Abstract
Aim
Onion is one of the commonly cultivated and consumed vegetables rich in nutrients and phytochemicals. Various nutraceuticals are found in the outer fleshy layers and dry peel of onion which usually is treated as a common biowaste. Diabetes mellitus is a leading non communicable disease causing hyperglycemia and increased production of free radicals that potentially disrupts antioxidant enzymatic activity. Considering global consumption of wheat, the present study was designed to evaluate the anti-hyperglycemic and antioxidant effects of wheat bread supplemented with onion peel extract (OPE) or onion powder (OP) on diabetic rats.
Methods
In this study, ethanolic extract of onion peel and onion bulb were prepared separately. Male Sprague Dawley rats were divided into 6 groups (n = 7). Different regimens of supplemented wheat bread (OPE (1% and 3%) and OP (5% and 7%)) were given to diabetic rats for eight weeks, plain bread was used as the control. Blood glucose level, body weight and activities of SOD, CAT, GPx, GR, GSH and MDA in the liver and kidney tissues were evaluated. Statistical analysis was performed using SPSS Version (25) and Dunnett’s multiple comparison test.
Results
Bread supplemented with 1% and 3% onion peel extract and 7% onion powder significantly reduced blood glucose levels and MDA in the treated rats compared with the control group diabetic rats. Body weight of diabetic rats was reduced for control group, while onion supplemented diet improved the body weight of treated rats. Onion supplementation also brought significant improvement in antioxidant enzyme activities among the treated diabetic rats.
Conclusion
These findings suggested that onion supplementation is effective in lowering blood glucose and could potentially aid in protecting organs from oxidative stress.
Keywords: Onion powder, Onion peel extract, Antioxidant activity, Hypoglycemic activity, Wheat bread, Phytochemicals
Introduction
Diabetes is characterized by impaired carbohydrate, protein, and fat metabolism that results from poor insulin secretion, insulin resistance, or both. Diabetes mellitus is currently one of the most prevalent and life-threatening metabolic disorder globally. Based on several estimations, it is suggested that its incidence rate will rise even more rapidly in the coming future [1]. Recurrent hyperglycemia, which is the major clinical manifestation in diabetic patients, is primarily associated with the progression of several acute and chronic diabetic complications. Keeping blood glucose under controlled levels, understanding the regulation of insulin response, and preventing diabetes-associated complications are the most important goals in managing diabetes [2]. Oxidative stress is one of the primary factors involved in the development of diabetes complications and is postulated to be linked with increased production of oxygen free radical [3]. Under normal physiological conditions, various antioxidant defense mechanisms work to protect the body from the dangers of free radical production [4]. In diabetes, the elevation of blood glucose levels promotes the production of reactive oxygen species (ROS) [5]. The mechanisms involved in ROS production not only includes auto-oxidative and non-enzymatic glycosylation but also the metabolic stress that results from altered energy metabolism, raised levels of inflammatory mediators, and poor status of the antioxidant defense system [6].
To understand the mechanism of diabetes and its relation to oxidative stress, several in vivo models were developed including alloxan-induced diabetes in mice. Alloxan, a pyrimidine derivative, is used to induce diabetes and produce cytotoxic free radicals that destroy animals’ pancreatic cells. These effects suggest that alloxan could influence the intrinsic antioxidant defense system in response to oxidative stress [7]. The hazardous effects of oxygen and hydroxy radicals can be prevented by various antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD). Glutathione (GSH) also works efficiently to neutralize the damaging electrophiles [8].
Several classes of drugs were developed to manage diabetes showing impressive results but many of the developed products resulted in side effects because the antidiabetic drugs are lifelong. Therefore, there is growing interest to incorporate medicinal plant-based products into the diet of the general population to reduce the medication use and thus their side effects and to obtain maximum preventive outcome [9]. Onion (Allium cepa L.) is a widely cultivated and consumed vegetable that serves as an excellent source of macro and micronutrients [10]. Along with the bulb, the peel of onion, which is generally regarded as a biowaste is rich in various phytonutrients. Although the management of diabetes with traditional plants has been a widespread practice for centuries [11], only a few remedies have been assessed scientifically. Many researchers demonstrated the antidiabetic, hypocholesterolemic, and antioxidant potential of onion peel extract and onion powder [12–18]. The evaluation and utilization of biowaste (onion peel) as a supplementary ingredient in food for target groups will have sustainable health, economic and environmental impact. Wheat (Triticum aestivum L.) is one of the most commonly consumed staple food [19], and there has been growing interest towards its fortification with micronutrients and nutraceuticals [20–22]. Hence, in the present study, the American Institute of Nutrition (AIN)-93G diet for rodents was modified to formulate bread using ingredients like wheat flour, onion powder/onion peel extract to evaluate the effect of their supplementation on blood glucose level, and the antioxidant activities of various enzymes in the kidney and liver homogenates of diabetic rats. The potential physiological influence of the onion’s outer peel and the edible bulb has not been compared yet in a scientific study. Considering all these points, the present study examined the potential impact and antioxidant activities of the peel extract and bulb powder of onion on the in vivo kidney and liver cell metabolism of diabetic rats.
Material and Methods
Animals
Male Sprague Dawley rats (10–12 weeks old) weighing 250–300 g, were purchased from the National Institute of Health (NIH) Islamabad-Pakistan and were acclimatized for 2 weeks in the animal house of the University of Lahore, Pakistan (March 2019–May 2019). Seven rats were kept in each cage and the environmental conditions such as humidity (55 ± 5%) and temperature (23 ± 2 °C) were sustained during the entire study with 12 h of light-dark period. Rats were given free access to the basal diet and water ad libitum. The rats were handled according to the international guidelines on the use and handling of experimental animals (US National Institute of Health) [23] and the guidelines provided by the ethical committee of the University of Lahore, Pakistan (Ref No: IRB-UOL-FAHS/435/2019).
Onion peel extract preparation
Excellent quality, even-sized, undamaged onions (Var. Desi Red, Phulkara) were procured from the local market. The outer dry and fleshy layers of onions were washed thoroughly using sterile water. Extraction was done with 60% ethanol at 50 °C for 3 h [13]. The extract was filtered, concentrated, and stored after freeze-drying. The extraction yield was 5.35 ± 0.12% while the total phenolic content was 397.85 ± 4.03 mg/g as determined by the Folin-Ciocalteu method [24].
Onion powder preparation
The obtained peeled onion bulbs were chopped using a sterile knife and placed in a drying oven at 50 °C for 3 days. The dried onion pieces were grounded into a fine powder using an electric grinder and the obtained powder was stored in an airtight container inside the refrigerator [25]. The yield of onion powder was 12 g/100 g of the raw onion.
Preparation of bread
Five different formulations of bread were prepared including (1) a control bread (B0) based on the basic AIN-93G diet without any supplementation; (2) bread with 1% onion peel extract (B1); (3) bread with 3% onion peel extract (B2); (4) bread with 5% onion powder (B3); and (5) bread with 7% onion powder (B4). The preparation of all mentioned types of bread was done by following the same procedure [26] and the composition of each bread is presented in Table 1.
Table 1.
Ingredients (g/100 g) of the experimental diets (breads) fed to rats for 56 days
| Composition | Control Bread (B0) | Bread with 1% OPE (B1) | Bread with 3% OPE (B2) | Bread with 5% onion powder (B3) | Bread with 7% onion powder (B4) |
|---|---|---|---|---|---|
| Wheat | 68.2 | 67.2 | 65.2 | 63.2 | 61.2 |
| Salt | 1.36 | 1.36 | 1.36 | 1.36 | 1.36 |
| Dry yeast | 1.36 | 1.36 | 1.36 | 1.36 | 1.36 |
| Improver | 0.68 | 0.68 | 0.68 | 0.68 | 0.68 |
| Casein | 14.58 | 14.58 | 14.58 | 14.58 | 14.58 |
| Soybean oil | 8.93 | 8.93 | 8.93 | 8.93 | 8.93 |
| Minerals | 3.38 | 3.38 | 3.38 | 3.38 | 3.38 |
| Vitamin mix | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 |
| L-cysteine | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 |
| Choline | 0.24 | 0.24 | 0.24 | 0.24 | 0.24 |
| TBHQ | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Onion powder | 0 | 0 | 0 | 5 | 7 |
| Onion peel extract | 0 | 1 | 3 | 0 | 0 |
The control wheat bread comprised 100% wheat flour, 70% water (vol/wt, flour basis), 2% dry yeast (wt/wt, flour basis) (Rossmoor, Pakistan), 2% salt (wt/wt, flour basis), and 1% dough improver (wt/wt, flour basis) (eka-300) [26]. For supplemented bread, the formulation was the same but for bread B1 and B2, the wheat flour was substituted with OPE at 1% and 3%, respectively. Whereas for bread B3 and B4 the wheat flour was substituted with onion powder at 5% and 7%, respectively [13, 27–29].
