Abstract
Diabetes mellitus is a metabolic disorder with several psychological problems such as anxiety, depression, and pain sense. This study aimed to evaluate the effect of Schiff base on the modulation of anxiety, depression, and pain behaviors in diabetic rats. Anxiety, depression, and pain behaviors were evaluated by elevated plus maze (EPM), forced swim test (FST), and hot-plate test, respectively. The results indicated that induction of diabetes decreased time spent in open arms (OAT) in the EPM whereas injection of insulin (1 ml/kg), glibenclamide (5 mg/kg), and Schiff base II (100 mg/kg) increased OAT in the EPM. So, induction of diabetes in rats caused an anxiogenic effect that this effect reversed by drug treatment. Interestingly, co-treatment of insulin and glibenclamide along with an ineffective dose of Schiff base II potentiated anxiolytic behavior in diabetic rats. Furthermore, induction of diabetes increased immobility time in the FST but administration of insulin (1 ml/kg), glibenclamide (5 mg/kg), and Schiff base II (25, 50, and 100 mg/kg) decreased immobility time in the FST which indicated depressant effect in diabetic rats without drug-treatment and antidepressant effect in diabetic rats with drug-treatment. Additionally, induction of diabetes decreased latency in the hot-plate test while injection of insulin (1 ml/kg), glibenclamide (5 mg/kg), Schiff base I (50 mg/kg), and Schiff base II (25, 50, and 100 mg/kg) enhanced latency in the hot-plate test which revealed hyperalgesic effect in diabetic rats without drug-treatment and analgesic effect in diabetic rats with drug-treatment. Consequently, induction of diabetes-induced anxiogenic, depressant, and hyperalgesia effects that administration of insulin, glibenclamide, Schiff bases I, and II reversed these effects.
Keywords: Diabetes, Schiff bases, Depression, Anxiety, Pain, Rat
Introduction
Diabetes mellitus is a complex chronic disease that is the main source of ill health worldwide in which an individual has a high blood sugar level, either because the pancreas does not secrete sufficient insulin, or cells do not react to the insulin which is secreted. This metabolic disease is recognized primarily by hyperglycemia and disorders in the metabolism of carbohydrate, fat, and protein, secondary to an absolute or comparative lack of the hormone insulin [1–4]. The chronic hyperglycemia of diabetes is related to long-term injury, dysfunction, and failure of various organs, particularly the eyes, kidneys, nerves, heart, and blood vessels [5]. Besides hyperglycemia, numerous other factors such as dyslipidemia or hyperlipidemia participate in the development of micro- and macro-vascular problems of diabetes which are chief reasons for morbidity and death metabolisms, secondary to an absolute or comparative lack of the hormone insulin [6].
Diabetes mellitus is not only an organic disease, it is a disorder with psychiatric and psychosocial dimensions [7]. The most common psychiatric disorders related to diabetes mellitus are anxiety and depression. In patients with diabetes, depression and anxiety are seen at a higher rate than in the general population [8–13]. Furthermore, diabetic peripheral neuropathic pain is a common problem of diabetes [14–16]. The pain related to diabetic peripheral neuropathy may be due to failure of the endogenous analgesic mechanisms in the descending spinal pathways which modulate pain transmission to the brain [17, 18].
Schiff bases are some of the most broadly used organic compounds which use in the pharmaceutical and biological field [19–22]. Schiff bases of isatin exhibit anticonvulsant, anti-influenza virus, antimicrobial, anti-HIV, and anti-inflammatory activities [23–26].
Insulin and oral hypoglycemic drugs similar glibenclamide, sulphonylureas and biguanides are still the agents of choice and as these drugs are to be used throughout the life and reduction of response after long use and induce side effects [27]. Because of the drawbacks of the existing drugs, there is continually a need to find novel anti-diabetic drugs. In this aspect, the current research was undertaken to assess the anti-diabetic effect of Schiff bases on the modulation of anxiety, depression, and pain behaviors in diabetic rats.
Materials and methods
Synthesis of Schiff bases
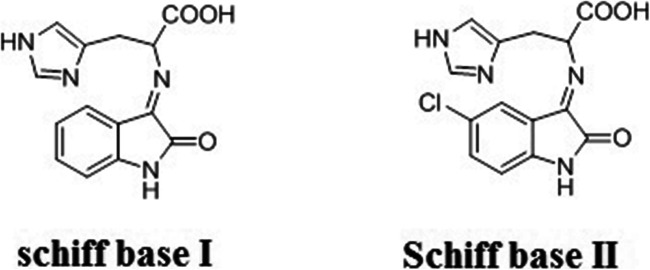
Schiff bases I and II were synthesized by the condensation of isatin (3.50 mmole), 5-chloroisatin (3.50 mmole), with the histidine (3.50 mmole) in 1: 1 molar ratio using ethanol (30 mL) as the reaction medium, and then it was refluxed for 4–5 h. The mixture of reactions was filtered and washed with hot ethanol (75%) to give remarkably pure powders of the products (Fig. 1). In all experimental groups, the LD50 value of the applied Schiff’s base was less than 5%.
Fig. 1.
Schematic structure of Schiff bases I and II
Animals
Animals were adult male Wistar rats weighing 220–250 g obtained from the Shahid Beheshti University (Tehran, Iran). The rats were kept 4 per Plexiglas cage in a room with controlled temperature (22 ± 2 °C) under 12/12 h light/dark cycles (lights on 07:00 a.m.). The rats had free access to food and water. Eight rats were used in each experimental group and each rat was only tested once. Behavioral tests were performed during the light phase of the light/dark cycle. All procedures in this research were conducted under the institutional guidelines for animal care and use.
Induction of diabetes
Diabetes was induced by a single intraperitoneal (i.p.) administration of streptozotocin (50 mg/kg of body weight) which dissolved in physiological serum [5, 28]. Diabetes was confirmed after the third day of streptozotocin injection by estimating serum glucose using a glucose peroxidase kit (Pars Azmoon Co., Tehran, Iran). Rats that developed serum glucose levels of more than 200 mg/dl were considered diabetic and were used for the research.
