Abstract
Question addressed by the study:
Methotrexate (MTX) is a key anchor drug for rheumatoid arthritis (RA) management. Fibrotic interstitial lung disease (ILD) is a common complication of RA. Whether MTX exposure increases the risk of ILD in patients with RA is disputed. We aimed to evaluate the association of prior MTX use with development of RA-ILD.
Methods:
Through a case–control study design with discovery and international replication samples, we examined the association of MTX exposure with ILD in 410 patients with chronic fibrotic ILD associated with RA (RA-ILD) and 673 patients with RA without ILD. Estimates were pooled over the different samples using meta-analysis techniques.
Results:
Analysis of the discovery sample revealed an inverse relationship between MTX exposure and RA-ILD (adjusted OR 0.46, 95% CI 0.24–0.90; p=0.022), which was confirmed in the replication samples ( pooled adjusted OR 0.39, 95% CI 0.19–0.79; p=0.009). The combined estimate using both the derivation and validation samples revealed an adjusted OR of 0.43 (95% CI 0.26–0.69; p=0.0006). MTX ever-users were less frequent among patients with RA-ILD compared to those without ILD, irrespective of chest high-resolution computed tomography pattern. In patients with RA-ILD, ILD detection was significantly delayed in MTX ever-users compared to never-users (11.4±10.4 years and 4.0±7.4 years, respectively; p<0.001).
Answer to the question:
Our results suggest that MTX use is not associated with an increased risk of RA-ILD in patients with RA, and that ILD was detected later in MTX-treated patients.
Introduction
Interstitial lung disease (ILD) is a severe manifestation of rheumatoid arthritis (RA) that affects 2.2–30% of RA patients based on high-resolution computed tomography (HRCT) chest scan findings [1, 2]. RA-ILD is the second leading cause of mortality in RA and contributes to death in 6.8–9.8% of RA patients [3, 4].
Methotrexate (MTX) is recommended as the first-line treatment of RA, as it effectively reduces disease activity, morbidity and mortality [5, 6]. MTX has long been suspected as a causative agent in lung disease, including fibrotic ILD [7–9], and many rheumatologists and pneumologists are reluctant to introduce or maintain MTX in patients with RA-ILD. However, other than acute or subacute hypersensitivity pneumonitis, which is a rare complication of MTX [10], the evidence for a cause-and-effect relationship in modern populations between MTX and chronic fibrotic ILD in a patient with RA (i.e. RA-ILD) is unsettled. Recent studies of the incidence of ILD among RA and non-RA populations have cast doubt on the causal role of MTX and some data have even suggested a possible protective effect of MTX against RA-ILD [11–15]. However, most of these studies had several potential biases: 1) the ILD status was not systematically assessed by HRCT chest scan in cases (RA-ILD) and controls (RA-noILD), resulting in potential misclassification (i.e. classification of patients with preclinical RA-ILD in the RA-noILD group) and precluding subanalyses according to the HRCT pattern; 2) identification of ILD based on data recorded on case report forms and death certificates without independent validation, leading to an underestimation of the RA-ILD incidence; 3) the design of the study did not take into account exposure to MTX before the diagnosis of ILD, thus leading to a potential bias of MTX use (i.e. non-initiation or discontinuation of MTX in patients with RA-ILD); and 4) the year of RA onset and the corresponding guidelines for the management of RA, thus influencing MTX use, were not considered. The aim of this study was to evaluate whether MTX exposure is associated with an increased risk of RA-ILD.
Methods
Study populations
This case–control association study included a discovery step and a replication step. The discovery sample included patients with chronic fibrotic ILD associated with RA (RA-ILD) (cases) and patients with RA who did not have ILD (RA-noILD) (controls), from the French RA-ILD network [16]. The replication step included patients from multi-ethnic case series from five countries (Italy, UK, Mexico, Brazil and United States). All the patients included in the study were investigated using a chest HRCT scan; data were collected through a systematic chart review. The date of inclusion in the study was defined as follows: date of ILD diagnosis for patients with RA-ILD and date of the chest HRCT scan excluding the ILD diagnosis in patients with RA-noILD. Patients with RA in whom ILD onset preceded RA onset were not included in the study. All cases fulfilled the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) and/or 1987 ACR revised criteria for RA and were included consecutively in each participating centre [17, 18]. Because of the known relationship between the risk of occurrence of ILD and RA duration [19], cases and controls (i.e. RA-ILD and RA-noILD) were matched according to the RA duration at the date of inclusion. The ILD status of patients with RA was established by chest HRCT images that were reviewed centrally by experienced radiologist and pulmonologist readers at each participating centre. The chest HRCT ILD pattern was classified as usual interstitial pneumonia (UIP), possible UIP or inconsistent with UIP according to international criteria [20], and all readers were blinded to the clinical data. In each participating centre, patients classified by a senior pulmonologist as having a diagnosis of MTX-related hypersensitivity pneumonitis according to previously published criteria were not included in the study [21]. The institutional review board at each institution approved all protocols.