Induction of diabetes in rats
Diabetes was induced in overnight fasted Sprague Dawley rats by injecting alloxan (120 mg/kg/BW) dissolved in normal saline, through the subcutaneous route. Glucose solution (20% (w/v)) was also given to these rats to prevent fatal post-alloxan hypoglycemia. After 48 h of alloxan administration, the rats with fasting blood glucose levels>250 mg/dl were considered diabetic and were included in the study [18, 30].
Treatment groups
The animals were randomly divided into six groups (7 rats each):
► Group 1 (NC): Normal rats fed with control bread.
► Group 2 (DC): Diabetic rats fed with control bread.
► Group 3 (OPE_1): Diabetic rats fed with bread supplemented with OPE (1%).
► Group 4 (OPE_3): Diabetic rats fed with bread supplemented with OPE (3%).
► Group 5 (OP_5): Diabetic rats fed with bread supplemented with onion powder (5%).
► Group 6 (OP_7): Diabetic rats fed with bread supplemented with onion powder (7%).
Before starting the trial, fasting blood glucose level (mg/dl) and body weight (g) were determined using a glucometer [31] and weighing scale, respectively. The modified diet was then given to the rats for 8 weeks. During the study, physical parameters such as water and bread consumption were measured daily (Appendix Table 4) while blood glucose was determined fortnightly. At the end of the trial, the rats fasted for 12 h and the final measurement of blood glucose level and body weight was taken [32]. All rats were anesthetized and sacrificed as per ethical committee guidelines and at 4 °C, the kidney and liver tissues were excised. Ice-cold saline was used to wash the isolated tissues that were later dipped in liquid nitrogen and instantly stored at −80 °C until further analysis [33].
Biochemical analysis
In the liver and kidney tissue homogenates, the activity of superoxide dismutase (SOD) was determined by the Misra and Fridovich method using a Hitachi U-2000 spectrophotometer for 4 min at 480 nm. The potential enzymatic activity is stated based on its ability to inhibit the epinephrine oxidation up to 50%, that is equivalent to 1 U per milligram of protein [34, 35]. The method of Aebi was used to assess the activity of catalase (CAT) in which absorbance was determined by UV spectrophotometer at 240 nm for 1 min [35, 36]. The method elaborated by Carlberg and Mannervik was used to determine the activity of Glutathione reductase (GR) and recommendations of Akerboom and Sies were followed to measure the reduced glutathione (GSH) concentration in the kidney and liver homogenates [37–40]. Hepatic and renal enzymatic activity of glutathione peroxidase (GPx) was evaluated following the Flohe and Gunzler method, using cumene hydrogen peroxide while the absorbance was calibrated at 340 nm [41, 42]. The procedure described by Ohkawa, Ohishi, and Yagi was used to determine the concentration of malondialdehyde (MDA), which indicates the extent of lipid peroxidation [43, 44]. Enzymatic activities were reported as per milligram of protein and the protocol of Lowry, Rosebrough, Farr, and Randall was used for estimation of tissue protein content using bovine serum albumin (BSA) as the standard [45, 46].
Statistical analysis
Data were analyzed using SPSS (Version 25.0) [47]. Dunnett’s multiple comparison test (p < 0.01) was performed to compare the antioxidants and enzymatic activity between the diabetic and control (DC) groups [33, 48].
Results
Effect of OPE and OP supplemented bread on the bodyweight of diabetic rats
The results obtained in the current study indicated that the rat’s body weight (BW) was substantially reduced after induction of diabetes. The incorporation of bread supplemented with onion peel or onion powder significantly improved the body weight, although the effect was statistically significant by onion peel supplementation (Table 2). The difference between the mean body weight of the treated and diabetic control group was initially insignificant (day 0) but during the later stages of the trial (day 28 and 56), a statistically significant increase in body weight of the treatment groups was observed (p < 0.01) (Table 2).
Table 2.
Effects of onion peel extract and onion powder supplemented breads on body weight changes in diabetic rats
| Groups | Body Weight (g) | Day 28 | Day 56 |
|---|---|---|---|
| Day 0 | |||
| NC | 257 ± 2.5 | 276 ± 3.0* | 295 ± 1.4* |
| DC | 258 ± 3.2 | 237 ± 2.1 | 222 ± 1.7 |
| OPE_1 | 266 ± 4.2 | 267 ± 4.6* | 270 ± 4.2* |
| OPE_3 | 266 ± 6.7 | 271 ± 6.2* | 282 ± 6.1* |
| OP_5 | 262 ± 6.2 | 256 ± 6.0* | 259 ± 5.9* |
| OP_7 | 258 ± 5.6 | 262 ± 5.7* | 266 ± 6.2* |
Values expressed as means ± SEM, (NC = normal control, DC = diabetic control, OPE_1 = group fed with bread containing 1% onion peel extract, OPE_3 = group fed with bread containing 3% onion peel extract, OP_5 = group fed with bread containing 1% onion powder, OP_7 = group fed with bread containing 7% onion powder), * shows the significant difference compared with the diabetic control group (p < 0.01) via Dunnett’s multiple comparison test
Effect of OPE and OP supplemented bread on blood glucose level of diabetic rats
Blood glucose levels were measured fortnightly and weekly variation in all the groups is presented in Table 3. The results showed a significant effect of the bread incorporated with onion peel extract and onion powder on the blood glucose level of alloxan-induced diabetic rats. The initial and final blood glucose levels in diabetic rats that were fed with the control bread did not vary significantly. In treatment groups that were either fed with 1%, OPE supplemented bread, 3% OPE supplemented bread, or 7% OP supplemented bread, the final blood glucose level (day 56) was significantly lower (p < 0.01) than the initial blood glucose levels (day 0). While comparing the treatment groups, it can be seen that in OPE_3 group blood glucose level dropped remarkably from 289 ± 5.8 on day 0 to 205 ± 3.3 on day 56 which is significantly high as compared to OPE_1 and OP_7 groups where the final day blood glucose level was 234 ± 6.2 and 230 ± 3.3 respectively. Whereas the glucose-lowering effect of 5% OP supplemented bread remained non-significant for most of the observation checkpoints except for the end-point measurement (day 56) (p < 0.01). The overall glucose-lowering effect of 3% OPE supplemented bread was more pronounced as compared to any other supplemented bread.
Table 3.
Effects of onion peel extract and onion powder supplemented breads on blood glucose level in diabetic rats
| Groups | Blood glucose level (mg/dl) | ||||
|---|---|---|---|---|---|
| Day 0 | Day 14 | Day 28 | Day 42 | Day 56 | |
| NC | 82 ± 0.4* | 86 ± 0.9* | 87 ± 1.0* | 93 ± 0.6* | 93 ± 0.7* |
| DC | 285 ± 5.8 | 288 ± 6.3 | 289 ± 5.7 | 290 ± 6.1 | 294 ± 4.8 |
| OPE_1 | 285 ± 7.9 | 280 ± 7.8 | 257 ± 5.9* | 241 ± 3.3* | 234 ± 6.2* |
| OPE_3 | 289 ± 5.8 | 275 ± 4.6 | 242 ± 4.4* | 219 ± 3.4* | 205 ± 3.3* |
| OP_5 | 289 ± 2.6 | 284 ± 2.7 | 280 ± 2.8 | 277 ± 2.8 | 268 ± 3.5* |
| OP_7 | 292 ± 5.4 | 282 ± 5.0 | 264 ± 5.2* | 246 ± 4.5* | 230 ± 3.3* |
Values expressed as means ± SEM, (NC = normal control, DC = diabetic control, OPE_1 = group fed with bread containing 1% onion peel extract, OPE_3 = group fed with bread containing 3% onion peel extract, OP_5 = group fed with bread containing 1% onion powder, OP_7 = group fed with bread containing 7% onion powder), * shows the significant difference compared to the diabetic control group (p < 0.01) via Dunnett’s multiple comparison test
Effect of OPE and OP supplemented bread on the antioxidant enzyme activities and MDA levels of diabetic rats
Significant (p < 0.01) rise in the level of malondialdehyde (MDA) and decline in catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GPx) activities, and glutathione (GSH) content were reported in the diabetic control group in comparison with the normal rats (Figs. 1, 2 and 3). In the 5% OP supplemented group, no significant changes were observed in GPx levels of the liver and kidney in comparison to the diabetic control group. Also, there was no significant variation in the kidney’s GR and GSH levels by the end of the 56 days treatment. However, in a similar group also supplemented with 5% OP there was a significant 29% and 35% reduction (p < 0.01) in the liver’s superoxide dismutase and catalase activity, respectively.
