Abstract
Objectives
This community-based cross-sectional study aimed to identify the frailty prevalence and associated socio-demographic factors among older adults in five cities of the south west of Iran.
Methods
We selected a random sample of adults aged 60 years and above from five Southwest cities in Iran. Data for this study were retrospectively collected from 540 community-dwelling older adults. To measure frailty, we utilized the frailty index of cumulative deficit (FICD). Data were collected from medical records and socio-demographic factors, including gender, age, marital status, education level, lifestyle, income, and job status. The chi-square test and Spearman’s correlation coefficient test were used to assess the relationship between the demographic variables and frailty status (SPSS version 22). Also, multiple binary logistic regression models were used to estimate the effects of demographic characteristics on the frailty recurrence.
Results
The overall frailty prevalence was as follows: 77 (14.3%) frail, 139 (25.7%) pre-frail, and 324 (60%) not frail. The findings showed that all variables except education level and marital status are significantly associated with frailty status (P < 0.05). Multiple ridge logistic regression model indicated that age, gender, marital status, job status have significant, and education level, living arrangement, and economic status have no considerable effect on the frailty.
Conclusions
This study has shown that age and gender significantly contributed to the frailty process in older adults. The research also has shown the syndrome’s occurrence affected by the aging process, and it supports the biological characteristics of frailty.
Keywords: Frailty, Iran, Older adults, Prevalence
Introduction
Due to economic and social development, the enhanced life expectancy, and improvements in medical treatment methods and technologies for most chronic diseases, the world’s inhabitants are aging fast. Unlike the developed countries, the developing countries have a higher aging rate so that half of the world’s older population inhabits these territories [1]. Nowadays, 9.3% of Iran’s inhabitants comprise older adults, and this figure is estimated to reach 26% by 2050 [2]. This trend led to significant outcomes for the population and older adults. A very challenging consequence is the growing prevalence of frailty amongst older adults [3].
Frailty is considered an increasing physiological weakening in multiple body systems and specifies by function loss, physiologic reserve loss, and intensified exposure to poor health outcomes [4]. Frailty develops as a consequence of physiological changes that are typically associated with aging, absence of physical activities, and malnutrition and can raise the risk of adverse effects such as disability, institutionalization, and death, as well as lowering the life quality of the affected older adults [5].
There are two leading attitudes to define frailty. One of these two approaches is the Frailty Phenotype (FP), which focuses on physical manifestations. Frailty appears when there are three or more criteria: an unintentional loss of weight, a weak strength of grip, self-reported exhaustion, slowness, and a low level of physical activity [6]. The cumulative deficit model is based on mathematics so that frailty is the ratio of deficits in a person and is characterized as a frailty index [7]. There are several methods reported in the literature that are quick and easy to assess frailty [8]. Most of these proposed tools are time-consuming, costly, and need complex equipment and healthcare experts to assess frailty. Most of these screening tools are self-reported, validated and non-health professionals can interpret the result. However, the diagnostic accuracy and value of some of these screening tools have not yet been validated. No research has accurately introduced an ideal screening tool that would help healthcare professionals assess frailty so far. Screening should be done to identify the risk and suggest the best intervention for people at frailty risk. From this viewpoint, using a screening tool that is not time-consuming, costly, and easy to use would guide healthcare professionals to develop the best interventions for people who are at risk of frailty. Among several screening tools proposed so far, the frailty index of cumulative deficit (FICD) is considered a more sensitive and multidimensional predictor. Using the deficit approach is more challenging due to more significant number of variables required. However, it was argued that, compared to Fried’s frailty index, a FICD is a more sensitive tool for adverse health outcomes due to its more sufficient graded scale and multidimensionality [9].
Research on frailty and its related factors is growing rapidly. Although psychosocial vulnerability has been linked to frailty [10], socio-demographic factors are also essential to assess. Exploring socio-demographic risk factors and their impact on frailty will be very useful to help healthcare professionals develop effective strategies to reduce medical costs in older adults and plan interventions to prevent mortality risk and morbidity [11]. Some socio-demographic factors are prominent amongst the factors affecting the development of frailty, even though the healthcare team often disregards them. In evaluating older adults, these factors should be systematically considered. The greater frailty rates in older adults have been detected in developing countries [12]. For instance, in Mexico and Peru, studies have shown the association between frailty and socio-demographic variables, such as gender and age [13]. Studies conducted on the frailty in the older adults population have been mostly in Western countries; however, similar studies have rarely been conducted in developing countries, including Iran, and the health conditions have been somewhat neglected in older age. Considering that the population of different countries could experience different levels of frailty due to cultural, regional, or political distinctions [14], older adults in Iran have low education and cannot gain adequate facilities and income. Therefore, considering the importance of old age and the growing trend of this group in the country, detecting frailty and related factors can help clarify the situation and better planning for older adults [15].
There are very few studies on frailty in Iran, and only two studies have been conducted in this field so far [16]. Thus, this study aimed to investigate the prevalence of frailty in the Iranian population, and the association between frailty syndrome and socio-demographic characteristics in them.
Materials and methods
Study design, setting, and population
This cross-sectional study was conducted on a proportional stratified sample consisting of 540 community-dwelling older adults (≥60 years) in five southwestern cities in Iran (Ahvaz, Bandar-e Mahshahr, Susangerd, Dezful, and Masjed Soleyman). We selected those old adults living in both the metropolitan and rural areas and had health-condition and medical records at healthcare centers. Older adults were randomly selected and invited to centers for interview. To measure frailty, the Accumulative Deficits Frailty Index (FI) was utilized. Data were collected from medical records and socio-demographics such as gender, age, marital status, education level, living arrangement, income, and job status. All participants were interviewed for the Reported Index of Accumulative Deficits (details are presented in Frailty definition). The participants read and signed the informed consent before participating and agreed to be available for further research. Well-trained interviewers conducted interviews with participants to reduce interviewer bias.
