Abstract
Adults with HIV on therapy can live a normal lifespan but exhibit advanced ageing which includes reduced cardiorespiratory fitness. Our objective was to determine the feasibility and effects of high-intensity interval training (HIIT) combined with resistance training (RT) in older adults with HIV. We conducted a cross-over pilot study within a randomized exercise trial in sedentary adults with HIV ≥50 years of age. First, participants were randomized to 4 months of continuous high-intensity aerobic exercise (AEX) and RT 3x/week or standard of care control. Then, the control group completed 4 months of HIIT + RT (3x/week). Among the 32 individuals enrolled, 26 eligible participants were randomized. Most participants were African American (63%) and male (95%) with a mean (SD) age of 61.5 (6.7) years and VO2peak of 24.5 (4.9) ml/kg/min. Attendance and adherence to both exercise training interventions were high. The clinically significant increases in VO2peak (ml/kg/min) after HIIT (3.09 ±1.04, p=0.02) and AEX (2.09 ±0.72, p=0.01) represented improvements of 17.1% and 7.7%, respectively. Both groups had improvements in exercise endurance (time on the treadmill) and strength (all p< 0.01). This pilot study supports HIIT as an efficient means to deliver high-intensity AEX to improve cardiorespiratory fitness toward the goal of attenuating the accelerated ageing process in adults with HIV.
Keywords: high-intensity interval training (HIIT), Aerobic Exercise, Cardiorespiratory Fitness, HIV, Ageing
Introduction
Cardiorespiratory fitness (CRF) is a physiological biomarker of ageing that independently predicts cardiovascular and all-cause mortality (Sui et al., 2007; Wei et al., 1999). Adults living with HIV have significantly reduced CRF that exemplifies the advanced ageing phenotype observed in adults with chronic HIV infection (Oursler & Sorkin, 2016). The risk of cardiovascular disease (CVD) is higher in adults with HIV and is independent of traditional risk factors which is mediated in part by chronic systemic inflammation (Hsue et al., 2012). A recent randomized trial in younger adults with HIV shows that the positive effect of rosuvastatin on lipid and inflammatory profiles was amplified by the addition of aerobic exercise (AEX) and resistance training (RT) (Zanetti et al., 2020). High-intensity AEX has a greater impact on ageing-related processes, including loss of CRF, and therefore should be a priority in older adults with HIV (Balducci et al., 2010; Chodzko-Zajko et al., 2009). The combination with RT is important to ameliorate frailty and sarcopenia, also observed with the HIV advanced ageing phenotype (Hawkins et al., 2017). Our overall goal is to develop exercise strategies that minimize the advanced ageing effect of HIV.
Two recent exercise trials in older adults with HIV (≥50 years of age) demonstrate the feasibility and efficacy of high-intensity aerobic exercise (AEX) training to increase CRF (Erlandson et al., 2018; Oursler et al., 2018). Yet, durable strategies for exercise training in older adults remain a challenge with time and motivation as common obstacles (CDC, 2013). Additional barriers in those with HIV include HIV symptoms and antiretroviral medication effects (Montoya et al., 2019). High-intensity interval training (HIIT) is an AEX approach that consists of periods of high-intensity, anaerobic exercise (≥85% heart rate reserve) alternating with low-intensity AEX (Batacan et al., 2017). HIIT may offer an advantage for sustainable exercise programs given less time commitment with similar or greater gains in CRF compared with moderate-intensity continuous training (MICT) (Batacan et al., 2017). In older patient populations without HIV (e.g., diabetes, CVD), HIIT increases CRF and improves CVD risk factors (Campbell et al., 2019). Further, HIIT appears safe across a wide range of populations (Martland et al., 2020) and increases CRF across the age span of 20–70 years (Storen et al., 2017). However, experience with HIIT in adults living with HIV is limited despite the extensive number of AEX trials and remains untested in older persons (O’Brien et al., 2016). The objective of this study was to conduct a pilot of HIIT in older adults with HIV. We hypothesized that HIIT combined with RT would be feasible and would increase CRF in older adults with HIV.
Methods
Study design
As a pilot study, we used HIIT as the modality of AEX training for the delayed entry exercise group in a randomized trial of continuous progressive high-intensity AEX training and resistance training (RT) versus sedentary controls. Participants in the HIIT pilot represent those individuals who were initially randomized to the sedentary standard of care control group and completed follow-up testing. Then, in a cross-over design, these participants entered delayed exercise training of 4 months of HIIT and RT and completed post-exercise testing at 32 weeks (Figure 1). In this pilot trial of HIIT, we present results of HIIT + RT and high-intensity continuous AEX + RT.
Figure 1.
