspring.
Retinal is the key molecule in the vision process but also further metabolized to retinoic acid which in its all-trans and 9-cis configuration binds as a ligand to the retinoic acid receptor (RAR) family. To form a functional transcription factor the RAR must dimerize with the retinoic X receptor (RXR) which is activated by the ligand 9-cis retinoic acid. RAR/RXR dimer control the transcription of genes associated with cell differentiation, proliferation, apoptosis, or embryonic development. Apart from the RAR a number of other nuclear receptors form hetero dimers with RXR for activation. Among them are peroxisome proliferator-activated receptors (PPARs), liver X receptors (LXRs), the farnesoid X receptor (FXR), the pregnane X receptor (PXR) and the constitutive androstan receptor (CAR) which play important roles in the regulation of growth and adaptation of energy metabolism as well as inflammation and inflammaging [41]. Thus, RXR is a key player in this regulatory machinery and it has been hypothesized that controlling the activity of RXR is an attractive approach to address cellular functions which are affected in diseases such as cancer, diabetes, Alzheimer's disease or Parkinson's disease [42]. Although the role of 9-cis retinoic acid as natural ligand of the RXR is still not completely understood, tight regulation of its cellular levels via control of retinoic acid synthesis and metabolism of carotenoid precursors are mandatory. Inadequate levels of this regulatory molecule likely disturb important cellular signalling pathways. Thus, the fate of the 9-cis β-carotene geometrical isomers is of considerable interest. Although our knowledge on factors that influence the metabolism of β-carotene and its geometrical isomers in vivo is limited, shifts in carotenoid levels or the carotenoid pattern might have impact and influence the response of the organism.
Retinoids and their parent carotenoids have also been shown to stimulate gap junctional intercellular communication (GJIC) which has been discussed as a possible biochemical mechanisms underlying the cancer-preventive properties of these compounds [43]. Among the major dietary carotenoids β-carotene, cryptoxanthine, zeaxanthin and lutein efficiently induce GJIC.
Under prooxidant conditions carotenoid epoxides and cyclic peroxides are generated which are chemically unstable and cleaved in subsequent reactions. Major decomposition products from carotenoids under oxidative conditions are ketones and aldehydes and it has been speculated that reactive cleavage products modify proteins via Schiff-base or Michael-type reactions. Thus, not only direct but also indirect prooxidant damage may occur. Cleavage products obtained in enzyme-mediated metabolic processes like apo-carotenals retain the structure of biologically active signalling molecules and may trigger signaling pathways [44]. Chemical modification of thiol groups in regulatory molecules triggers further transcription systems, such as the electrophile-antioxidant response element pathway and nuclear factor-κB [45]. The complex interaction of carotenoids and their metabolites with cellular targets involved in adipose tissue biology has recently been reviewed [46].
3.3. Carotenoids and cognition
Carotenoids are differentially distributed in various organs of the human body. Interestingly, xanthophylls account for 66–77% of the total carotenoids in the frontal and occipital lobes of the human brain, whereas less than 40% of the total carotenoids in most tissues and plasma are reported to be xanthophylls [29]. Among the carotenoids, lutein and zeaxanthin are the only two that cross the blood-retina barrier to form macular pigment in the eye [37] and lutein is the dominant carotenoid in human brain tissue [37,47]. Lutein is the major carotenoid in brain tissue despite not being the major carotenoid in matched serum, indicating the preferential uptake into brain tissue [48]. Lutein and zeaxanthin in macula were found to be significantly correlated with their levels in matched brain tissue [49], Macular pigment can be therefore used as a biomarker in brain tissue, and in fact a significant correlation was found between macular pigment density and global cognitive function in healthy older adults [50,51]. Examination of a relationship between cognition and lutein levels in brain tissue of decedents from a population-based study of adults found that lutein was consistently associated with a wide range of cognitive measures that included executive function, language, learning, and memory, which are all associated with specific brain regions [47]. Similarly, a relationship was recently found between levels serum and brain levels of carotenoids (lutein, zeaxanthin, cryptoxanthin, β-carotene), α-, γ-tocopherols, total n-3 polyunsaturated fatty acids (PUFAs), and n-6/n-3 PUFA in participants in the Georgia Centenarian Study [52]. As no significant relationship was identified between serum and brain retinol, total saturated fatty acid, total monounsaturated fatty acid, and trans-fatty acid levels, the authors concluded that serum carotenoids, tocopherols, total n-3 PUFAs, and n-6/n-3 PUFA ratio reflect levels in brain and might be used as surrogate biomarkers in older persons. Others have shown that chronic administration of the oxocarotenoid lycopene significantly restores the mitochondrial respiratory enzyme activities in Aβ42 treated rats and attenuated mitochondrial oxidative stress [53], suggesting a role for carotenoids in maintaining mitochondrial integrity in the brain.