Inclusion criteria
Community-dwelling older people age 60 years and above, those who were willing to participate in the study, those who were able to provide voluntary informed consent, and those who were able to communicate were included.
Exclusion criteria
Those individuals who were dissatisfied with continuing to study, individuals with a terminal illness, individuals with the bed or wheelchair restrictions, individuals having a severe hearing loss or visual impairment, and other acute disease at the time of examination were excluded.
Sample size estimation
The following formula was used to calculate the sample size
Given the global prevalence of 10.7 and the power of 97%, we needed at least 408 samples. So, by adding 10%, samples selected for shedding were 449; however, 540 samples were finally selected.
Measurement of frailty
To measure frailty, we utilized the Accumulative Deficits Frailty Index (FI). The frailty instrument was the “Index of Accumulative Deficits” developed by Kenneth Rockwood. A frailty index counts deficits in health. These deficits were defined as symptoms, signs, disabilities, and diseases. All health deficits, including Activities Daily Living (ADL, is a term used in healthcare to refer to people’s daily self-care activities) [17] and Instrumental ADL (IADL, include more complex activities that are related to the ability to live independently in the community. This would include activities such as e.g., managing finances and medications, food preparation, housekeeping, laundry) [18], impairments in general cognition (a person has trouble remembering, learning new things, concentrating, or making decisions that affect their everyday life) [19], physical performance (such as walk speed, grip strength, and chair rise time are useful as part of the frailty assessments and powerful individual predictors of adverse outcomes in a wide range of older populations) [20], co-morbidity (two or more disorders or illnesses occurring in the same person) [21], self-rated health (also called Self-reported health, self-assessed health, or perceived health refers to both a single question such as “in general, would you say that your health is excellent, very good, good, fair, or poor?”) [22], and depression/mood (is a state of low mood and aversion to activity. It can affect a person’s thoughts, behavior, motivation, feelings, and sense of well-being) [22] were evaluated [8].
All binary variables were recorded using the convention that ‘0’ indicated the absence of the deficit, and ‘1’ was the presence of a deficit. For variables that included a single intermediate response (e.g. ‘sometimes’ or ‘maybe’), the additional value of ‘0.5’ was used. Frailty index variables can also accommodate ordinal and continuous variables as deficits.
To do so requires grading the continuum or rank into a score between 0 (where no deficit is present) and 1 (where the given variable maximally expresses the deficit). For some variables, this re-coding is self-evident. Consider the widely used Self-rated Health Question (“How would you rate your health? Excellent, Very Good, Good, Fair, Poor”). To grade this between ‘0’ and ‘1’, each lower self-rating of health was coded to represent a larger deficit (“Excellent = 0”, “Very Good = 0.25”, “Good = 0.5”, “Fair = 0.75” and “Poor = 1”). Similarly, recognized cut-points can be used for ordinal and continuous variables, such as the rapid walk test. For the MMSE, we recorded deficits, according to the severity of impairment. We assigned 1 for scores less than 10, denoting severe dementia, 0.75 for scores ≥10 and ≤ 17, denoting moderate dementia, 0.5 for scores ≥18 and ≤ 20, denoting mild dementia, 0.25 for scores >20 and < 24, denoting mild cognitive impairment (MCI), 0 for scores ≥24, and denoting no cognitive impairment. Some readers might object that a score of ‘1’ seems something of a discount (not a sufficiently high count) for severe dementia and that losing only one point for it, compared with 0.25 points for MCI, is not valid on its face. It was considered that a person with severe dementia is likely to have many more deficits than a person with MCI, e.g., more disability, poorer physical performance, higher degrees of behavioral problems, and so forth [8].
Statistical analysis
The demographic characteristics are presented as frequencies in the contingency table. The chi-square test and Spearman’s correlation coefficient test were used to assess the relationship between the demographic variables and frailty status. Multiple binary logistic regression models were used to estimate the effects of demographic characteristics on the frailty’s recurrence. For modeling, multicollinearity between predictor variables was assessed using the variance inflation factor (VIF). Owing to this problem, multiple ridge logistic regression model is used instead of traditional multiple binary logistic regression model for estimating the adjusted effects of demographic characteristics on the occurrence of the frailty (frailty was categorized as a binary outcome variable based on two categories: nonfrail and prefrail/frail). Hosmer-Lemeshow goodness of fit (GOF) test was used to assess the goodness of fit of the logistic regression model. Discrimination performance measures such as sensitivity, specificity, positive and negative predictive value, Youden’s Index, accuracy, area under the curve (AUC) were used to determine the discrimination power of the logistic regression model. This model has good discrimination performance, if it has sensitivity, specificity, positive and negative predictive value, Youden’s Index, and accuracy near to one. Also, the receiver operating characteristic curve analysis was used to calculate the AUC of the model. AUC indicates an overall good performance measure, and a perfect diagnostic discrimination model has an AUC equal to one. Differences were regarded as statistically significant when p < 0.05. Data analysis was done using SPSS version 22 and R3.5.3 software.
Ethical issues
The study is based on the approval of the Medical Ethics Committee of University of Social Welfare and Rehabilitation Sciences, Iran (reference number: IR.USWR.REC.1398.365). All study subjects signed informed consent. All methods were performed in accordance with the relevant guidelines and the institution regulations.
