Abstract
Neurological complications are well described in SARS-CoV-2, but for the first time we report a case of unilateral diaphragm paralysis occurring early in mechanical ventilation for respiratory failure due to such an infection. The patient subsequently required tracheostomy and ventilator support for 37 days, and had increased breathlessness and an elevated diaphragm at clinic review 9 months later. Dynamic chest radiography demonstrated persistent diaphragm paralysis with an accompanying postural change in lung volumes, and he subsequently underwent surgical plication. This case demonstrates that although persistent dyspnoea is a common feature following SARS-CoV-2 infection and is usually due to deconditioning or persistent parenchymal involvement, it can be due to other causes and needs to be investigated appropriately.
Keywords: COVID-19, mechanical ventilation, lung function, radiology
Background
Lower respiratory tract involvement is a common feature of infection with the SARS-CoV-2 virus, most notably respiratory failure due to viral pneumonitis, but as the COVID-19 pandemic continues, long-term complications are emerging. Among these are involvement of the neurological tract1 2 and chronic lung disease, chiefly pulmonary fibrosis.3 We report a case of unilateral diaphragmatic paralysis in an individual with SARS-CoV-2 infection, and explore the possible contributory factors and learning points of this novel case.
Case presentation
A 54-year-old Caucasian man was admitted with rapidly progressive dyspnoea due to PCR positive SARS-CoV-2 infection. He had a history of insulin dependent type 2 diabetes mellitus, obstructive sleep apnoea (OSA) managed with home continuous positive airway pressure (CPAP), primary hypertension and a raised body mass index (38.1 kg/m2). He reported no other respiratory history and was a non-smoker. After 3 days, due to refractory type 2 respiratory failure despite CPAP, he was intubated and placed on lung protective airway pressure release ventilation with intense neuromuscular blockade. He also developed acute renal failure, for which he received temporary veno-venous renal replacement therapy. Standard drug treatment in use for SARS-CoV-2 pneumonia at the authors’ unit at the time was given: intravenous broad-spectrum antibiotics, anticoagulation and corticosteroids.
Investigations
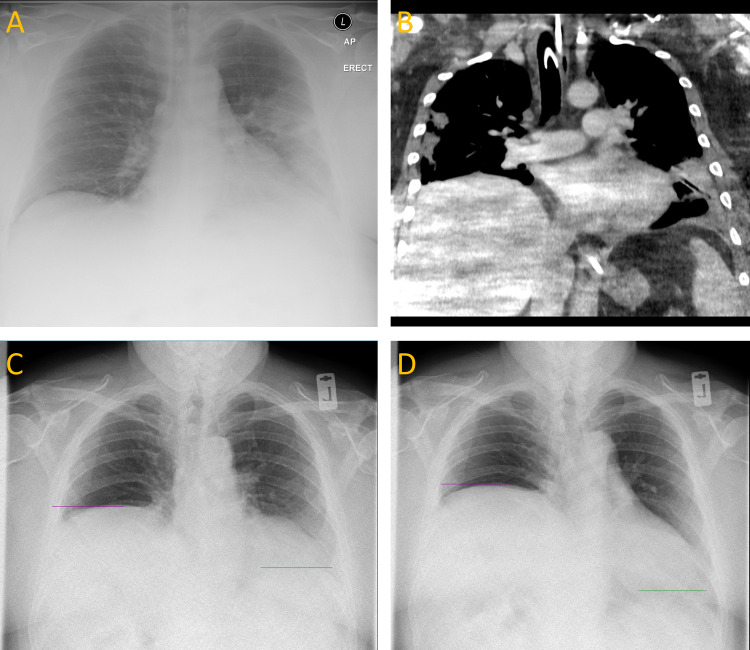
The initial chest radiograph showed ground glass consolidation in the left lung, with normal bilateral haemidiaphragm position (figure 1A), but this progressed rapidly to bilateral, peripherally predominant ground glass change consistent with SARS-CoV-2 pneumonia. Bilateral consolidation and volume loss were seen early in the admission, with a progressively elevated right haemidiaphragm from day 7. He had received a right jugular central venous catheter (CVC) at the beginning of his admission, but this was placed without complication, replaced soon after by a contralateral jugular haemofiltration line and the elevated right haemidiaphragm did not become apparent for 4 days after placement. A tracheostomy was performed at 2 weeks, followed by a prolonged respiratory wean supported with CPAP, and he was liberated from mechanical ventilator support after 37 days. No iatrogenic injury to the neck was noted during this period. CT thorax at this point showed bilateral multifocal consolidation worse on the right, with an elevated right haemidiaphragm (figure 1B).
Figure 1.
(A) Initial anteroposterior (AP) chest radiograph demonstrating left mid-zone and lower zone consolidation, with both haemidiaphragms in a conventional position. (B) Coronal CT thorax showing bilateral multifocal peripheral consolation with a raised right haemidiaphragm and no mediastinal mass. (C, D) Still frames from a posteroanterior (PA) DCR during sniff test at expiration (C) and inspiration (D) showing further elevation and paradoxical motion of the right haemidiaphragm. Resolution of the lung parenchymal changes has occurred. DCR, dynamic chest radiography.