In line with these findings, we showed that selected tocopherols and carotenoids are associated to global cognition measures and biomarkers of oxidative stress in healthy persons independent of age, sex and fruit/vegetable intake, suggesting a protective role of these substances even in the absence of disease [54,55]. More recently, longitudinal data from 1251 participants in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study (Age at visit 1 in 2004–2009 (v1): 30–65 years) showed an interaction between total (and individual) carotenoids for three of 11 cognitive tests at v1, with only one meeting the statistical significance upon multiple testing correction whereby vitamin E was linked with greater verbal memory performance in the uppermost total carotenoid tertile (p = 0.002), a synergism largely driven by carotenoid lycopene [56]. In another study, among 927 participants from the Rush Memory and Aging Project free from AD at baseline and followed up for a mean of 7 y, a higher intake of total carotenoids was associated with substantially lower hazard of AD after controlling for age, sex, education, ApoE-ε4, participation in cognitively stimulating activities, and physical activity level [57]. In this study, among the deceased participants, consumers of higher total carotenoids had less global AD pathology (b: -0.10; SE = 0.04; P-trend = 0.01). While lutein-zeaxanthin and lycopene were inversely associated with brain global pathology, lutein-zeaxanthin showed inverse associations also with neuritic plaque severity as well as neurofibrillary tangle density and severity [57]; the authors concluded that the possible beneficial role of total carotenoid consumption, in particular lutein/zeaxanthin, on AD incidence may be related to the inhibition of brain β-amyloid deposition and fibril formation.
The systemic metabolic deficit that frequently accompanies the development of dementia has also been explored in respect to circulating carotenoid concentrations. Consistently, generic markers of lipid peroxidation are increased and carotenoids are depleted in AD serum. Our own work has shown that lutein, lycopene, and zeaxanthin concentrations were significantly lower in AD patients with vascular co-morbidities compared to healthy subjects [58,59].
A comparison of serum lipid oxidation between AD and age-matched control subjects before and after six-month carotenoid supplementation showed higher serum levels of the novel oxidized phospholipid biomarker 1-palmitoyl-2-(5′-oxo-valeroyl)-sn-glycero-3-phosphocholine (POVPC) analysed using electrospray ionisation tandem mass spectrometry (MS) with multiple reaction monitoring (MRM) compared to age-matched controls, (p = 0.017). Cognitive function was correlated inversely with POVPC (r = −0.37; p = 0.04). After six months of carotenoid intervention, serum POVPC was not different in AD patients compared to healthy controls. However, POVPC was significantly higher in control subjects after six months of carotenoid intervention compared to their baseline (p = 0.03) [60]. In agreement with this, 20 μM POVPC was recently shown to induce loss of GSH and a mitochondrial bioenergetic deficit in neuronal cells that was not mitigated by oxocarotenoids [61]. In this study, since oxocarotenoids are normally metabolized in mitochondria, we investigated whether non-toxic POVPC concentrations impair mitochondrial metabolism in differentiated (d)SH-SY5Y neuronal cells and whether there is any protective role for oxocarotenoids against mitochondrial dysfunction. Following delivery of lutein (0.1–1 μM) and zeaxanthin (0.5–5 μM) over 24 h in vitro, the increase in mitochondrial ROS production induced by POVPC was prevented. We observed that carotenoids protected against uncoupling although did not restore ATP production that was induced by high concentrations of POVPC [61].