Results
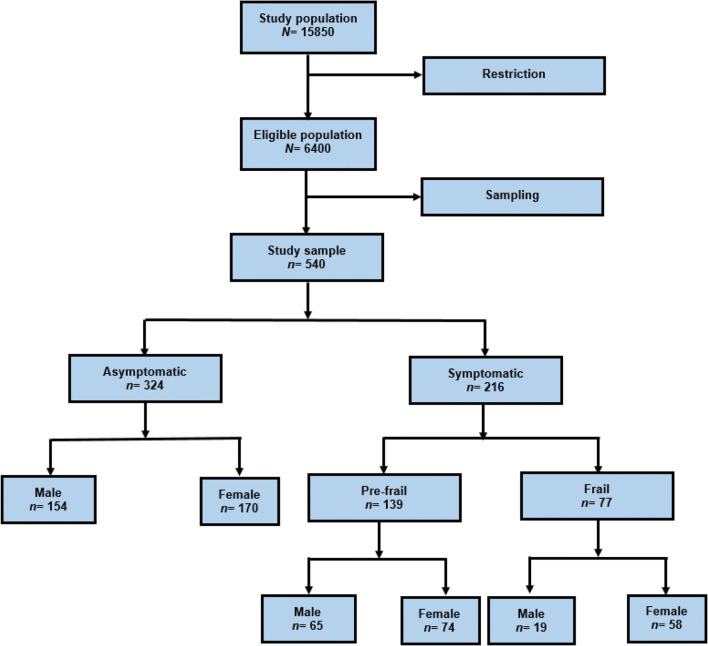
Total 540 older adults with a mean (±SD) age of 72.61 ± 8.72 with an age range of 60 to 93 participated in this study. Also, 302 (55.93%) and 238 (44.07%) of patients were women and men, respectively (Fig. 1). The prevalence of frailty in older adults was 77 (14.3%), and 139 (25.7%) of the participants were pre-frail and 324 (60%) of them were not frail. The characteristics of the study participants are shown in (Table 1). A total of 62.59% of the participants were married. A total of 58.7% of the participants had a middle school or high school degree, and 6.7% of them were living alone. Also, 57.1% of the participants reported an income average (Table 1). Spearman’s correlation coefficient test showed that there is a statistically significant positive weak correlation between age and frailty status (r = 0.435, P < 0.001) (Table 1). This test also indicated a non-significant negative correlation between economic status and frailty status (r = −0.02, P = 0.645). Table 1 indicated that there is no significant association between marital status (χ2(1) = 0.02, P = 0.887) and education level (r = −0.084, P = 0.051) with frailty status. Also, this table showed that there is a statistically significant association between some variables such as gender (χ2(1) = 9.605, P = 0.002), job status (χ2(1) = 15.024, P < 0.001) and living arrangement status (χ2(1) = 7.323, P = 0.007) with frailty status.
Fig. 1.
Flow diagram for patient selection
Table 1.
Association between frailty and sociodemographic characteristics in participants by levels of frailty
| Variable | Category | n (%) | no frailty(%) | pre-frailty(%) | frailty(%) | p value |
|---|---|---|---|---|---|---|
| Age | 60–74 | 308 (57.04) | 232 (71.6) | 64 (46) | 12 (15.6) | < 0.001* |
| 75–84 | 157 (29.07) | 75 (23.1) | 54 (38.8) | 28 (36.4) | ||
| > = 85 | 75(13.89) | 17 (5.2) | 21 (15.1) | 37 (48.1) | ||
| Gender | Female | 302(55.93) | 170 (52.5) | 74 (53.2) | 58 (75.3) | 0.002** |
| male | 238(44.07) | 154 (47.5) | 65 (46.8) | 19 (24.7) | ||
| Marital status | Married | 338(62.59) | 216 (67.7) | 90 (64.7) | 32 (41.6) | 0.887** |
| Single | 38(7.04) | 16 (4.9) | 8 (5.8) | 14 (18.2) | ||
| Widowed | 164(30.37) | 92 (28.4) | 41 (29.5) | 31 (40.3) | ||
| Education level | Elementary or below | 112(20.7) | 63 (19.4) | 29 (20.9) | 20 (26) | 0.051* |
| Middle school or high school | 317(58.7) | 187 (57.7) | 81 (58.3) | 49 (63.6) | ||
| College or above | 111(20.6) | 74 (22.8) | 29 (20.9) | 8 (10.4) | ||
| Living arrangement | Alone | 36(6.7) | 16 (4.9) | 9 (6.5) | 11 (14.3) | 0.007** |
| Living with others | 504(93.3) | 308 (95.1) | 130 (93.5) | 66 (85.7) | ||
| Economic status | Weak | 129 (23.9) | 74 (22.8) | 33 (23.7) | 22 (28.6) | 0.645 |
| Average | 198(36.7) | 121 (37.3) | 54 (38.8) | 23 (29.9) | ||
| Good | 213(39.4) | 129 (39.8) | 52 (37.4) | 32 (41.6) | ||
| Job status | Part−/full-time employment | 90(16.7) | 73 (22.5) | 15 (10.8) | 2 (2.6) | < 0.001* |
| Homemaker | 245(45.4) | 141 (43.5) | 62 (44.6) | 42 (54.5) | ||
| Unemployed/retired | 205(38) | 110 (34) | 62 (44.7) | 33 (42.9) |
* Trend chi-square test
** Spearman’s coefficient correlation test
The results of univariate binary logistic regression and multiple ridge binary logistic regression for estimating unadjusted and adjusted odds ratios (ORs) are shown in Table 2. Table 2 indicated that the age variable has a significant effect on the occurrence of frailty. The occurrence of frailty is equal to 1.625 times for participants with an age range of 74–85 compared to participants with an age range of 60–74 (OR = 1.625,95%CI: 1.08–2.83, P < 0.001). The occurrence of frailty is equal to 2.719 times for participants with age higher than 85 compared to participants with an age range of 60–74 (OR = 2.719, P < 0.001). According to this table, OR of occurrence of frailty is equal to 1.202 times for women as compared to men, and the variable gender has no significant effect on OR of occurrence of frailty (OR = 1.202, 95%CI: 0.98–2.21, P = 0.051). The findings in Table 2 indicated that homemaker older adults are more likely to be frailer than part−/full-time employment persons (OR = 1.178, 95%CI: 0.42–0.97, P = 0.052) and OR of occurrence of frailty in unemployed/retired persons is significantly more than persons with part−/full-time employment (OR = 1.303, 95%CI: 1.49–3.39, P = 0.002). Single older adults are significantly more vulnerable than married persons (OR = 1.175, P = 0.042) and OR of occurrence of frailty is equal to 1.011 times for single Elderly as compared to widow persons (OR = 1.202, P = 0.051). Also, multiple ridge logistic regression model indicated that other predictor variables such as education level, living arrangement and economic status have not significant effect on the occurrence of the frailty (P > 0.05) (Table 2).