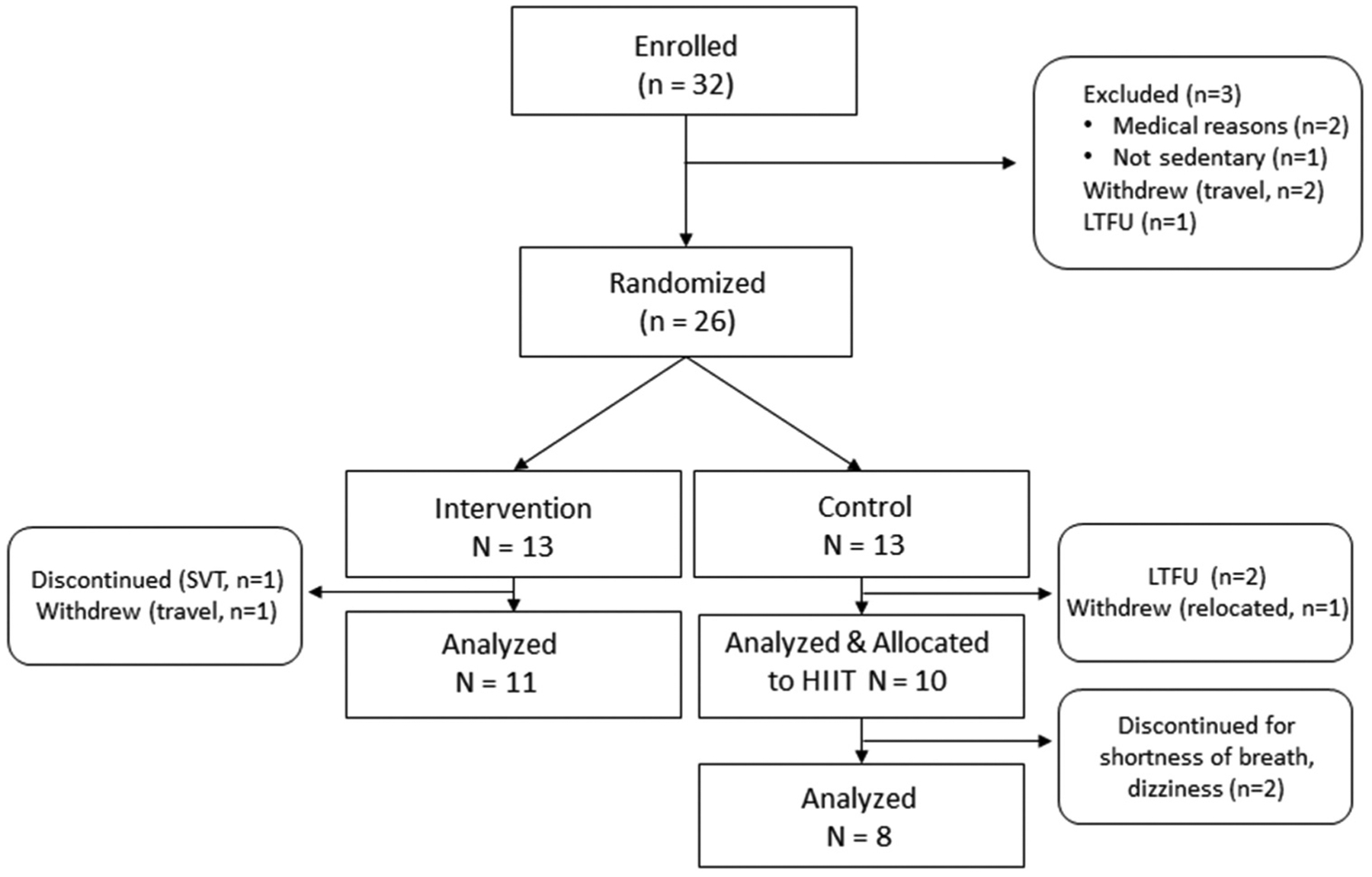
Participant flow diagram HIIT, high-intensity interval training; LTFU, loss to follow-up; SVT, supraventricular tachycardia.
Participants
Sedentary adults living with HIV infection who were ≥50 years of age and receiving antiretroviral therapy (ART) were eligible for the exercise trial. To minimize confounding by other physical activities, sedentary was defined as participating in no more than one structured physical activity session per week. Exclusion criteria were based on safety criteria for high-intensity AEX per the American College of Sports Medicine (ACSM) and included poorly controlled blood pressure, angina and claudication (American College of Sports Medicine, 2018). HIV specific eligibility criteria included a consistent ART regimen and absence of AIDS-defining conditions in the prior 6 months. Use of medication that affected heart rate (e.g., β-blockers) was also a criterion for exclusion to facilitate HR-based AEX training. Patients were recruited from local veteran and community-based clinics and provided written informed consent. The study was approved by the Salem VAMC Institutional Review Board and the Research and Development Committee and registered with clinicaltrials.gov (NCT02101060).
Outcomes
Data on all outcomes are presented before and after the exercise intervention. The primary outcome measure was cardiorespiratory fitness defined as the utilization of oxygen at peak exercise performance, VO2peak. Participants performed a graded exercise treadmill test (GXT) with a modified Bruce protocol while the gas exchange was measured with a True One 2400 Metabolic Measurement cart (Parvo Medics, Salt Lake City, UT). VO2peak was selected from the highest 10-s averaged value in the last minute of exercise. Exercise endurance was measured as the total duration of the GXT. The treadmill test was terminated when participants reached voluntary exhaustion or ACSM safety criteria. One participant had an initial treadmill test that met Minnesota Criteria for ischemia and was discontinued from the study (Figure 1).
Strength was measured by one-repetition maximum (1-RM) on selectorized resistance equipment using a standardized approach (American College of Sports Medicine, 2018). Participants were asked to lift the maximum weight possible using proper form after a warm-up of 6–10 submaximal repetitions. Participants were given 5 trials with 3 min of rest between each trial to arrive at 1-RM for each exercise, which included chest press, seated row, pull down, dual leg press, knee extension and knee flexion.
Dual-energy x-ray absorptiometry (DXA) (Hologic Horizon-A) was used to measure total and regional lean mass and fat mass after an overnight fast. Appendicular lean mass (ALM) and per cent body fat were calculated. Waist circumference, weight and height were measured using the protocol established in the Third National Health and Nutrition Examination Survey (National Health and Nutrition Examination Survey (NHANES) III, 1988).