The above cited association of circulating carotenoids with cognitive performance in healthy persons [55] and AD patients with and without vascular comorbidities [56,58,59,62], therefore, appears to be at least in part mediated by protective effects exerted by carotenoids against lipid peroxidation [60,61]. Together with these observations and with the report of a preferential uptake of specific carotenoids in the central nervous system [32,37,44,[47], [48], [49], [50], [51], [52], [53]], the association between carotenoid intake and dementia risk prior to AD onset [57] strongly supports a physiological protective role of carotenoids in the brain beyond the expected function of nutritional indicator. Indeed, our own research showed that plasma concentrations of lutein, zeaxanthin and α-carotene are similarly depleted in patients with AD and with MCI, considered the prodromic phase of AD [63], pointing at carotenoid deficiency not simply as an epiphenomenon of advanced neurodegeneration.
Carotenoids appear therefore to possess a privileged position among micronutrients as far as central nervous system pathophysiology and protection against dementia are concerned, and recent studies deeper explored their role in this sense. Among the up to 331 candidate (bio)markers investigated in the MARK-Age study in 2220 randomly recruited age-stratified persons, lower levels of β-cryptoxanthin and zeaxanthin were found, in those who were physically, cognitively or psychologically frail [64]. In this study, after adjustment for confounders, levels of these carotenoids were inversely associated with the risk of being affected by cognitive frailty, i.e. when scoring below the 10th percentile on global cognitive functioning. Frailty, defined as a state of increased vulnerability of the aging body due to diminished homeostatic reserves and resistance to cope with endogenous stressors [65], is in the meanwhile recognized as a multidimensional, dynamic condition [66,67]. Although frailty is strongly associated with adverse clinical outcomes and is therefore associated with a high individual and socioeconomic burden [66,67], it is not systematically diagnosed in clinical routine and therefore no prevention and therapy is structurally implemented. Due to the recent advances in the pathophysiology and molecular biology of frailty, however, carotenoids are recognized as relevant biomarkers of frailty, also in the frame of anorexia of aging and as indicators of inflammation and oxidative stress involvement [66,67]. Nutritional interventions, along with physical exercise and other lifestyle strategies, are highly recommended to restore robustness [66,67]. In line with this evidence, recently, of the 121 patients with MCI included in the NeuroExercise study at the German Sports University in Cologne, Germany, 56 had the full dataset including neuropsychological assessment, physical fitness analysis as well as plasma levels of micronutrients including retinol, six carotenoids and two tocopherols [68]. Significant correlations independently of fruit and vegetable intake were found between plasma levels of β-cryptoxanthin and physical performance measured by the Timed Up-and-Go Test (p < 0.05), γ-tocopherol and number of daily steps (p < 0.01), as well as four out of six measured carotenoids – lutein, zeaxanthin, β-cryptoxanthin and β-carotene with cognitive performance measured by means of the International Shopping List Test (p < 0.01) [68]. In this study, plasma concentrations of several carotenoids were strongly correlated with tasks of the CogState battery, a valid gamified computerized testing method sensitively and reliably measuring verbal learning and memory [69]. Interestingly, a significant association was previously found in persons with subjective cognitive impairment (SCI) between the ISLT and the endothelial peripheral arterial tonometry index (EndoPAT Index), a measure of endothelial function [70], considered an important mediator of cognitive impairment [[6], [7], [8]]. Interestingly, in this study, we found that plasma levels of β-cryptoxanthin were also correlated with the TUG test, a marker of balance and increased fall risk and of physical frailty [71].