Materials
Streptozotocin was purchased from the Sigma Chemical Company (St Louis, USA). Insulin and glibenclamide were purchased from the Exir Company and Minoo Company (Tehran, Iran) respectively.
Experimental design
The rats were randomly divided into 10 groups with 8 rats each. Group 1, Normal control group treated with saline (1 ml/kg); Group 2, Diabetic rats treated with saline (1 ml/kg); Group 3, Diabetic rats treated with insulin (1 ml/kg); Group 4, Diabetic rats treated with glibenclamide (5 mg/kg); Group 5: Diabetic rats treated with Schiff base I (50 mg/kg); Group 6: Diabetic rats treated with Schiff base II (25 mg/kg); Group 7: Diabetic rats treated with Schiff base II (50 mg/kg); Group 8: Diabetic rats treated with Schiff base II (100 mg/kg); Group 9: Diabetic rats treated with Schiff base II (25 mg/kg) along with insulin (1 ml/kg); Group 10: Diabetic rats treated with Schiff base II (25 mg/kg) along with glibenclamide (5 mg/kg). The diabetes induction day was determined as Day 0. Days 1–3 were considered for confirmation of diabetes induction. Drug treatment was carried out intraperitoneally (i.p.) at a period of 14 days (from Day 4, after confirmation of diabetes induction, to Day 17). On Day 17, 30 min after drug treatment, behavioral tests (Elevated plus maze (EPM) test, Forced swim test (FST), and Hot-plate) were carried out. All experiments were done by someone blind to the responses and statistical analysis. The experimental groups were clarified in Table 1.
Table 1.
The table explained experimental groups
| Experiments | Fig. | Drug injection (Intraperitoneally) | Effect on anxiety | Effect on depression | Effect on pain |
|---|---|---|---|---|---|
| 1 | A | Saline 1 ml/kg, Insulin 1 ml/kg, Glibenclamide 5 mg/kg, Schiff base I 50 mg/kg, Schiff base II 25, 50, 100 mg/kg |
Diabetes(anxiogenic) Drugs (anxiolytic) |
- | - |
| B | Saline 1 ml/kg, Insulin 1 ml/kg, Glibenclamide 5 mg/kg, Schiff base I 50 mg/kg, Schiff base II 25, 50, 100 mg/kg | No effect | - | - | |
| C | Saline 1 ml/kg, Insulin 1 ml/kg, Glibenclamide 5 mg/kg, Schiff base I 50 mg/kg, Schiff base II 25, 50, 100 mg/kg | No effect | - | - | |
| 2 | A | Saline 1 ml/kg, Insulin 1 ml/kg, Glibenclamide 5 mg/kg, Schiff base II 25, Schiff base II 25 mg/kg + Insulin 1 ml/kg, Schiff base II 25 mg/kg + Glibenclamide 5 mg/kg | No effect | - | - |
| B | Saline 1 ml/kg, Insulin 1 ml/kg, Glibenclamide 5 mg/kg, Schiff base II 25, Schiff base II 25 mg/kg + Insulin 1 ml/kg, Schiff base II 25 mg/kg + Glibenclamide 5 mg/kg | Anxiolytic | - | - | |
| C | Saline 1 ml/kg, Insulin 1 ml/kg, Glibenclamide 5 mg/kg, Schiff base II 25, Schiff base II 25 mg/kg + Insulin 1 ml/kg, Schiff base II 25 mg/kg + Glibenclamide 5 mg/kg | No effect | - | - | |
| 3 | A | Saline 1 ml/kg, Insulin 1 ml/kg, Glibenclamide 5 mg/kg, Schiff base I 50 mg/kg, Schiff base II 25, 50, 100 mg/kg | - | Diabetes(depressant) Drugs(antidepressant) | - |
| B | Saline 1 ml/kg, Insulin 1 ml/kg, Glibenclamide 5 mg/kg, Schiff base II 25, Schiff base II 25 mg/kg + Insulin 1 ml/kg, Schiff base II 25 mg/kg + Glibenclamide 5 mg/kg | - | No effect | - | |
| 4 | A | Saline 1 ml/kg, Insulin 1 ml/kg, Glibenclamide 5 mg/kg, Schiff base I 50 mg/kg, Schiff base II 25, 50, 100 mg/kg | - | - | Diabetes(hyperalgesia) Drugs (analgesia) |
| B | Saline 1 ml/kg, Insulin 1 ml/kg, Glibenclamide 5 mg/kg, Schiff base II 25, Schiff base II 25 mg/kg + Insulin 1 ml/kg, Schiff base II 25 mg/kg + Glibenclamide 5 mg/kg | - | - | No effect |
Behavioral tests
Elevated plus maze (EPM) test
The apparatus made of four arms two of which had no side or end walls (open arms; 50 × 10 cm). The other two arms had side walls and end walls, but were open on the top (closed arms; 50 × 10 × 40 cm). Where the four arms crossed, there was a square platform of 10 × 10 cm. Rats were located in the experimental room at least 1 h before the test. Rats were individually located in the center of the apparatus facing a closed arm and allowed 5 min of free exploration. The number of entries into the open arms, the number of entries into the closed arms, the total time spent in the open arms, and the total time spent in the closed arms were recorded via a video camera through a monitor and a computer recording system were installed in a next room. The test room was illuminated via two 60-W bulbs placed 1.5 m above the apparatus. Raw data were used to manually evaluate the anxiety-like behaviors. Entry was considered as putting all four paws in the arms and calculated via a hand counter. The time spent in open arms (OAT) and the numbers of entry to the open arms (OAE) was recorded as an amount for anxiety. Moreover, the number of total arm entries was measured as an amount for locomotor activity. Drugs that act on anxiety-like behavior may either enhance or reduce OAT and OAE showing anxiolytic-like or anxiogenic like behaviors respectively [29].