Exercise training
Exercise training consisted of 48 sessions performed 3 times a week over 4 months at the Salem VA Medical Center under individual-level supervision by an exercise physiologist. Progressive continuous AEX training was performed using heart rate values from the treadmill test to calculate heart rate reserve (HRR = maximal HR−resting HR). Target heart rate (THR) for exercise sessions was calculated as (HRR × % intensity) + HR rest. In the AEX group, over the first 12 training sessions, participants started at 50–60% HRR for 15 min and were progressed until they reached at least 30 min at 60% HRR. Over the last 36 sessions, the intensity was increased as tolerated to 70–80% HRR, and duration was titrated to the goal of 30–40 min of high-intensity AEX.
Participants randomized to the delayed entry group began HIIT after follow-up testing was complete at the end of the 4-month sedentary period. The 4-month treadmill test constituted the pre-exercise evaluation and was used to calculate training HRR and HRmax. During the first 12 sessions, the same protocol for progressive continuous AEX training was used. Starting with session 13, HIIT was performed for a minimum of 36 sessions. Each HIIT session consisted of a 10-min warm-up at 50–60% HRmax followed by four 4-min intervals at 90–95% HRmax. Each 4-min interval was separated by a 3-min active recovery at 50–70% HRmax. The training session concluded with a 3-min cool-down at 50–60% HRmax. Total prescribed exercise time of high-intensity AEX during the intervals was 16 min.
For all AEX training sessions, a Polar A300 heart rate monitor (Polar Electro Inc., Lake Success, NY) was used to continuously monitor and collect the heart rate. Data collected per second were downloaded to the Polar website and aggregated by the target heart rate zones per participant. The duration of high-intensity exercise was defined as the time in target heart rate zone for each session (HIIT, ≥90%HRmax; AEX, ≥60%HRR). To quantitate the amount of high-intensity exercise, we adapted the approach by MacInnis and Gibala (MacInnis & Gibala, 2017). Energy expenditure in kilocalories was calculated per session by multiplying minutes of exercise by estimated kilocalorie per min. For each participant, an estimated kilocalorie per min was derived using the following ACSM formula: (METs × 3.5 × bodyweight kg)/200 (American College of Sports Medicine, 2018). METs were calculated for each session based on heart rate zone as % of baseline VO2peak. The total training volume of high-intensity AEX per participant was calculated as the sum of energy expenditure in sessions 13–48. In addition, detailed written exercise logs were maintained for each session that included target heart rate training zone, exercise type and session duration. The primary exercise modality was motorized treadmill. However, the physiologist substituted other modalities if needed to minimize joint pain, including elliptical trainers, upright bicycle ergometers and recumbent cross trainers (NuStep, Ann Arbor, MI). Substitutions were at the discretion of the physiologist and based on the participant’s capacity to meet target intensity while considering their preference. Overall, after the first 12 sessions, these substitutions were not necessary, and high-intensity AEX training was conducted by a treadmill in both groups.
Supervised resistance training (RT) followed the AEX training for both groups. Three upper body (chest press, seated row, pulldown) and three lower body exercises (leg press, knee extension, knee flexion) were performed in each RT session using selectorized strength equipment (Life Fitness, Rosemont IL). Initial intensity was set at 50% of the 1-RM. Each exercise was performed for two sets of 8–12 repetitions with 60 s of rest between sets. Weight progression was made once a participant could comfortably perform two sets of 12 repetitions. The increase in training weight was by an amount that caused the muscle group to fatigue after 8 repetitions (~10%). This cycle of increasing repetitions and training weight was continued over the 4-month training period. If a participant reached their initial 1-RM, they were subjected to a re-max test on that exercise using the initial testing procedures to allow for continued progression. Supervised stretching of the major muscle groups followed each RT session.
Statistical analyses
The Shapiro–Wilk test and histograms were performed to assess the distribution of data. Baseline differences between groups were examined using Student’s t-test or rank-sum test and Pearson’s chi-squared test or Fisher’s exact test. Differences between groups in attendance and high-intensity AEX energy expenditure and time were tested by the Student’s t-test or rank-sum test. The effect of exercise training within each group was tested using paired t-tests for data with a normal distribution. Data with a skewed distribution were tested by the Wilcoxon signed rank-sum test. Based on our observed mean change in VO2peak within-group, a comparison trial with 80% power and alpha of 0.05 would require more than 100 participants per group to detect a between-group difference in continuous high-intensity AEX and HIIT. Since the study was not designed to compare the effect of different modalities of high-intensity AEX, no between-group tests were performed considering the large type 2 error. A two-tailed p<0.05 was taken to indicate statistical significance.
Results
Among the 32 individuals who were enrolled, 26 participants were randomized 1:1 into AEX+RT or control (delayed exercise) groups (Figure 1). Among the six participants who were not randomized, two were excluded for cardiopulmonary disease, one was not sedentary, and three withdrew or were lost to follow-up. These participants were on average 6 years younger than those randomized (p=0.03) but similar in race and sex. All control participants who completed follow-up testing entered 4 months of HIIT + RT. Attrition during exercise training was 20% (2/10) for the HIIT group and 15% (2/13) for the AEX group. Reasons for discontinuation included cardiopulmonary disease (2) and dizziness (1) and one withdrawal due to travel (Figure 1). Exercise training in both groups was well tolerated and no serious exercise-related adverse events occurred. Characteristics of the participants who completed 16 weeks of exercise training are provided in Table 1. There were no differences in demographic, clinical, or lifestyle characteristics by group.
Table 1.