In spite of this convincing evidence for a role of carotenoids for brain and cognitive integrity, intervention studies with single carotenoid compounds against onset and progression of cognitive impairment have yield conflicting results [[72], [73], [74], [75]]. This is largely due to the above mentioned multifactoriality of the ageing process [2,22,23] as well as to considerable methodological drawbacks of intervention studies, including, among others, use of single compounds in heterogeneous populations, different time windows and length of the application, different doses, and dishomogeneous inclusion criteria [75,76]. One important issue is certainly the timeframe in which antioxidant supplementation begins, in fact, as dementia's symptomes become overt years after begin of the neuropathologic alterations, early administration, at the latest during the SCI, appears a very meaningful strategy. In 59 healthy persons between 18 and 25 years participating in a 6-month, double-blind, placebo-controlled trial to evaluate the effects of xantophyll supplementation (13 mg or 27 mg/day) on cognitive performance, scores for composite memory, verbal memory, sustained attention, psychomotor speed, and processing speed all improved significantly in the treated compared to the placebo group [77]. In this study, change in serum lutein was found to be significantly correlated to change in verbal memory, composite memory, and sustained attention, while change in serum zeaxanthin isomers was significantly correlated with change in verbal memory. For these findings and the significant relationship observed between change in BDNF and IL-1β over the course of the study, the authors concluded that regular consumption of xantophylls may interrupt inflammatory processes and enhance cognition [77]. In line with the benefit of early administration during the course of life and of cognitive decline, recently a composite supplement containing food-derived astaxanthin and sesamin or placebo was administered to 21 MCI patients [78]. In this RCT, treated patients showed higher psychomotor speed and processing speed compared to the placebo group [78]. These beneficial effects on cognitive performance were confirmed in further investigations and randomized controlled trials in older persons. In a double-masked, randomized, placebo-controlled trial in 51 older adults randomized into groups receiving either 12 mg lutein and zeaxanthin or placebo, participants receiving the active supplement but not controls had statistically significant increases in macular pigment optical density as well as improvements in complex attention, cognitive flexibility and composite memory [79]. These and other investigations also showing effects on brain regions by functional imaging [[80], [81], [82]] demonstrate the special role that some carotenoids may play with respect to other micronutrients in cognitive performance and warrant a great deal of attention as far as preventive and therapeutic measures are concerned.
4. Conclusions and research outlook
A PubMed search on „carotenoids and cognition“ on December 30th, 2020, shows that 1 to 7 papers per year where published between 1977 and 2007, while 60 articles were published on the topic only in 2019. This increase of attention is largely due to the demographic explosion and related growing prevalence of cognitive decline [3,23] increasing awareness on a major public health priority of our time. A fundamental factor paving the way to the consolidation of the field of nutritional cognitive neuroscience of aging, however, is constituted by rapid advances in the research on lipophilic micronutrients. In particular, carotenoids exert biological activities of special importance for the optimal function of the brain and maintenancy of robustness and healthy aging [83]. To overcome recurrent inconsistencies across within-model observational and pathophysiological investigations (in vitro, animal models, humans) as well as across intervention studies, a number of actions should be taken (see also Weber et al., Redox biomarkers in dietary interventions and nutritional observation studies - From new insights to old problems, this issue). First of all, studies on micronutrients, nutrition and lifestyle should take into account the multifactoriality of aging, nutrigenetics, geographical, social and cultural aspects. Secondly, novel multidimensional and personalized designs as well as innovative technology including artificial intelligence and deep learning should be considered whenever possible in human studies to help disentangling the multifactoriality of aging. Furthermore, upcoming studies on the effects of carotenoid supplementation should take into account the multiple interactions with other (micro)nutrients and involve a broader spectrum of robust biomarkers of both antioxidant status and free radical-induced damage. Finally, to ensure a correct interpretability of findings, a rigorous clinical characterization of study participants, including demographics, frailty status, comorbidities, habits, and laboratory values should always be performed.
Declaration of competing interest
No conflict of interest.
Acknowledgements
Work of WS was partially funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project STA699/3-1.