MTX exposure assessment
MTX exposure was assessed through a systematic chart review of all the patients included in the study. To avoid any bias resulting in MTX withdrawal secondary to ILD detection in patients with RA, MTX exposure was systematically assessed during the period encompassing the date of RA onset (year or year and month, when available) to the date of ILD diagnosis (year or year and month, when available) for patients with RA-ILD, and to the date of ILD-negative HRCT scan (year or year and month, when available) for the patients with RA-noILD. This period was termed “MTX exposure duration”. MTX exposure was evaluated by including the MTX ever-/never-use status for the period defined (i.e. MTX exposure duration). Due to the retrospective design of the study, the accuracy of both MTX doses and MTX duration exposure were considered to be low. Consequently, the cumulative dose of MTX (exposure duration × mean MTX dose) information was not considered sufficiently robust; therefore, corresponding p-values were considered as descriptive.
Statistical analysis
The association between MTX ever-use and the occurrence of ILD in patients with RA was expressed in terms of odds ratio and analysed in the discovery and in all replication samples. Following the same principles as the two-stage approach to individual patients’ meta-analysis, the association in all replication samples was then pooled by a random effects meta-analysis model with inverse variance weighting to obtain a single estimate of the replication odds ratio. Discovery and replication odds ratios were then pooled similarly to obtain an overall (combined) odds ratio. Given the risk for sparse data bias in cohorts with small number of unexposed subjects, Firth’S [22] penalised logistic regression was used. Analyses were adjusted (adj) for age at RA onset, sex, ever-smoking, biologics ever-use, study site, MTX exposure duration and periods of RA onset.
To avoid bias secondary to MTX practice patterns at the year of RA onset of each patient included, we defined and considered four distinct periods of MTX use at RA onset. According to major publications and available guidelines these four periods were defined as follows: unlikely (before 1985) [23], less often (1985–1995) [24, 25], often (1996–2007) [26] and standard of care (since 2008). These four periods were considered as covariates when adjusting for periods of MTX use at year of RA onset. Missing data were handled by multiple imputations using the chained equations method, with 30 independent imputed datasets generated and analysed separately. All adjustment factors were considered in the imputation model, as well as MTX ever-use and RA-ILD status. Results were then pooled over imputed datasets using Rubin’s rule. Sensitivity analyses were carried out by 1) using a logistic model with random sample effects (one-stage individual patient data meta-analysis); 2) removing the replication samples from Italy and Mexico in which very few patients were unexposed to MTX; and 3) by repeating the analysis on complete cases, and by adding MTX doses and durations in the imputation model. Of note, in one of the replication samples (UK population), all patients were exposed to MTX. This sample was therefore omitted from the statistical analyses assessing the association of MTX ever-use and ILD. Other analyses involved mixed-effects multivariable models, with sample as a random effect. All analyses were carried out using R 3.6.1 statistical software (The R Foundation of Statistical Computing, Vienna, Austria).
Results
This case–control study included 1083 patients with RA, 410 with ILD and 673 without ILD. We computed that, given the correlation between MTX use and adjustment variables and the proportions of patients exposed to MTX, the study would have 62% power to detect an odds ratio of 0.5 in the discovery sample, 78% in the replication sample and 96% in the combined analysis.
Characteristics of the RA population
The discovery case series included 100 RA-ILD cases and 165 RA-noILD controls. The replication step comprised a multi-ethnic case series and included 310 RA-ILD and 508 RA-noILD patients. Characteristics of the overall population of 1083 patients with RA are summarised in table 1. Characteristics of patients with RA-ILD and patients with RA-noILD in each case series are summarised in supplementary table S1. As compared with RA-noILD, patients with RA-ILD were more frequently male, older, older at RA onset, ever-smokers and had a shorter MTX exposure duration (tables 1 and 2). RA-ILD and RA-noILD patients did not differ in rheumatoid factor and/or anti-citrullinated protein antibody (ACPA) positivity, RA duration, periods of MTX use at year of RA onset and biologic disease-modifying anti-rheumatic drug (DMARD) use (tables 1 and 2). The frequency of UIP or possible UIP pattern on HRCT ranged from 36.4% to 55.6% (supplementary table S1). Overall, 45.1% of patients with RA-ILD had UIP or possible UIP pattern (table 1).
TABLE 1.