Clinical and demographic characteristics between the groups.
| Characteristicsa | Total N= 19 |
Exercise Group | ||
|---|---|---|---|---|
| High-intensity Interval Training (HIIT) N= 8 |
High-intensity Continuous Aerobic Exercise (AEX) N= 11 |
p value* | ||
| Age, years | 61.5 (6.7) | 63.4 (7.1) | 60.1 (6.3) | 0.30 |
| Race (n, %) | 0.07 | |||
| African American | 12 (63.1%) | 3 (37.5%) | 9 (81.9%) | |
| Caucasian | 7 (36.8%) | 5 (62.5%) | 2 (18.2%) | |
| Sex, Male (n,%) | 18 (94.7%) | 8 (100%) | 10 (91.9%) | 1.00 |
| Smoking, current (n,%) | 5 (27.8%) | 2 (25.0%) | 3 (30.0%) | 1.00 |
| Cocaine/Heroin history (n,%) | 11 (61.1%) | 4 (50.0%) | 7 (70.0%) | 0.63 |
| Alcohol ≥14 drinks/week (n,%) | 1 (5.6%) | 1 (12.5%) | 0 | 0.44 |
| HCV antibody positive (n,%) | 2 (10.5%) | 1 (12.5%) | 1 (9.1%) | 1.00 |
| Hypertension (n,%) | 6 (31.6%) | 3 (37.5%) | 3 (27.3%) | 1.00 |
| Diabetes (n,%) | 4 (21.1%) | 3 (37.5%) | 1 (9.1%) | 0.26 |
| COPD (n,%) | 2 (10.5%) | 1 (12.5%) | 1 (9.1%) | 0.68 |
| Body Mass Index (BMI, kg · m−2)(n, %) | ||||
| Normal (BMI 18.6–24.9) | 9 (47.4%) | 4 (50.0%) | 5 (45.5%) | 1.00 |
| Overweight and obese (BMI ≥ 25.0) | 10 (52.6%) | 4 (50.0%) | 6 (54.6%) | |
| Prior AIDS-defining illness (n,%) | 6 (31.6%) | 2 (25.0%) | 4 (36.4%) | 1.00 |
| ARV current regimen (n,%): | ||||
| NRTI | 17 (89.5%) | 8 (100.0%) | 9 (81.8%) | 0.48 |
| NNRTI | 7 (36.8%) | 2 (25.0%) | 5 (45.5%) | 0.63 |
| Protease Inhibitor | 3 (15.8%) | 2 (25.0%) | 1 (9.1%) | 0.55 |
| ISTI | 11 (57.9%) | 4 (50.0%) | 7 (63.6%) | 0.66 |
| CD4 count (cells/μL) | 711 (323) | 648 (363) | 757 (301) | 0.48 |
| HIV-1 RNA <20 c/ml (n,%) | 18 (94.7%) | 8 (100.0%) | 10 (91.9%) | 1.00 |
| Duration of HIV Infection, years | 20.4 (7.9) | 18.8 (7.4) | 21.5 (8.3) | 0.46 |
Data shown as mean (SD) unless otherwise indicated.
Continuous data tested by Student’s t-test. For categorical variables, Chi-square test or Fisher’s exact test was used.
ARV, antiretroviral; COPD, chronic obstructive pulmonary disease; HCV, hepatitis C virus; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; ISTI, integrase strand transfer inhibitor.
The attendance rate of scheduled training sessions (median (IQR)) was not different between the HIIT group (91% (11.5%)) and the AEX group (89% (9.5%), p=0.73). As expected, duration of high-intensity AEX per session was different by group during the last 36 sessions (mean minutes (SE); 12.3 (0.7) vs. 31.4 (1.3), p<0.001). Correspondingly, training volume of high-intensity AEX per session was lower in HIIT compared with continuous AEX (median kcal (IQR); 105.3 (23.6) vs. 257.0 (53.2), p<0.01). The mean ±SE of outcomes before and after exercise training is provided in Table 2. The per cent change in VO2peak (mean L/min ± SE) significantly increased in the HIIT group by 17.1% ±4.5% (p<0.01) and in the continuous high-intensity AEX group by 7.7% ±2.4% (p<0.01). There was a significant per cent increase in exercise endurance (time on treadmill) in both the HIIT group (14.6% ±2.4%, p<0.01) and the AEX group (24.2% ±3.8%, p<0.01). The per cent increase in upper and lower body strength was 32% or greater in both groups (all p<0.01). The trend for an increase in muscle mass measured by kilograms of appendicular lean mass in the HIIT group (0.62 ±0.44, p=0.20) and the AEX group (0.97 ±0.42, p=0.05) was not statistically significant. Cardiovascular disease risk factors did not change significantly in either group.
Table 2.
Effect of exercise training by group on cardiorespiratory fitness, strength, body composition, and CVD risk factors in older adults with HIV.