References
- 1.Zamroziewicz M.K., Barbey A.K. Nutritional cognitive neuroscience: innovations for healthy brain aging. Front. Neurosci. 2016;10:240. doi: 10.3389/fnins.2016.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polidori M.C., Section . Ageing Medicine and Geriatrics. In: Gu D., Dupre M., editors. Springer; 2021. (Encyclopedia of Gerontology and Population Aging). [Google Scholar]
- 3.Polidori M.C. Dementia. In: Rattan S., editor. Elsevier; 2019. (Encyclopedia of Biomedical Gerontology). [Google Scholar]
- 4.Petersen R.C. Aging, memory, and mild cognitive impairment. Int. Psychogeriatr. 1997;9(Suppl 1):65–69. doi: 10.1017/s1041610297004717. [DOI] [PubMed] [Google Scholar]
- 5.Reisberg B., Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer's disease. Int. Psychogeriatr. 2018;20:1–16. doi: 10.1017/S1041610207006412. [DOI] [PubMed] [Google Scholar]
- 6.Polidori M.C., Pientka L., Mecocci P. A review of the major vascular risk factors related to Alzheimer's disease. J. Alzheimers Dis. 2012;32:521–530. doi: 10.3233/JAD-2012-120871. [DOI] [PubMed] [Google Scholar]
- 7.Polidori M.C., Schulz R.J. Nutritional contributions to dementia prevention: main issues on antioxidant micronutrients. Genes Nutr. 2014;9:382. doi: 10.1007/s12263-013-0382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polidori M.C., Pientka L. Bridging the pathophysiology of Alzheimer's disease with vascular pathology: the feed-back, the feed-forward, and oxidative stress. J. Alzheimers Dis. 2012;28:1–9. doi: 10.3233/JAD-2011-111034. [DOI] [PubMed] [Google Scholar]
- 9.Mangialasche F., Polidori M.C., Section . Ageing medicine and geriatrics. In: Gu D., Dupre M., editors. Prevention of Age-Related Cognitive Impairment, Alzheimer's Disease, and Dementia Encyclopedia of Gerontology and Population Aging. Springer; 2021. [DOI] [Google Scholar]
- 10.Kivipelto M. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018;14(11):653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 11.Fanò G. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J. Muscle Res. Cell Motil. 2001;22:345–351. doi: 10.1023/a:1013122805060. [DOI] [PubMed] [Google Scholar]
- 12.Mecocci P. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic. Biol. Med. 2000;28:1243–1248. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]
- 13.Polidori M.C. Different antioxidant profiles in Italian centenarians: the Sardinian peculiarity. Eur. J. Clin. Nutr. 2007;61:922–924. doi: 10.1038/sj.ejcn.1602596. [DOI] [PubMed] [Google Scholar]
- 14.Dias I.H.K. Inflammation, lipid (Per)oxidation, and redox regulation. Antioxidants Redox Signal. 2020;33(3):166–190. doi: 10.1089/ars.2020.8022. [DOI] [PubMed] [Google Scholar]
- 15.Dias I.H.K. Oxidized LDL lipids increase β-amyloid production by SH-SY5Y cells through glutathione depletion and lipid raft formation. Free Radic. Biol. Med. 2014;75:48–59. doi: 10.1016/j.freeradbiomed.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L. Oxidative LDL modification is increased in vascular dementia and is inversely associated with cognitive performance. Free Radic. Res. 2010;44:241–248. doi: 10.3109/10715760903440153. [DOI] [PubMed] [Google Scholar]
- 17.Mecocci P. A long journey into aging, brain aging, and alzheimer's disease following the oxidative stress tracks. J. Alzheimers Dis. 2018;62:1319–1335. doi: 10.3233/JAD-170732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polidori M.C. Hallmarks of protein oxidative damage in neurodegenerative diseases: focus on Alzheimer's disease. Amino Acids. 2007;32:553–559. doi: 10.1007/s00726-006-0431-x. [DOI] [PubMed] [Google Scholar]
- 19.Polidori M.C. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: relevance to Alzheimer disease and vascular dementia. Dement. Geriatr. Cognit. Disord. 2004;18:265–270. doi: 10.1159/000080027. [DOI] [PubMed] [Google Scholar]
- 20.Sies H., Jones D.P. Reactive oxygen species as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 21.Sierra F. Geroscience and the role of aging in the etiology and management of Alzheimer's disease. J. Prev. Alzheimers Dis. 2020;7:2–3. doi: 10.14283/jpad.2019.49. [DOI] [PubMed] [Google Scholar]
- 22.Polidori M.C. Physiology of Aging as Basis of Complexity in Ageing Medicine and Geriatrics. In: Gu D., Dupre M., editors. Springer; 2021. (Encyclopedia of Population Aging and Geriatrics). [Google Scholar]