Characteristics of patients with rheumatoid arthritis (RA) at inclusion
| Discovery |
Pooled replication |
Combined |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RA-ILD | RA-noILD | p-value# | RA-ILD | RA-noILD | p-value# | RA-ILD | RA-noILD | p-value# | |
| Patients | 100 | 165 | 310 | 508 | 410 | 673 | |||
| Female | 56 (56.0) | 131 (79.4) | <0.0001 | 186 (60.0) | 421 (82.9) | <0.001 | 242 (59.0) | 552 (82.0) | <0.0001 |
| Age at inclusion years | 60 (53–68) | 55 (46–65) | 0.002 | 66 (57–73) | 65 (56–72) | 0.13 | 64 (55–73) | 62 (53–71) | 0.006 |
| Age at RA onset years | 54 (44–62) | 42 (30–52) | <0.0001 | 55 (45–66) | 48 (37–58) | <0.001 | 54 (44–66) | 46 (36–56) | <0.0001 |
| RA duration years | 10 (4–18) | 10 (4–16) | 0.53 | 13 (7–21) | 15 (8–22) | 0.14 | 12 (7–20) | 13 (7–21) | 0.35 |
| Ever-smoker | 59 (59.0) | 70 (45.2) | 0.040 | 176 (57.9) | 208 (41.8) | <0.0001 | 235 (58.2) | 278 (42.6) | <0.0001 |
| Tobacco exposure packs per year | 13.8±17.0 | 10.0±21.2 | 0.002 | 16.1±22.8 | 8.7±18.0 | <0.0001 | 15.5±21.3 | 9.0±18.8 | <0.0001 |
| Biologic ever-use | 37 (57.8) | 86 (55.5) | 0.77 | 149 (48.9) | 256 (50.9) | 0.61 | 186 (50.4) | 342 (52.0) | 0.65 |
| RA autoimmunity | |||||||||
| ACPA-positive | 83 (85.6) | 140 (88.1) | 0.57 | 204 (80.0) | 346 (78.3) | 0.63 | 287 (81.5) | 486 (80.9) | 0.86 |
| RF-positive | 71 (77.2) | 120 (77.4) | >0.99 | 229 (85.4) | 346 (78.1) | 0.018 | 300 (83.3) | 466 (77.9) | 0.046 |
| UIP or possible UIP HRCT pattern | 47 (52.8) | 133 (42.9) | 180 (45.1) | ||||||
| Pulmonary function testing | |||||||||
| FVC % pred | 88 (66–102) | 76 (61–90) | 78 (62–93) | ||||||
| DLCO % pred | 59 (50–70) | 55 (41–68) | 56 (43–69) | ||||||
| TLC % pred | 81 (69–94) | 77 (67–87) | 79 (68–88) | ||||||
Data are presented as n, n (%), median (interquartile range) or mean±SD, unless otherwise stated. RA-ILD: patients with RA-associated interstitial lung disease; RA-noILD: RA patients without interstitial lung disease; ACPA: anti-citrullinated protein antibody; RF: rheumatoid factor; UIP: usual interstitial pneumonia; HRCT: high-resolution computed tomography; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; TLC: total lung capacity.
unadjusted p-values obtained by Wilcoxon rank-sum tests (continuous variables) and Fisher’s exact tests (categorical variables).
TABLE 2.
Methotrexate (MTX) use in patients with rheumatoid arthritis (RA) with or without interstitial lung disease (ILD)
| Discovery |
Pooled replication |
Combined |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RA-ILD | RA-noILD | p-value | ORadj (95% CI) |
RA-ILD | RA-noILD | p-value | ORadj (95% CI) |
RA-ILD | RA-noILD | p-value | ORadj (95% CI) |
|
| MTX exposure duration# years | 4 (1–13) | 10 (4–16) | <0.0001 | 6 (2–15) | 15 (8–22) | <0.0001 | 6 (1–14) | 13 (7–21) | <0.0001 | |||
| Periods of MTX use at year of RA onset | 0.17 | 0.97 | 0.68 | |||||||||
| Unlikely | 6 (6.0) | 19 (11.5) | 25 (8.1) | 39 (7.7) | 31 (7.6) | 58 (8.6) | ||||||
| Less often | 18 (18.0) | 24 (14.5) | 51 (16.5) | 79 (15.6) | 69 (16.8) | 103 (15.3) | ||||||
| Often | 42 (42.0) | 81 (49.1) | 126 (40.6) | 213 (41.9) | 168 (41.0) | 294 (43.7) | ||||||
| Standard of care | 34 (34.0) | 41 (24.8) | 108 (34.8) | 177 (34.8) | 142 (34.6) | 218 (32.4) | ||||||
| MTX ever-use¶ | 60 (60.0) | 137 (83.0) | 0.022 | 0.46 (0.24–0.90) | 247 (79.7) | 485 (95.5) | 0.009 | 0.39 (0.19–0.79) | 307 (74.9) | 622 (92.4) | 0.0006 | 0.43 (0.26–0.69) |
| MTX cumulative dose g | 0.1 (0.0–3.1) | 2.2 (0.2–5.5) | <0.0001 | 1.5 (0.0–4.7) | 4.5 (1.3–7.8) | <0.0001 | 1.3 (0.0–4.2) | 3.9 (1.0–7.4) | <0.0001 | |||
Data are presented as median (interquartile range) or n (%), unless otherwise stated. For MTX ever-use, odds ratio (ORadj) p-values are adjusted for age at RA onset, sex, ever-smoking, biologic ever-use, MTX exposure duration and periods of MTX use at year of RA onset, and obtained by random-effects meta-analysis of pooled estimates over multiply imputed datasets. p-values are unadjusted for MTX exposure duration, periods of MTX use at year of RA onset and MTX cumulative dose. RA-ILD: patients with RA-associated ILD; RA-noILD: RA patients without ILD.
to avoid any bias resulting in MTX withdrawal secondary to ILD co-occurrence in patients with RA, the MTX exposure for patients with RA-ILD was established during the period before the diagnosis of ILD;
UK excluded from the pooled replication and combined analyses.