Table 2.
Ridge binary logistic regression model to assess the effects of socio-demographic characteristics on the occurrence of frailty (OR: Odds Ratio, CI: Confidence Interval)
| Variable | Categories | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|
| β |
OR (95% CI) |
P value** | β |
OR (95% CI) |
P value*** | ||
| Age | 75–84 | 1.205 | 3.338 (2.23–5.03) | < 0.001 | 0.486 | 1.625 (1.08–2.83) | < 0.001 |
| >85 | 2.343 | 10.415 (5.84–19.46) | < 0.001 | 1.0004 | 2.719 (2.60–4.11) | < 0.001 | |
| 60–74* | |||||||
| Gender | Female | 0.353 | 1.424 (1.01–2.02) | 0.048 | 0.184 | 1.202 (0.98–2.21) | 0.051 |
| *Male | |||||||
| Marital Status | Married | - 0.889 | 0.411 (0.21–0.81) | 0.011 | - 0.162 | 0.851 (0.42–0.97) | 0.042 |
| Widow | - 0.564 | 0.569 (0.28–1.16) | 0.122 | - 0.011 | 0.989 (0.43–1.21) | 0.896 | |
| Single* | |||||||
| Education Level | Middle school or high school | - 0.112 | 0.894 (0.58–1.39) | 0.613 | - 0.009 | 0.991 (0.56–1.49) | 0.923 |
| College or above | - 0.442 | 0.643 (0.37–1.10) | 0.111 | - 0.088 | 0.916 (0.54–1.86) | 0.419 | |
| Elementary or below* | |||||||
| Living arrangement | Living with others | - 0.675 | 0.509 (0.25–1) | 0.052 | - 0.248 | 0.781 (0.37–1.81) | 0.187 |
| *Alone | |||||||
| Economic status | Average | - 0.155 | 0.856 (0.55–1.35) | 0.499 | - 0.039 | 0.961 (0.53–1.47) | 0.664 |
| Good | - 0.132 | 0.876 (0.56–1.37) | 0.559 | - 0.031 | 0.969 (0.56–1.55) | 0.729 | |
| Weak* | |||||||
| Job status | Homemaker | 1.153 | 3.167 (1.79–5.84) | < 0.001 | 0.164 | 1.178 (0.92–3.16) | 0.052 |
| Unemployed/retired | 1.311 | 3.709 (2.09–6.89) | < 0.001 | 0.265 | 1.303 (1.49–3.39) | 0.002 | |
| Part−/full-time employment* | |||||||
*: Reference category; **: Unadjusted effects are obtained from binary logistic regression model; ***: Adjusted effects are obtained from multiple ridge binary logistic regression model
Hosmer-Lemeshow goodness of fit test showed that goodness of fit of model is established (χ2(8) = 10.001, P = 0.265). Discrimination performance measures such as sensitivity, specificity, positive and negative predictive value, Youden’s Index, Accuracy, area under the curve (AUC) for determining the discrimination power of the logistic regression model are shown in Table 3 and according to AUC value, the model has good discrimination power. Also, Fig. 2 indicates the ROC curve for multiple ridge logistic regression models.
Table 3.
Discrimination performance measures with a 95% confidence interval for multiple binary logistic regression model
| Discrimination performance measures | Value | 95% CI |
|---|---|---|
| Area Under Curve (AUC) | 0.738 | 0.693 −0.782 |
| Sensitivity | 0.560 | 0.491 −0.628 |
| Specificity | 0.827 | 0.782 −0.867 |
| Positive Predictive Value (PPV) | 0.684 | 0.609 −0.751 |
| Negative Predictive Value (NPV) | 0.738 | 0.689−0.783 |
| Youden’s Index | 0.387 | 0.273 − 0.494 |
| Accuracy | 0.720 | 0.680 − 0.758 |
Fig. 2.