Fig. 1.
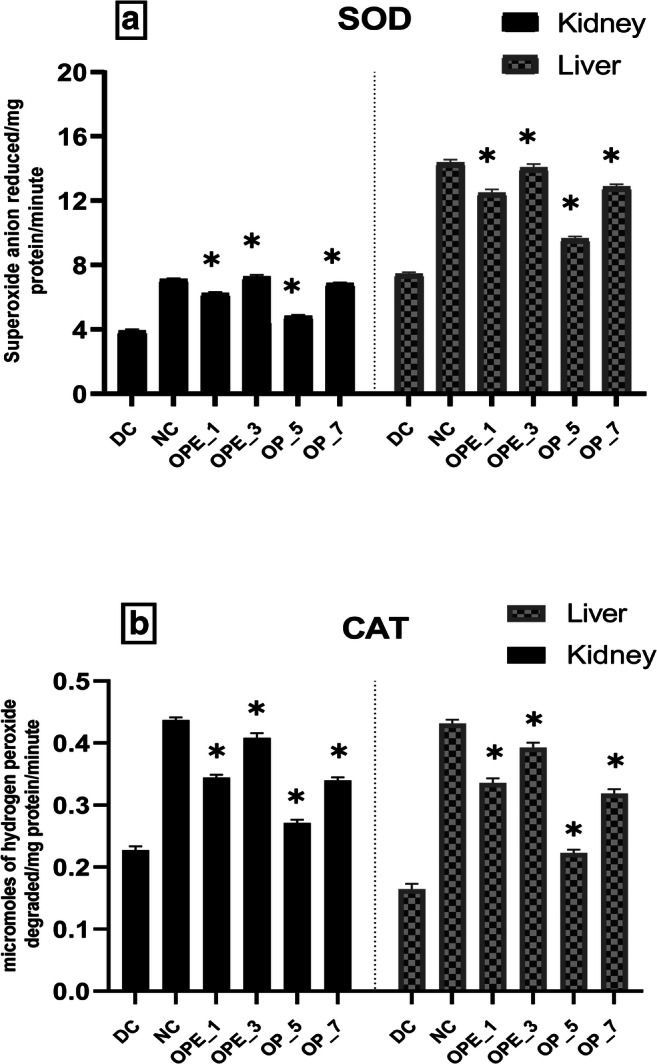
Effects of onion peel extract and onion powder supplemented bread on SOD (a) and CAT (b) activities in the liver and kidney tissues of normal and experimental rats. NC = normal control, DC = diabetic control, OPE_1 = group fed with bread containing 1% onion peel extract, OPE_3 = group fed with bread containing 3% onion peel extract, OP_5 = group fed with bread containing 1% onion powder, OP_7 = group fed with bread containing 7% onion powder. *shows the significant difference compared with the diabetic control group (p < 0.01) via Dunnett’s multiple comparison test, while ‘ns’ shows a non-significant difference
Fig. 2.
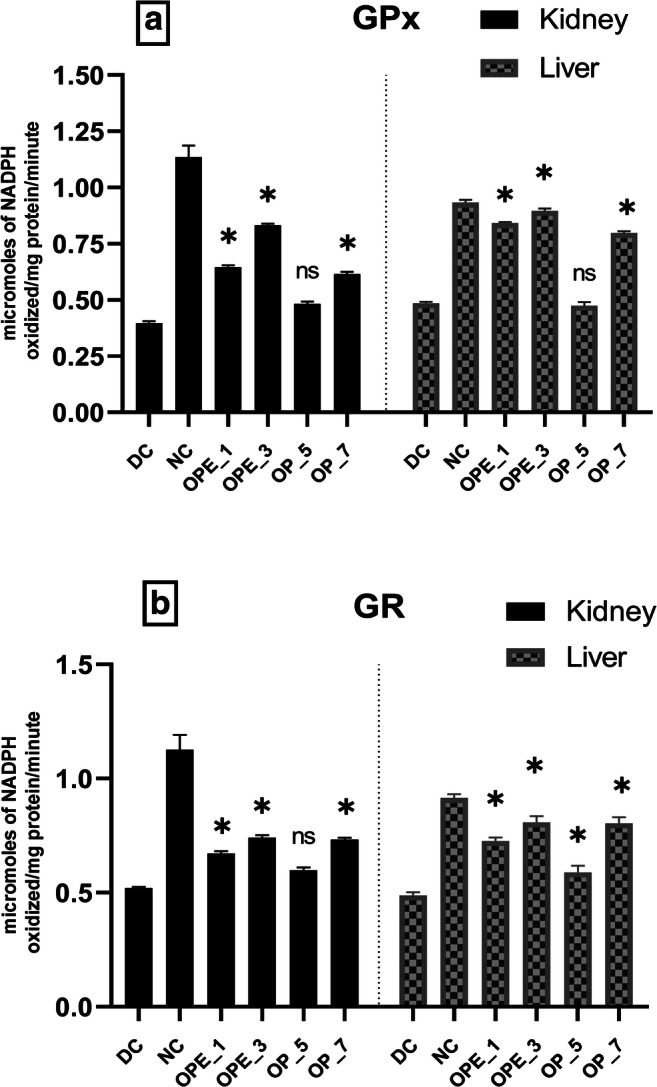
Effects of onion peel extract and onion powder supplemented bread on GPx (a) and GR (b) activities in the liver and kidney tissues of normal and experimental rats. NC = normal control, DC = diabetic control, OPE_1 = group fed with bread containing 1% onion peel extract, OPE_3 = group fed with bread containing 3% onion peel extract, OP_5 = group fed with bread containing 1% onion powder, OP_7 = group fed with bread containing 7% onion powder.*shows the significant difference compared with the diabetic control group (p < 0.01) via Dunnett’s multiple comparison test, while ‘ns’ shows a non-significant difference
Fig. 3.
