Abstract
Poplar 84 K (Populus alba x P. tremula var. glandulosa) is a good resource for genetic engineering due to its rapid growth and wide adaptability, and it is also an excellent ornamental tree species. In this study, we used 84 K plantlets grown in the nitrogen-limited medium as experimental materials to explore the molecular mechanism in 84 K leaves under nitrogen deficiency. A total of 5,868 differentially expressed genes (DEGs) were identified using the transcriptional information from RNA-seq data. GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment results revealed that the DEGs were mainly involved in energy metabolism and anthocyanin biosynthesis. We then identified differentially expressed transcription factors (TFs) and constructed TF centered gene co-expression networks for chlorophyll and anthocyanin biosynthesis pathway genes. Twenty potential regulators were finally identified. We speculated the transcription factors that control the pigmentation in leaves with the MYB-bHLH-WD40 (MBW) pigment regulatory model. Such identification will clarify the genetic basis of the secondary metabolism in 84 K, and being a source of candidate genes for future plant genetic engineering. Our work broadens the researchers' understanding of the regulation of anthocyanin synthesis in trees and provides new perspectives for ornamental 84 K poplar breeding.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01012-3.
Keywords: 84 K, Nitrogen deficiency, RNA-seq, Chlorophyll, Anthocyanin, Proanthocyanidin
Background
Nitrogen is an essential nutritional element in tree growth, development and forestry production. But due to the deficiency and low utilization efficiency of nitrogen in most plants, nitrogen deficiency stress has become one of the most common abiotic stresses in forestry. In recent years, many studies have been implemented to investigate the molecular mechanism of plants under nitrogen deficiency stress. Most of the researches focus on the effect of nitrogen deficiency on roots of plants (Carvalhais et al. 2011; Hsieh et al. 2018; Miller and Cramer 2005; Zhao et al. 2014), although a few studies have explored both shoots and roots (Sinha et al. 2018; Sevanthi et al. 2020). Only a limited number of studies on nitrogen deficiency have been reported in plant leaves. Plant leaves are the principal organs for photosynthesis, which generate carbohydrates and energy for the growth and cellular respiration of plants. A previous study (Nguyen and Dal Cin 2009) has shown that nitrogen stress has a strong impact on plant leaf development, especially on chlorophyll synthesis and photosynthesis. Chlorophylls and anthocyanins are the main typical pigments in plants, which are usually distributed in the cells of flowers, fruits, stems and leaves (Holton and Cornish 1995; Winkel-Shirley 2001). Pigments of plants not only contribute to the different colors of leaves but also participate in several physiological and biochemical processes in plants including UV protection, insect attraction, herbivore defense and symbiosis (Koes et al. 2005; Peters and Constabel 2002). Zhao et al. (2005) reported that N-deficiency affects plant hyperspectral reflectance properties, and giving the leaves reddening of sorghum. Schaberg et al. (2003) reported that leaves of sugar maple trees with low foliar N concentrations turned red earlier and more completely than those of trees with high foliar N concentrations. Afrousheh et al. (2010) reported that the petioles and midribs of N-deficient leaves will become red in pistachios.
Poplar 84 K (Populus alba x P. tremula var. glandulosa) is a fast-growing poplar hybrid. It originated in South Korea and resulted from a breeding program. It was introduced into China in 1984 and extensively cultivated in northern China (Qiu et al. 2019). Nitrogen deficiency activates a range of adaptive responses of trees due to nitrogen’s diverse roles in plant physiology and development (Horgan and Wareing 1980). However, the specific mechanism in 84 K leaves under nitrogen deficiency stress is unclear as of now. In recent years, more plant genomes have been sequenced, and they have had a major impact on plant research and improvement in a short time. However, most of the sequenced plant genomes are herbaceous, while whole-genome sequences of woody trees are still limited. Fortunately, the genome sequence of 84 K has become available in the last few years, which can be exploited in numerous ways. So, the purpose of the present study was to investigate it by transcriptome analysis and identification of the changes in the gene expression. This study will improve our understanding of the molecular mechanism in 84 K during nitrogen deficiency stress, and also lays the foundation for breeding ornamental varieties via molecular breeding.
Results
Transcriptome sequencing of 84 K
To obtain a global view of transcriptional profiles of 84 K under nitrogen deficiency stress, a total of 18 cDNA libraries from six time points, three replicates for each time point, were constructed for paired-end sequencing on Illumina platform. A total of 419,374,675 raw sequencing reads were generated from these cDNA libraries. After filtration of ambiguous nucleotides, low-quality and adapter sequences, a total of 417,599,086 clean reads were finally obtained. The clean reads were aligned to the 84 K reference genome (Qiu et al. 2019) using STAR (Spliced Trans Alignment to a Reference) (Dobin et al. 2013) and average of 81.60% reads were mapped to the reference genome in each sample. A summary of the alignment statistics for each sample is listed in Table 1. In addition, the RNA-Seq data set of the 18 samples were subjected to Principal component analysis (PCA) and the result is shown in Figure S1. The data showed a clear grouping of replicate samples, indicating similar gene expression between samples within treatment groups. The first principal component (PC1) accounted for 37.2% of the variance in gene expression between samples, and the second principal component (PC2) accounted for 16.1% of the total variance in gene expression between samples.
Table 1.
The summary of the alignment statistics
| Sample ID | Clean reads | Mapped reads | Mapped reads (%) |
|---|---|---|---|
| CK_rep1 | 33,289,042 | 29,514,406 | 88.66% |
| CK_rep2 | 29,416,612 | 26,564,956 | 90.31% |
| CK_rep3 | 35,990,830 | 32,632,266 | 90.67% |
| 2_days_rep1 | 21,939,024 | 18,169,486 | 82.82% |
| 2_days_rep2 | 20,943,478 | 17,165,358 | 81.96% |
| 2_days_rep3 | 21,706,290 | 18,117,634 | 83.47% |
| 4_days_rep1 | 20,057,432 | 15,724,726 | 78.40% |
| 4_days_rep2 | 19,834,662 | 15,983,400 | 80.58% |
| 4_days_rep3 | 21,723,402 | 17,425,938 | 80.22% |
| 6_days_rep1 | 29,294,264 | 23,504,584 | 80.24% |
| 6_days_rep2 | 22,484,540 | 18,762,168 | 83.44% |
| 6_days_rep3 | 22,001,258 | 17,301,192 | 78.64% |
| 8_days_rep1 | 20,035,224 | 16,333,940 | 81.53% |
| 8_days_rep2 | 20,462,966 | 17,016,138 | 83.16% |
| 8_days_rep3 | 23,561,108 | 19,452,752 | 82.56% |
| 10_days_rep1 | 24,755,934 | 15,465,218 | 62.47% |
| 10_days_rep2 | 23,795,572 | 19,325,304 | 81.21% |
| 10_days_rep3 | 25,243,756 | 19,825,704 | 78.54% |
Identification of differentially expressed genes
Differentially expressed genes (DEGs) in pairwise comparisons between nitrogen deficiency treated samples and control samples were identified using edgeR (Robinson et al. 2010). Using FDR < 0.05 and |log2FC|> 1 as thresholds, a total of 640 genes (321 up-regulated, 319 down-regulated) were identified as DEGs in 84 K after nitrogen deficiency treatment for 2 days, 179 genes (94 up-regulated, 85 down-regulated) in 4 days, 2,101 genes (652 up-regulated, 1,449 down-regulated) in 6 days, 5,311 genes (1,794 up-regulated, 3,517 down-regulated) in 8 days and 2,905 genes (1,013 up-regulated, 1,892 down-regulated) in 10 days, respectively (Fig. 1 and Table 2). According to the DEG numbers, a significant transcriptional reprogram was observed at 8 days after nitrogen deficiency stress. A total of 2,245 DEGs were specific to this time point. A relatively high number of DEGs were detected at 10 days after nitrogen deficiency stress. We also found that the transcriptional changes in 8 days and 10 days under nitrogen deficiency stress were similar, a total of 2,607 DEGs were detected at both of the two time points.
Fig. 1.

The Venn maps of differentially expressed genes (DEGs) of 84 K under nitrogen deficiency. 2 days: The number of DEGs compared between nitrogen deficiency treatment 2 days and the control group. 4 days: The number of DEGs compared between nitrogen deficiency treatment 4 days and the control group. 6 days: The number of DEGs compared between nitrogen deficiency treatment 6 days and the control group. 8 days: The number of DEGs compared between nitrogen deficiency treatment 8 days and the control group. 10 days: The number of DEGs compared between nitrogen deficiency treatment 10 days and the control group
Table 2.