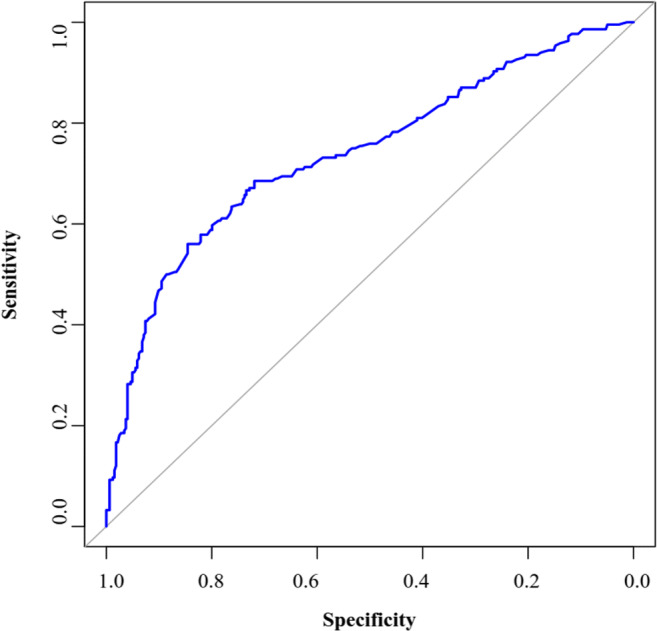
ROC curve for multiple ridge logistic regression model
Discussion
The present study results indicated that the prevalence rates of frailty and pre-frailty in the Iranian population were equal to 14.3% and 25.7%, respectively. Frailty is the main topic of the health care systems of older adults in an aging population, and this outcome has a negative effect on the quality of life of these people [23]. Frailty is related to increased risk of some adverse events such as disability, falls, dependency, hospitalization, institutionalization, and death [24–26]. Diagnosis of frailty is essential because it is a prevalent cause of death in community-dwelling [27].
Evidence from the latest systematic reviews and meta-analyses suggested a wide range of interventions, from physical activity, nutrition, geriatric assessment, or a combination of these delivered in community settings, and reported studies that integrated a physical activity element were reliably the most effective at improving frailty status or functional ability [28].
As a result of the exploration of effective factors on the occurrence of frailty is an important topic. Several studies have been conducted about frailty, but little is known about this problem in Iran. Therefore, the present study’s main aim was to estimate the prevalence of the level of frailty and examine the association between frailty status and demographic factors in the Iranian older population.
Different tools are defined to measure frailty status, and the present study used Rockwood’s frailty index for classifying frailty. According to this definition, the current study results indicated that the prevalence of frailty and pre-frailty were found equal to 14.3% and 25.7% in the Iranian population, respectively. Also, the prevalence of frailty levels has different values in different people due to various tools to measure frailty, differences between the study populations (inclusion and exclusion criteria), different study designs, sample sizes, and geographic regions. A systematic review and meta-analysis studies about the prevalence of frailty showed that frailty prevalence was from 8.6% to 50.9% [29] or 3.9% to 51.4%, and pre-frailty prevalence was from 13.4% to 71.6% [30].
It is not easy to compare the present study findings with other studies because each study reported different values for frailty prevalence using various tools to measure frailty. For example, Cedillo et al. estimated the frailty phenotype prevalence equal to 15.7% among 1933 older adults Mexican adults aged 60 years or older. Like the present study, their study indicated that female adults, older age, less educated, living alone, less income level, and live with others increase the incidence of frailty [31]. Also, using the Comprehensive Geriatric Assessment Frailty Index, Tang et al. indicated that the prevalence of frailty was equal to 9.9% among China’s 5844 dwelling peoples [32].
Simultaneous adjustment using multiple binary logistic regression models in the present study indicated that the only age predictor variable has a statistically significant effect on the occurrence of frailty, and indeed, the frequency of frail older adults significantly increases with age. This result was supported by other studies [31–33]. For example, in a cross-sectional survey, Kolankiewicz et al. showed that adjusting only the age variable has a statistically significant effect on frailty occurrence. They also showed that frailty prevalence using Fried’s criteria was equal to 17.7% among 555 Brazilian older adults aged ≥60 years [33]. Also, a longitudinal study on German adults showed this finding [34]. Association between age and frailty status supports the biological characteristics of frailty. Unlike other studies, Grden et al. showed that age is not significantly associated with frailty status among 243 Brazilian older adults. The prevalence of frailty in their study was reported equal to 14.8% [35].
Furthermore, Bergman et al. showed that the association between frailty status and sociodemographic variables (sex, education level, income level, and marital status) significant difference after adjusting the age variable [36]. Their study’s frailty prevalence was equal to 7.4% among 740 older adults aged 75 years and over. The present study also indicated that individuals with older age, women living alone, lower-income, and lower education levels increase the frailty. These findings were reported in other longitudinal studies and cross-sectional studies [37–39]. Though most of the studies which showed that individuals with older age, women, living alone, lower-income and lower education levels may increase risk of the frailty were cross-sectional, also cohort studies [40], as well as systematic reviews and meta-analyses [15, 41] confirmed it.
The association between education level and frailty status showed that education level was associated with frailty status, and these results and the frequency of frail older adults will increase with a lower level of education [42–44]. However, other studies did not show this relation [45]. As state in many previous studies, we found no significant association between education level and frailty. The association between education level is somewhat controversial, of which some studies showed is not associated with a higher number of frailty indicators [46], while some claimed that the education variables appeared as significant for the group of frail elderlies, frailty was related to the sociodemographic variables education, finally patients in the frail group had significantly lowest education level [34, 47]. The low education level reflects the deprivation of opportunities and inequality in people’s health conditions, especially in elder ages. Unsuitable socioeconomic situations such as a low level of education is a feature that is present in most debilitated people, who are more susceptible to health problems such as frailty [48].
Similarly, different studies reported that women are more vulnerable than men [49]. Unlike the present study, some studies did not show a significant effect of sex on the occurrence of frailty before or after adjusting other predictor variables [46], whereas other studies showed against this result [31]. We know that women have less muscle mass than men, and this problem interferes with functional physical capacity and other reasons such as hormonal changes and risk of osteoporosis and sarcopenia. So, women are becoming more vulnerable than men.