| Continuous High-intensity AEX + Resistance Training N= 11 |
High-intensity Interval Training + Resistance Training N= 8 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Before Exercise Training | After Exercise Training | Change | p-value* | Before Exercise Training | After Exercise Training | Change | p-value* |
| Treadmill Test | ||||||||
| VO2peak, L/min | 2.04 ±0.34 | 2.21 ±0.40 | 0.16± 0.15 | 0.01 | 1.92 ±0.30 | 2.25 ±043 | 0.32 ±0.25 | 0.01 |
| VO2peak, ml/kg/min | 24.4 ±1.7 | 26.4 ±2.0 | 2.1 ±0.7 | 0.02 | 24.7 ±1.8 | 27.7 ±2.4 | 3.1 ±1.04 | 0.02 |
| Resting HR, beats/min. | 73.9 ±5.0 | 66.7 ±3.7 | −7.2 ±3.0 | 0.04 | 81.0 ±5.0 | 77.0 ±5.0 | −4.0 ±3.6 | 0.30 |
| Time on treadmill, min. | 12.4 ±0.7 | 15.3 ±0.8 | 2.9 ±0.5 | 0.00 | 13.5 ±0.8 | 15.5 ±0.8 | 1.9 ±0.3 | 0.00 |
| Strength | ||||||||
| Leg Press, lbs | 229.1 ±19.8 | 345.9 ±19.0 | 116.8 ±17.3 | 0.00 | 230.0 ±15.9 | 356.9 ±24.8 | 126.9 ±20.7 | 0.00 |
| Chest Press, lbs | 140.0 ±14.93 | 190.4 ±15.3 | 50.4 ±4.5 | 0.00 | 148.8 ± 8.4 | 198.1 ±17.6 | 49.4 ±12.6 | 0.00 |
| Body composition | ||||||||
| Weight, kg | 84.4 ±3.3 | 84.7 ±3.4 | 0.3 ±0.9 | 0.72 | 78.5 ±4.8 | 79.0 ±5.0 | 0.6 ±1.1 | 0.61 |
| Waist circumference, cm | 97.1 ±3.0 | 94.9 ±3.7 | −2.2 ±1.6 | 0.21 | 93.7 ±5.4 | 93.0 ±5.8 | −0.68 ±1.7 | 0.70 |
| DXA: | ||||||||
| Appendicular lean mass, kg | 26.6 ±0.8 | 27.6 ±0.8 | 0.97 ±0.42 | 0.05 | 24.1 ±0.1.0 | 24.7 ±1.0 | 0.63 ±0.4 | 0.20 |
| Fat mass, kg | 22.6 ±2.3 | 21.7 ±2.6 | −0.9 ±0.6 | 0.19 | 20.1 ±3.0 | 20.1 ±3.2 | 0.02 ±0.7 | 0.98 |
| Body fat, percent | 26.0 ±1.9 | 24.6 ±2.3 | −1.4 ±0.7 | 0.07 | 24.4 ±2.4 | 24.1 ±2.6 | −0.4 ±0.8 | 0.68 |
| CVD Risk Factors | ||||||||
| Systolic blood pressure | 123 ±3 | 126 ±5 | 3 ±5 | 0.53 | 136 ±4 | 134 ±5 | −2 ±4 | 0.73 |
| Diastolic blood pressure | 78 ±2 | 75 ±2 | −3±2 | 0.13 | 77 ±3 | 79 ±4 | 2 ±4 | 0.57 |
| Total Cholesterol, mg/dl | 195.3 ±18.7 | 174.9 ±13.6 | −20.4 ±13.6 | 0.17 | 160.5 ±15.0 | 156.0 ±9.3 | −4.5 ±8.4 | 0.61 |
| HDL-C, mg/dl | 58.7 ±4.8 | 58.1 ±5.0 | −0.63 ± 1.7 | 0.72 | 46.3 ±4.3 | 48.9 ±3.9 | 2.6 ±2.0 | 0.23 |
| LDL-C, mg/dl | 110.6 ±16.2 | 92.5 ±12.4 | −18.0 ±11.9 | 0.16 | 82.3 ±9.5 | 69.8 ± 6.9 | 12.5 ±7.8 | 0.15 |
| Triglycerides, mg/dl (median, IQR) | 102 (73, 203) | 98 (59,158) | −13 (−27,0) | 0.15 | 141 (78,190) | 167 (99,263) | 1.5 (−17, 73) | 0.49 |
All values are mean ± SE unless otherwise indicated.
Paired t-test or Wilcoxon signed rank-sum test (triglycerides).
DXA, Dual-energy X-ray absorptiometry; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Discussion
This pilot study of HIIT provides results on the feasibility and effects of high-intensity interval training in older adults living with HIV for the first time to our knowledge. We found that HIIT was well tolerated with an attrition rate and attendance rate that was similar to continuous high-intensity AEX training conducted within the same study population. HIIT produced significant increases in CRF and endurance. In addition, RT conducted in conjunction with the HIIT generated substantial gains in strength. This combination of HIIT + RT in an older patient population is an important finding since the combined modality targets CRF and weakness, both key for healthy ageing.
The longitudinal decline in CRF with age has not been studied in adults with HIV, despite the known associations with poor metabolic health and shorter life expectancy. In a cross-sectional study of older adults (median age 57 years, range 50–82), we found VO2peak was reduced by 42% in adults with HIV compared to healthy adults without HIV, which was consistent across the age span (Oursler et al., 2006). A combination of central and peripheral factors contributes to the low CRF observed with HIV infection that includes diastolic dysfunction (Oursler et al., 2019) and reduced skeletal muscle oxidative capacity (Ortmeyer et al., 2016). Numerous AEX trials in younger adults with HIV show an increase in VO2peak of ≥2 ml/kg/min (O’Brien et al., 2016). Two recent AEX trials in older adults (≥50 years) with HIV demonstrate greater gains in VO2 peak with high-intensity AEX compared with moderate-intensity AEX (Erlandson et al., 2018; Oursler et al., 2018). Early in the HIV epidemic when most adults with HIV were younger (age <40 years), several trials with HIIT were conducted. Yet, overall attrition rates were high (32–76%), which likely is explained by advanced HIV disease prior to effective ART (MacArthur et al., 1993; Perna et al., 1999; Terry et al., 1999). Among those compliant with HIIT in these studies (≥50% sessions attended), VO2peak increased 12–24% (MacArthur et al., 1993; Perna et al., 1999). A randomized trial of HIIT versus moderate-intensity AEX in adults with HIV (mean age 31 years) found significant increases in exercise endurance with larger gains in the HIIT group but did not measure CRF (Terry et al., 1999). In 1990, LaPerriere et al. conducted a HIIT intervention in young men with HIV (n=10) and without HIV (n=19) and found a 9% increase in estimated VO2peak in both groups (LaPerriere et al., 1990). More recently, Lindegaard et al. conducted a 4-month HIIT intervention of 10 men with HIV and lipoatrophy, average age 53 years, but excluded those with age-related diseases, including diabetes and arthritis (Lindegaard et al., 2008). They reported a 14% increase in VO2 peak and 20% attrition rate. In comparison, we found HIIT increased VO2peak 17% in adults with HIV who were on average 10 years older (mean age 63 years), many with age-related comorbidities such as diabetes and hypertension. While HIV was well controlled in our participants who had a CD4 cell count in the normal reference range, the average duration of HIV infection was 20 years, and several had prior AIDS-defining conditions. Therefore, our results apply to adults ageing with chronic HIV infection. Overall, our HIIT protocol was well tolerated with a high attendance rate and attrition of 20%. We acknowledge that the results from our two high-intensity AEX training groups cannot be tested for differences due to the small sample size and lack of direct randomization. However, our findings demonstrate the feasibility of HIIT in older adults with HIV and suggest at least comparable effects on CRF and endurance. Further, data on training volume support the efficiency of HIIT. The significant gains in strength in both groups which were combined with RT is an important finding since high-intensity aerobic training is seldom combined with resistance training in exercise trials of older patient populations.