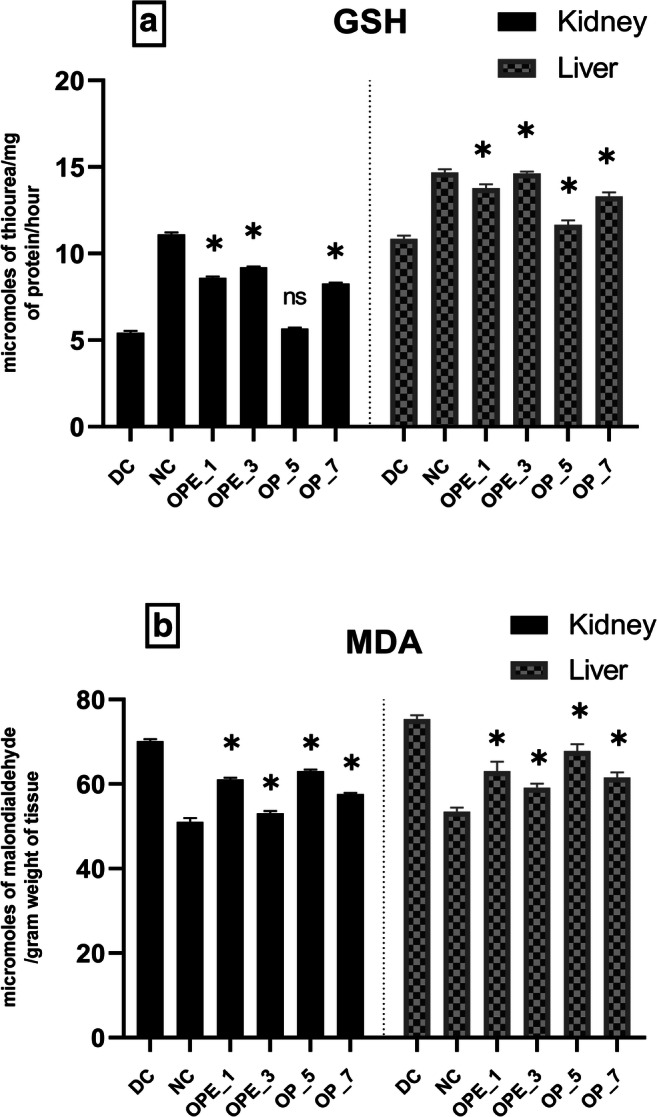
Effects of onion peel extract and onion powder supplemented bread on GSH (a) and MDA (b) levels activities in the liver and kidney tissues of normal and experimental rats. NC = normal control, DC = diabetic control, OPE_1 = group fed with bread containing 1% onion peel extract, OPE_3 = group fed with bread containing 3% onion peel extract, OP_5 = group fed with bread containing 1% onion powder, OP_7 = group fed with bread containing 7% onion powder.*shows the significant difference compared with the diabetic control group (p < 0.01) via Dunnett’s multiple comparison test, while ‘ns’ shows a non-significant difference
Diabetic rat groups that were treated with OPE (1% and 3%) and OP (7%) showed a significant (p < 0.01) increase in the activities of GPx, GR, CAT, SOD, and GSH levels but the effect was more pronounced in the group supplemented with 3% OPE bread. It was observed that MDA levels were reduced by (21%) in the liver of the 3% OPE treated group in comparison with the diabetic control. Along with the significant improvement in GPx, GR, and GSH levels, the similar group presented a prominent threefold increase in SOD and CAT levels in the liver as compared with the diabetic control group showing the restoration of the altered antioxidant defense system near to the normal level.
Discussion
We observed a significant increase in the blood glucose level with alloxan induction. This might be due to the destruction of Langerhans islets β-cells by alloxan which also elevates oxidative stress by compromising the intrinsic antioxidant mechanism and by increasing the production of free radicals [18, 49, 50]. Oxidative damage induced by reactive oxygen species can promote negative consequences including uncontrolled hyperglycemia [51].
The rats’ body weights were reduced after induction of diabetes (Table 2). This kind of weight reduction was observed in alloxan-induced diabetes in rodents [52]. The breakdown of lipids stored in adipose tissues and the disruption of the skeletal muscle proteins might have contributed to this weight loss [53]. When supplemented bread was added to the diet of alloxan-induced diabetic rats, their body weight increased along with their body’s ability to regulate blood glucose levels. However, the effect was more significant in rats that were given bread supplemented with 3% of onion peel extract.
The effect of onion supplementation in reducing blood glucose level was also reported in some recent studies [54–56]. Phytochemicals such as quercetin and allyl-propyl disulfides found in onion peel and bulb might be responsible for the beneficial effect on blood glucose level by up-regulating the expression of insulin receptors and glucose transporters, improving insulin sensitivity and promoting glucose metabolism in peripheral tissues in diabetic rats [13]. Kim et al. (2011) had reported that supplementation of onion peel extract brought significant reduction in blood glucose level by slowing down the glucose absorption rate via inhibition of sucrase enzyme in the intestinal tract [57]. The uptake of glucose is carried out by series of events from binding by insulin to the receptors present on cell surface, and later the ability of insulin to increase glucose transport inside muscle tissue by GLUT [58]. Hence, it is anticipated that phytochemicals present in onion might improve insulin sensitivity by amending the insulin receptor and glucose transporters expression and by promoting glucose metabolism in peripheral tissues of diabetic rats [13]. In the present research, both onion peel and onion powder supplemented breads brought reduction in blood glucose level. However, it was observed that effect of even smaller percentages of onion peel extract was more pronounced as compared to the relatively higher percentages of onion bulb powder. Previous studies have demonstrated that in vitro antioxidant potential of onion peel is fairly high as compared to the inner bulb [17, 59]. Phytochemicals such as quercetin, isorhamnetin and kaempferol which possess strong antidiabetic potential are much more abundant in the outer dry scales and fleshy peels than the inner layers [60, 61] and this might be the reason behind more potent antihyperglycemic and antioxidant activity of the onion peel as compared to the bulb [62].
Among human subjects, few studies have been conducted which demonstrated that Allium cepa possess strong potential in lowering blood glucose level of normal subjects as well as diabetic patients [63–65], but antidiabetic potential of onion bulb and onion peel extract has not been compared previously. Although, the use of onion powder and the peel as food supplements has been analyzed on basis of sensorial acceptability by trained panel indicating a potential use of such supplemented breads in future [66]. Therefore, current study serves as a steppingstone towards the development of onion powder and peel based functional foods. In the present research, the activities of studied enzymes of the antioxidative defense system such as CAT and SOD were significantly reduced while the level of malondialdehyde (MDA) was increased in alloxan-induced diabetic rats. The manifestation of oxidative stress occurs when ROS production increases abruptly, or the ROS-scavenging capacity of the body gets attenuated [67]. In the alloxan-induced diabetic rats, weak antioxidant enzymatic activities, elevated lipid peroxidation, and lowered non-enzymatic antioxidant levels were reported in previous studies [68–71]. To protect the body against ROS-mediated oxidative damage, antioxidant enzymes are released including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) [72].
In the present research, the studied antioxidative defense system enzymes such as CAT and SOD displayed lower activities in the evaluated kidney and liver tissues (Fig. 1) and these results agreed with earlier published results [73, 74]. Excess blood glucose and recurrent non-enzymatic glycation of proteins promote the generation of O2 and H2O2 and this might be the reason behind the reduced activities of CAT and SOD [75]. The limited activation of antioxidant enzymes due to the presence of H2O2 and other hydrogen radicals was recently reported [76]. Another reason behind the decreased activity of CAT and SOD might be the reduction of the protein expression levels that is a common phenomenon in hyperglycemia [77].
The activity of another important antioxidant enzyme, glutathione peroxidase (GPx), was also reduced in the hepatic and renal tissues of diabetic rats indicating impaired scavenging activity of lipid hydroperoxides and H2O2. A similar finding was observed in a previous investigation [78]. When diabetic rats were fed with bread supplemented with onion peel extract or onion powder, the altered activities of the antioxidant enzymes such as SOD, CAT, GPx, and GR were significantly restored (p < 0.01) (Figs. 1 and 2). The antioxidant potential of various nutraceuticals such as quercetin, isorhamnetin, kaempferol, and allyl-propyl disulfides might have contributed to this effect. All these constituents previously showed a potent effect in reducing blood glucose level and oxidative stress as well by modulating various antioxidant enzymes in diabetic rats [79, 80].
Also, it was observed in our present study that the enzymatic activity of GR was significantly reduced in diabetic rats due to a reduction in its production [81]. In groups receiving 3% of onion peel extract or 7% onion powder, the GR activity was raised significantly (p < 0.01) as shown in Fig. 2. It was previously reported that the plasma antioxidant capacity was improved in diabetic rats following onion treatment [80]. Glutathione (GSH) also serves as a marker of oxidative damage as it is an important non-enzymatic antioxidant agent involved in preserving the integrity of the cellular systems [82]. The present study indicated that in the renal and hepatic tissues of alloxan-induced diabetic rats, the GSH level decreased (Fig. 3). The downfall in tissue GSH levels increases the progression of cellular damage initiated by free radicals. The increased rate by which GSH is used in diabetic rats highlights its important activity against ROS [83]. When diabetic rats were fed with onion supplemented bread it restored the GSH to almost normal levels (Fig. 3) but, the effect was more pronounced in the 3% OPE group as compared with other groups suggesting that onion peel extract can be beneficial against oxidative stress. Previous studies indicated the potent antioxidant potential of onion towards increasing the GSH level and upholding the antioxidant enzymatic activities within the normal range [84, 85].