The summary of the differentially expressed genes (DEGs)
| Group | DEGs | Up | Down |
|---|---|---|---|
| 2 days | 640 | 321 | 319 |
| 4 days | 179 | 94 | 85 |
| 6 days | 2,101 | 652 | 1,449 |
| 8 days | 5,311 | 1,794 | 3,517 |
| 10 days | 2,905 | 1,013 | 1,892 |
In addition, the Venn diagram displayed that 88 common DEGs (47 up-regulated, 41 down-regulated) were identified in the above five comparison groups. We also found a similar trend in the expression of these genes (Fig. 2). Furthermore, a total of 5,868 genes were differentially expressed in at least one time point, of which 2,130 and 3,738 genes were up-regulated and down-regulated, respectively. These DEGs were further mapped to biological processes to unveil the mechanism under nitrogen deficiency stress in 84 K.
Fig.2.
Differential expression profiles of the 84 K common DEGs under nitrogen deficiency. The samples were placed at a nitrogen-limited medium, and the total transcriptome information at 2 days, 4 days, 6 days, 8 days and 10 days was measured. CK is a control group and is cultured at MS medium. a 47 genes up-regulated out of 88 common DEGs in response to nitrogen deficiency stress. b 41 genes down-regulated out of 88 common DEGs in response to nitrogen deficiency stress
GO and KEGG enrichment analysis of the DEGs
GO enrichment has been widely used to reveal the molecular mechanism underlying transcriptional alterations (Gang et al. 2019; Wang et al. 2019, 2020). We performed GO enrichment analysis Wang for the up-regulated genes and down-regulated genes (Table S1), respectively. GO enrichment results of the 2130 up-regulated DEGs revealed that a total of nine biological processes were significantly enriched (Fig. 3), including starch metabolic process, cellular carbohydrate metabolic process, cellular aldehyde metabolic process, glucan catabolic process, cellular chemical homeostasis, cellular polysaccharide metabolic process, cellular polysaccharide catabolic process, response to gibberellin and proanthocyanidin biosynthesis process. A total of seven terms were significantly enriched in the molecular function domain, including UDP-glycosyltransferase activity, transferase activity, anion transmembrane transporter activity, inorganic molecular entity transmembrane transporter activity, protein binding, DNA-binding transcription factor activity and DNA binding. A total of nine terms were significantly enriched in the cellular component domain, including vacuolar membrane, endosome, trans-Golgi network, an integral component of membrane, plasmodesma, cytosol, endoplasmic reticulum, plasma membrane and nucleus. The results indicated that the expression of genes related to energy metabolism has been significantly affected under nitrogen deficiency stress. Of these GO terms, the cellular carbohydrate metabolic process is the most enriched one. Simultaneously, the enrichment of the amylase activity in the molecular function verifies that under nitrogen deficiency stress the demand for amylolysis activity is increased.
Fig. 3.
Upset plot of GO enrichment intersections between sets of genes induced (FDR < 0.05, log2FC > 2) by nitrogen-limited condition compared to the nitrogen-rich condition. The upper bar chart indicates the intersection size between sets of genes up-regulated with each GO term. Dark connected dots on the bottom panel indicate which substrates are considered for each intersection
Nitrogen-deficient stress reduces the overall cell activity and also affects macro-development such as cotyledon and flower organs to a certain extent, which leads to slowing plant growth. GO enrichment results of the 3,738 down-regulated DEGs indicated that a total of 69 biological processes were significantly enriched. Many pathways were reduced to varying degrees, including carbon fixation, quinone biosynthesis process, steroid biosynthesis process and chlorophyll biosynthesis process, etc. A total of 15 GO terms were significantly enriched in the molecular function category, including ATP-dependent microtubule motor activity, chlorophyll binding, microtubule binding, serine-type endopeptidase activity, isomerase activity, protein threonine kinase activity, protein serine kinase activity, lyase activity, hydrolase activity, hydrolyzing O-glycosyl compounds, oxidoreductase activity and metal ion binding, etc. A total of 22 terms were significantly enriched in the cellular component domain, including NAD(P)H dehydrogenase complex (plastoquinone), MCM complex, chloroplast thylakoid lumen, photosystem II oxygen evolving complex, chloroplast thylakoid membrane protein complex, kinesin complex, photosystem I and chloroplast nucleoid, etc. The lack of nitrogen sources prevents large amounts of organic carbon from participating in the cycle properly, disrupting the carbon–nitrogen balance and causing a significant weakening of the carbon fixation pathway (Hu et al. 2017). In addition, the reduction in chlorophyll directly leads to damage to the photosynthesis system. The GO enrichment results revealed that photosystem related biological processes, including photosynthesis, photosynthetic electron transport chain, photorespiration, light reaction, light harvesting, were significantly repressed. The enrichments of photosystem and plastoquinone in the cellular component also indicated that the photosynthetic system was disturbed under nitrogen deficiency. Due to insufficient carbon provided by the photosynthetic system, plants have to improve the decomposition capacity of materials such as starch to increase the total amount of their available resources.
In addition, the transcriptome of 84 K was gradually reprogramed after nitrogen deficiency stress according to the DEG numbers (Figure S2). Although a small number of DEGs were identified within four days of nitrogen deficiency, drastic changes of transcriptome were observed after six days of nitrogen deficiency. GO enrichment was used to identify overrepresented biological processes of the DEGs at different time points during nitrogen deficiency stress. Four nitrogen related biological processes were identified after 2 days and 4 days of nitrogen deficiency stress, including cellular response to organonitrogen compound, cellular response to nitrogen compound, reactive nitrogen species metabolic process and nitrogen compound metabolic process.
The biological processes of DEGs in different time periods were further examined to explore the pattern of carbohydrate metabolism during nitrogen deficiency stress. The results show that biological processes of carbohydrate phosphorylation and transport were significantly affected within four days. The carbohydrate biosynthetic process and cellular response to carbohydrate stimulus were then significantly affected when nitrogen deficiency stress continued to six and ten days. According to the GO enrichment results, carbon–nitrogen metabolism related genes including PagASPGB1 (Pop_A02G066107), PagNIC1 (Pop_A07G070513), PagURE (Pop_A08G045981), PagPYD2 (Pop_A09G028846) and PagARGAH1 (Pop_A14G000085) are significantly differentially expressed under nitrogen deficiency stress. PagASPGB1 encodes asparaginase, PagNIC1 encodes a nicotinamidase, PagURE encodes a nickel-containing urea hydrolase involved in nitrogen recycling, PagPYD2 encodes a protein with dihydropyrimidine amidohydrolase activity, and PagARGAH1 encodes an arginase involved in polyamine biosynthesis.
The molecular function of DEGs in different time periods was further examined to explore the expression pattern of N-metabolizing related enzymes during nitrogen deficiency stress. The GO enrichment results indicate that molecular functions of the DEGs at different time points were altered with the prolonging of nitrogen stress. Phosphotransferase, transferase and ligase activity were significantly enriched within four days of nitrogen deficiency stress, while hydrolase activity was also significantly enriched with the prolonging of nitrogen stress. Phosphotransferase and transferase will affect the number of nitrogen-containing compounds, while ligase can form new carbon–nitrogen bonds, and hydrolase can act on carbon–nitrogen (but not peptide) bonds in linear amides. These results all suggest that 84 K could control N-metabolizing related enzyme activity by affecting gene expression during nitrogen deficiency stress.
We also performed a GO enrichment analysis for the 88 DEGs throughout the whole nitrogen deficiency stress (Table S1). Eight biological processes were enriched, including carbon fixation, protein peptidyl-prolyl isomerization, peptidyl-proline modification, phylloquinone biosynthetic process, phylloquinone metabolic process, quinone biosynthetic process, quinone metabolic process and ketone biosynthetic process. In addition, the 88 DEGs were also enriched on peptidyl-prolyl cis–trans isomerase activity in molecular function domain, and chloroplast envelope in cellular component domain.