Recent reviews and meta-analyses demonstrate that HIIT increases CRF in healthy adults and other patient populations (Batacan et al., 2017; Campbell et al., 2019). A large single-arm 8-week intervention of HIIT in 94 healthy adults with age ranging from 20 to 83 years shows that baseline fitness, not age, predicted training response and increase in VO2peak (Storen et al., 2017). Specifically in healthy older adults, HIIT interventions with intervals of 2–4 min and intensity of ≥80% VO2peak or ≥90% HRmax show 9–13% increases in VO2peak (Bruseghini et al., 2015; Østerås et al., 2005; Storen et al., 2017). Using a similar HIIT protocol, we found that VO2peak increased 17% in adults with a mean age of 63 years, a robust response which is likely due to their lower baseline fitness.
In the setting of cardiac rehabilitation of adults without HIV, HIIT has clear positive physiological effects (Guiraud et al., 2012), but the impact on metabolic CVD risk factors is variable and frequently compared to continuous moderate AEX (Keating et al., 2017; Kessler et al., 2012). HIIT appears to have the greatest impact on metabolic diseases (glucose, lipids) and weight/visceral fat loss in patients with diabetes (Jelleyman et al., 2015). Our HIIT pilot found no change in fasting glucose and lipids, which is consistent with prior reports of continuous high-intensity AEX in older adults with HIV (Erlandson et al., 2018; Oursler et al., 2018). However, it is possible that weight loss is necessary considering that numerous trials of moderate-intensity AEX with calorie restriction and weight loss in younger adults with HIV have demonstrated positive effects on CVD risk factors (O’Brien et al., 2016). Further research in older adults with HIV is needed to determine the ideal exercise strategies which may need to be tailored to the primary goal of the individual with regards to gain of muscle mass and loss of body fat. In particular, strategic approaches may be needed for racial and ethnic minorities which represent the fastest-growing group of HIV infection in the United States (Centers for Disease Control and Prevention, 2020).
Adults with HIV have an increased risk of CVD and heart failure, especially heart failure with preserved ejection fraction (HFpEF) (Butler et al., 2018; So-Armah & Freiberg, 2014). In the general geriatric population, HFpEF is the most common form of heart failure that appears to be driven by diastolic dysfunction and manifests as exercise intolerance (Kitzman et al., 2002). With limited pharmacologic treatment options, exercise training is key but the ideal combination of modality and intensity is still being studied (Fleg et al., 2015). However, interval training can improve diastolic function and exercise tolerance (Alves et al., 2012; Guiraud et al., 2012). This question is a priority in HIV research given the increased risk of diastolic dysfunction HFpEF and its association with CRF (Oursler et al., 2019) and chronic inflammation (Butler et al., 2018). Our results support further research in HIIT as an efficient and effective means to deliver high-intensity AEX toward the goal of attenuating the accelerated ageing process in adults with HIV.
This study is limited by its small size and narrow demographic characteristics of the participants; results may be different in women. Similarly, findings must be interpreted with caution in those with more severe cardiometabolic or HIV-related disease. Yet, characteristics of the study population, including older age, length of HIV infection and prevalent age-related comorbidity strengthens the importance of our findings. While the cross-over design limits the comparison of the effects of HIIT and continuous high-intensity AEX, it does not impact the integrity of the HIIT results.
Conclusions
Our findings show the feasibility and efficacy of HIIT combined with RT in older adults with stable HIV. The rigorous conduct of the intervention with continuous heart rate monitoring and workload evaluation adds to the significance of our findings. Our pilot study supports HIIT as an efficient means to deliver high-intensity AEX to improve cardiorespiratory fitness toward the goal of attenuating the accelerated ageing process in adults with HIV. Future research is needed to develop targeted exercise rehabilitation strategies for this complex and quickly growing population of older adults with HIV.
Acknowledgments
The authors thank the dedicated participants. Also, the authors acknowledge and appreciate assistance from Chani Jain for biostatistics and tables, Kim Birkett for references and graphics, Timothy Wamsley for study design and Dr. Dorothy Garner for recruitment.
Funding
This research was supported by The Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service [I01 RX000667] and the National Institute on Aging Claude D. Pepper Older Americans Independence Center [P30AG028747].
Footnotes
Declaration of Interest Statement
The authors have no conflict of interest or competing financial interests to declare.