MTX ever-use and risk of RA-ILD
In the discovery sample, the frequency of MTX ever-use was 60.0% in RA-ILD patients and 83.0% in RA-noILD patients. After controlling for age at RA onset, sex, ever-smoking, MTX exposure duration, periods of MTX use at RA onset and biologic use, a negative association was found between the ever-use of MTX and RA-ILD when compared to RA-noILD (ORadj 0.46, 95% CI 0.24–0.90; padj=0.022) (figure 1, table 2). A similar association was found in the pooled replication population, where fewer MTX ever-users were significantly observed among the patients with RA-ILD compared to those with RA-noILD (79.7% and 95.5%, respectively, ORadj 0.39, 95% CI 0.19–0.79; padj=0.009). The inverse relationship between the MTX ever-use and occurrence of ILD among RA patients was confirmed in the combined population (ORadj 0.43, 95% CI 0.26–0.69; padj=0.0006) (figure 1, table 2). Of note, no heterogeneity was found among the replication samples (I2=0%), and no difference was found between the discovery and replication samples (I2=0%, test for between-group differences p=0.73). Sensitivity analyses yielded similar results (supplementary table S2).
FIGURE 1.
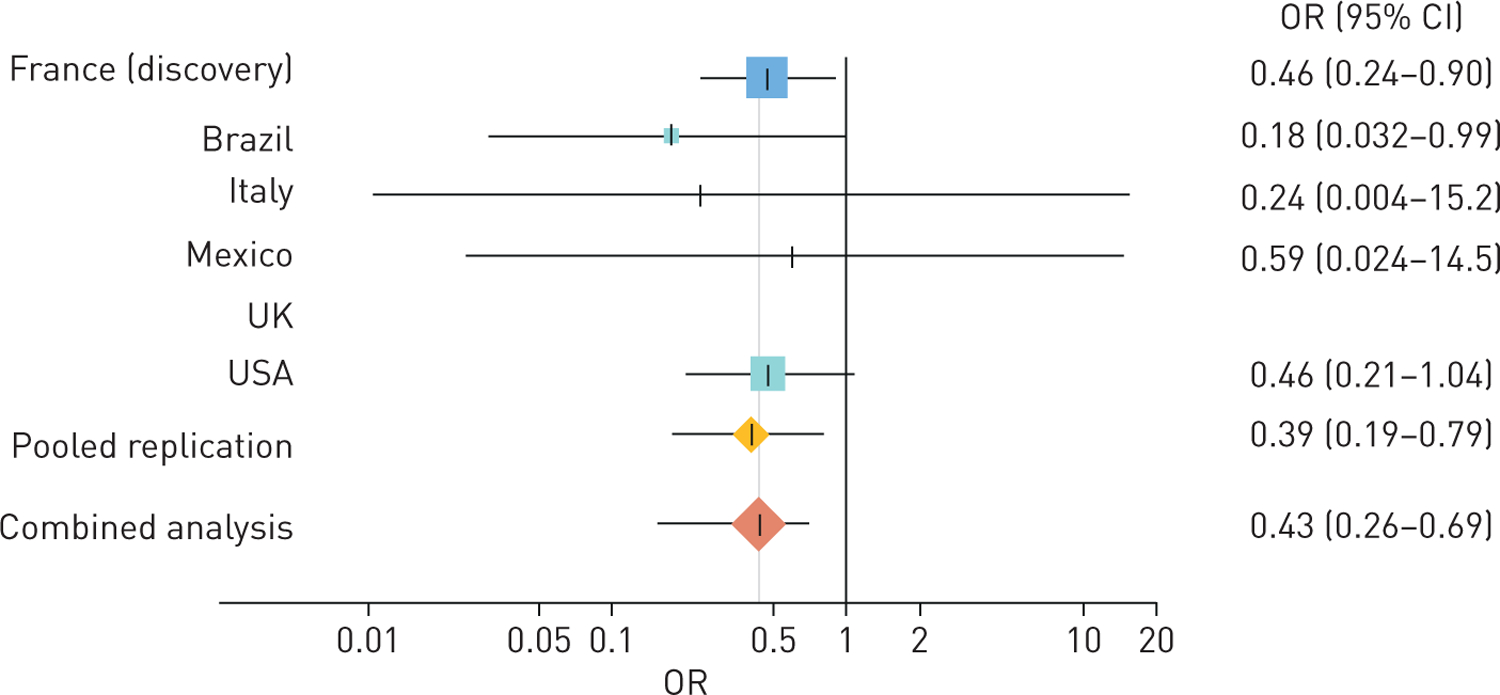
Methotrexate (MTX) ever-use and risk of rheumatoid arthritis (RA)-associated interstitial lung disease. Forest plot of odds ratios for interstitial lung disease among patients with RA according to MTX ever-use. The square boxes indicate odds ratios, and the horizontal lines indicate 95% confidence intervals for each sample. Diamonds display the pooled estimates. The black vertical line represents a mean odds ratio of 1. Odds ratios were adjusted for age at RA onset, sex, ever-smoking, case series origin, biologic ever-use, MTX exposure duration and periods of MTX use at year of RA onset.