Lipid peroxidation is another crucial factor that is strongly associated with the integrity of cellular membranes and it helps in evaluating the composition and structure of the tissue lipids. In diabetes, the raised level of free radicals in certain tissues may lead to a rise in the rate of lipid peroxidation [86]. Hence malondialdehyde (MDA), which is a product of lipid peroxidation was measured in tissues of diabetic rats as an indicator of the raised rate of lipid peroxidation in their kidneys and liver [87]. Nonetheless, the elevated MDA level observed in the tissues of alloxan-induced rats was found to be declined after treatment with onion in the form of both onion peel extract as well as onion bulb powder. Another benefit that is offered by onion supplementation was the prevention of membrane lipid peroxidation. Previous studies proved that onion possesses strong hypolipidemic properties [88–91]. The observed decline in the concentration of MDA and the overall improved antioxidant status in the onion peel extract and onion powder treated diabetic rats highlights their strong potential against oxidative stress and lipid peroxidation.
The current study suggested that both onion peel and bulb may have beneficial effects against diabetes by lowering glucose levels and suppressing oxidative stress when given in a certain amount and this holds the hope of a new generation of functional foods. Onion peel is commonly treated as agro-biowaste but it contains useful phytochemicals that need the attention of the scientific community as well as the food industry. However, the present study has some limitations. The phenolic content and antioxidant capacity vary among different onion varieties [92] and the biochemical properties of different wheat flour types can affect the final product. Therefore, standardization of both the constituents is needed before any further in vivo experimentation is planned to account for such variations. In earlier studies, the concentration of various phenolic acids and flavonoids in onion extracts was determined by HPLC [93, 94]. Phenols possess weak thermal stability and the baking process increases the amount of the free phenolic acids and decreases the concentration of bound phenolic compounds [95, 96] and thus it is recommended to assess the concentration of phytochemicals in the developed bread samples as well. Moreover, the current study also suffered from being an animal study, and the obtained findings cannot be exactly applied in humans in varying states of health and with diverse dietary patterns. Therefore, it is suggested to conduct a human trial to examine the postprandial metabolic and appetitive responses to the developed bread. If similar findings are projected to human trials, it will open fascinating possibilities for formulating frequently consumed foods like bread with health-promoting effects.
Appendix
Table 4.
Daily feed intake (g) of all the studied rat groups
| Days | DC | NC | OPE_1 | OPE_1 | OP_5 | OP_7 |
|---|---|---|---|---|---|---|
| Day 1 | 111 | 106 | 110 | 113 | 109 | 110 |
| Day 2 | 107 | 105 | 107 | 105 | 104 | 106 |
| Day 3 | 112 | 111 | 109 | 111 | 112 | 107 |
| Day 4 | 116 | 100 | 101 | 104 | 110 | 105 |
| Day 5 | 101 | 105 | 103 | 106 | 107 | 108 |
| Day 6 | 109 | 100 | 104 | 111 | 114 | 110 |
| Day 7 | 114 | 112 | 109 | 108 | 104 | 111 |
| Day 8 | 100 | 105 | 109 | 110 | 107 | 113 |
| Day 9 | 115 | 111 | 112 | 111 | 106 | 112 |
| Day 10 | 115 | 113 | 114 | 112 | 114 | 110 |
| Day 11 | 110 | 110 | 114 | 113 | 117 | 111 |
| Day 12 | 113 | 109 | 107 | 110 | 109 | 113 |
| Day 13 | 108 | 113 | 115 | 116 | 115 | 116 |
| Day 14 | 110 | 108 | 106 | 107 | 111 | 112 |
| Day 15 | 111 | 114 | 115 | 116 | 114 | 115 |
| Day 16 | 113 | 113 | 116 | 118 | 115 | 113 |
| Day 17 | 116 | 114 | 117 | 117 | 114 | 116 |
| Day 18 | 112 | 110 | 112 | 113 | 115 | 110 |
| Day 19 | 110 | 111 | 117 | 115 | 117 | 113 |
| Day 20 | 112 | 110 | 115 | 116 | 112 | 116 |
| Day 21 | 116 | 117 | 114 | 115 | 114 | 117 |
| Day 22 | 117 | 119 | 117 | 118 | 118 | 118 |
| Day 23 | 119 | 118 | 116 | 117 | 117 | 117 |
| Day 24 | 111 | 118 | 115 | 118 | 118 | 118 |
| Day 25 | 121 | 116 | 117 | 115 | 115 | 115 |
| Day 26 | 113 | 114 | 113 | 113 | 113 | 113 |
| Day 27 | 110 | 111 | 110 | 116 | 116 | 116 |
| Day 28 | 114 | 117 | 116 | 117 | 117 | 115 |
| Day 29 | 119 | 121 | 118 | 115 | 116 | 113 |
| Day 30 | 118 | 117 | 121 | 119 | 113 | 116 |
| Day 31 | 117 | 114 | 114 | 119 | 120 | 116 |
| Day 32 | 118 | 115 | 114 | 120 | 117 | 121 |
| Day 33 | 117 | 118 | 117 | 118 | 114 | 119 |
| Day 34 | 119 | 113 | 115 | 116 | 117 | 115 |
| Day 35 | 121 | 118 | 116 | 117 | 114 | 117 |
| Day 36 | 115 | 117 | 116 | 120 | 119 | 118 |
| Day 37 | 120 | 113 | 114 | 118 | 117 | 121 |
| Day 38 | 119 | 115 | 113 | 117 | 113 | 118 |
| Day 39 | 120 | 118 | 119 | 114 | 115 | 119 |
| Day 40 | 116 | 115 | 116 | 113 | 122 | 118 |
| Day 41 | 121 | 119 | 120 | 119 | 116 | 115 |
| Day 42 | 122 | 122 | 119 | 118 | 113 | 120 |
| Day 43 | 120 | 124 | 121 | 119 | 118 | 119 |
| Day 44 | 119 | 116 | 115 | 120 | 118 | 117 |
| Day 45 | 122 | 119 | 117 | 122 | 117 | 121 |
| Day 46 | 121 | 120 | 121 | 119 | 115 | 118 |
| Day 47 | 119 | 121 | 116 | 117 | 115 | 120 |
| Day 48 | 122 | 118 | 119 | 118 | 117 | 117 |
| Day 49 | 118 | 114 | 115 | 116 | 119 | 117 |
| Day 50 | 123 | 121 | 119 | 120 | 118 | 119 |
| Day 51 | 126 | 119 | 121 | 118 | 122 | 123 |
| Day 52 | 119 | 118 | 122 | 120 | 119 | 118 |
| Day 53 | 125 | 119 | 117 | 116 | 116 | 115 |
| Day 54 | 118 | 121 | 119 | 118 | 121 | 117 |
| Day 55 | 123 | 119 | 121 | 121 | 118 | 121 |
| Day 56 | 124 | 118 | 120 | 122 | 117 | 118 |
| Mean | 116.02 | 114.50 | 114.74 | 115.54 | 114.82 | 115.40 |
Values presented as the difference between diet provided and the leftover by the end of 24 h for each individual study group; (NC = normal control, DC = diabetic control, OPE_1 = group fed with bread containing 1% onion peel extract, OPE_3 = group fed with bread containing 3% onion peel extract, OP_5 = group fed with bread containing 1% onion powder, OP_7 = group fed with bread containing 7% onion powder).
Authors contributions
Conceptualization: Sara Masood, Shahid Bashir, Muhammad Imran and Attiq ur Rehman, Methodology: Sara Masood and Attiq ur Rehman, Software and Formal Analysis: Attiq ur Rehman, Investigation: Sara Masood, Palwasha Khalil, Faiza Iftikhar, Hafiza Madfiha Jaffar, Tara Khursheed, Resources: Sara Masood, Shahid Bashir, Muhammad Imran, Data curation: Sara Masood, Palwasha Khalil, Faiza Iftikhar, Hafiza Madfiha Jaffar, Tara Khursheed, Writing—original draft preparation: Sara Masood, and Attiq ur Rehman, Writing—review and editing; Mohamed El Shazly, Supervision: Shahid Bashir and Muhammad Imran, Project administration: Shahid Bashir and Muhammad Imran. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by Natural Resources Institute Finland (LUKE).
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sara Masood, Email: sarah.13494@gmail.com.
Attiq ur Rehman, Email: attiq.rehman@luke.fi.