In the same line the other studies, the association between income level and frailty status showed that low income level no significantly increases the occurrence of frailty [32, 33], in contrast, other studies showed that this factor has no significant effect on the occurrence of frailty [33]. This could be explained that greater access to quality of healthcare confer a lower risk of frailty, which may relatively clarify the disparity in frailty incidence between low-middle income and high-income countries, giving chance to prevent or delay the beginning of chronic pathologies related to higher frailty risk [50].
Our findings showed that the age of participant may significantly associated with frailty, while Muszalik et al [50], reported non-significant correlation between age and frailty, hence, another two recent studies suggested a significant correlation between age and frailty [51, 52]. We know that frailty risk increases in association with age, which could be due to the biological than the chronological age of individuals. There is consequent an erosion of the homeostatic reserve and vulnerability to disproportionate changes in health status after relatively minor stress events. There is a continuous loss of strength and aerobic resistance, which causes a decrease in functional independence and makes the older adult frail. In general, frailty is superior to age in identifying at-risk older people [45, 53].
Similar to other studies, the findings showed that live alone increases the occurrence of frailty, and this factor has no significant effect on the occurrence of frailty [33], whereas other studies indicated that this variable decreases the occurrence of frailty and can explain that these individuals are more independent [31].All studies present that single persons are more likely to be frailer than married persons. Several studies indicated that marital status has a significant effect on the occurrence of frailty [31–33]; it is in contrast to earlier findings [46].
Limitations
The results of the present study can be useful to identify at-risk persons. This current survey, like other studies, has some Limitations. First, other study designs, such as cohort study or longitudinal study are necessary to obtain valuable information about the relationship between demographic characteristics and frailty status. Since this study was a cross-sectional study, associations between the outcome variable and predictor variables were assessed simultaneously. So, this design used in the present study cannot establish causal relationships. Hence, more studies are required for identifying frailty prevalence and its association with predictor variables in the Iranian population. A second and important limitation that in this study, is the multiple ridge logistic regression model was used to estimate the effects of adjusted demographic characteristics on the frailty event. The frailty status was also defined as a binary variable (non-frail and pre-frail/frail). Because the number of frail individuals in this study was low, 77 (14.3%), to estimate the adjusted effects of demographic characteristics on the occurrence of frailty, pre-frail individuals 139 (25.7%) and frail individuals in one class were defined. Otherwise, by defining the outcome variable as a categorical variable in the fit of the logistic model, the convergence of regression parameters was not possible. Third and important limitation was assessing conditions that could contribute to findings such as sarcopenia and cancer and their associated factors for instances energy and protein intake and physical activity. Considering that none of the included patients had sarcopenia, and only a very small fraction of patients (n = 5) had malignancy, therefore, our findings are likely generalizable to other settings.
Implications for clinical practice
In Iran, frailty has been less studied; so, timely identification of vulnerable seniors in order to determine the different needs of these individuals by specialists, physicians, and treatment staff is absolutely essential [53]. Also, it may hide the light on their need for a standard tool for designing and planning the appropriate interventions to prevent frailty in the older adults [28]. Given the considerable prevalence of frailty and its association with falls and fears of falling, it is important to identify frailty among the older adult population. Moreover, since frailty is reversible, early screening at the primary care center is recommended; moreover, planning for preventive interventions such as nutritional enrichment, regular and codified exercise program and physical activity, cognitive therapy, or a combination of the above-mentioned interventions is recommended [45]. Therefore, conducting prospective, national studies with larger sample size and long-term follow-up to evaluate the effectiveness of interventions in reducing falls or improving daily activities among the older adults population reported in Western populations, to extend in the Iranian population are recommended.
Conclusion
According to the findings, the prevalence of frailty in the older adults was close to 15%, which could be a warning sign to propose frailty as an important risk factor for older adults. Because the prevalence of frailty in older women is higher than in men, and this has been proven in most studies, the need to pay special attention to this group is of particular importance physiologically, socially and psychologically. Among other factors that had a significant relationship with the prevalence of frailty, low income, which should be considered measures in this regard, may lead to special consideration for strengthening the financial situation of older adults, as well as attracting the support of legislators and policymakers. Therefore, considering the undesirable consequences of frailty seems necessary to design interventions to prevent it. The results of this study can be useful for healthcare professionals to deliver intervention strategies and to delay the frailty status. Thus, these results may help to more accurately diagnose older adults who are at risk for frailty. The findings indicated that female gender, older age, single marital status, low income level, job status, and living alone may increase the incidence of frailty.
Acknowledgements
The authors wish to thank the participants for their involvement in this research.
Authors’ contributions
SS contributed to the concept and design, acquisition of data, analysis and interpretation of the data, drafting and revising the article. AD contributed to the acquisition of data, analysis and interpretation of the data, drafting and revising the article. NZ contributed to the concept and design, supervising acquisition of data, and revising the article critically for important intellectual content. YAM contributed to the concept and design, supervising acquisition of data, and revising the article critically for important intellectual content. The author(s) read and approved the final manuscript.