MTX ever-use and risk of RA-ILD according to chest HRCT scan patterns
In the combined population, the frequency of MTX ever-use was 70.0% and 79.9% in cases of RA-UIP and RA-nonUIP, respectively, compared to 92.4% in cases of RA-noILD (RA-UIP ORadj 0.34, 95% CI 0.19–0.61, p=0.0003; RA-nonUIP ORadj 0.44, 95% CI 0.24–0.81, p=0.008). Details are given in table 3. In the combined population, the comparison of adjusted odds ratios for RA-UIP versus RA-noILD to RA-nonUIP versus RA-noILD did not reach statistical significance ( p=0.54).
TABLE 3.
Association of the methotrexate (MTX) ever-use with rheumatoid arthritis (RA)-related interstitial lung disease (ILD), according to chest high-resolution computed tomography (HRCT) scan patterns
| Discovery |
Pooled replication# |
Combined# |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RA-UIP | RA-nonUIP | RA-noILD | RA-UIP | RA-nonUIP | RA-noILD | RA-UIP | RA-nonUIP | RA-noILD | |
| Patients | 47 | 42 | 165 | 133 | 177 | 508 | 180 | 219 | 673 |
| MTX ever-use | 26 (55.3) | 28 (66.7) | 137 (83.0) | 100 (75.2) | 147 (83.1) | 485 (95.5) | 126 (70.0) | 175 (79.9) | 622 (92.4) |
| ORadj (95% CI) | 0.44 (0.20–0.98) | 0.41 (0.18–0.95) | 0.25 (0.11–0.59) | 0.48 (0.20–1.12) | 0.36 (0.19–0.61) | 0.44 (0.24–0.81) | |||
| Adjusted p-value | 0.044 | 0.038 | 0.002 | 0.090 | 0.0003 | 0.008 | |||
Data are presented as n or n (%), unless otherwise stated. UIP: usual interstitial pneumonia; RA-noILD: RA patients without ILD. The RA-UIP subset includes RA-ILD patients with the following HRCT scan patterns: UIP and possible UIP. The RA-nonUIP subset includes RA-ILD patients having the following HRCT scan patterns: nonspecific interstitial pneumonia, organising pneumonia, unclassifiable ILD. Adjusted odds ratios (ORadj) and their corresponding p-values are adjusted for age at RA onset, sex, ever-smoking, biologic ever-use, MTX exposure duration and periods of MTX use at year of RA onset, and obtained by random-effects meta-analysis of pooled estimates over multiply imputed datasets. ORadj are adjusted odds ratios for RA-UIP or RA-nonUIP versus RA-noILD.
UK excluded from the pooled replication and combined analyses.
Effect of MTX ever-use on delay of detection of RA-ILD
In the discovery population and after adjusting for covariates, ILD was detected later in MTX ever-users as compared to never-users (padj=0.001). These findings were replicated in the replication multi-ethnic case series sample ( padj<0.001), leading in the combined analysis to a mean delay of detection of 11.4±10.4 years in MTX ever-users compared to 4.0±7.4 years in MTX never-users in the combined analysis (padj<0.001) (supplementary table S3).
Discussion
MTX is currently recommended as the first-line disease-modifying treatment for RA [5, 6]. Even though fibrotic ILD is a well-recognised extra-articular complication of RA (i.e. RA-ILD), the effect of MTX on the development of RA-ILD remains unsettled, as conflicting results have been published [11–15]. Interpretation of previous studies is complicated by a number of biases affecting the association of MTX ever-use and development of ILD, one of the main sources of bias being the absence of systematic evaluation of the lung phenotype with chest HRCT. Both MTX-related hypersensitivity pneumonitis and the putative association between MTX and RA-ILD [15, 27] have led rheumatologists and pulmonologists to withdraw MTX therapy, not only in patients with established prior history of MTX-related hypersensitivity pneumonitis, but also in patients with RA who developed ILD, and to limit the use of MTX in patients with RA-ILD.
In this study, we found a lower frequency of MTX ever-use in RA-ILD patients compared to RA-noILD patients within the French discovery population and within the international replication population. One of the major strengths of our study is that the inverse relationship was consistent in all studied populations, which is reassuring (figure 1 and supplementary table S1), and sensitivity analyses did not change the direction or magnitude of the association signal of MTX exposure on risk of ILD among patients with RA (supplementary table S2). In addition, we observed that the HRCT ILD detection was delayed by 3.6 years in MTX ever-users compared to never-users, supporting the hypothesis of a possible inverse relationship between MTX ever-use and ILD development in RA patients [13].