Forced swim test (FST)
FST is commonly used to assess depressant like behaviors in rodents. FST is upon the hypothesis that when a rat is located in a container filled with water; it will first attempt to escape. Nonetheless, the rat finally will show immobility which may be determined as a measure of behavioral despair. Rats were subjected to the FST apparatus which made of a cylindrical glass container (height = 30 cm and diameter = 20 cm) filled with water (25 °C). Each rat was individually located in the apparatus for 6 min. Because great agitation is usually perceived during the first 2 min, only the immobility times observing during the last 4 min were recorded. A rat was detected immobile when it stopped its swimming tries and sustained floating motionless in the water, making only those movements necessary to keep its head above water [30].
Hot plate test
The hot plate test is an assessment of pain behavior in rodents which is alike to the tail-flick test. The hot plate test is performed to evaluate the pain threshold and to examine the effectiveness of the analgesic by detecting the response to the heat produced pain. Eddy and Leimbach suggested the hot plate test in 1953 [31]. In this protocol, behaviors including jumping are induced after a noxious thermal stimulus. Jumping determines a more elaborated response, with latency, and contains an emotional component of escaping. The plate was surrounded by four Plexiglas walls. Consequently, the rat could not escape. The rat was removed from the plate rapidly after jumping or no response within 50 s.
Statistical analysis
The results were statistically assessed by the one-way variance analysis (ANOVA) followed by Tukey’s multiple comparisons to analyze drug action, and two-way variance analysis ANOVA was used to analyze the interactions between the drug combination in comparison to the individual treatment groups. Mean ± SD indicates the possible different outcomes among the experimental groups and their corresponding controls. Following a significant F value, Post-hoc analysis (Tukey test) was performed to assess differences between groups. P-value was less than 0.05 showing statistical significance.
Results
Effects of insulin, glibenclamide and Schiff bases on anxiety behavior in diabetic rats
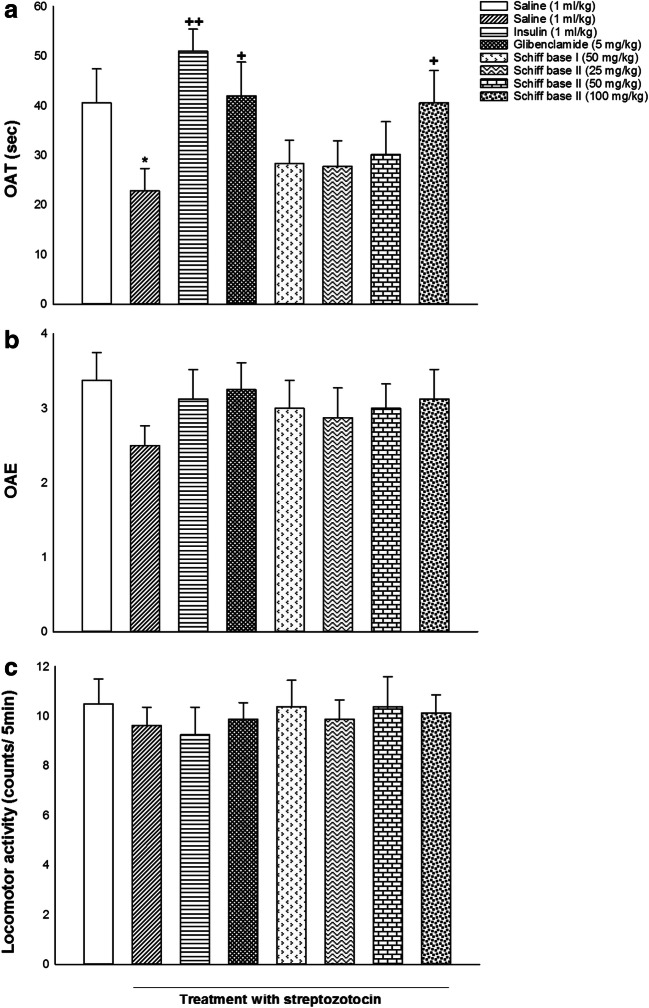
Figure 2 indicated the effects of i.p. administrations of insulin, glibenclamide, Schiff base I, and Schiff base II on anxiety behavior in male diabetic rats. One-way ANOVA and post hoc analysis exhibited that i.p. administration of saline (1 ml/kg), insulin (1 ml/kg), glibenclamide (5 mg/kg), Schiff base I (50 mg/kg), Schiff base II (25, 50, 100 mg/kg) changed OAT [F (7, 56) = 2.726, P = 0.017; Fig. 2a] but did not alter OAE [F (7, 56) = 0.525, P = 0.812; Fig. 2b] and locomotor activity [F (7, 56) = 0.210, P = 0.982; Fig. 2c] in comparison with the saline group. Post-hoc analysis displayed that induction of diabetes decreased OAT while administration of insulin (1 ml/kg), glibenclamide (5 mg/kg), and Schiff base II (100 mg/kg) increased OAT in the EPM in comparison with saline group, presenting anxiogenic effect in diabetic rats without drug treatment and anxiolytic effect in diabetic rats with drug treatment.
Fig. 2.