Outcome and follow-up
Following a period of rehabilitation, he was discharged at 61 days. At 4-month clinic review, he reported persistent dyspnoea and orthopnoea. CT revealed significant improvement of the consolidation, with a raised right haemidiaphragm and minor residual upper lobe linear atelectasis.
His symptoms persisted, and at 9 months dynamic chest radiography (a real-time large-field-of-view thoracic imaging system) demonstrated clear lung fields but a raised right haemidiaphragm with ipsilateral paradoxical motion on sniff manoeuvre (figure 1C, D). Spirometry showed a postural reduction in forced vital capacity of 43.5% from standing to lying. He subsequently underwent surgical plication.
Discussion
The diaphragm is the primary muscle of respiration, and each haemidiaphragm is supplied by the phrenic nerve. Damage to this nerve or intrinsic weakness of the diaphragm muscle fibres can lead to diaphragmatic palsy, which may be traumatic, malignant, iatrogenic, neurological, inflammatory or idiopathic.4
The SARS-CoV-2 virus has neuroinvasive potential,5 and infection is associated with numerous neuromuscular complications such as myasthenia gravis, Guillain-Barré syndrome and anosmia.1 6 To the authors’ knowledge, there has been only one previous case report of diaphragm paralysis following SARS-CoV-2 infection,7 not associated with mechanical ventilation.
Prolonged intubation and mechanical ventilation are associated with diaphragm weakness, likely as a consequence of critical illness polyneuropathy8 9 or mechanical trauma, and diaphragm dysfunction in ventilated patients carries a high mortality and morbidity.10 11 However, in our case, diaphragmatic paralysis occurred early in the disease course and no proning manoeuvres or neck trauma took place, suggesting that it was not due tomechanical causes or critical illness. Phrenic neuropathy is well described in diabetes,12 and this may have been contributory. Although phrenic nerve palsy may be associated with trauma during jugular CVC insertion,13 this is extremely rare, and unlikely given the uncomplicated insertion and lack of temporal association with the development of haemidiaphragm paralysis.
Learning points.
SARS-CoV-2 infection may be associated with diaphragm paralysis.
Diabetes and raised body mass index are risk factors for diaphragm paralysis.
Persistent dyspnoea in the absence of persistent lung parenchymal change following SARS-CoV-2 infection should prompt further investigation.
Spirometry and real-time imaging (such as dynamic chest radiography) should be utilised in these cases.
Footnotes
Contributors: TSF was the lead author of this work. CM, JG and MW helped write the work. MW provided expert opinion on interpretation of pulmonary physiology. CM provided images and expert thoracic radiological advice. JG provided overall guidance for the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–90. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guadarrama-Ortiz P, Choreño-Parra JA, Sánchez-Martínez CM, et al. Neurological aspects of SARS-CoV-2 infection: mechanisms and manifestations. Front Neurol 2020;11:1039. 10.3389/fneur.2020.01039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020;30:3306–9. 10.1007/s00330-020-06731-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kokatnur L, Rudrappa M. Diaphragmatic palsy. Diseases 2018;6:16. 10.3390/diseases6010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y-C, Bai W-Z, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020;92:552–5. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paliwal VK, Garg RK, Gupta A, et al. Neuromuscular presentations in patients with COVID-19. Neurol Sci 2020;41:3039–56. 10.1007/s10072-020-04708-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurier F, Godbert B, Perrin J. Respiratory distress in SARS-CoV-2 without lung damage: phrenic paralysis should be considered in COVID-19 infection. Eur J Case Rep Intern Med 2020;7:001728. 10.12890/2020_001728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dres M, Goligher EC, Heunks LMA, et al. Critical illness-associated diaphragm weakness. Intensive Care Med 2017;43:1441–52. 10.1007/s00134-017-4928-4 [DOI] [PubMed] [Google Scholar]
- 9.Supinski GS, Morris PE, Dhar S, et al. Diaphragm dysfunction in critical illness. Chest 2018;153:1040–51. 10.1016/j.chest.2017.08.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medrinal C, Prieur G, Frenoy Éric, et al. Respiratory weakness after mechanical ventilation is associated with one-year mortality - a prospective study. Crit Care 2016;20:231. 10.1186/s13054-016-1418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demoule A, Jung B, Prodanovic H, et al. Diaphragm dysfunction on admission to the intensive care unit. prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med 2013;188:213–9. 10.1164/rccm.201209-1668OC [DOI] [PubMed] [Google Scholar]
- 12.Yesil Y, Ugur-Altun B, Turgut N, et al. Phrenic neuropathy in diabetic and prediabetic patients without neuromuscular complaint. Acta Diabetol 2013;50:673–7. 10.1007/s00592-012-0371-8 [DOI] [PubMed] [Google Scholar]
- 13.Ahn EJ, Baek CW, Shin HY, et al. Phrenic nerve palsy after internal jugular venous catheter placement. Korean J Anesthesiol 2012;63:183–4. 10.4097/kjae.2012.63.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]