Most importantly, the lung phenotype (ILD or noILD) was systematically assessed with chest HRCT scan, avoiding the misclassification of patients with pre-clinical RA-ILD at the time of inclusion, and allowing us to demonstrate that the inverse relationship was found whatever the pattern of ILD (UIP or non-UIP). Even if the temporality of the chest HRCT scan (median RA duration time of 13 (7–21) years) is relevant according to the reported mean time of ILD occurrence in RA [28], a late occurrence of ILD in some individuals classified as RA-noILD cannot be fully excluded, which is one limitation of the study. In addition, there is a possibility that patients with ILD or respiratory symptoms would be less likely to be prescribed MTX. However, to take this into account the MTX exposure was systematically assessed before the detection of ILD. Nonetheless, these findings are consistent with three previously reported meta-analyses of randomised controlled trials in RA and non-RA inflammatory diseases in which MTX was not associated with noninfectious respiratory adverse events and with two British cohorts in which MTX was not associated with an increased risk of incident RA-ILD [11, 13, 29, 30].
Beside the practical implications for RA-ILD management, these results may have important implications for the interpretation of ongoing and future clinical trials addressing RA-ILD or other connective tissue disease related ILD. Putative beneficial effects of other agents studied for their effect on RA-ILD in the context of MTX therapy may be misattributed to the study drug rather than MTX.
Although an association between ACPA positivity and RA-ILD has been suggested previously [31, 32], concordant with previously reported large studies [33, 34], we did not detect such association. Several arguments would explain this apparent discrepancy of our findings: 1) a multi-ethnic and larger population of patients was investigated; 2) the ILD status was defined using chest HRCT scan, avoiding misclassification; and 3) the use of the ACR/EULAR 2010 classification criteria, which include the seropositivity for ACPA [35]. In our multi-ethnic case–control study the lack of association of ACPA positivity with RA-ILD was constantly observed in each investigated population, strengthening our finding.
Because of the retrospective design of this study, the inverse relationship between MTX exposure and the risk of RA-ILD should be interpreted with caution. First, age, sex and smoking history was statistically different between the patients with and without ILD and may have influenced MTX prescription. Second, information about biological treatment (drugs, dosage, treatment duration…) could not be assessed in this retrospective study. Third, due to missing data, the cumulative dose of MTX could only be considered as descriptive. Fourth, the ratio of patients with and without ILD varies between countries resulting in a potential bias of confounding by centre. Fifth, some physicians may perform pulmonary function testing before MTX initiation in order to avoid MTX prescription in patients with abnormal lung function. Such evaluation was not assessed in our study, inducing a possible prescription bias. In order to avoid such bias, confounders such as age at RA onset, sex, ever-smoking, biologics ever-use, participating centre, MTX exposure duration and periods of RA onset were taken into account using a regression adjustment. However, it is possible that all the confounding factors that affect the observed inverse relationship between MTX ever-use and RA-ILD could not be taken into account.
Altogether, these results suggest that MTX could be considered as having a disease-modifying effect on RA-ILD that could result from distinct anti-inflammatory mechanisms, involving 1) a direct immune-suppressive effect of MTX specifically targeting the lung, similar to that observed in some other immune-mediated pulmonary diseases such as sarcoidosis [36]; and 2) an indirect effect related to MTX-driven decrease of RA-related systemic inflammation. Indeed, previous studies have reported that higher RA disease activity is associated with an increased risk of extra-articular manifestations including ILD [37–39] and a recent study confirmed that active RA was associated with an increased risk for developing RA-ILD [40]. It is worth noting that we did not observe a difference in biological agents ever-use between RA-ILD and RA-noILD patients, suggesting that the inverse relationship detected in this study may be specific to MTX as compared to other DMARDs. However, even if the strength of the inverse relationship and the reproducibility in the different populations investigated are in favour of a causal relationship, our study only describes a statistical relationship and did not provide definitive evidence for causality. Indeed, the observed effect may be due to an unexpected confounder that was not taken into account in our statistical analysis. Although we acknowledge that a prospective study would be required to further establish that MTX ever-use delays the onset of RA-ILD, such a trial is unlikely to be performed due to the central role of MTX in the management of RA.
Our results suggest that MTX use is not associated with an increased risk of RA-ILD in patients with RA, and that ILD was detected later in MTX-treated patients.
Supplementary Material
Acknowledgments
V.M. Holers reports grants from NIH/NIAID (U01 Grant), during the conduct of the study. C. Boileau has nothing to disclose. M-P. Debray reports personal fees and non-financial support for travel to meetings from Boehringer Ingelheim and Roche, outside the submitted work. R. Porcher has nothing to disclose. D.A. Schwartz reports grants from NIH-NHLBI (P01 HL092870, R01 HL097163, R33 HL120770 and UH2 HL123442) and DOD Focused Program (W81XWH-17-1-0597), during the conduct of the study; personal fees for consultancy and advisory board work from NuMedii, Inc., and is an employee of Eleven P15, Inc., outside the submitted work; and has a patent Compositions and Methods of Treating or Preventing Fibrotic Diseases pending, a patent Biomarkers for the Diagnosis and Treatment of Fibrotic Lung Disease pending, and a patent Methods and Compositions for Risk Prediction, Diagnosis, Prognosis, and Treatment of Pulmonary Disorders issued. R. Vassallo reports grants from Pfizer, Bristol-Myers-Squibb and SunPharma, outside the submitted work. B. Crestani reports grants from Apellis and MedImmune, grants and personal fees for lectures from Boehringer Ingelheim and Roche, personal fees for lectures from AstraZeneca and Sanofi, outside the submitted work. P. Dieudé reports fees for consultancy from Pfizer, Abbvie and MSD, grants and personal fees for consultancy and lectures from Roche, Chugai and BMS, outside the submitted work.