The effects of insulin, glibenclamide, Schiff base I, and Schiff base II on behavioral despair in the OAT (a), OAE (b), and locomotor activity (c). Values are expressed as mean ± S.E.M. from 8 rats and were analyzed by one-way ANOVA followed by Tukey’s post hoc test. * p < 0.05 in comparison to the saline group. ++ p < 0.01 and + p < 0.05 in compared with the saline/streptozotocin group
Effects of co-treatment of insulin and glibenclamide along with Schiff bases II on anxiety behavior in diabetic rats
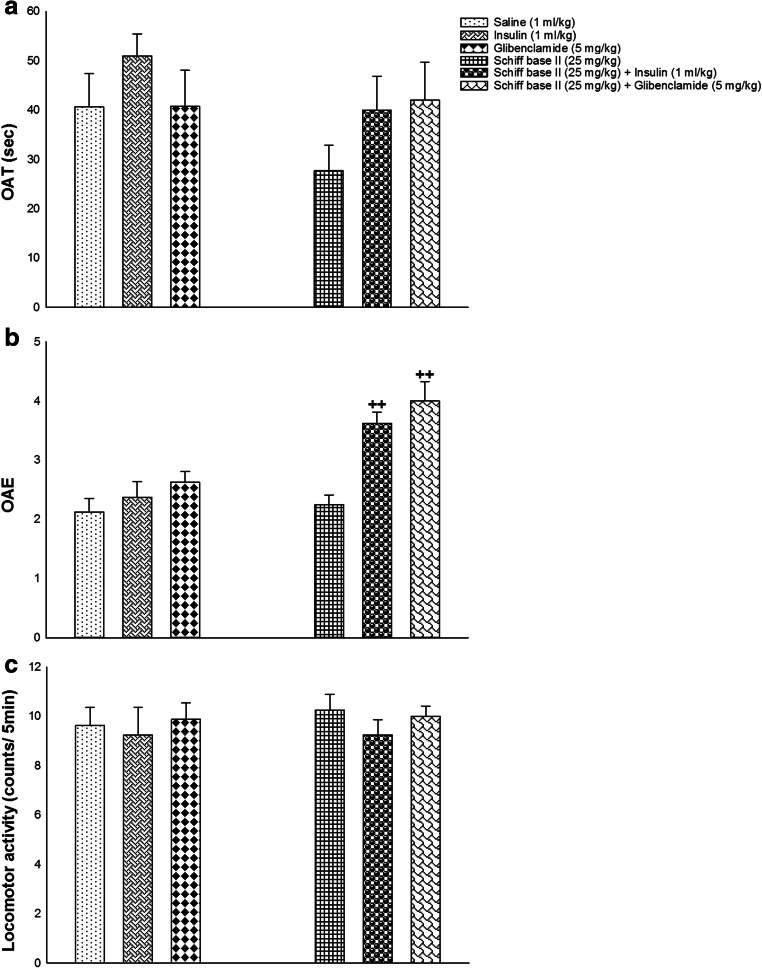
In Fig. 3 are seen the effects of co-administrations of insulin and glibenclamide along with ineffective dose of Schiff base II on anxiety behavior in male diabetic rats. Two-way ANOVA showed a significant difference between diabetic rats without drug treatment in comparison to drug treatment in OAE [treatment effect: F (1,42) = 23.548, P = 0.000, dose effect: F (2,42) = 12.590, P = 0.000, treatment–dose interaction: F (2,42) = 4.424, P = 0.018; Fig. 3b] but not for OAT [treatment effect: F (1,42) = 2.022, P = 0.162, dose effect: F (2,42) = 1.554, P = 0.223, treatment–dose interaction: F (2,42) = 0.697, P = 0.504; Fig. 3a] and locomotor activity [treatment effect: F (1,42) = 0.176, P = 0.677, dose effect: F (2,42) = 0.591, P = 0.558, treatment–dose interaction: F (2,42) = 0.103, P = 0.903; Fig. 3c]. Thus, co-administration of insulin and glibenclamide along with not-effective dose of Schiff base II increased anxiolytic behavior in diabetic rats.
Fig. 3.
The co-administrations of insulin and glibenclamide along with a not-effective dose of Schiff base II on behavioral despair in the OAT (a), OAE (b), and locomotor activity (c). Values are indicated as mean ± S.E.M. from 8 rats and were analyzed by two-way ANOVA followed by Tukey’s post hoc test. ++ p < 0.01 in compared with respective group
Effects of alone and co-injection of insulin, glibenclamide and Schiff bases on depression behavior in diabetic rats
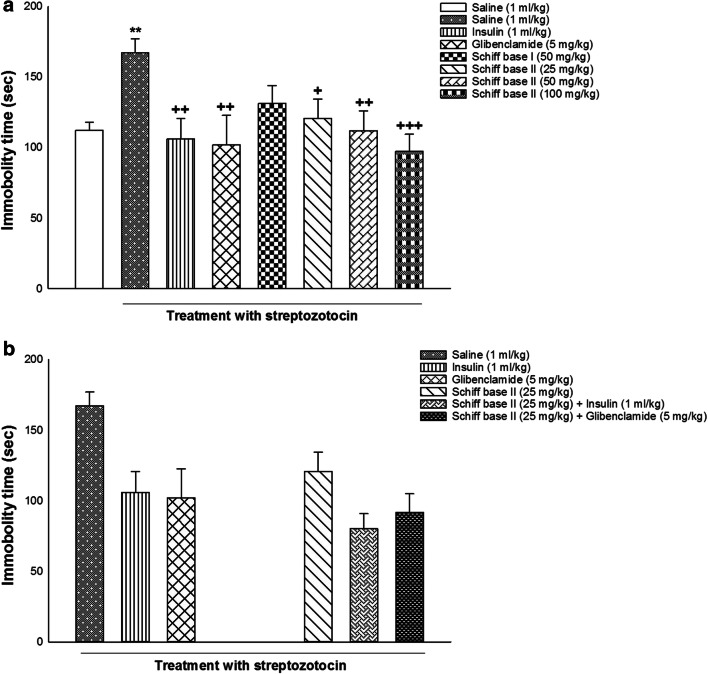
Figure 4 showed the effects of alone and co-injection of insulin, glibenclamide, Schiff base I, and Schiff base II on depression behavior in diabetic rats. One-way ANOVA and post hoc analysis indicated that alone injection of saline (1 ml/kg), insulin (1 ml/kg), glibenclamide (5 mg/kg), Schiff base I (50 mg/kg), Schiff base II (25, 50, 100 mg/kg) modified immobility time in the FST [F (7, 56) = 2.739, P = 0.016; Fig. 4a]. Post-hoc analysis revealed that induction of diabetes enhanced immobility time whereas injection of insulin (1 ml/kg), glibenclamide (5 mg/kg), and different doses of Schiff base II (25, 50, and 100 mg/kg) decreased immobility time in the FST in comparison with saline group, indicating depressant response in diabetic rats without drug treatment and antidepressant response in diabetic rats with drug treatment.