The KEGG information was obtained from the KAAS (KEGG Automatic Annotation Server) tools (Moriya et al. 2007). The KEGG results (Table S2) showed that metabolic pathways involved in activities of cells, such as DNA replication proteins, cell cycle and defense system were also down-regulation, were significantly decreased under nitrogen deficiency stress. The 2,130 up-regulated DEGs were significantly enriched in six KEGG pathways, including valine, leucine and isoleucine degradation, methane metabolism, beta-alanine metabolism, citrate cycle (TCA cycle), glycerolipid metabolism, and endocytosis. The KEGG pathway with the most enriched genes was endocytosis, which related to the lack of elements under adverse environments. The up-regulation of the above pathways provides sufficient raw materials for the synthesis of anthocyanins and procyanidins. The 3,738 down-regulated differential genes were significantly enriched in 36 KEGG pathways. Consistent with the GO enrichment results, pathways such as porphyrin and chlorophyll metabolism, cytochrome P450, and nitrogen metabolism were significantly down-regulated. Photosynthesis related components including photosynthesis proteins, photosynthesis-antenna proteins, carbon fixation in photosynthetic organisms were significantly reduced.
Chlorophyll biosynthesis related genes in 84 K under nitrogen deficiency stress
Nitrogen and magnesium are the main components of chlorophyll molecules and the deficiency of them will affect chlorophyll biosynthesis. The GO enrichment results indicated that energy metabolism of 84 K may be affected under nitrogen deficiency stress (Fig. 4). Therefore, we further investigated the expressional profiles of genes related to chlorophyll biosynthesis. A total of 43 analogues of the genes (Table 3) involved in the chlorophyll biosynthetic pathway of A. thaliana were identified using BLASTP. Of these genes, 21 were differentially expressed under nitrogen deficiency stress, including PagHEMA2, PagGSA2, PagHEMB, PagHEMC1, PagHEMC2, PagHEMD, PagHEME2, PagHEMF, PagHEMG2, PagCHLH2, PagCHLH4, PagCHLI1, PagCHLD3, PagCHLM1, PagCRD1, PagCRD2, PagDVR, PagPORA, PagPORC, PagCHLG1 and PagCAO2. As expected, all these genes were down-regulated in 84 K leaves under nitrogen deficiency stress.
Fig. 4.
Core pathway for chlorophyll biosynthesis, showing related pathways for chlorophyll a and chlorophyll b synthesis. The chlorophyll biosynthesis is catalyzed by glutamyl-tRNA reductases (HEMAs), glutamate-1-semialdehyde aminomutases (GSAs), 5-aminolevulinate dehydratases (HEMBs), hydroxymethylbilane synthases (HEMCs), uroporphyrinogen III synthases (HEMDs), uroporphyrinogen III decarboxylases (HEMEs), coproporphyrinogen III oxidases (HEMFs), protoporphyrinogen oxidases (HEMGs), magnesium chelatase H subunits (CHLHs), magnesium chelatase I subunits (CHLIs), magnesium chelatase D subunits (CHLDs), magnesium protoporphyrin IX methyltransferases (CHLMs), Mg-protoporphyrin IX monomethyl ester cyclases (CRDs), 3,8-divinyl protochlorophyllide a 8-vinyl reductases (DVRs), protochlorophyllide oxidoreductases (PORs), chlorophyll synthases (CHLGs) and chlorophyllide-a oxygenases (CAOs)
Table 3.
Putative 84 K chlorophyll biosynthesis genes in 17 enzyme families
| Gene family | Gene name | Gene ID (84 K) |
|---|---|---|
| HEMA | PagHEMA1 | Pop_A01G005975 |
| PagHEMA2 | Pop_A02G005576 | |
| PagHEMA3 | Pop_A09G015363 | |
| GSA (HEML) | PagGSA1 | Pop_A12G067962 |
| PagGSA2 | Pop_A15G049131 | |
| HEMB | PagHEMB | Pop_A02G088548 |
| HEMC | PagHEMC1 | Pop_A05G011522 |
| PagHEMC2 | Pop_A07G006294 | |
| HEMD | PagHEMD | Pop_A14G000379 |
| HEME | PagHEME1 | Pop_A01G024372 |
| PagHEME2 | Pop_A19G052799 | |
| HEMF | PagHEMF | Pop_A11G091327 |
| HEMG | PagHEMG1 | Pop_A01G032128 |
| PagHEMG2 | Pop_A02G012523 | |
| PagHEMG3 | Pop_A14G045042 | |
| CHLH | PagCHLH1 | Pop_A06G061868 |
| PagCHLH2 | Pop_A06G061869 | |
| PagCHLH3 | Pop_A16G055319 | |
| PagCHLH4 | Pop_UnG075846 | |
| PagCHLH5 | Pop_UnG075847 | |
| PagCHLH6 | Pop_UnG075848 | |
| CHLI | PagCHLI1 | Pop_A04G019634 |
| PagCHLI2 | Pop_A11G056632 | |
| CHLD | PagCHLD1 | Pop_A01G057020 |
| PagCHLD2 | Pop_A01G057021 | |
| PagCHLD3 | Pop_A09G029036 | |
| CHLM | PagCHLM1 | Pop_A12G067917 |
| PagCHLM2 | Pop_A15G084415 | |
| PagCHLM3 | Pop_A15G084416 | |
| PagCHLM4 | Pop_A15G084417 | |
| PagCHLM5 | Pop_UnG084501 | |
| PagCHLM6 | Pop_UnG084502 | |
| PagCHLM7 | Pop_UnG084503 | |
| CRD (ACSF) | PagCRD1 | Pop_A06G062043 |
| PagCRD2 | Pop_A16G035326 | |
| DVR | PagDVR | Pop_A08G046041 |
| POR | PagPORA | Pop_A01G074950 |
| PagPORB | Pop_A11G020351 | |
| PagPORC | Pop_A13G031221 | |
| CHLG | PagCHLG1 | Pop_A06G089405 |
| PagCHLG2 | Pop_A16G090118 | |
| CAO (CHL) | PagCAO1 | Pop_A02G088637 |
| PagCAO2 | Pop_A05G056114 |
Gene information in bold is for the genes most probably encode chlorophyll biosynthesis enzymes
The GluTR encoded by the PagHEMA2 is a catalyst for ALA synthesis, which determines the total flux of the chlorophyll biosynthetic pathway. The expression of PagHEMA2 was gradually reduced during the nitrogen deficiency stress treatment, reached the lowest point on the 8th day, and recovered to the expression level of the 6th day on the 10th day. PagGSA2, PagHEMB, PagHEMC1, PagHEMC2, PagHEME2, PagHEMF, PagHEMG2, PagCHLH4, PagCHLI1, PagCHLD3, PagCHLM1, PagCRD1 and PagCRD2 showed similar expression patterns with PagHEMA2, which were gradually reduced in the first eight days, and recovered on the 10th day. The expression levels of PagPORA, PagPORC and PagCAO2 were slightly different, which showed an up-regulated trend on the 2nd day of nitrogen deficiency stress. This up-regulated peak was shifted to 4th day in PagHEMD, PagCHLH2 and PagCHLG1, and then the expression levels of these genes were decreased. The expression level of all these 84 K chloroplast genes has a callback process when the 10th day of nitrogen deficiency treatment is approaching. However, PagDVR is an exception with the above genes, the expression of which was immediately decreased after nitrogen deficiency treatment, and gradually resumed as the duration of the stress.