References
- Alves AJ, Ribeiro F, Goldhammer E, Rivlin Y, Rosenschein U, Viana JL, Duarte JA, Sagiv M, & Oliveira J. (2012). Exercise training improves diastolic function in heart failure patients. Medicine and science in sports and exercise, 44(5), 776–785. 10.1249/MSS.0b013e31823cd16a [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. (2018). ACSM’s Guidelines for Exercise Testing and Prescription: Tenth Edition (Riebe D, Ehrman JK, Liguori G, & Magal M ed; Tenth ed.). Wolters Kluwer Health. [Google Scholar]
- Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, Fallucca S, Alessi E, Letizia C, Jimenez A, Fallucca F, & Pugliese G. (2010). Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutrition, metabolism, and cardiovascular diseases: NMCD, 20(8), 608–617. 10.1016/j.numecd.2009.04.015 [DOI] [PubMed] [Google Scholar]
- Batacan RBJ, Duncan MJ, Dalbo VJ, Tucker PS, & Fenning AS (2017). Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. British journal of sports medicine, 51(6), 494–503. 10.1136/bjsports-2015-095841 [DOI] [PubMed] [Google Scholar]
- Bruseghini P, Calabria E, Tam E, Milanese C, Oliboni E, Pezzato A, Pogliaghi S, Salvagno GL, Schena F, Mucelli RP, & Capelli C. (2015). Effects of eight weeks of aerobic interval training and of isoinertial resistance training on risk factors of cardiometabolic diseases and exercise capacity in healthy elderly subjects. Oncotarget, 6(19), 16998–17015. 10.18632/oncotarget.4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J, Kalogeropoulos AP, Anstrom KJ, Hsue PY, Kim RJ, Scherzer R, Shah SJ, Shah SH, Velazquez EJ, Hernandez AF, Desvigne-Nickens P, & Braunwald E. (2018). Diastolic Dysfunction in Individuals With Human Immunodeficiency Virus Infection: Literature Review, Rationale and Design of the Characterizing Heart Function on Antiretroviral Therapy (CHART) Study. Journal of cardiac failure, 24(4), 255–265. 10.1016/j.cardfail.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WW, Kraus WE, Powell KE, Haskell WL, Janz KF, Jakicic JM, Troiano RP, Sprow K, Torres A, Piercy KL, & Bartlett DB. (2019). High-Intensity Interval Training for Cardiometabolic Disease Prevention. Medicine and science in sports and exercise, 51(6), 1220–1226. 10.1249/MSS.0000000000001934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2013). Adult participation in aerobic and muscle-strengthening physical activities–United States, 2011. MMWR. Morbidity and mortality weekly report, 62(17), 326–330. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a2.htm [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2020). Estimated HIV incidence and prevalence in the United States, 2014–2018. HIV surveillance supplemental report, 25(1). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, & Skinner JS. (2009). American College of Sports Medicine position stand. Exercise and physical activity for older adults. Medicine and science in sports and exercise, 41(7), 1510–1530. 10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- Erlandson KM, MaWhinney S, Wilson M, Gross L, McCandless SA, Campbell TB, Kohrt WM, Schwartz R, Brown TT, & Jankowski CM. (2018). Physical function improvements with moderate or high-intensity exercise among older adults with or without HIV infection. AIDS, 32(16), 2317–2326. 10.1097/QAD.0000000000001984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleg JL, Cooper LS, Borlaug BA, Haykowsky MJ, Kraus WE, Levine BD, Pfeffer MA, Piña IL, Poole DC, Reeves GR, Whellan DJ, & Kitzman DW. (2015). Exercise training as therapy for heart failure: current status and future directions. Circulation. Heartfailure , 8(1), 209–220. 10.1161/CIRCHEARTFAILURE.113.001420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, & Bosquet L (2012). High-intensity interval training in cardiac rehabilitation. Sports Medicine, 42(7), 587–605. 10.2165/11631910-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Hawkins KL, Brown TT, Margolick JB, & Erlandson KM (2017). Geriatric syndromes: new frontiers in HIV and sarcopenia. AIDS, 31 (Suppl2) , S137–S146. 10.1097/QAD.0000000000001444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsue PY, Deeks SG, & Hunt PW (2012). Immunologic basis of cardiovascular disease in HIV-infected adults. The Journal of infectious diseases, 205(Suppl), S375–82. 10.1093/infdis/jis200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelleyman C, Yates T, O’Donovan G, Gray LJ, King JA, Khunti K, & Davies MJ. (2015). The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obesity reviews, 16(11), 942–961. 10.1111/obr.12317 [DOI] [PubMed] [Google Scholar]
- Keating SE, Johnson NA, Mielke GI, & Coombes JS (2017). A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obesity reviews, 18(8), 943–964. 10.1111/obr.12536 [DOI] [PubMed] [Google Scholar]
- Kessler HS, Sisson SB, & Short KR (2012). The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Medicine, 42(6), 489–509. 10.2165/11630910000000000-00000 [DOI] [PubMed] [Google Scholar]
- Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, & Stewart KP (2002). Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA, 288(17), 2144–2150. 10.1001/jama.288.17.2144 [DOI] [PubMed] [Google Scholar]
- LaPerriere AR, Antoni MH, Schneiderman N, Ironson G, Klimas N, Caralis P, & Fletcher MA. (1990). Exercise intervention attenuates emotional distress and natural killer cell decrements following notification of positive serologic status for HIV-1. Biofeedback and self-regulation, 15(3), 229–242. 10.1007/BF01011107 [DOI] [PubMed] [Google Scholar]