Fig. 4.
The effects of alone and co-administration of insulin, glibenclamide, Schiff base I, and Schiff base II on behavioral despair in the immobility time in the FST (a and b). Values are presented as mean ± S.E.M. from 8 rats and were analyzed by one- and two-way ANOVA followed by Tukey’s post hoc test. ** p < 0.01 in comparison to saline group. +++ p < 0.001, ++ p < 0.01 and + p < 0.05 in compared with the saline/streptozotocin group
Moreover, two-way ANOVA showed no significant effect of drugs co-injection in immobility time of the FST [treatment effect: F (1,42) = 5.627, P = 0.022, dose effect: F (2,42) = 7.975, P = 0.001, treatment–dose interaction: F (2,42) = 0.821, P = 0.447; Fig. 4b].
Effects of alone and co-administration of insulin, glibenclamide and Schiff bases on pain behavior in diabetic rats
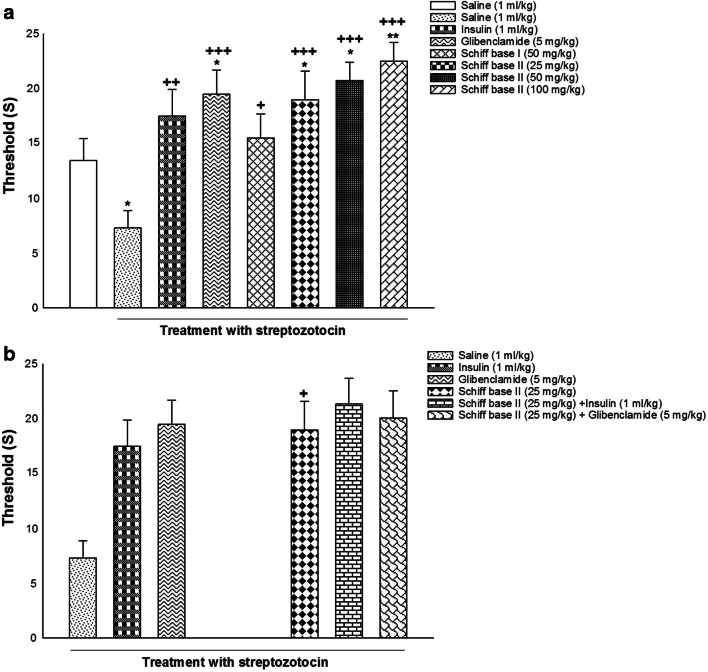
The effects of alone and co-administration of insulin, glibenclamide, Schiff base I, and Schiff base II on pain behavior in diabetic rats are illustrated in Fig. 5. The results determined using one-way ANOVA displayed that alone administration of saline (1 ml/kg), insulin (1 ml/kg), glibenclamide (5 mg/kg), Schiff base I (50 mg/kg), Schiff base II (25, 50, 100 mg/kg) significantly altered latency in the hot-plate test [F (7, 56) = 5.399, P = 0.000; Fig. 5a]. Post-hoc analysis displayed that induction of diabetes decreased threshold of latency but the injection of insulin (1 ml/kg), glibenclamide (5 mg/kg), Schiff base I (50 mg/kg), and diverse doses of Schiff base II (25, 50, and 100 mg/kg) increased threshold of latency in the hot-plate test in comparison with saline group, suggesting hyperalgesic effect in diabetic rats without drug treatment and analgesic effect in diabetic rats with drug treatment.
Fig. 5.
The effects of alone and co-injection of insulin, glibenclamide, Schiff base I, and Schiff base II on behavioral despair in the latency of the hot-plate test (a and b). Values are displayed as mean ± S.E.M. from 8 rats and were analyzed by one- and two-way ANOVA followed by Tukey’s post hoc test. ** p < 0.01 and * p < 0.05 in comparison to saline group. +++ p < 0.001, ++ p < 0.01 and + p < 0.05 in compared with the saline/streptozotocin group
Furthermore, two-way ANOVA exhibited no meaningful effect of drugs co-administration in latency of the hot-plate test [treatment effect: F (1,42) = 8.336, P = 0.005, dose effect: F (2,42) = 5.294, P = 0.009, treatment–dose interaction: F (2,42) = 2.689, P = 0.080; Fig. 5b].
Discussion
In the present research, diabetes mellitus was induced in rats via a streptozotocin injection which destroys the β-cells of Langerhans islets, as reported by many researchers [2, 32, 33]. In addition to the problems of type 2 diabetes, some psychological syndromes are also very common in these patients [7]. The results of the present research indicated that the induction of type 2 diabetes mellitus produced anxiogenic, depressant, and hyperalgesia effects in male rats. The association between anxiety and depression with diabetes has been extensively analyzed [34]. Anxiety and depression are usual disorders in patients suffering from type 2 diabetes [7, 35, 36]. Evidence revealed that symptoms of anxiety and depression often remain unrecognized in diabetes diseases. As diabetes has enhanced worldwide recently, it is essential to decrease the prevalence of factors which are related to anxiety and depression in diabetes patients [35]. Our results support early detection of anxiety and depression in patients with diabetes. The occurrence of anxiety and depressive diseases can be as high as two-fold greater in individuals suffering from type 2 diabetes [8–11, 13]. Evidence demonstrated a bidirectional correlation between diabetes mellitus, and anxiety and depressive disorders. So that patients with anxiety symptoms may be at enhanced risk of developing type 2 diabetes and vice versa [37]. Also, there are many controlled researches indicating the enhanced prevalence of depression in patients with type 2 diabetes mellitus [34]. Indeed, there are many biological reasons why diabetes and anxiety/depression enhance each other’s prevalence [14]. Studies revealed that inflammatory markers-related to diabetes increased in diabetes diseases which along with hyperglycemia and maybe hyperinsulinemia caused a net pro-inflammatory state in numerous tissues. Access of pro-inflammatory markers to the brain may then lead to activation of the processes leading to the progress of psychological disorders such as anxiety and depression. Moreover, type 2 diabetes mellitus is linked with decreased size of the brain area such as the hippocampus and amygdala, providing evidence for the proposition that type 2 diabetes mellitus provide a biological risk factor for anxiety and depression [38]. Additionally, microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular (cardiovascular and cerebrovascular) problems are common in type 2 diabetes mellitus [16, 39–41] which is possible due to failure of the endogenous analgesic system in the descending spinal pathway [17, 18].