Anthocyanin/proanthocyanidin biosynthesis related genes in 84 K leaves under nitrogen deficiency stress
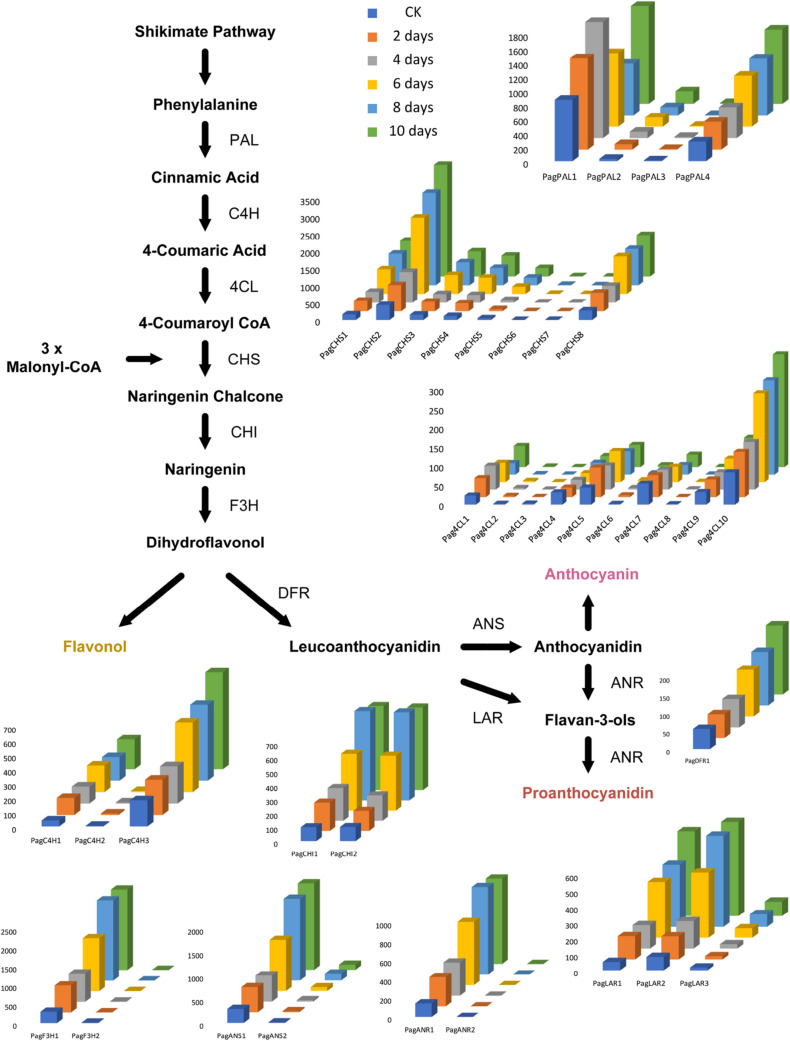
According to the GO enrichment results, anthocyanin/proanthocyanidin biosynthesis related genes were differentially expressed under nitrogen deficiency stress (Fig. 5). During the past decades, studies centered on flavonoid pigments have resulted in the most important scientific discoveries, and this process has been thoroughly studied in several plants (Santos-Buelga et al. 2014). We identified 37 genes that may be involved in the anthocyanin/proanthocyanidin biosynthetic pathway (Table 4). Of these genes, 17 were differentially expressed, including PagPAL1, PagPAL4, PagC4H1, PagC4H3, Pag4CL1, Pag4CL10, PagCHS1, PagCHS2, PagCHS8, PagCHI1, PagCHI2, PagF3H1, PagDFR1, PagANS1, PagANR1, PagLAR1 and PagLAR2. Most of these potential anthocyanin/proanthocyanidin biosynthesis genes are expressed abundantly in red organs/tissues than in non-red organs/tissues (Espley et al. 2013). As expected, all these 17 genes were up-regulated in 84 K leaves after nitrogen deficiency stress.
Fig. 5.
Core pathway for anthocyanin biosynthesis, showing related pathways for flavonol and flavone synthesis. The phenylpropane metabolic pathway is catalyzed by phenylalanine ammonia-lyases (PALs), cinnamate 4-hydroxylases (C4Hs) and 4-coumaryol CoA ligase (4CLs). Enzymes involved in flavonoid biosynthesis are chalcone synthases (CHSs), chalcone isomerizes (CHIs) and flavanone 3-hydroxylases (F3Hs). Anthocyanins are synthesized by dihydroflavonol 4-reductases (DFRs) and anthocyanidin synthases (ANSs), proanthocyanidins are synthesized by anthocyanidin reductases (ANRs) and leucoanthocyantin reductases (LARs)
Table 4.
Putative 84 K anthocyanin/proanthocyanidin biosynthesis genes in 10 enzyme families
| Gene family | Gene name | Gene ID (84 K) |
|---|---|---|
| PAL | PagPAL1 | Pop_A06G085807 |
| PagPAL2 | Pop_A08G002346 | |
| PagPAL3 | Pop_A10G046757 | |
| PagPAL4 | Pop_A16G091238 | |
| C4H | PagC4H1 | Pop_A13G031180 |
| PagC4H2 | Pop_A18G034210 | |
| PagC4H3 | Pop_A19G055094 | |
| 4CL | Pag4CL1 | Pop_A01G080036 |
| Pag4CL2 | Pop_A03G020013 | |
| Pag4CL3 | Pop_A03G050296 | |
| Pag4CL4 | Pop_A04G026309 | |
| Pag4CL5 | Pop_A05G002097 | |
| Pag4CL6 | Pop_A06G064757 | |
| Pag4CL7 | Pop_A12G068073 | |
| Pag4CL8 | Pop_A15G049039 | |
| Pag4CL9 | Pop_A18G018804 | |
| Pag4CL10 | Pop_A19G052806 | |
| CHS | PagCHS1 | Pop_A01G079912 |
| PagCHS2 | Pop_A01G079914 | |
| PagCHS3 | Pop_A03G019870 | |
| PagCHS4 | Pop_A03G019871 | |
| PagCHS5 | Pop_A03G019873 | |
| PagCHS6 | Pop_A05G056081 | |
| PagCHS7 | Pop_A12G007961 | |
| PagCHS8 | Pop_A14G045758 | |
| CHI | PagCHI1 | Pop_A10G047182 |
| PagCHI2 | Pop_A19G052939 | |
| F3H | PagF3H1 | Pop_A05G073152 |
| PagF3H2 | Pop_A13G054224 | |
| DFR | PagDFR1 | Pop_A05G001883 |
| ANS | PagANS1 | Pop_A01G059975 |
| PagANS2 | Pop_A03G050513 | |
| ANR | PagANR1 | Pop_A04G018182 |
| PagANR2 | Pop_A11G056528 | |
| LAR | PagLAR1 | Pop_A08G063978 |
| PagLAR2 | Pop_A10G069055 | |
| PagLAR3 | Pop_A15G064510 |
Gene information in bold is for the genes most probably encode anthocyanin/proanthocyanidin biosynthesis enzymes
The expression levels of most anthocyanin/proanthocyanidin genes were gradually increased during the nitrogen deficiency stress treatment, including PagPAL4, PagC4H3, Pag4CL10, PagCHS1, PagCHS2, PagCHS8, PagF3H1, PagDFR1, PagANS1, PagLAR1 and PagLAR2. The expression patterns of PagCHI1, PagCHI2 and PagANR1 were slightly different. The expression levels of these three genes were peaked on the 8th day and down-regulated on the 10th day. The expression pattern of PagPAL1 is most distinctive from other anthocyanin/proanthocyanidin genes. This gene’s expression level is steadily up-regulated in the first four days, then continuously down-regulated from the 6th day to the 8th day under nitrogen deficiency stress, and finally recovered on the 10th day.
Transcription factors in 84 K under nitrogen deficiency stress
Transcription factors (TFs) play vital roles in modulating plant adaptations to biotic stresses (Table S3). A total of 287 TFs in 52 families were up-regulated, and a total of 484 TFs in 63 families were down-regulated under nitrogen deficiency treatment, respectively. It is obvious that more TFs were down-regulated under nitrogen deficiency stress. C2H2 was the most influenced TF family by nitrogen deficiency stress, including 51 up-regulated and 44 down-regulated members, respectively. Another significantly differentially expressed family is MYB-HB-like, of which 37 and 33 were up-regulated and down-regulated, respectively. Most of the TF families were significantly down-regulated. In bHLH family, 34 members were down-regulated and only 12 were up-regulated. Similarly, in TF families of Homobox-WOX, Hap3/NF-YB, AP2-EREBP, PHD, NAM, GRAS and HD-ZIP, more genes were down-regulated than up-regulated. Only in some TF families, more genes were up-regulated than down-regulated, such as CCHC (10 up-regulated, 6 down-regulated) and C2C2-Dof (4 up-regulated, 2 down-regulated).
Co-expression network between TFs and nitrogen deficiency responsive genes
The gene co-expression network analysis is a system biology method for describing the correlation patterns among genes across RNA-Seq samples. We constructed a TF-centered co-expression network by WGCNA to identify regulators of chlorophyll and anthocyanin biosynthetic pathway genes. The results showed that a total of 20 differentially expressed TFs were related to the pigment synthesis (Fig. 6). Among them, 13 TFs were involved in the process of chlorophyll synthesis, and 7 TFs were involved in the process of anthocyanin/anthocyanidin synthesis.
Fig. 6.