Shahid Bashir, Email: shahid.bashir@rsmi.uol.edu.pk.
Mohamed El Shazly, Email: mohamed.elshazly@pharma.asu.edu.eg.
Muhammad Imran, Email: mic_1661@yahoo.com.
Palwasha Khalil, Email: palwasha5993@gmail.com.
Faiza Ifthikar, Email: faizaifthikar@gmail.com.
Hafiza Madiha Jaffar, Email: madiha.jaffar@dnsc.uol.edu.pk.
Tara Khursheed, Email: tara.khursheed@dnsc.uol.edu.pk.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017; 128:40–50. 10.1016/j.diabres.2017.03.024. [DOI] [PubMed]
- 2.Jadidoleslami M, Nejad MA, Shahraki MR. The effect of Aloe vera aqueous extract on glucose and lipid levels in diabetic male rats, Iran. J. Diabetes Lipid Disord. 2006; 6(2):E18.
- 3.Elangovan V, Shohami E, Gati I, Kohen R. Increased hepatic lipid soluble antioxidant capacity as compared to other organs of streptozotocin-induced diabetic rats: A cyclic voltammetry study. Free Radic Res. 2000; 32(2):125–34. 10.1080/10715760000300131. [DOI] [PubMed]
- 4.Cui X, Lin Q, Liang Y. Plant-Derived Antioxidants Protect the Nervous System From Aging by Inhibiting Oxidative Stress. Frontiers in Aging Neuroscience. 2020; 12:209. 10.3389/fnagi.2020.00209. [DOI] [PMC free article] [PubMed]
- 5.Molehin OR, Adefegha SA, Adeyanju AA. Role of oxidative stress in the pathophysiology of type 2 diabetes and cardiovascular diseases. In Role of oxidative stress in pathophysiology of diseases. 2020.10.1007/978-981-15-1568-2.
- 6.Kirtonia A, Sethi G, Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci. 2020; 77(22):4459–4483. 10.1007/s00018-020-03536-5. [DOI] [PMC free article] [PubMed]
- 7.Godwin II, Adebisi KE, Osagie VE. Analysis of certain biochemical indices on alloxan induced diabetic rats administered with protein isolated and purified from Vernonia amygdalina. African J Biol. Sci. 2019; 01(02):60. 10.33472/AFJBS.1.2.2019.60-67.
- 8.Ramalingam S, Karuppiah M, Thiruppathi M, Palanivelu S, Panchanatham S. Antioxidant potential of biflavonoid attenuates hyperglycemia by modulating the carbohydrate metabolic enzymes in high fat diet/streptozotocin induced diabetic rats. Redox Rep. 2020; 25(1):1–10. 10.1080/13510002.2020.1722914. [DOI] [PMC free article] [PubMed]
- 9.Matsui T, Ogunwande I, Abesundara K, Matsumoto K. Anti-hyperglycemic potential of natural products. Mini-Reviews Med Chem. 2006; 6(3):349–56. 10.2174/138955706776073484. [DOI] [PubMed]
- 10.Bhattacharjee S. Analysis of the proximate composition and energy values of two varieties of onion (Allium cepa L.) bulbs of different origin: A comparative study. Int J Nutr Food Sci. 2013; 2(5):246. 10.11648/j.ijnfs.20130205.16.
- 11.Kesari AN, Gupta RK, Watal G. Hypoglycemic effects of Murraya koenigii on normal and alloxan-diabetic rabbits. J Ethnopharmacol. 2005; 97(2):247–51. 10.1016/j.jep.2004.11.006. [DOI] [PubMed]
- 12.Kang M, Kim JH, Choi HN, Kim MJ, Han JH, Lee JH, Kim JI. Hypoglycemic effects of welsh onion in an animal model of diabetes mellitus. 2010; 4(6):486–491. 10.4162/nrp.2010.4.6.486. [DOI] [PMC free article] [PubMed]
- 13.Jung JY, Lim Y, Moon MS, Kim JY, Kwon O. Onion peel extracts ameliorate hyperglycemia and insulin resistance in high fat diet/streptozotocin-induced diabetic rats. Nutr Metab. 2011;8:1–18. 10.1186/1743-7075-8-18. [DOI] [PMC free article] [PubMed]
- 14.Takahashi M, Shibamoto T. Chemical compositions and antioxidant/anti-inflammatory activities of steam distillate from freeze-dried onion (Allium cepa L.) sprout. J Agric Food Chem. 2008; 26;56(22):10462–7. 10.1021/jf801220b. [DOI] [PubMed]
- 15.Boots AW, Drent M, de Boer VCJ, Bast A, Haenen GRMM. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011; 30(4):506–12. 10.1016/j.clnu.2011.01.010. [DOI] [PubMed]
- 16.Aguirre L, Arias N, Macarulla MT, Gracia A, Portillo MP. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 2011; 4:189–198. 10.2174/1876396001104010189.
- 17.Kim MH, Jo SH, Jang HD, Lee MS, Kwon YI. Antioxidant activity and α-glucosidase inhibitory potential of onion (Allium cepa L.) extracts. Food Sci Biotechnol. 2010; 1;19(1):159–64. 10.1007/s10068-010-0022-1.
- 18.El-Demerdash FM, Yousef MI, El-Naga NIA. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43(1):57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Rehman AU, Khalil IH, Ali I. Genetic diversity and traits association in tetraploid and hexaploid wheat genotypes in Khyber Pakhtunkhwa Province of Pakistan. Sarhad J of Agric. 2020; 36(4): 1112–9. http://doi.org/10.17582/journal.sja/2020/36.4.1112.1119.
- 20.Tablante EC, Pachón H, Guetterman HM, Finkelstein JL. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database Syst Rev. 2019; 1;7(7):CD012150. 10.1002/14651858.CD012150. [DOI] [PMC free article] [PubMed]
- 21.Rehman, AU, Masood, S, Khan, NU, Abbasi, ME, Hussain, Z, Ali, I. Molecular basis of Iron Biofortification in crop plants; A step towards sustainability. Plant Breed. 2021; 140: 12– 22. 10.1111/pbr.12886.
- 22.Lai WT, Nicholas MHK, Sue SL, Yen YH, Biow IS, Kah YL, Oi ML. A review: Modified agricultural by-products for the development and fortification of food products and nutraceuticals. Trends Food Sci Technol. 2017; 1(59):148–60. 10.1016/j.tifs.2016.11.014.
- 23.NIH. Public health service policy on humane care and use of laboratory animals. 2015.
- 24.Jeong CH, Kwak JH, Kim JH, Choi GN, Kim DO, Heo HJ. Neuronal cell protective and antioxidant effects of phenolics obtained from Zanthoxylum piperitum leaf using in vitro model system. Food Chem. 2011; 125(2):417–422. 10.1016/j.foodchem.2010.09.022.
- 25.Arslan D, Özcan MM. Study the effect of sun, oven and microwave drying on quality of onion slices. LWT - Food Sci Technol. 2010; 43(7):1121–1127. 10.1016/j.lwt.2010.02.019.
- 26.Brites CM, Trigo MJ, Carrapiço B, Alviña M, Bessa RJ. Maize and resistant starch enriched breads reduce postprandial glycemic responses in rats. Nutr Res. 2011; 31(4):302–8. 10.1016/j.nutres.2011.02.001. [DOI] [PubMed]
- 27.Yoshinari O, Shiojima Y, Igarashi K. Anti-obesity effects of onion extract in Zucker diabetic fatty rats. Nutrients. 2012; 22;4(10):1518–26. 10.3390/nu4101518. [DOI] [PMC free article] [PubMed]
- 28.Lee JO, Lee SA, Kim KH, Choi JJ, Yook HS. Quality characteristics of cookies added with hot-air dried yellow and red onion powder. J Korean Soc Food Sci Nutr. 2008;37(3):342–347. doi: 10.3746/jkfn.2008.37.3.342. [DOI] [Google Scholar]
- 29.Bang M-A, Kim H-A, Cho Y-J. Alterations in the blood glucose, serum lipids and renal oxidative stress in diabetic rats by supplementation of onion (Allium cepa. Linn). Nutr Res Pract. 2009;3(3):242–46. 10.4162/nrp.2009.3.3.242. [DOI] [PMC free article] [PubMed]
- 30.Ozougwu J, Nwachi U, Eyo J. Comparative Hypolipidaemic Effects of Allium cepa, Allium sativum and Zingiber officinale Aqueous Extracts on Alloxan-Induced Diabetic Rattus novergicus. Bio-Research. 2009; ;6(2):384–91.