Furthermore, our results indicated that the administration of insulin, glibenclamide, Schiff bases I, and Schiff base II induced anxiolytic, antidepressant, and analgesic effects in diabetic rats. Previous researches have revealed that events of psychological nature are key factors interfering with insulin secretion causing poor diabetic stability. According to reports, the relationship between these conditions is bi-directional. It is reported that patients with anxiety, depression, and diabetes, compared to diabetic patients alone, have been linked with more diabetes complications. The incidence of anxiety and depression in persons with diabetes appears to be linked with physical activity, socioeconomic status, family status, obesity, and smoking habits [8, 42]. As the disease’s additional progress and complications of diabetes ensue, particularly neuropathy, nephropathy, and sexual dysfunction, the incidence of depression in the patient further increases [43–45]. Insulin and glibenclamide as hypoglycemic drugs are still the drugs of choice. These drugs are used throughout life that reduction of response after long use may produce side effects [27]. Therefore, finding new anti-diabetic drugs that potentiate the anti-diabetic effects of low-dose of insulin and/or glibenclamide will be a good achievement in the medicine for diabetes management. In this respect, Schiff bases are some of the most generally used organic compounds that indicated anti-diabetic activity [22]. In keeping with our findings, Khan and colleagues (2009) reported that Schiff bases of isatin indicated a variety of biological activities including antidepressant, anti-inflammatory, antimicrobial, and effects on the central nervous system [46]. Schiff bases of isatin are capable of crossing the blood–brain-barrier. Also, isatins induce antiglycation activity [46]. So that numerous Schiff bases show α-glucosidase inhibitory properties [19–21]. α-Glucosidase is a membrane-bound enzyme that hydrolyzes terminal non-reducing 1–4 connected α-glucose residues to release monomeric glucose molecules that is principally responsible to induce hyperglycemia [47, 48]. Blocking of α-glucosidase action can delay carbohydrate absorption and used as one of the therapeutic procedures for the treatment of diabetes [48, 49]. It has been reported that Schiff bases of isatins possessed antiglycation activity [46, 50] and can restore insulin signaling in the muscle, liver, and fat cells [50, 51]. Schiff bases I and II via inhibition of α-glucosidase function likely blocked problems induced by diabetes for example anxiogenic, depressant, and hyperalgesia effects. Interestingly, our data indicated that co-administration of Schiff base II and insulin or glibenclamide induced anxiolytic effect in diabetic rats, suggesting an interaction between these drugs on the modulation of anxiety behavior. However, further studies are needed to determine Schiff base II therapeutic potential against diabetes complications.
Acknowledgements
We are thankful to all contributors for their participation.
Compliance with ethical standards
The study was carried out under ethical standards in all aspects.
Conflict of interest
No financial or other conflicts of interest are declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nirmala M, Suhasini GE, Venkata Lakshmi K, Archanagiri B, Solomon Sunder R. ANTIDIABETIC ACTIVITY OF NEW ISATIN DERIVATIVE – N’- ( 7- Chloro- 2- oxo -2,3- dihydro- 1H - indol- 3-yl ) BENZOHYDRAZIDE IN ALLOXAN-INDUCED DIABETIC RATS. Int J Pharm Biol Sci. 2013;3(4):107–21. [Google Scholar]
- 2.Venkateshwarlu E. Evaluation of anti-diabetic and hypolipidemic activity of isatin derivatives in streptozotocin-nicotinamide induced type ii diabetic rats. Adv Biol Res. 2013;7(6):288–95. [Google Scholar]
- 3.Ajani HB, Patel HP, Shah GB, Acharya SR, Shah SK. Evaluation of antidiabetic effect of methanolic extract of inula racemosa root in rats. Pharmacologyonline. 2009;3:118–29. [Google Scholar]
- 4.Radenkovic M, Stojanovic M, Prostran M. Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J Pharmacol Toxicol Methods 78:13–31. [DOI] [PubMed]
- 5.Chundi V, Challa SR, Garikapati DR, Juvva G, Jampani A, Pinnamaneni SH, et al. Biochanin-A attenuates neuropathic pain in diabetic rats. J Ayurveda Integr Med. 2016;7(4):231–7. doi: 10.1016/j.jaim.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tejasree Ch, Kiran G, Rajyalakshmi G. Antidiabetic activity of 1-(4-(dimethylamino)benzylidene)-5-(2-oxo indolin-3-ylidene) thiocarbohydrazone in rats. Int J Pharm Sci Res. 2014;5(7):2738–43. [Google Scholar]
- 7.Oguz N. Anxiety and depression in diabetic patients. Eurasian J Med Investig. 2018;2(4):174–7. [Google Scholar]
- 8.Palizgir M, Bakhtiari M, Esteghamati A. Association of depression and anxiety with diabetes mellitus type 2 concerning some sociological factors. Iran Red Crescent Med J. 2013;15(8):644–8. [DOI] [PMC free article] [PubMed]
- 9.Nichols GA, Brown JB. Unadjusted and adjusted prevalence of diagnosed depression in type 2 diabetes. Diabetes Care. 2003;26(3):744–9. doi: 10.2337/diacare.26.3.744. [DOI] [PubMed] [Google Scholar]