The transcription factor regulatory network calculated by WGCNA. The purple dots were transcription factors, the green dots were chlorophyll structural genes, and the red dots were anthocyanin/proanthocyanidin structural genes. a chlorophyll regulation network (green line). b anthocyanin regulatory network (red line)
As shown in Fig. 6, the chlorophyll structural genes were formed two co-expression networks with TFs, which from the five TFs families of ARF, HMG, IAA, NAM and NF-YB (HAP3). There were 8 chlorophyll structure genes were co-expressed with PagIAA6, PagIAA14, PagNAM107, PagNAM113, PagNF-YB15, PagNF-YB16 and PagNF-YB74. PagARF6, PagARF22, PagHMG7, PagHMG20, PagIAA18 and PagNAM122 were co-expressed with the other 10 chlorophyll structure genes. ARF and IAA were the common auxin-related TFs, and the NAM was related to control chlorophyll degradation (Czerpak et al. 2002; Zhou et al. 2013). These chlorophyll related genes were down-regulated under a nitrogen-deficient environment. As for anthocyanin structural genes, they also formed two co-expression networks with TFs. These TFs were from the three TFs families of MYB-HB, bHLH, and WD40, which are involved in the regulation of anthocyanins by previous studies (Lloyd et al. 2017). A total of 13 anthocyanin structure genes co-expressed with PagMYB-HB187, PagMYB-HB343, PagMYB-HB359, and PagWD40-15. In addition, the PagbHLH23, PagMYB-HB124, and PagMYB-HB171 all were co-expressed with PagPAL4. These anthocyanin related genes were up-regulated under a nitrogen-deficient environment.
Validation of DEGs identified by RNA-seq using qRT-PCR
We used qRT-PCR to validate the expression profiles of the genes most probably encode anthocyanin/proanthocyanidin biosynthesis enzymes after N-starvation treatment. A total of 9 N-responsive genes were selected for qRT-PCR analysis. As shown in Fig. 7, the qRT-PCR results indicated that all the 9 N-responsive genes were up-regulated by N-starvation stress, which was consistent with the results derived from the RNA-seq data. N-responsive genes including PagPAL4, Pag4CL1, Pag4CL10, PagCHS1, PagCHI1, PagF3H1, PagANS1, PagANR1 and PagLAR1 were up-regulated when exposed to N-starvation stress for 8 days and 10 days. Overall, this qRT-PCR result supports the reliability of the RNA-seq analysis.
Fig. 7.
Expression analysis of the genes most probably encode anthocyanin/proanthocyanidin biosynthesis enzymes using quantitative real-time RT-PCR (RT-qPCR). The 84 K seedlings that 20 days old were incubated at N-starvation media for the indicated time. Data represent means ± SD in three replicates
Discussion
The molecular mechanism of 84 K under nitrogen deficiency
The principal results of this study are the changes during response under nitrogen deficiency of 84 K. Jia et al. (2015) reported that the polysaccharides containing glucose increased in Nannochloropsis oceanica under nitrogen-depletion conditions. The GO enrichment results also showed that cellular carbohydrate metabolic was the most increased biological process in our study, which indicated a large amount of free glucose in 84 K. The part of excess free glucose was converted into enol-type phosphopyruvate through the Embden-Meyerhof-Parnas pathway, the others were converted into erythrose-4-P through the hexose monophosphate pathway. And these biomacromolecules were then be involved in the oxalate pathway used to produce phenylalanines with β-alanine. But these phenylalanines cannot participate in protein synthesis due to the lack of essential elements (N), instead degraded by PAL and transferred to the flavonoid biosynthetic pathway, and increase the anthocyanins and proanthocyanidins content finally. The enrichment results of the proanthocyanidin biosynthesis process validated our conjecture. On the other hand, the KEGG result confirmed that the slowdown of chlorophyll metabolism, which provides an opportunity for anthocyanin display, otherwise chlorophyll will cover the color of anthocyanins.
In addition, AtHHO2 (AT1G68670) is an HRS1 homolog, which is involved in the N cross-regulation of P signaling. It functions as transcriptional repressors of SPX1, SPX2, and SPX4 as part of a cascade to regulate nitrogen and phosphorus balance. Transcriptional repressors that function with other NIGT genes as an important hub in the nutrient signaling network associated with the acquisition and use of nitrogen and phosphorus. The homology of AtHHO2 in 84 K is PagHHO2 (Pop_A08G063989), which was highly up-regulated after nitrogen deficiency stress. AtHHO3 (AT1G25550) is also a member of the HHO/HRS GARP type transcriptional repressor family, which is involved in P uptake and P starvation signaling. PagHHO3 (Pop_A10G069044), the homology of AtHHO3 in 84 K, was highly up-regulated in 84 K during nitrogen deficiency stress. This gene family has been shown to have a co-expression relationship with the MYB transcription factor (Nagarajan et al. 2016).
Chlorophyll regulatory network in 84 K leaves coloration
As described previously (Deluc et al. 2006; Morohashi et al. 2012; Tohge et al. 2005), the pigment biosynthetic pathway requires the participation of multiple enzymes to complete, so the entire process is controlled by both structural genes and regulatory genes (Jaakola 2013). The most notable in the chlorophyll biosynthesis pathway is the first step, which uses glutamyl-tRNA to synthesize δ-aminolevulinic acid (ALA). The rate of ALA synthesis is a rate-limiting factor in determines the total flux of the chlorophyll biosynthetic pathway, and this process is mainly regulated by GluTR (Cornah et al. 2003). This protein serves the purpose of changing the NADPH-dependent reduction of Glu-tRNA (Glu) into glutamate 1-semialdehyde (GSA) with the release of free tRNA, thereby controlling the total flux of the chlorophyll biosynthetic pathway (Cornah et al. 2003). From the results of the five groups of differentially expressed genes in our study, the expression of PagHEMA2 (GluTR) in 84 K was found to be significantly down-regulated under nitrogen deficiency conditions. Kumar and Soll (2000) reported that when the antisense HEMA was integrated into the chromosome, levels of ALA ranged from 21 to 56% and levels of total chlorophyll ranged from 23 to 82% in the tested plants compared with the control. This phenomenon indicates that the antisense HEMA can inhibit the synthesis of ALA, and cause chlorophyll deficiency in A. thaliana. Goslings et al. (2004) reported that the FLU can interact with the coiled-coil domain at the C-terminal end of GluTR, thereby negatively regulating chlorophyll biosynthesis. By referring to the above research, we speculate that the content of chlorophyll in 84 K can be reduced by antisense PagHEMA2 or increasing the expression of negative regulatory factors PagFLU (Pop_A01G003835).
PORs is another pivotal control point in chlorophyll biosynthesis, it can catalyze the light-dependent reduction of protochlorophyllide to chlorophyllide. The expression of PagPORA was found to be significantly down-regulated under nitrogen deficiency conditions, indicating that this gene also plays a positive regulatory role in chlorophyll biosynthesis. Zhang et al. (2017a) found that the expression of PORA, PORB, and PORC has significantly increased in the AtBRM (AT2G46020) mutants. The dark-grown knockout and knockdown mutants and RNA interference transgenic seedlings of AtBRM had higher greening rates, accumulated less protochlorophyllide and produced less reactive oxygen species than did A. thaliana wild-type plants upon light exposure. The expression of PagBRM1 was found to be significantly up-regulated under nitrogen deficiency conditions in our study, which proves the above results. In addition, Paik et al. (2012) found that the cytosolic protein PNT1 can bind to PORA mRNA in vivo and recruits the Pfr form of phytochrome to the 5’-UTR of PORA mRNA to inhibits the translation of this mRNA. The most similar 84 K protein to AtBRM and AtPNT1 is PagBRM1 (Pop_A02G012268) and PagPNT1 (Pop_A13G074166), we speculate that PagPORA expression in 84 K can also be reduced by increasing the expression of these two genes. In addition, light is critical in the ability of plants to accumulate chlorophyll. The wild-type seedlings will fail to green in a response known as the far-red block of greening when exposed to far-red light and then grown in white light in the absence of sucrose. This response is controlled by PHYA through repression of PORs by far-red light coupled with irreversible plastid damage. (Alameldin et al. 2020).
In addition, the chlorophyll synthesis process requires the participation of various auxin-related TFs, they were decreased in 84 K under nitrogen deficiency stress such as IAA and ARF. Interestingly, some chlorophyll degradation TFs were also co-expressed with chlorophyll structural genes such as NAM. We speculate that because of the insufficient chlorophyll content, and the trees decreased the chlorophyll degradation pathways to ensure chlorophyll content as much as possible.