Funding
This work was supported by University of Social Welfare and Rehabilitation Sciences.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Corriere M, Rooparinesingh N, Kalyani RR. Epidemiology of diabetes and diabetes complications in the elderly: an emerging public health burden. Curr Diab Rep. 2013;13(6):805–813. doi: 10.1007/s11892-013-0425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noroozian M. The elderly population in Iran: an ever growing concern in the health system. Iran J Psychiatry Behav Sci. 2012;6(2):1–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Hamdi O, Chalouf MA, Ouattara D, Krief F. eHealth: survey on research projects, comparative study of telemonitoring architectures and main issues. J Netw Comput Appl. 2014;46:100–112. doi: 10.1016/j.jnca.2014.07.026. [DOI] [Google Scholar]
- 4.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 6.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World J. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nutakor JA, Gavu AK. Frailty screening tools: frail detection to primary assessment. Elderly Health Journal. 2020;6(1):63–68. [Google Scholar]
- 8.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 9.Andrew MK. Frailty and social vulnerability. In: Frailty in Aging, vol. 41: Karger Publishers; 2015. p. 186–95. 10.1159/000381236. [DOI] [PubMed]
- 10.de Labra C, Maseda A, Lorenzo-López L, López-López R, Buján A, Rodríguez-Villamil JL, Millán-Calenti JC. Social factors and quality of life aspects on frailty syndrome in community-dwelling older adults: the VERISAÚDE study. BMC Geriatr. 2018;18(1):66. doi: 10.1186/s12877-12018-10757-12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sánchez-García S, Sánchez-Arenas R, García-Peña C, Rosas-Carrasco O, Avila-Funes JA, Ruiz-Arregui L, Juárez-Cedillo T. Frailty among community-dwelling elderly Mexican people: prevalence and association with sociodemographic characteristics, health state and the use of health services. Geriatr Gerontol Int. 2014;14(2):395–402. doi: 10.1111/ggi.12114. [DOI] [PubMed] [Google Scholar]
- 12.Runzer-Colmenares FM, Samper-Ternent R, Al Snih S, Ottenbacher KJ, Parodi JF, Wong R. Prevalence and factors associated with frailty among Peruvian older adults. Arch Gerontol Geriatr. 2014;58(1):69–73. doi: 10.1016/j.archger.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harttgen K, Kowal P, Strulik H, Chatterji S, Vollmer S. Patterns of frailty in older adults: comparing results from higher and lower income countries using the Survey of Health, Ageing and Retirement in Europe (SHARE) and the Study on Global AGEing and Adult Health (SAGE) PloS one. 2013;8(10):e75847. doi: 10.71371/journal.pone.0075847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos-Eggimann B, Cuénoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64(6):675–681. doi: 10.1093/gerona/glp1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grden CRB, Lenardt MH, Sousa JAV, Kusomota L, Dellaroza MSG, Betiolli SE. Associations between frailty syndrome and sociodemographic characteristics in long-lived individuals of a community. Rev Lat Am Enfermagem. 2017;25:e2886. doi: 10.1590/1518-8345.1770.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mousavisisi M, Shamshirgaran SM, Rezaeipandari H, Matlabi H. Multidimensional approach to frailty among rural older people: applying the Tilburg frailty indicator. Elderly Health J. 2019;5(2):92–101. [Google Scholar]
- 17.Edemekong PF, Bomgaars DL, Levy SB: Activities of daily living (ADLs). 2017: https://www.ncbi.nlm.nih.gov/books/NBK470404/. [PubMed]
- 18.Guo HJ, Sapra A: Instrumental Activity of Daily Living (IADL). 2020: https://www.ncbi.nlm.nih.gov/books/NBK553126/. [PubMed]
- 19.Borson S. Cognition, aging, and disabilities: conceptual issues. Phys Med Rehabil Clin N Am. 2010;21(2):375–382. doi: 10.1016/j.pmr.2010.1001.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witham MD, Davies JI, Bärnighausen T, Bountogo M, Manne-Goehler J, Payne CF, et al. Frailty and physical performance in the context of extreme poverty: a population-based study of older adults in rural Burkina Faso. Wellcome Open Res. 2019;4. 10.12688/wellcomeopenres.15455.12681 PMID: 32280791; PMCID: PMC37137808. [DOI] [PMC free article] [PubMed]
- 21.Valderas J, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: Understanding health implication for and health services. Ann Fam Med. 2009;7(4):357–363. doi: 10.1370/afm.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bombak AE. Self-rated health and public health: a critical perspective. Public Health Front. 2013;1:15. doi: 10.3389/fpubh.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chand S, Arif H: Depression.[Updated 2019 Jan 7]. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing 2018:Available from: https://www.ncbi.nlm.nih.gov/books/NBK430847/.
- 24.Buckinx F, Rolland Y, Reginster J-Y, Ricour C, Petermans J, Bruyère O. Burden of frailty in the elderly population: perspectives for a public health challenge. Arch Public Health. 2015;73(1):19. doi: 10.1186/s13690-015-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C. Searching for an operational definition of frailty: a Delphi method based consensus statement. The frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 27.Morley JE, Vellas B, Van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea W, Doehner W, Evans J. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Mañas L, Fried LP. Frailty in the clinical scenario. Lancet. 2015;385(9968):e7–e9. doi: 10.1016/S0140-6736(14)61595-6. [DOI] [PubMed] [Google Scholar]
- 29.Dedeyne L, Deschodt M, Verschueren S, Tournoy J, Gielen E. Effects of multi-domain interventions in (pre) frail elderly on frailty, functional, and cognitive status: a systematic review. Clin Interv Aging. 2017;12:873–896. doi: 10.2147/CIA.S130794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puts MTE, Toubasi S, Andrew MK, Ashe MC, Ploeg J, Atkinson E, Ayala AP, Roy A, Rodríguez Monforte M, Bergman H. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46(3):383–392. doi: 10.1093/ageing/afw247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kidd T, Mold F, Jones C, Ream E, Grosvenor W, Sund-Levander M, Tingström P, Carey N. What are the most effective interventions to improve physical performance in pre-frail and frail adults? A systematic review of randomised control trials. BMC Geriatr. 2019;19(1):184. doi: 10.1186/s12877-12019-11196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apóstolo J, Cooke R, Bobrowicz-Campos E, Santana S, Marcucci M, Cano A, Vollenbroek-Hutten M, Germini F, D’Avanzo B, Gwyther H. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database System Rev Implement Rep. 2018;16(1):140–232. doi: 10.11124/JBISRIR-2017-003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha-Balcázar LJ, Cortes-Sarmiento DS, Castellanos-Perilla N, Núñez-Aguirre S, Salinas-Martínez R, Pérez-Zepeda MU: Systematic review and meta-analysis of frailty prevalence in Mexican older adults. 2018.