- 10.Knol MJ, Heerdink ER, Egberts AC, Geerlings MI, Gorter KJ, Numans ME, et al. Depressive symptoms in subjects with diagnosed and undiagnosed type 2 diabetes. Psychosom Med. 2007;69(4):300–5. doi: 10.1097/PSY.0b013e31805f48b9. [DOI] [PubMed] [Google Scholar]
- 11.Huang CJ, Hsieh HM, Tu HP, Jiang HJ, Wang PW, Lin CH. Generalized anxiety disorder in type 2 diabetes mellitus: prevalence and clinical characteristics. Braz J Psychiatry. 2020. [DOI] [PMC free article] [PubMed]
- 12.Khan P, Qayyum N, Malik F, Khan T, Khan M, Tahir A. Incidence of anxiety and depression among patients with type 2 diabetes and the predicting factors. Cureus. 2019;11(3):e4254. doi: 10.7759/cureus.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salinero-Fort MA, Gomez-Campelo P, San Andres-Rebollo FJ, Cardenas-Valladolid J, Abanades-Herranz JC, Carrillo de Santa Pau E, et al. Prevalence of depression in patients with type 2 diabetes mellitus in Spain (the DIADEMA Study): results from the MADIABETES cohort. BMJ Open. 2018;8(9):e020768. doi: 10.1136/bmjopen-2017-020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain R, Jain S, Raison CL, Maletic V. Painful diabetic neuropathy is more than pain alone: examining the role of anxiety and depression as mediators and complicators. Curr Diabetes Rep. 2011;11(4):275–84. doi: 10.1007/s11892-011-0202-2. [DOI] [PubMed] [Google Scholar]
- 15.Raafat K, El-Lakany A. Acute and subchronic in-vivo effects of Ferula hermonis L. and Sambucus nigra L. and their potential active isolates in a diabetic mouse model of neuropathic pain. BMC Complement Altern Med. 2015;15:257. doi: 10.1186/s12906-015-0780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raafat K, Samy W. Amelioration of diabetes and painful diabetic neuropathy by Punica granatum L. extract and its spray dried biopolymeric dispersions. Evid Based Complement Alternat Med. 2014;2014:180495. doi: 10.1155/2014/180495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy MK, Kuriakose AS, Varma SK, Jacob LA, Beegum NJ. A study on comparative efficacy and cost effectiveness of Pregabalin and Duloxetine used in diabetic neuropathic pain. Diabetes Metab Syndr. 2017;11(1):31–5. doi: 10.1016/j.dsx.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Tanenberg RJ, Irving GA, Risser RC, Ahl J, Robinson MJ, Skljarevski V, et al. Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomized, noninferiority comparison. Mayo Clinic Proc. 2011;86(7):615–26. doi: 10.4065/mcp.2010.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyazaki R, Yasui H, Yoshikawa Y. α-Glucosidase inhibition by new Schiff base complexes of Zn(II) Open J Inorg Chem. 2016;6:114–24. doi: 10.4236/ojic.2016.62007. [DOI] [Google Scholar]
- 20.Rahim F, Malik F, Ullah H, Wadood A, Khan F, Javid MT, et al. Isatin based Schiff bases as inhibitors of alpha-glucosidase: Synthesis, characterization, in vitro evaluation and molecular docking studies. Bioorg Chem 2015;60:42–8 [DOI] [PubMed]
- 21.Ullah H, Rahim F, Taha M, Hussain R, Tabassum N, Wadood A, et al. Aryl-oxadiazole Schiff bases: Synthesis, a-glucosidase in vitro inhibitory activity and their in silico studies. Arab J Chem. 2020;13:4904–15. doi: 10.1016/j.arabjc.2020.01.005. [DOI] [Google Scholar]
- 22.Abdel-Rahman LH, El-Khatib RM, Nassr LA, Abu-Dief AM, Lashin Fel D. Design, characterization, teratogenicity testing, antibacterial, antifungal and DNA interaction of few high spin Fe(II) Schiff base amino acid complexes. Spectrochim Acta A Mol Biomol Spectrosc. 2013;111:266–76. doi: 10.1016/j.saa.2013.03.061. [DOI] [PubMed] [Google Scholar]
- 23.Verma M, Pandeya SN, Singh KN, Stables JP. Anticonvulsant activity of Schiff bases of isatin derivatives. Acta Pharm. 2004;54(1):49–56. [PubMed] [Google Scholar]
- 24.Almutairi MS, Zakaria AS, Al-Wabli RI, Joe IH, Abdelhameed AS, Attia MI. Synthesis. Spectroscopic Identification and Molecular Docking of Certain N-(2-{[2-(1H-Indol-2-ylcarbonyl) Hydrazinyl](oxo)Acetylphenyl)Acetamides and N-[2-(2-{[2-(Acetylamino)Phenyl](oxo)Acetylhydrazinyl)-2-Oxoethyl]-1H-Indole-2-Carboxamides: New Antimicrobial Agents. Molecules (Basel, Switzerland). 2018;23(5):1043. [DOI] [PMC free article] [PubMed]
- 25.Pandeya SN, Sriram D, Nath G, de Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin and its derivatives with triazole. Arzneimittelforschung. 2000;50(1):55–9. doi: 10.1055/s-0031-1300164. [DOI] [PubMed] [Google Scholar]
- 26.Nirmal R, Prakash C, Meenakshi K, Shanmugapandiyan P. Synthesis and pharmacological evaluation of novel schiff base analogues of 3-(4-amino) phenylimino) 5-fluoroindolin-2-one. J Young Pharm. 2010;2(2):162–8. doi: 10.4103/0975-1483.63162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414(6865):821–7. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- 28.Annapurna A, Reddy CS, Akondi RB, Rao SR. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 2009;61(10):1365–74. doi: 10.1211/jpp.61.10.0014. [DOI] [PubMed] [Google Scholar]