Anthocyanin/proanthocyanidin regulatory network in 84 K leaves coloration
Anthocyanins have many benefits for the trees, so there were many studies on anthocyanin-rich horticultural plants in a few years. The biosynthesis of anthocyanins in plants was originally from phenylalanine through the phenylpropane metabolic pathway to synthesize coumaroyl CoA via the turnover of phenylalanine ammonia-lyases (PAL). Rohde et al. (2004) and Olsen et al. (2008) reported that the disruption of AtPAL1 will promote the phenylpropanoid biosynthesis, and accumulate flavonoids in A. thaliana. Aiqin et al. (1997) have shown that increasing MdPAL1 activity can effectively inhibit the protein biosynthetic pathway, so that excess phenylalanine can participate in the pathway of anthocyanin synthesis, which causes the Malus domestica peels to turn red. In the anthocyanin/proanthocyanidin pathway of 84 K, the expression of PagPAL4 was also found to be significantly up-regulated under nitrogen deficiency conditions, indicating that this gene plays the same positive regulatory role in the 84 K anthocyanin/proanthocyanidin biosynthesis. PagCHS2 was also significantly increased in the nitrogen-deficient environment. This protein was annotated as chalcone synthase and the most similar to PhCHS (TT4) in Petunia hybrida. Chalcone synthase is active in the second stage of flavonoid metabolism, which is a critical period for anthocyanin/proanthocyanidin biosynthesis. The chalcone synthase can catalyze the first step of flavonoid biosynthesis by directing carbon flux from general phenylpropanoid metabolism to the flavonoid pathway, and coumaroyl CoA entered the flavonoid biosynthetic pathway to synthesize chalcone with three molecules of malonyl-CoAs (Zhang et al. 2017b). Hanumappa et al. (2007) reported that overexpression of PhCHS can effectively increase the amount of anthocyanin, and modulate the flower color intensity of P. hybrida. They were also proved the utility of dominant-negative PhCHS enzymes in plant metabolism engineering can regulating flavonoid biosynthesis.
The expression of PagMYB-HB124, PagMYB-HB171, PagMYB-HB340, PagWRKY82, PagbHLH23 and PagWD40-371 were all be found to up-regulated under nitrogen deficiency conditions in our study. The transcription factor most similar to PagMYB-HB124 is MYB123 (TT2) in A. thaliana. MYB123 (TT2) can promote the anthocyanin pathway and directly increase WRKY44 (TTG2) transcription factor expression in A. thaliana (Ishida et al. 2007). The most similar transcription factor to WRKY44 (TTG2) is PagWRKY82 in 84 K, which expression was also significantly up-regulated in our study. The most similar transcription factor to PagMYB-HB171 is MYBD in A. thaliana, MYBD can increase anthocyanin accumulation via repression of MYBL2 (Nguyen et al. 2015). The PagMYB-HB340 was most similar to MYB6 in Populus tomentosa, which promotes anthocyanin and proanthocyanidin biosynthesis but inhibits secondary cell wall formation. WD40 and bHLH were also considered to be involved anthocyanin biosynthesis and seed coat pigment accumulation (An et al. 2012; Xie et al. 2016). The transcription factor of PagWD40-371 most similar to A. thaliana is TTG1, which regulates trichome differentiation and anthocyanin biosynthesis (Walker et al. 1999). PagbHLH23 is a bHLH transcription factor and most similar to bHLH42 (TT8) in A. thaliana. Li et al. (2016) reported that over-expression of bHLH42 induced increases in the production of anthocyanins in M. truncatula hairy roots and suggesting that bHLH42 is a critical factor in stimulating proanthocyanidin production.
In addition, TFs tend to cooperatively bind the other TFs as large complexes, or clusters (Burley and Kamada 2002). Based on comparison with the transcription factor regulatory networks of anthocyanins in A. thaliana, we speculated that the PagMYB-HB124, PagbHLH23 and PagWD40-371 may form the MBW (TT2/TT8/TTG1) complex, which was the most extensive transcription factor complex affecting anthocyanin synthesis (Lloyd et al. 2017). When the anthocyanin biosynthetic pathway is overloaded, excess PagWRKY82 (TTG2) then interacts with PagWD40-371 (TTG1) to form an MBWW (TT2-TT8-TTG1-TTG2) quartet. The existence of this quartet model will narrow greatly the target range of the MBW complex in the flavonoid pathway, and then reversely regulate the anthocyanin synthesis rate (Lloyd et al. 2017).
Conclusions
In this study, a total of about 62.24 Gb raw RNA-Seq data was generated by high-throughput sequencing and dynamic changes in transcriptome expression under nitrogen deficiency treatments were observed. Differential gene analysis identified 5,868 nitrogen stress-responsive genes, and GO and KEGG analysis revealed a large number of pathways related to chlorophyll synthesis and anthocyanin synthesis in 84 K. Based on the expression analyses of candidate transcripts, we proposed a molecular mechanism in 84 K leaves under nitrogen deficiency, identified candidate TFs for the MBW model and constructed the associated gene co-expression networks. The transcriptome and digital expression profiling of 84 K provide a valuable resource for the functional evaluation of nitrogen deficiency stress response genes, which is assumed to hold great potential for genetic engineering of resistance and ornamental breeding.
Methods
Plant materials and stress treatments
A time-series experiment of nitrogen deficiency was designed to understand the effect of nitrogen on 84 K. Plantlets were pre-grown on MS rooting medium (MS medium with 0.5 mg/L 6-BA, 0.1 mg/L NAA, 25 g/L sucrose, 7.5 g/L agar, pH 5.8). The cultivation environment was a controlled indoor greenhouse, the light conditions were 16 h light/8 h darkness. After 20 days of cultivation, the plantlets were transferred to N-starvation media (MS medium with 0.5 mg/L 6-BA, 0.1 mg/L NAA, 25 g/L sucrose, 7.5 g/L agar, pH 5.8, without NH4NO3 and with 1.9 g/L KCl instead of KNO3). The leaves were sampled every two days till the 10th day, and all the leaves were harvested and immediately frozen in liquid nitrogen for RNA extraction. Plants grown on MS rooting medium were used as controls. For each time point, three biological replicates were generated and each replicate was mixed from five individuals.
Sample preparation, cDNA library construction and illumina sequencing
Total RNA of the samples were extracted using the traditional CTAB (Cetyltrimethylammonium Bromide) method (Doyle 1990) referring to previous studies (Yu et al. 2020; Xu et al. 2019; Wei et al. 2017; Berg et al. 2019). The RNA samples used for cDNA libraries construction were assessed using the Qubit Fluorometer and the Agilent 2100 Bioanalyzer. The qualified RNA samples were then used for cDNA libraries construction according to the user manual of RNA-Seq Library Preparation Kit (Illumina) and subsequent sequencing. The paired-end read sequencing was constructed by BGI-Wuhan (BGI, Wuhan, China) using the MGISEQ-2000 platform. After filtering out low-quality reads, the clean sequencing data were all has been submitted to the NCBI SRA (Sequence Read Archive) database (Accession No. PRJNA629424).
Identification of differentially expressed genes (DEGs)
Gene expression analysis was performed by using RSEM (RNA-Seq by Expectation–Maximization) pipeline (Li and Dewey 2011), which is a sophisticated procedure for transcriptome data analysis. Spliced Transcripts Alignments to a Reference (STAR) (Dobin et al. 2013) was used as aligner to map the sequencing reads to the reference genome with parameters recommended by RSEM. Gene expression levels were then determined by using the mapping results of STAR. After calculating gene expressions for all the samples, these data were combined using TMM normalization to eliminate the influence on the expression of calculated genes. The differential gene calculation phase using edgeR software (Robinson et al. 2010). All samples were compared with the control group to calculate the differential genes with the threshold was set to FDR < 0.05 and log2FC > 1. The fold changes of DEGs were the average TMM of each nitrogen deficiency treatment group divided by the average TMM of the control group, and the minimum value of count per million was 1 to filter out samples with low expression.
Functional annotation of sequence
The 84 K genome (Qiu et al. 2019; Huang et al. 2020) was downloaded and used for the reference genome. In order to obtain the most complete annotation results, we used three functional annotation software in this step. GO annotation was complete by AHRD (Consortium 2012), all 84 K genes were searched in Uniprot's Swiss-Prot database and filter out the reliable results. The search program was BLASTP (Camacho et al. 2009), and the cut-off value was set as an E-value threshold of 1e-5. KEGG annotation was used the KAAS tool (Moriya et al. 2007) provided by KEGG officials. The assignment method was SBH (single-directional best hit), the search program was GHOST X and the genes data set was selected eukaryotes. COG/EggNOG annotation was completed by EggNOG-mapper (Huerta-Cepas et al. 2017), and the search program was used diamond. These three sets of data were then be manually merged and formatted for subsequent analysis.