- 31.“Accu-Chek®Aviva System,” Diabetes Educ., vol. 31, no. 6, pp. 901–902, Nov. 2005.
- 32.Sari MI, Ilyas S, Widyawati T, Antika MA. Effect of lawsonia innermis (Linn) leaves ethanolic extract on blood glucose and malondialdehyde level in alloxan-induced diabetic rats. IOP Conf Series: Earth Environ Sci. 2018;130:012034. 10.1088/1755-1315/130/1/012034.
- 33.Shanmugam KR, Mallikarjuna K, Nishanth K, Kuo CH, Reddy KS. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2011; 124(4):1436–42. 10.1016/j.foodchem.2010.07.104.
- 34.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972; 25;247(10):3170–5. [PubMed]
- 35.Faisal MA, Maulida S, Mairudi SI, Suhartono E. Superoxide dismutase and catalase activity in cataract lens of diabetes mellitus. In AIP conference proceedings. 2019; (Vol. 2108, No. 1, p. 020041).
- 36.Aebi H. Methods In Enzymology, Volume. 105, Catalase in Vitro. Chance, Britt - Acta Chem Scand. 1984; 1(105):121–6. [DOI] [PubMed]
- 37.Carlberg I, Mannervik B. [59] glutathione reductase. Methods Enzymol. 1985; 1;113:484–90. [DOI] [PubMed]
- 38.Chauhan P, Sharma H, Kumar U, Mayachari A, Sangli G, Singh S. Protective effects of Glycyrrhiza glabra supplementation against methotrexate-induced hepato-renal damage in rats: an experimental approach. J Ethnopharmacol. 2020; 5;263:113209. 10.1016/j.jep.2020.113209. [DOI] [PubMed]
- 39.Akerboom TPM, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol., 1981; 77:373–82. https://doi.org/10.1016/s0076-6879(81)77050–2 [DOI] [PubMed]
- 40.Sahyon HA, Al-Harbi SA. Chemoprotective role of an extract of the heart of the Phoenix dactylifera tree on adriamycin-induced cardiotoxicity and nephrotoxicity by regulating apoptosis, oxidative stress and PD-1 suppression. Food Chem Toxicol. 2020; 135:111045. 10.1016/j.fct.2019.111045. [DOI] [PubMed]
- 41.Flohé WA, Günzler L. Assays of glutathione peroxidase. Oxygen radicals in biological systems. Methods Enzymol. 1984; 105:114–21. 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed]
- 42.Mona FAD, Mohamed MA. Insights into the oxidative status and antioxidative responses of germinating broccoli (Brassica oleracea var. italica L.) seeds in tungstate contaminated water. Chemosphere. 2020; 261. 10.1016/j.chemosphere.2020.127585. [DOI] [PubMed]
- 43.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95(2):351–8. 10.1016/0003-2697(79)90738-3. [DOI] [PubMed]
- 44.Uppuluri S, Sowjanya K, Vaishnavi V, Kundana Bhavani J, Jennifer M, Stefi Seles K. Nephro-protective activity of berry powder of ‘Hippophae rhamnoides’ against cisplatin induced nephrotoxicity. Int J Pharm Res. 2020; 12: 2223–9. 10.31838/ijpr/2020.SP1.324.
- 45.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1):265–75 [PubMed]
- 46.Akkoyun MB, Ozdemir S, Kilinc E, Birhanli E, Aygün A, Sen F. Resistance, removal, and bioaccumulation of Ni (II) and co (II) and their impacts on antioxidant enzymes of Anoxybacillus mongoliensis. Comp Biochem Physiol Part - C Toxicol Pharmacol. 2020; ;235:108790. 10.1016/j.cbpc.2020.108790. [DOI] [PubMed]
- 47.I. SPSS. Version 25.O. Armonk,NY:IBM Corp. 2017.
- 48.Salkind N. Encyclopedia of research design. Thousand Oaks, California. 2010.
- 49.Roghani M, Aghaie M.The effect Allium ampeloprasum feeding on serum level of glucose, triglyceride, and total cholesterol of diabetic rats. Koomesh. 2007; 8(2):73–8
- 50.García-Sánchez A, Miranda-Díaz AG, Cardona-Muñoz EG. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- And Antioxidant Properties in Chronic Diseases. Oxidative Med Cell Long. 2020; 23;2020:2082145. 10.1155/2020/2082145. [DOI] [PMC free article] [PubMed]
- 51.Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C. Oxidative stress, nitric oxide, and diabetes. Rev Diabetic Stud. 2010; 7(1): 15–25. 10.1900/RDS.2010.7.15. [DOI] [PMC free article] [PubMed]
- 52.Fasanmade AA, Alabi OT. Differential effect of honey on selected variables in alloxan-induced and fructose-induced diabetic rats. African J Biomed Res. 2008; 11(2). 10.4314/ajbr.v11i2.50706.
- 53.Ahmad FM, Syed MK, Syed SG, Syeda SM, Shaik RA, Shaik MA. Antidiabetic activity of Vinca rosea extracts in alloxan-induced diabetic rats. Int J Endocrinol. 2010:841090. 10.1155/2010/841090. [DOI] [PMC free article] [PubMed]
- 54.Benítez V, Mollá E, Martín-Cabrejas MA, Aguilera Y, Esteban RM. Physicochemical properties and in vitro antidiabetic potential of fibre concentrates from onion by-products. J Funct Foods. 2017;36:34–42. 10.1016/j.jff.2017.06.045.
- 55.Abouzed TK, Contreras MDM, Sadek KM, Shukry M, H Abdelhady D, Gouda WM, Abdo W, Nasr NE, Mekky RH, Segura-Carretero A, Kahilo KA, Abdel-Sattar E. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in Wistar rats in relation to their metabolite fingerprint. Diabetes Res Clin Pract. 2018; 140:253–64. 10.1016/j.diabres.2018.03.042. [DOI] [PubMed]
- 56.Lolok N, Mashar HM, Annah I, Saleh A, Yuliastri WO, Isrul M. Antidiabetic effect of the combination of garlic peel extract (Allium sativum) and onion peel (Allium cepa) in rats with oral-glucose tolerance method. Res J Pharm Technol 2019; 12. 10.5958/0974-360X.2019.00357.3.
- 57.Kim SH, Jo SH, Kwon YI, Hwang JK. Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. Int J Mol Sci. 2011;12(6):3757–69. 10.3390/ijms12063757. [DOI] [PMC free article] [PubMed]
- 58.Kwon O, Eck P, Chen S, Corpe CP, Lee JH, Kruhlak M, Levine M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007; ;21(2):366–77. 10.1096/fj.06-6620com. [DOI] [PubMed]
- 59.Jeong CH, Heo HJ, Choi SG, Shim KH. Antioxidant and anticancer properties of methanolic extracts from different parts of white, yellow, and red onion. Food Sci Biotechnol. 2009; 18(1):108–12
- 60.Beesk N, Perner H, Schwarz D, George E, Kroh LW, Rohn S. Distribution of quercetin-3,4′-O-diglucoside, quercetin-4′-O-monoglucoside, and quercetin in different parts of the onion bulb (Allium cepa L.) influenced by genotype. Food Chem. 2010; 122(3): 566–71.10.1016/j.foodchem.2010.03.011.
- 61.Lee J, Mitchell AE. Quercetin and isorhamnetin glycosides in onion (Allium cepa L.): varietal comparison, physical distribution, coproduct evaluation, and long-term storage stability. J Agric Food Chem. 2011; 9;59(3):857–63. 10.1021/jf1033587. [DOI] [PubMed]
- 62.Ola-mudathir F, Abdul-Wahab A, Moshood A, Obuotor E. Comparative evaluation of antioxidant properties of methanol extracts of Allium cepa bulb, Allium cepa bulb peels and Allium fistulosum. Kragujev J Sci. 2018. 131–41. 10.5937/KgJSci1840131O.