Support statement:
Supported by grants from Société Française de Rhumatologie, National Heart, Lung, and Blood Institute (K23-HL138131 and K23-HL119558). Funding information for this article has been deposited with the Crossref Funder Registry.
Footnotes
@ERSpublications
This multi-ethnic case–control study showed that methotrexate use is not associated with an increased risk of interstitial lung disease in patients with rheumatoid arthritis https://bit.ly/3fC8skd
Conflict of interest: P-A. Juge has nothing to disclose. J.S. Lee reports grants from NIH, personal fees for advisory board work from Genentech and Celgene, outside the submitted work. J. Lau has nothing to disclose. L. Kawano-Dourado has nothing to disclose. J. Rojas-Serrano has nothing to disclose. M. Sebastiani has nothing to disclose. G. Koduri has nothing to disclose. E. Matteson has nothing to disclose. K. Bonfiglioli has nothing to disclose. M. Sawamura has nothing to disclose. R. Kairalla has nothing to disclose. L. Cavagna has nothing to disclose. E. Bozzalla Cassione has nothing to disclose. A. Manfredi has nothing to disclose. M. Mejia has nothing to disclose. P. Rodríguez-Henriquez has nothing to disclose. M.I. González Pérez has nothing to disclose. R. Falfán-Valencia has nothing to disclose. I. Buendia-Roldán has nothing to disclose. G. Pérez-Rubio has nothing to disclose. E. Ebstein reports personal fees from Sanofi, outside the submitted work. S. Gazal has nothing to disclose. R. Borie reports grants and personal fees for lectures from Roche and Boehringer Ingelheim, outside the submitted work. S. Ottaviani has nothing to disclose. C. Kannengiesser has nothing to disclose. B. Wallaert reports grants and personal fees for advisory board work and meeting attendance from Boehringer Ingelheim and Roche, outside the submitted work. Y. Uzunhan reports personal fees from Roche and Boehringer Ingelheim, non-financial support from Oxyvie, outside the submitted work. H. Nunes has nothing to disclose. D. Valeyre reports personal fees for advisory board work from Roche and Boehringer Ingelheim, personal fees for lectures from AstraZeneca, outside the submitted work. N. Saidenberg-Kermanac’h has nothing to disclose. M-C. Boissier has nothing to disclose. L. Wemeau-Stervinou reports personal fees for lectures and travel support from Roche, personal fees for lectures and advisory board work, and travel support from Boehringer-Ingelheim, personal fees for lectures from Janssen-Cilag and Bristol-Myers-Squibb, outside the submitted work. R.M. Flipo reports grants and personal fees from Roche Chugai, Abbvie and Pfizer, personal fees from Bristol-Meyers Squibb, outside the submitted work. S. Marchand-Adam reports fees for research, lectures, meeting attendance, consultancy and advisory board work from Roche, Boehringer Ingelheim and Novartis, outside the submitted work. P. Richette reports personal fees from Ipsen/Menarini, AstraZeneca, Savient and Grünenthal, outside the submitted work. Y. Allanore reports personal fees from Actelion, Bayer, Bristol-Myers Squibb, Boehringer and Inventiva, grants from Sanofi and Roche, outside the submitted work. C. Dromer has nothing to disclose. M-E. Truchetet has nothing to disclose. C. Richez has nothing to disclose. T. Schaeverbeke has nothing to disclose. H. Lioté has nothing to disclose. G. Thabut reports personal fees from AstraZeneca, outside the submitted work. K.D. Deane has nothing to disclose. J. Solomon has nothing to disclose. T. Doyle has nothing to disclose. J.H. Ryu has nothing to disclose. I. Rosas reports personal fees for advisory board work from Genentech, Boehringer and Three Lakes Partners, outside the submitted work.