- 34.Casale-Martínez RI, Navarrete-Reyes AP, Ávila-Funes JA. Social determinants of frailty in elderly Mexican community-dwelling adults. J Am Geriatr Soc. 2012;60(4):800–802. doi: 10.1111/j.1532-5415.2011.03893.x. [DOI] [PubMed] [Google Scholar]
- 35.Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2018;8(3):e018195. doi: 10.1136/bmjopen-2017-018195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sánchez-García S, Sánchez-Arenas R, García-Peña C, Rosas-Carrasco O, Ávila-Funes JA, Ruiz-Arregui L, Juárez-Cedillo T. Frailty among community-dwelling elderly M exican people: prevalence and association with sociodemographic characteristics, health state and the use of health services. Geriatr Gerontol Int. 2014;14(2):395–402. doi: 10.1111/ggi.12114. [DOI] [PubMed] [Google Scholar]
- 37.Jürschik P, Nunin C, Botigué T, Escobar MA, Lavedán A, Viladrosa M. Prevalence of frailty and factors associated with frailty in the elderly population of Lleida, Spain: the FRALLE survey. Arch Gerontol Geriatr. 2012;55(3):625–631. doi: 10.1016/j.archger.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Poli S, Cella A, Puntoni M, Musacchio C, Pomata M, Torriglia D, Vello N, Molinari B, Pandolfini V, Torrigiani C. Frailty is associated with socioeconomic and lifestyle factors in community-dwelling older subjects. Aging Clin Exp Res. 2017;29(4):721–728. doi: 10.1007/s40520-016-0623-5. [DOI] [PubMed] [Google Scholar]
- 39.Vaingankar JA, Chong SA, Abdin E, Picco L, Chua BY, Shafie S, Ong HL, Chang S, Seow E, Heng D. Prevalence of frailty and its association with sociodemographic and clinical characteristics, and resource utilization in a population of Singaporean older adults. Geriatr Gerontol Int. 2017;17(10):1444–1454. doi: 10.1111/ggi.12891. [DOI] [PubMed] [Google Scholar]
- 40.Hajek A, Brettschneider C, Posselt T, Lange C, Mamone S, Wiese B, Weyerer S, Werle J, Fuchs A, Pentzek M. Predictors of frailty in old age–results of a longitudinal study. J Nutr Health Aging. 2016;20(9):952–957. doi: 10.1007/s12603-015-0634-5. [DOI] [PubMed] [Google Scholar]
- 41.Wong CH, Weiss D, Sourial N, Karunananthan S, Quail JM, Wolfson C, Bergman H. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: a cross-sectional study. Aging Clin Exp Res. 2010;22(1):54–62. doi: 10.1007/BF03324816. [DOI] [PubMed] [Google Scholar]
- 42.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 43.Avila-Funes JA, Helmer C, Amieva H, Barberger-Gateau P, Goff ML, Ritchie K, Portet F, Carrière I, Tavernier B, Gutiérrez-Robledo LM. Frailty among community-dwelling elderly people in France: the three-city study. J Gerontol A Biol Sci Med Sci. 2008;63(10):1089–1096. doi: 10.1093/gerona/1063.1010.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, Raat H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: a systematic review of longitudinal studies. PLoS One. 2017;12(6):e0178383. doi: 10.1371/journal.pone.0178383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walston J, Buta B, Xue Q-L. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med. 2018;34(1):25–38. doi: 10.1016/j.cger.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenardt MH, Carneiro NHK, Binotto MA, Setoguchi LS, Cechinel C. The relationship between physical frailty and sociodemographic and clinical characteristics of elderly. Escola Anna Nery. 2015;19(4):585–592. doi: 10.5935/1414-8145.20150078. [DOI] [Google Scholar]
- 47.Dokuzlar O, Soysal P, Isik AT. Association between serum vitamin B12 level and frailty in older adults. North Clin Istanb. 2017;4(1):22. doi: 10.14744/nci.12017.82787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fugate Woods N, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative observational study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 49.Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, Gasevic D, Ademi Z, Korhonen MJ, LoGiudice D. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Network Open. 2019;2(8):e198398. doi: 10.1001/jamanetworkopen.2019.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muszalik M, Gurtowski M, Doroszkiewicz H, Gobbens RJ, Kędziora-Kornatowska K. Assessment of the relationship between frailty syndrome and the nutritional status of older patients. Clin Interv Aging. 2019;14:773. doi: 10.2147/CIA.S201835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero-Ortuno R, Kenny RA. The frailty index in Europeans: association with age and mortality. Age Ageing. 2012;41(5):684–689. doi: 10.1093/ageing/afs1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji L, Qiao X, Jin Y, Si H, Liu X, Wang C. Age differences in the relationship between frailty and depression among community-dwelling older adults. Geriatr Nurs. 2020. 10.1016/j.gerinurse.2020.1001.1021 Epub 2020 Feb 1020. [DOI] [PubMed]
- 53.Council NR. Preparing for an aging world: the case for cross-national research: National Academies Press (US); 2001. [PubMed]