- 29.Rezayof A, Hosseini SS, Zarrindast MR. Effects of morphine on rat behaviour in the elevated plus maze: the role of central amygdala dopamine receptors. Behav Brain Res. 2009;202(2):171–8. doi: 10.1016/j.bbr.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 30.Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. Journal of visualized experiments: JoVE. 2015(97):52587. [DOI] [PMC free article] [PubMed]
- 31.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107(3):385–93. [PubMed] [Google Scholar]
- 32.Cameron-Smith D, Habito R, Barnett M, Collier GR. Dietary guar gum improves insulin sensitivity in streptozotocin-induced diabetic rats. J Nutr. 1997;127(2):359–64. doi: 10.1093/jn/127.2.359. [DOI] [PubMed] [Google Scholar]
- 33.Ferner RE. Drug-induced diabetes. Baillieres Clin Endocrinol Metab. 1992;6(4):849–66. doi: 10.1016/S0950-351X(05)80170-3. [DOI] [PubMed] [Google Scholar]
- 34.Anaforoğlu I, Ramazanoğulları I, Algün E, Kutanis R. Depression, anxiety and quality of life of family caregivers of patients with type 2 diabetes. Med Princ Pract. 2012;21(4):360–5. doi: 10.1159/000334622. [DOI] [PubMed] [Google Scholar]
- 35.Dehesh T, Dehesh P, Shojaei S. Prevalence and Associated Factors of Anxiety and Depression Among Patients with Type 2 Diabetes in Kerman, Southern Iran. Diabetes Metab Syndr Obes Targets Ther. 2020;13:1509–17. [DOI] [PMC free article] [PubMed]
- 36.Liu X, Haagsma J, Sijbrands E, Buijks H, Boogaard L, Mackenbach JP, et al. Anxiety and depression in diabetes care: longitudinal associations with health-related quality of life. Sci Rep. 2020;10(1):8307. doi: 10.1038/s41598-020-57647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woon LS-C, Sidi HB, Ravindran A, Gosse PJ, Mainland RL, Kaunismaa ES, et al. Depression, anxiety, and associated factors in patients with diabetes: evidence from the anxiety, depression, and personality traits in diabetes mellitus (ADAPT-DM) study. BMC Psychiatry. 2020;20(1):227. doi: 10.1186/s12888-020-02615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palizgir M, Bakhtiari M, Esteghamati A. Association of depression and anxiety with diabetes mellitus type 2 concerning some sociological factors. Iran Red Crescent Med J. 2013;15(8):644–8. [DOI] [PMC free article] [PubMed]
- 39.Stopford R, Winkley K, Ismail K. Social support and glycemic control in type 2 diabetes: a systematic review of observational studies. Patient Educ Couns. 2013;93(3):549–58. doi: 10.1016/j.pec.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Alkhatatbeh MJ, Abdul-Razzak KK, Khasawneh LQ, Saadeh NA. Prevalence of musculoskeletal pain in association with serum 25-hydroxyvitamin D concentrations in patients with type 2 diabetes mellitus. Biomed Rep. 2018;8(6):571–7. doi: 10.3892/br.2018.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Q, Chen Y, Gong N, Wang YX. Methylglyoxal mediates streptozotocin-induced diabetic neuropathic pain via activation of the peripheral TRPA1 and Nav1.8 channels. Metabolism 2016;65(4):463–74. [DOI] [PubMed]
- 42.Bouwman V, Adriaanse MC, van ‘t Riet E, Snoek FJ, Dekker JM, Nijpels G. Depression, anxiety and glucose metabolism in the general dutch population: the new Hoorn study. PLoS One 2010;5(4):e9971. [DOI] [PMC free article] [PubMed]
- 43.Bajaj S, Agarwal SK, Varma A, Singh VK. Association of depression and its relation with complications in newly diagnosed type 2 diabetes. Indian J Endocrinol Metab. 2012;16(5):759–63. doi: 10.4103/2230-8210.100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 45.van Steenbergen-Weijenburg KM, van Puffelen AL, Horn EK, Nuyen J, van Dam PS, van Benthem TB, et al. More co-morbid depression in patients with Type 2 diabetes with multiple complications. An observational study at a specialized outpatient clinic. Diabet Med 2011;28(1):86–9. [DOI] [PubMed]
- 46.Khan KM, Khan M, Ali M, Taha M, Rasheed S, Perveen S, et al. Synthesis of bis-Schiff bases of isatins and their antiglycation activity. Bioorg Med Chem. 2009;17(22):7795–801. doi: 10.1016/j.bmc.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 47.van de Laar FA. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc Health Risk Manag. 2008;4(6):1189–95. doi: 10.2147/VHRM.S3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie Z, Wang G, Wang J, Chen M, Peng Y, Li L, et al. Synthesis, biological evaluation, and molecular docking studies of novel isatin-thiazole derivatives as alpha-glucosidase inhibitors. Molecules. 2017;22(4):659. doi: 10.3390/molecules22040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joshi SR, Standl E, Tong N, Shah P, Kalra S, Rathod R. Therapeutic potential of alpha-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin Pharmacother. 2015;16(13):1959–81. doi: 10.1517/14656566.2015.1070827. [DOI] [PubMed] [Google Scholar]
- 50.Aftab MF, Afridi SK, Mughal UR, Karim A, Haleem DJ, Kabir N, et al. New isatin derivative inhibits neurodegeneration by restoring insulin signaling in brain. J Chem Neuroanat. 2017;81:1–9. doi: 10.1016/j.jchemneu.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Afridi SK, Aftab MF, Murtaza M, Ghaffar S, Karim A, Mughal UR, et al. A new glycotoxins inhibitor attenuates insulin resistance in liver and fat cells. Biochem Biophys Res Commun. 2016;476(4):188–95. doi: 10.1016/j.bbrc.2016.05.085. [DOI] [PubMed] [Google Scholar]