- 23.Polidori M.C. Cognitive Decline. In: Roller-Wirnsberger R.E., Singler K., Polidori M.C., editors. Springer; 2018. (Learning Geriatric Medicine). [Google Scholar]
- 24.Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepka K. Iron-sulfur glutaredoxin 2 protects oligodendrocytes against damage induced by nitric oxide release from activated microglia. Glia. 2017;65:1521–1534. doi: 10.1002/glia.23178. [DOI] [PubMed] [Google Scholar]
- 26.Griffiths H.R. Oxidised LDL lipids, statins and a blood-brain barrier. Free Radic. Biol. Med. 2014;75(Suppl 1):S15–S16. doi: 10.1016/j.freeradbiomed.2014.10.591. [DOI] [PubMed] [Google Scholar]
- 27.Dias I.H.K. Hypercholesterolaemia-induced oxidative stress at the blood-brain barrier. Biochem. Soc. Trans. 2014;42(4):1001–1005. doi: 10.1042/BST20140164. [DOI] [PubMed] [Google Scholar]
- 28.Bohn T. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017:61. doi: 10.1002/mnfr.201600685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polidori M.C., Stahl W. Biological Activity of Carotenoids: Implications for Cognitive Decline. In: Martin C., Preedy V., editors. Academic Press; Cambridge, MA, USA: 2014. (Diet and Nutrition in Dementia and Cognitive Decline). [Google Scholar]
- 30.Britton G. Carotenoid research history and new perspectives for chemistry in biological systems. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865 doi: 10.1016/j.bbalip.2020.158699. 158699. [DOI] [PubMed] [Google Scholar]
- 31.Gruszecki T., Strzałka K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta. 2005;1740:108–115. doi: 10.1016/j.bbadis.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Widomska A. Can xanthophyll-membrane interactions explain their selective presence in the retina and brain? Foods. 2016:5. doi: 10.3390/foods5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edge R., Truscott T.G. Singlet oxygen and free radical reactions of retinoids and carotenoids—a review. Antioxidants. 2018;7 doi: 10.3390/antiox7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohn T. Carotenoids and markers of oxidative stress in human observational studies and intervention trials: implications for chronic diseases. Antioxidants. 2019;8 doi: 10.3390/antiox8060179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Black F. vol. 9. 2020. (The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-oxidative Mechanisms-A Comprehensive Review Antioxidants). Basel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto S. Singlet molecular oxygen generated by biological hydroperoxides. J. Photochem. Photobiol., B. 2014;139:24–33. doi: 10.1016/j.jphotobiol.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 37.Arunkumar R. The macular carotenoids: a biochemical overview. BBA. 2020;1865 doi: 10.1016/j.bbalip.2020.158617. 158617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grune T. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010;140:2268S–2285S. doi: 10.3945/jn.109.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison R.E. Kopec, Enzymology of vertebrate carotenoid oxygenases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865 doi: 10.1016/j.bbalip.2020.158653. 158653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linda D, Thomas, Sepalika Bandara, VipulkumarParmar M, Ramkumar Srinivasagan, Nimesh Khadka, Marcin Golczak, PhilipKiser D, Johannes von Lintig The human mitochondrial enzyme BCO2 exhibits catalytic activity toward carotenoids and apocarotenoids. J Biol Chem. 2020;13295Nov):4615553–4615565. doi: 10.1074/jbc.RA120.015515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brtko Z. Dvorak. Natural and synthetic retinoid X receptor ligands and their role in selected nuclear receptor action. Biochimie. 2020;179:157–168. doi: 10.1016/j.biochi.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 42.de Almeida R.N., Conda-Sheridan M. A review of the molecular design and biological activities of RXR agonists. Med. Res. Rev. 2019;39:1372–1397. doi: 10.1002/med.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stahl W., Ale-Agha N., Polidori M.C. Non-antioxidant properties of carotenoids. Biol. Chem. 2002;383:553–558. doi: 10.1515/BC.2002.056. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Concepcion M. A global perspective on carotenoids: metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018:70. doi: 10.1016/j.plipres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Sharoni K. Carotenoids and apocarotenoids in cellular signaling related to cancer: a review. Mol. Nutr. Food Res. 2012;56:259–269. doi: 10.1002/mnfr.201100311. [DOI] [PubMed] [Google Scholar]