References
- 1.Hyldgaard C, Hilberg O, Pedersen AB, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 2017; 76: 1700–1706. [DOI] [PubMed] [Google Scholar]
- 2.Doyle TJ, Lee JS, Dellaripa PF, et al. A roadmap to promote clinical and translational research in rheumatoid arthritis-associated interstitial lung disease. Chest 2014; 145: 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavagna L, Monti S, Grosso V, et al. The multifaceted aspects of interstitial lung disease in rheumatoid arthritis. Biomed Res Int 2013; 2013: 759760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011; 183: 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017; 76: 960–977. [DOI] [PubMed] [Google Scholar]
- 6.Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res 2016; 68: 1–25. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan RL, Waite DH. Progressive interstitial lung disease from prolonged methotrexate therapy. Arch Dermatol 1978; 114: 1800–1802. [PubMed] [Google Scholar]
- 8.Phillips TJ, Jones DH, Baker H. Pulmonary complications following methotrexate therapy. J Am Acad Dermatol 1987; 16: 373–375. [DOI] [PubMed] [Google Scholar]
- 9.Carson CW, Cannon GW, Egger MJ, et al. Pulmonary disease during the treatment of rheumatoid arthritis with low dose pulse methotrexate. Semin Arthritis Rheum 1987; 16: 186–195. [DOI] [PubMed] [Google Scholar]
- 10.Sathi N, Chikura B, Kaushik VV, et al. How common is methotrexate pneumonitis? A large prospective study investigates. Clin Rheumatol 2012; 31: 79–83. [DOI] [PubMed] [Google Scholar]
- 11.Conway R, Low C, Coughlan RJ, et al. Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheumatol 2014; 66: 803–812. [DOI] [PubMed] [Google Scholar]
- 12.Sebastiani M, Manfredi A, Cerri S, et al. Radiologic classification of usual interstitial pneumonia in rheumatoid arthritis-related interstitial lung disease: correlations with clinical, serological and demographic features of disease. Clin Exp Rheumatol 2016; 34: 564–565. [PubMed] [Google Scholar]
- 13.Kiely P, Busby AD, Nikiphorou E, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open 2019; 9: e028466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gochuico BR, Avila NA, Chow CK, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med 2008; 168: 159–166. [DOI] [PubMed] [Google Scholar]
- 15.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 2009; 68: 1100–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juge PA, Borie R, Kannengiesser C, et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J 2017; 49: 1602314. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315–324. [DOI] [PubMed] [Google Scholar]
- 18.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010; 69: 1580–1588. [DOI] [PubMed] [Google Scholar]
- 19.Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology 2010; 49: 1483–1489. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Searles G, McKendry RJ. Methotrexate pneumonitis in rheumatoid arthritis: potential risk factors. Four case reports and a review of the literature. J Rheumatol 1987; 14: 1164–1171. [PubMed] [Google Scholar]
- 22.Firth D Bias reduction of maximum likelihood estimates. Biometrika 1993; 80: 27–38. [Google Scholar]
- 23.Weinblatt ME, Coblyn JS, Fox DA, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 1985; 312: 818–822. [DOI] [PubMed] [Google Scholar]
- 24.Kwoh CK, Simms RW, Anderson CJ, et al. Guidelines for the management of rheumatoid arthritis: American College of Rheumatology ad hoc Committee on Clinical Guidelines. Arthritis Rheum 1996; 39: 713–722. [PubMed] [Google Scholar]
- 25.Weinblatt ME. Methotrexate for chronic diseases in adults. N Engl J Med 1995; 332: 330–331. [DOI] [PubMed] [Google Scholar]
- 26.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008; 59: 762–784. [DOI] [PubMed] [Google Scholar]
- 27.Imokawa S, Colby TV, Leslie KO, et al. Methotrexate pneumonitis: review of the literature and histopathological findings in nine patients. Eur Respir J 2000; 15: 373–381. [DOI] [PubMed] [Google Scholar]
- 28.Hyldgaard C, Ellingsen T, Hilberg O, et al. Rheumatoid arthritis-associated interstitial lung disease: clinical characteristics and predictors of mortality. Respiration 2019; 98: 455–460. [DOI] [PubMed] [Google Scholar]
- 29.Conway R, Low C, Coughlan RJ, et al. Methotrexate use and risk of lung disease in psoriasis, psoriatic arthritis, and inflammatory bowel disease: systematic literature review and meta-analysis of randomised controlled trials. BMJ 2015; 350: h1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fragoulis GE, Conway R, Nikiphorou E. Methotrexate and interstitial lung disease: controversies and questions. A narrative review of the literature. Rheumatology 2019; 58: 1900–1906. [DOI] [PubMed] [Google Scholar]
- 31.Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics – a large multicentre UK study. Rheumatology 2014; 53: 1676–1682. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Zhou Y, Chen X, et al. A metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J Rheumatol 2014; 41: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 33.Juge PA, Lee JS, Ebstein E, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med 2018; 379: 2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manfredi A, Cassone G, Cerri S, et al. Diagnostic accuracy of a velcro sound detector (VECTOR) for interstitial lung disease in rheumatoid arthritis patients: the InSPIRAtE validation study (INterStitial pneumonia in rheumatoid ArThritis with an Electronic device). BMC Pulm Med 2019; 19: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 36.Baughman RP, Grutters JC. New treatment strategies for pulmonary sarcoidosis: antimetabolites, biological drugs, and other treatment approaches. Lancet Respir Med 2015; 3: 813–822. [DOI] [PubMed] [Google Scholar]
- 37.Restrepo JF, del Rincón I, Battafarano DF, et al. Clinical and laboratory factors associated with interstitial lung disease in rheumatoid arthritis. Clin Rheumatol 2015; 34: 1529–1536. [DOI] [PubMed] [Google Scholar]
- 38.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010; 62: 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyhäll-Wåhlin BM, Petersson IF, Nilsson JA, et al. High disease activity disability burden and smoking predict severe extra-articular manifestations in early rheumatoid arthritis. Rheumatology 2009; 48: 416–420. [DOI] [PubMed] [Google Scholar]
- 40.Sparks JA, He X, Huang J, et al. Rheumatoid arthritis disease activity predicting incident clinically apparent RA-associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol 2019; 71: 1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