- Lindegaard B, Hansen T, Hvid T, van Hall G, Plomgaard P, Ditlevsen S, Gerstoft J, & Pedersen BK (2008). The effect of strength and endurance training on insulin sensitivity and fat distribution in human immunodeficiency virus-infected patients with lipodystrophy. The Journal of clinical endocrinology and metabolism, 93(10), 3860–3869. 10.1210/jc.2007-2733 [DOI] [PubMed] [Google Scholar]
- MacArthur RD, Levine SD, & Birk TJ (1993). Supervised exercise training improves cardiopulmonary fitness in HIV-infected persons. Medicine and science in sports and exercise, 25(6), 684–688. 10.1249/00005768-199306000-00006 [DOI] [PubMed] [Google Scholar]
- MacInnis MJ, & Gibala MJ (2017). Physiological adaptations to interval training and the role of exercise intensity. The Journal of physiology, 595 (9), 2915–2930. 10.1113/JP273196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martland R, Mondelli V, Gaughran F, & Stubbs B (2020). Can high-intensity interval training improve physical and mental health out-comes? A meta-review of 33 systematic reviews across the lifespan. Journal of sports sciences, 38(4), 430–469. 10.1080/02640414.2019.1706829 [DOI] [PubMed] [Google Scholar]
- Montoya JL, Jankowski CM, O’Brien KK, Webel AR, Oursler KK, Henry BL, Moore DJ, & Erlandson KM. (2019). Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS, 33(6), 931–939. 10.1097/QAD.0000000000002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health and Nutrition Examination Survey (NHANES) III. (1988). Body measurements (anthropometry) manual. Rockville, MD: Westat, Inc. https://wwwn.cdc.gov/nchs/nhanes/nhanes3/ManualsAndReports.aspx. [Google Scholar]
- O’Brien KK, Tynan A-M, Nixon SA, & Glazier RH (2016). Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC infectious diseases, 16, 182. 10.1186/s12879-016-1478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østerås H, Hoff J, & Helgerud J (2005). Effects of High-Intensity Endurance Training on Maximal Oxygen Consumption in Healthy Elderly People. Journal of applied gerontology: the official journal of the Southern Gerontological Society, 24(5), 377–387. 10.1177/0733464804273185 [DOI] [Google Scholar]
- Ortmeyer HK, Ryan AS, Hafer-Macko C, & Oursler KK (2016). Skeletal muscle cellular metabolism in older HIV-infected men. Physiological reports, 4(9), e12794. 10.14814/phy2.12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oursler KA, & Sorkin JD (2016). HIV and Aging. International journal of infectious diseases, 53, 59–60; (2017) 55, 139. 10.1016/j.ijid.2016.11.414 [DOI] [PubMed] [Google Scholar]
- Oursler KK, O’Boyle HM, Briggs BC, Sorkin JD, Jarmukli N, Katzel LI, Freiberg MS, & Ryan AS. (2019). Association of Diastolic Dysfunction with Reduced Cardiorespiratory Fitness in Adults Living with HIV. AIDS Patient Care and STDs, 33(12), 493–499. 10.1089/apc.2019.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oursler KK, Sorkin JD, Ryan AS, & Katzel LI (2018). A pilot randomized aerobic exercise trial in older HIV-infected men: Insights into strategies for successful aging with HIV. PLoS One, 13(6), e0198855. 10.1371/journal.pone.0198855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oursler KK, Sorkin JD, Smith BA, & Katzel LI (2006). Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS research and human retroviruses, 22(11), 1113–1121. 10.1089/aid.2006.22.1113 [DOI] [PubMed] [Google Scholar]
- Perna FM, LaPerriere A, Klimas N, Ironson G, Perry A, PAVONE J, Goldstein A, Majors P, Makemson D, Talutto C, Schneiderman N, Ann Fletcher M, Meijer OG, & Koppes L. (1999). Cardiopulmonary and CD4 cell changes in response to exercise training in early symptomatic HIV infection. Medicine and science in sports and exercise, 31(7), 973–979. 10.1097/00005768-199907000-00009 [DOI] [PubMed] [Google Scholar]
- So-Armah K, & Freiberg MS (2014). Cardiovascular disease risk in an aging HIV population: not just a question of biology. Current opinion in HIV and AIDS, 9(4), 346–354. 10.1097/COH.0000000000000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storen O, Helgerud J, Saebo M, Støa EM, Bratland-Sanda S, Unhjem RJ, Hoff J, & Wang E. (2017). The Effect of Age on the V O2max Response to High-Intensity Interval Training. Medicine and science in sports and exercise, 49(1), 78–85. 10.1249/MSS.0000000000001070 [DOI] [PubMed] [Google Scholar]
- Sui X, LaMonte MJ, & Blair SN (2007). Cardiorespiratory fitness and risk of nonfatal cardiovascular disease in women and men with hypertension. Journal of the American Society of Hypertension. Discipline. Hypertension, 20(6), 608–615. 10.1016/j.amjhyper.2007.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry L, Sprinz E, & Ribeiro JP (1999). Moderate and high intensity exercise training in HIV-1 seropositive individuals: a randomized trial. International journal of sports medicine, 20(2), 142–146. 10.1055/s-2007-971108 [DOI] [PubMed] [Google Scholar]
- Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS Jr., & Blair SN (1999). Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA, 282(16), 1547–1553. 10.1001/jama.282.16.1547 [DOI] [PubMed] [Google Scholar]
- Zanetti HR, Goncalves A, Teixeira Paranhos Lopes L, Mendes EL, Roever L, Silva-Vergara ML, Neves FF, & Resende ES. (2020). Effects of Exercise Training and Statin Use in People Living with Human Immunodeficiency Virus with Dyslipidemia. Medicine and science in sports and exercise, 52(1), 16–24. 10.1249/MSS.0000000000002120 [DOI] [PubMed] [Google Scholar]