- 46.Bonet J. Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: pre-clinical and human studies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865 doi: 10.1016/j.bbalip.2020.158676. 158676. [DOI] [PubMed] [Google Scholar]
- 47.Craft N.E. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J. Nutr. Health Aging. 2004;8:156–162. [PubMed] [Google Scholar]
- 48.Johnson E.J. Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J. Aging Res. 2013;2013 doi: 10.1155/2013/951786. 951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vishwanathan R. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr. Neurosci. 2013;16:21–29. doi: 10.1179/1476830512Y.0000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vishwanathan R. Macular pigment optical density is related to cognitive function in the elderly. Age Ageing. 2013;43:271–275. doi: 10.1093/ageing/aft210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feeney J. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol. Aging. 2013;34:2449–2456. doi: 10.1016/j.neurobiolaging.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Tanprasertsuk J. Serum carotenoids, tocopherols, total n-3 polyunsaturated fatty acids, and n-6/n-3 polyunsaturated fatty acid ratio reflect brain concentrations in a cohort of centenarians. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:306–314. doi: 10.1093/gerona/gly125. [DOI] [PubMed] [Google Scholar]
- 53.Kelly D. Cognitive function and its relationship with macular pigment optical density and serum concentrations of its constituent carotenoids. J. Alzheimers. Dis. 2015;48:261–277. doi: 10.3233/JAD-150199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polidori M.C. Plasma micronutrient status is improved after a 3-month dietary intervention with 5 daily portions of fruits and vegetables: implications for optimal antioxidant levels. Nutr. J. 2009;8:10. doi: 10.1186/1475-2891-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polidori M.C. High fruit and vegetable intake is positively correlated with antioxidant status and cognitive performance in healthy subjects. J. Alzheimers Dis. 2009;17:921–927. doi: 10.3233/JAD-2009-1114. [DOI] [PubMed] [Google Scholar]
- 56.Beydoun M.A. Association of antioxidant vitamins A, C, E and carotenoids with cognitive performance over time: a cohort study of middle-aged adults. Nutrients. 2020;12:3558. doi: 10.3390/nu12113558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan C. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: a community-based cohort of older adults. Am. J. Clin. Nutr. 2020;113:200–208. doi: 10.1093/ajcn/nqaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polidori M.C. Influence of vascular comorbidities on the antioxidant defense system in Alzheimer's disease. Dtsch. Med. Wochenschr. 2012;137:305–308. doi: 10.1055/s-0031-1298883. [DOI] [PubMed] [Google Scholar]
- 59.Dias I.H.K. Plasma levels of HDL and carotenoids are lower in dementia patients with vascular comorbidities. J. Alzheimers Dis. 2014;40:399–408. doi: 10.3233/JAD-131964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ademowo O.S. Phospholipid oxidation and carotenoid supplementation in Alzheimer's disease patients. Free Radic. Biol. Med. 2017;108:77–85. doi: 10.1016/j.freeradbiomed.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ademowo O.S. Partial mitigation of oxidized phospholipid-mediated mitochondrial dysfunction in neuronal cellas by oxocarotenoids. J. Alzheimers Dis. 2020;74:113–126. doi: 10.3233/JAD-190923. [DOI] [PubMed] [Google Scholar]
- 62.Polidori M.C. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: relevance to Alzheimer disease and vascular dementia. Dement. Geriatr. Cognit. Disord. 2004;18:265–270. doi: 10.1159/000080027. [DOI] [PubMed] [Google Scholar]
- 63.Rinaldi P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer's disease. Neurobiol. Aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 64.Rietman M.L. Antioxidants linked with physical, cognitive and psychological frailty: analysis of candidate biomarkers and markers derived from the MARK-AGE study. Mech. Ageing Dev. 2019;177:135–143. doi: 10.1016/j.mad.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Ferrucci L. Frailty. In: Halter J.B., editor. MacGraw Hill; 2017. (Hazzard's Geriatric Medicine and Gerontology, 7e). [Google Scholar]