GO and KEGG enrichment analysis of the DEGs
About the enrichment analysis, we used AnnotationForge (Carlson and Pagès 2019) to pack the 84 K annotation results into an OrgDb package, and the ClusterProfiler program (Yu et al. 2012) was then be used to undertake the enrichment analysis of the significant differentially expressed RNA. An over-representation test based on the hypergeometric distribution of the GO terms was performed to identify enriched GO terms, and we measured the statistical significance of each enriched GO term. The allocated ontology term option used was biological processes, and the P-value of each pathway was calculated and adjusted using the Benjamin-Hochberg method (Benjamini and Hochberg 1995). The KEGG analysis was then be performed in the same way. GO and KEGG terms with corrected adjusted P-value less than 0.1 were considered significantly enriched by differential expressed genes.
Identification of TFs and molecular regulation genes responsible for red 84 K leaves
The TFs of 84 K were identified by PlantTFcat (Dai et al. 2013), and the NCBI (National Center for Biotechnology Information) Conserved Domain Database (Marchler-Bauer et al. 2015) was used to determine whether they were correctly annotated. The relevant gene families of anthocyanin biosynthetic pathways in A. thaliana were then be collected via TAIR (Lamesch et al. 2012). And the proanthocyanidin synthesis genes (ANR, LAR) were collected in Vitis vinifera whose rich in proanthocyanidins (Tanner et al. 2003; Yu et al. 2019). We compare it to the 84 K genome and select the results with a similarity greater than 60% using BLASTP. The results were divided into ten subgroups based on their functional type in A. thaliana and V. vinifera. Further filtering was performed based on the DEG results, we selected the genes whose expression was significantly increased (log2FC > 1) in nitrogen deficiency treatment and whose expression for more than 10% of its gene family as predictive genes. The E-value threshold for all the above steps was all set to 1e-5.
Co-expression network construction for red 84 K leaves
We identified the co-expressed networks of anthocyanin biosynthetic pathway genes by using WGCNA (Weighted Correlation Network Analysis), and the relationships were displayed by Cytoscape. The data used as input for the co-expression network construction was the gene expression data derived from the 84 K time-series experiment under nitrogen deficiency. The optimal β (soft thresholding power) value was determined to be 9 after 20 iterations, we then calculated the correlation coefficient by the “Pearson” algorithm and made a similarity matrix used this β-value. The similarity matrix was transformed into an adjacency matrix, and then transformed into a TOM (topological overlap measure) matrix with the signed network type. The “deepSplit” value was set to 2 during clustering, the “minModuleSize” value was set to 30, and the “mergeCutHeight” value was set to 0.15. Among the correlated gene pairs of WGCNA results, the anthocyanin-related TF genes were extracted to reconstruct the TF-target network. Cytoscape was then be used to display the results, and irrelevant or low-relevant genes have been hidden.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Authors contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Caixia Liu, Song Chen and Sui Wang. Conceived and supervised were performed by Xiyang Zhao, Kailong Li, Su Chen and Guan-zheng Qu.
Funding
This research was supported by the National Natural Science Foundation of China (31670675), the Fundamental Research Funds for the Central Universities (2572018CL02) and the Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
Availability of data and material
The RNA datasets generated during the current study are available in the NCBI SRA (Sequence Read Archive) database (Accession No. PRJNA629424).
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Su Chen, Email: chensu@nefu.edu.cn.
Guan-zheng Qu, Email: quguanzheng@yahoo.com.
References
- Afrousheh M, Ardalan M, Hokmabadi H. Visual deficiency and multi-deficiency symptoms of macro and micro nutrients element in pistachio seedling (Pistacia vera) Options Méditérr. 2010;94:37–52. [Google Scholar]
- Aiqin Z, Jun Z, Jiping S, Lin S, Zhaojiang S (1997) The Relationship of Anthocyanidin Formation, PAL Activity and Protein Content During Apple Colouring. Journal of China Agricultural University 3
- Alameldin HF, Oh S, Hernandez AP, Montgomery BL. Nuclear-encoded sigma factor 6 (SIG6) is involved in the block of greening response in Arabidopsis thaliana. Am J Bot. 2020;107:329–338. doi: 10.1002/ajb2.1423. [DOI] [PubMed] [Google Scholar]
- An X-H, Tian Y, Chen K-Q, Wang X-F, Hao Y-J. The apple WD40 protein MdTTG1 interacts with bHLH but not MYB proteins to regulate anthocyanin accumulation. J Plant Physiol. 2012;169:710–717. doi: 10.1016/j.jplph.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (methodol) 1995;57:289–300. [Google Scholar]
- Berg M, Monnin D, Cho J, Nelson L, Crits-Christoph A, Shapira M. TGFβ/BMP immune signaling affects abundance and function of C. elegans gut commensals. Nat Commun. 2019;10:1–12. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley SK, Kamada K. Transcription factor complexes. Curr Opin Struct Biol. 2002;12:225–230. doi: 10.1016/S0959-440X(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:1–9. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Pagès H (2019) AnnotationForge: Tools for Building SQLite-Based Annotation Data Packages. R package version 1
- Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wirén N. Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J Plant Nutr Soil Sci. 2011;174:3–11. doi: 10.1002/jpln.201000085. [DOI] [Google Scholar]
- Consortium TG The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 2012;485:635. doi: 10.1038/nature11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornah JE, Terry MJ, Smith AG. Green or red: what stops the traffic in the tetrapyrrole pathway? Trends Plant Sci. 2003;8:224–230. doi: 10.1016/S1360-1385(03)00064-5. [DOI] [PubMed] [Google Scholar]
- Czerpak R, Dobrzyn P, Krotke A, Kicinska E. The Effect of Auxins and Salicylic Acid on Chlorophyll and Carotenoid Contents. Polish J Environ Stud. 2002;11:231–235. [Google Scholar]
- Dai X, Sinharoy S, Udvardi M, Zhao PX. PlantTFcat: an online plant transcription factor and transcriptional regulator categorization and analysis tool. BMC Bioinformatics. 2013;14:321. doi: 10.1186/1471-2105-14-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc L, et al. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006;140:499–511. doi: 10.1104/pp.105.067231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Espley RV, et al. Analysis of genetically modified red-fleshed apples reveals effects on growth and consumer attributes. Plant Biotechnol J. 2013;11:408–419. doi: 10.1111/pbi.12017. [DOI] [PubMed] [Google Scholar]
- Gang H, Li R, Zhao Y, Liu G, Chen S, Jiang J. Loss of GLK1 transcription factor function reveals new insights in chlorophyll biosynthesis and chloroplast development. J Exp Bot. 2019;70:3125–3138. doi: 10.1093/jxb/erz128. [DOI] [PubMed] [Google Scholar]
- Goslings D, Meskauskiene R, Kim C, Lee KP, Nater M, Apel K. Concurrent interactions of heme and FLU with Glu tRNA reductase (HEMA1), the target of metabolic feedback inhibition of tetrapyrrole biosynthesis, in dark-and light-grown Arabidopsis plants. Plant J. 2004;40:957–967. doi: 10.1111/j.1365-313X.2004.02262.x. [DOI] [PubMed] [Google Scholar]
- Hanumappa M, Choi G, Ryu S, Choi G. Modulation of flower colour by rationally designed dominant-negative chalcone synthase. J Exp Bot. 2007;58:2471–2478. doi: 10.1093/jxb/erm104. [DOI] [PubMed] [Google Scholar]
- Holton TA, Cornish EC. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 1995;7:1071. doi: 10.2307/3870058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan J, Wareing P. Cytokinins and the growth responses of seedlings of Betula pendula Roth. and Acer pseudoplatanus L. to nitrogen and phosphorus deficiency. J Exp Bot. 1980;31:525–532. doi: 10.1093/jxb/31.2.525. [DOI] [Google Scholar]
- Hsieh P-H, Kan C-C, Wu H-Y, Yang H-C, Hsieh M-H. Early molecular events associated with nitrogen deficiency in rice seedling roots. Sci Rep-Uk. 2018;8:1–23. doi: 10.1038/s41598-018-30632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Coomer TD, Loka DA, Oosterhuis DM, Zhou Z. Potassium deficiency affects the carbon-nitrogen balance in cotton leaves. Plant Physiol Bioch. 2017;115:408–417. doi: 10.1016/j.plaphy.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Huang X et al. (2020) An improved draft genome sequence of hybrid Populus alba × Populus glandulosa. Journal of Forestry Research 1–10