- 66.Dent E. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 67.Hoogendijk E.O. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 68.Gerger P. Associations of lipophilic micronutrients with physical and cognitive fitness in persons with mild cognitive impairment. Nutrients. 2019;11:902. doi: 10.3390/nu11040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson T.A. Sensitivity and test–retest reliability of the international shopping list test in assessing verbal learning and memory in mild Alzheimer's disease. Arch. Clin. Neuropsychol. 2011;26:412–424. doi: 10.1093/arclin/acr039. [DOI] [PubMed] [Google Scholar]
- 70.Nelles G. Endothelium-mediated changes in vascular tone and cognitive function in patients with subjective cognitive impairment: a pilot study. Neurology. 2015;84:14S. [Google Scholar]
- 71.Ansai J.H. Performance of different timed up-and-go subtasks in frailty syndrome. J. Geriatr. Phys. Ther. 2019;42:287–293. doi: 10.1519/JPT.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 72.Polidori M.C. Preventive benefits of natural nutrition and lifestyle counseling against Alzheimer's disease onset. J. Alzheimers Dis. 2014;42:S475–S482. doi: 10.3233/JAD-141539. [DOI] [PubMed] [Google Scholar]
- 73.Polidori M.C. Conflict of evidence: carotenoids and other micronutrients in the prevention and treatment of cognitive impairment. Biofactors. 2012;38:167–171. doi: 10.1002/biof.1001. [DOI] [PubMed] [Google Scholar]
- 74.Rutjes A.W. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Libr. Syst. 2018;12 doi: 10.1002/14651858.CD011906.pub2. CD011906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang F.F. Health effects of vitamin and mineral supplements. BMJ. 2020;369 doi: 10.1136/bmj.m2511. m2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mecocci P., Polidori M.C. Antioxidant clinical trials in mild cognitive impairment and Alzheimer's disease. Biochim. Biophys. Acta. 2012;1822:631–638. doi: 10.1016/j.bbadis.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 77.Stringham N.T. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol. Behav. 2019;211 doi: 10.1016/j.physbeh.2019.112650. 112650. [DOI] [PubMed] [Google Scholar]
- 78.Ito N., Saito H., Seki S., Ueda F., Asada T. Effects of composite supplement containing astaxanthin and sesamin on cognitive functions in people with mild cognitive impairment: a randomized, double-blind, placebo-controlled trial. J. Alzheimers Dis. 2018;62:1767–1775. doi: 10.3233/JAD-170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hammond B.R., Miller L.S., Bello M.O., Lindbergh C.A., Mewborn C.M. Effects of a lutein/zeaxanthin intervention on cognitive function: a randomized, double-masked, placebo- controlled trial of community dwelling older adults. Front. Aging Neurosci. 2017;9:1–9. doi: 10.3389/fnagi.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindbergh C.A., Renzi-Hammond L.M., Hammond B.R., Terry D.P., Mewborn C.M., Puente A.N., Miller L.S. Lutein and zeaxanthin influence brain function in older adults: a randomized controlled trial. J. Int. Neuropsychol. Soc. 2018;24(1):77–90. doi: 10.1017/S1355617717000534. [DOI] [PubMed] [Google Scholar]
- 81.Mewborn C.M., Lindbergh C.A., Robinson T.L., Gogniat M.A., Terry D.P., Jean K.R., Hammond B.R., Renzi-Hammond L.M., Miller S. Lutein and zeaxanthin are positively associated with visual-spatial functioning in older adults: an fMRI study. Nutrients. 2018;10(458):1–16. doi: 10.3390/nu10040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lindbergh C.A., Li J., Zhao Y., Mewborn C.M., Puente A.N., TerryRenzi-Hammond D.P.L.M., Hammond B.R., Liu T., Miller L.S. The effects of lutein and zeaxanthin on resting state functional connectivity in older Caucasian adults: a randomized controlled trial. Brain Imag. Behav. 2019:1–14. doi: 10.1007/s11682-018-00034-y. [DOI] [PubMed] [Google Scholar]
- 83.Bartali B., Semba R.D. Carotenoids and healthy aging: the fascination continues. Am. J. Clin. Nutr. 2021;113:259–260. doi: 10.1093/ajcn/nqaa364. [DOI] [PMC free article] [PubMed] [Google Scholar]