- Huerta-Cepas J, Forslund K, Coelho LP, Szklarczyk D, Jensen LJ, von Mering C, Bork P. Fast Genome-Wide Functional Annotation through Orthology Assignment by eggNOG-Mapper. Mol Biol Evol. 2017;34:2115–2122. doi: 10.1093/molbev/msx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, et al. Arabidopsis TRANSPARENT TESTA GLABRA2 is directly regulated by R2R3 MYB transcription factors and is involved in regulation of GLABRA2 transcription in epidermal differentiation. Plant Cell. 2007;19:2531–2543. doi: 10.1105/tpc.107.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013;18:477–483. doi: 10.1016/j.tplants.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Jia J, Han D, Gerken HG, Li Y, Sommerfeld M, Hu Q, Xu J. Molecular mechanisms for photosynthetic carbon partitioning into storage neutral lipids in Nannochloropsis oceanica under nitrogen-depletion conditions. Algal Res. 2015;7:66–77. doi: 10.1016/j.algal.2014.11.005. [DOI] [Google Scholar]
- Koes R, Verweij W, Quattrocchio F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005;10:236–242. doi: 10.1016/j.tplants.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Söll D. Antisense HEMA1 RNA expression inhibits heme and chlorophyll biosynthesis in Arabidopsis. Plant Physiol. 2000;122:49–56. doi: 10.1104/pp.122.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamesch P, et al. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 2012;40:D1202–D1210. doi: 10.1093/nar/gkr1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, et al. Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bHLH transcription factor MtTT8. New Phytol. 2016;210:905–921. doi: 10.1111/nph.13816. [DOI] [PubMed] [Google Scholar]
- Lloyd A, Brockman A, Aguirre L, Campbell A, Bean A, Cantero A, Gonzalez A. Advances in the MYB–bHLH–WD repeat (MBW) pigment regulatory model: addition of a WRKY factor and co-option of an anthocyanin MYB for betalain regulation. Plant Cell Physiol. 2017;58:1431–1441. doi: 10.1093/pcp/pcx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, et al. CDD: NCBI's conserved domain database. Nucleic Acids Res. 2015;43:D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Cramer M (2005) Root nitrogen acquisition and assimilation. In: Root physiology: From gene to function. Springer, pp 1–36
- Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35:W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K, et al. A genome-wide regulatory framework identifies maize pericarp color1 controlled genes. Plant Cell. 2012;24:2745–2764. doi: 10.1105/tpc.112.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan VK, Satheesh V, Poling MD, Raghothama KG, Jain A. Arabidopsis MYB-related HHO2 exerts a regulatory influence on a subset of root traits and genes governing phosphate homeostasis. Plant Cell Physiol. 2016;57:1142–1152. doi: 10.1093/pcp/pcw063. [DOI] [PubMed] [Google Scholar]
- Nguyen P, Dal Cin V. The role of light on foliage colour development in coleus (Solenostemon scutellarioides (L.) Codd) Plant Physiol Bioch. 2009;47:934–945. doi: 10.1016/j.plaphy.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Nguyen NH, Jeong CY, Kang GH, Yoo SD, Hong SW, Lee H. MYBD employed by HY5 increases anthocyanin accumulation via repression of MYBL2 in Arabidopsis. Plant J. 2015;84:1192–1205. doi: 10.1111/tpj.13077. [DOI] [PubMed] [Google Scholar]
- Olsen KM, Lea US, Slimestad R, Verheul M, Lillo C. Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental-triggered flavonoid synthesis. J Plant Physiol. 2008;165:1491–1499. doi: 10.1016/j.jplph.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Paik I, Yang S, Choi G. Phytochrome regulates translation of mRNA in the cytosol. Proc Natl Acad Sci. 2012;109:1335–1340. doi: 10.1073/pnas.1109683109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DJ, Constabel CP. Molecular analysis of herbivore-induced condensed tannin synthesis: cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides) Plant J. 2002;32:701–712. doi: 10.1046/j.1365-313X.2002.01458.x. [DOI] [PubMed] [Google Scholar]
- Qiu D, et al. The genome of Populus alba x Populus tremula var. glandulosa clone 84K. DNA Res. 2019;26:423–431. doi: 10.1093/dnares/dsz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde A, et al. Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell. 2004;16:2749–2771. doi: 10.1105/tpc.104.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Buelga C, Mateus N, De Freitas V (2014) Anthocyanins. Plant pigments and beyond. ACS Publications, 6879–6884 [DOI] [PubMed]
- Schaberg PG, Van-den-Berg AK, Murakami PF, Shane JB, Donnelly JR. Factors influencing red expression in autumn foliage of sugar maple trees. Tree Physiol. 2003;23:325–333. doi: 10.1093/treephys/23.5.325. [DOI] [PubMed] [Google Scholar]
- Sevanthi AM, et al. Integration of dual stress transcriptomes and major QTLs from a pair of genotypes contrasting for drought and chronic nitrogen starvation identifies key stress responsive genes in rice. Res Squ. 2020;1:1. doi: 10.1186/s12284-021-00487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha SK, Sevanthi VAM, Chaudhary S, Tyagi P, Venkadesan S, Rani M, Mandal PK. Transcriptome analysis of two rice varieties contrasting for nitrogen use efficiency under chronic N starvation reveals differences in chloroplast and starch metabolism-related genes. Genes. 2018;9:206. doi: 10.3390/genes9040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner GJ, Francki KT, Abrahams S, Watson JM, Larkin PJ, Ashton AR. Proanthocyanidin biosynthesis in plants purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J Biol Chem. 2003;278:31647–31656. doi: 10.1074/jbc.M302783200. [DOI] [PubMed] [Google Scholar]
- Tohge T, et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 2005;42:218–235. doi: 10.1111/j.1365-313X.2005.02371.x. [DOI] [PubMed] [Google Scholar]
- Walker AR, et al. The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell. 1999;11:1337–1349. doi: 10.1105/tpc.11.7.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen S, Liang D, Qu G-Z, Chen S, Zhao X. Transcriptomic analyses of Pinus koraiensis under different cold stresses. BMC Genomics. 2020;21:1–14. doi: 10.1186/s12864-019-6419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S et al (2019) Negative feedback loop between BpAP1 and BpPI/BpDEF heterodimer in Betula platyphylla × B. pendula. Plant Sci 289:110280 [DOI] [PubMed]
- Wei M, Xu X, Li C. Identification and expression of CAMTA genes in Populustrichocarpa under biotic and abiotic stress. Scientific Rep. 2017;7:1–10. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–493. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Tan H, Ma Z, Huang J. DELLA proteins promote anthocyanin biosynthesis via sequestering MYBL2 and JAZ suppressors of the MYB/bHLH/WD40 complex in Arabidopsisthaliana. Molecular Plant. 2016;9:711–721. doi: 10.1016/j.molp.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Xu Q, Tang C, Wang X, Sun S, Zhao J, Kang Z, Wang X. An effector protein of the wheat stripe rust fungus targets chloroplasts and suppresses chloroplast function. Nature Commun. 2019;10:1–13. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GC, Wang LG, Han YY, He QY. clusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters. Omics. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Jun JH, Duan C, Dixon RA. VvLAR1 and VvLAR2 Are Bifunctional Enzymes for Proanthocyanidin Biosynthesis in Grapevine. Plant Physiol. 2019;180:1362–1374. doi: 10.1104/pp.19.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, et al. Metabolite signatures of diverse Camellia sinensis tea populations. Nature Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Li Y, Zhang X, Zha P, Lin R. The SWI2/SNF2 chromatin-remodeling ATPase BRAHMA regulates chlorophyll biosynthesis in Arabidopsis. Molecular plant. 2017;10:155–167. doi: 10.1016/j.molp.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Abrahan C, Colquhoun TA, Liu C-J. A proteolytic regulator controlling chalcone synthase stability and flavonoid biosynthesis in Arabidopsis. The Plant Cell. 2017;29:1157–1174. doi: 10.1105/tpc.16.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Reddy KR, Kakani VG, Reddy VR. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. European J Agron. 2005;22:391–403. doi: 10.1016/j.eja.2004.06.005. [DOI] [Google Scholar]
- Zhao X, Zheng H, Li S, Yang C, Jiang J, Liu G. The rooting of poplar cuttings: a review. New For. 2014;45:21–34. doi: 10.1007/s11056-013-9389-1. [DOI] [Google Scholar]
- Zhou Y, Huang W, Liu L, Chen T, Zhou F, Lin Y. Identification and functional characterization of a rice NAC gene involved in the regulation of leaf senescence. Bmc Plant Biol. 2013;13:132. doi: 10.1186/1471-2229-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA datasets generated during the current study are available in the NCBI SRA (Sequence Read Archive) database (Accession No. PRJNA629424).