- 63.Tjokroprawiro A, Pikir BS, Budhiarta AA, Pranawa, Soewondo H, Donosepoetro M, Budhianto FX, Wibowo JA, Tanuwidjaja SJ, Pangemanan M, Widodo H, Sujadhana A. Metabolic effects of onion and green beans on diabetic patients. Tohoku J Exp Med. 1983; 671–6. 10.1620/tjem.141.suppl_671. [DOI] [PubMed]
- 64.Kumari K, Mathew BC, Augusti KT. Antidiabetic and hypolipidemic effects of S-methyl cysteine sulfoxide isolated from Allium cepa Linn. Indian J Biochem Biophys. 1995; 32(1):49–54. [PubMed]
- 65.Eldin IMT, Ahmed EM, Abd EHM. Preliminary Study of the Clinical Hypoglycemic Effects of Allium cepa (Red Onion) in Type 1 and Type 2 Diabetic patients. Environ Health Insights. 2010; 14(4): 71–7. 10.4137/EHI.S5540. [DOI] [PMC free article] [PubMed]
- 66.Masood S, Attiq UR, Shahid B, Muhammad I, Palwasha K, Tara K, Faiza I, Hafiza MJ, Sana F, Bahisht R, Nida J. Proximate and sensory analysis of wheat bread supplemented with onion powder and onion Peel extract. Biosci Res. 2020;17:4071–8.
- 67.Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radical Biol Med. 2019; 20(132):73–82. 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed]
- 68.Ceretta LB, Réus GZ, Abelaira HM, Ribeiro KF, Zappellini G, Felisbino FF, Steckert AV, Dal-Pizzol F, Quevedo J. Increased oxidative stress and imbalance in antioxidant enzymes in the brains of alloxan-induced diabetic rats. Exp Diabetes Res. 2012;302682. 10.1155/2012/302682. [DOI] [PMC free article] [PubMed]
- 69.Bhattacharya S, Gachhui R, Sil PC. Effect of Kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan induced diabetic rats. Food Chem Toxicol. 2013; 60:328–40. 10.1016/j.fct.2013.07.051. [DOI] [PubMed]
- 70.Chaudhry J, Ghosh NN, Roy K, Chandra R. Antihyperglycemic effect of a new thiazolidinedione analogue and its role in ameliorating oxidative stress in alloxan-induced diabetic rats. Life Sci. 2007; 27;80(12):1135–42. 10.1016/j.lfs.2006.12.004. [DOI] [PubMed]
- 71.Gao D, Li Q, Li Y, Liu Z, Fan Y, Liu Z, Zhao H, Li J, Han Z. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-induced diabetic rats. Phyther Res. 2009; 23(9):1257–62. 10.1002/ptr.2603. [DOI] [PubMed]
- 72.Sacan O, Turkyilmaz IB, Bayrak BB, Mutlu O, Akev N, Yanardag R. Protective role of zinc in liver damage in experimental diabetes demonstrated via different biochemical parameters. J Biochem Mol Toxicol. 2021; 35:e22617. 10.1002/jbt.22617. [DOI] [PubMed]
- 73.Santhakumari P, Prakasam A, Pugalendi KV. Modulation of oxidative stress parameters by treatment with Piper betle leaf in streptozotocin induced diabetic rats. Indian J. Pharmacol. 2003; 35(6):373–8.
- 74.Pari L, Amarnath Satheesh M. Antidiabetic activity of Boerhaavia diffusa L.: effect on hepatic key enzymes in experimental diabetes. J. Ethnopharmacol. 2004; 91(1):109–13. 10.1016/j.jep.2003.12.013. [DOI] [PubMed]
- 75.Karigidi KO, Olaiya CO. Curculigo pilosa mitigates against oxidative stress and structural derangements in pancreas and kidney of streptozotocin-induced diabetic rats. J Complement Integr Med. 2020. 10.1515/jcim-2019-0217. [DOI] [PubMed]
- 76.Bauer G. The synergistic effect between hydrogen peroxide and nitrite, two long-lived molecular species from cold atmospheric plasma, triggers tumor cells to induce their own cell death. Redox Biol. 2019; 20(101291). 10.1016/j.redox.2019.101291. [DOI] [PMC free article] [PubMed]
- 77.Sindhu RK, Koo JR, Roberts CK, Vaziri ND. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: response to insulin and antioxidant therapies. Clin Exp Hypertens. 2004; 26(1):43–53. 10.1081/ceh-120027330. [DOI] [PubMed]
- 78.Friesen NTE, Büchau AS, Schott-Ohly P, Lgssiar A, Gleichmann H. Generation of hydrogen peroxide and failure of antioxidative responses in pancreatic islets of male C57BL/6 mice are associated with diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2004; 47(4):676–85. 10.1007/s00125-004-1367-x. [DOI] [PubMed]
- 79.Senyigit A, Durmus S, Mirzatas EB, Ozsobacı NP, Gelisgen R, Tuncdemir M, Ozcelik D, Simsek G, Uzun H. Effects of quercetin on lipid and protein damage in the liver of streptozotocin-induced experimental diabetic rats. J Med Food. 2019; 22(1):52-56. 10.1089/jmf.2018.0030. [DOI] [PubMed]
- 80.Campos KE, Diniz YS, Cataneo AC, Faine LA, Alves MJQF, Novelli ELB. Hypoglycaemic and antioxidant effects of onion, Allium cepa: dietary onion addition, antioxidant activity and hypoglycaemic effects on diabetic rats. Int J Food Sci Nutr. 2003;54(3):241–6. 10.1080/09637480120092062. [DOI] [PubMed]
- 81.Ulusu NN, Sahilli M, Avci A, Canbolat O, Ozansoy G, Ari N, Bali M, Stefek M, Stolc S, Gajdosik A, Karasu C. Pentose phosphate pathway, glutathione -dependent enzymes and antioxidant defense during oxidative stress in diabetic rodent brain and peripheral organs: effects of stobadine and vitamin E. Neurochem Res. 2003; 28: 815–23. 10.1023/A:1023202805255. [DOI] [PubMed]
- 82.El-Hafidi M, Franco M, Ramírez AR, Sosa JS, Flores JAP, Acosta OL, Salgado MC, Cardoso-Saldaña G. Glycine increases insulin sensitivity and glutathione biosynthesis and protects against oxidative stress in a model of sucrose-induced insulin resistance. Oxid Med Cell Longev. 2018:2101562. 10.1155/2018/2101562. [DOI] [PMC free article] [PubMed]
- 83.Kanikarla-Marie P, Micinski D, Jain SK. Hyperglycemia (high-glucose) decreases l-cysteine and glutathione levels in cultured monocytes and blood of Zucker diabetic rats. Mol Cell Biochem. 2019; 459: 151–56. 10.1007/s11010-019-03558-z. [DOI] [PubMed]
- 84.Pradeep SR, Srinivasan K. Alleviation of oxidative stress-mediated nephropathy by dietary fenugreek (: Trigonella foenum-graecum) seeds and onion (Allium cepa) in streptozotocin-induced diabetic rats. In: Food and function 2018; 9(1):134–48. 10.1039/c7fo01044c. [DOI] [PubMed]
- 85.Fredotović Ž, Soldo B, Šprung M, Marijanović Z, Jerković I, Puizina J. Comparison of organosulfur and amino acid composition between triploid onion Allium cornutum Clementi ex visiani, 1842, and common onion Allium cepa L., and evidences for antiproliferative activity of their extracts. Plants. 2020; 9(1):98. doi:10.3390/plants9010098. [DOI] [PMC free article] [PubMed]
- 86.Quine SD, Raghu PS. Effects of (−)-epicatechin, a flavonoid on lipid peroxidation and antioxidants in streptozotocin-induced diabetic liver, kidney and heart. Pharmacol Reports. 2005; 57(5):610–5. [PubMed]
- 87.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal. Biochem. 2017; 1(524):13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed]
- 88.Vidyavati HG, Manjunatha H, Hemavathy J, Srinivasan K. Hypolipidemic and antioxidant efficacy of dehydrated onion in experimental rats. J Food Sci Technol. 2010; 47(1):55-60. doi: 10.1007/s13197-010-0015-3. [DOI] [PMC free article] [PubMed]
- 89.Kim J, Noh SK. Hypolipidemic effect of onion peel extract in rats exposed to cigarette smoke extract with a high-fat diet. J Korean Soc Food Sci Nutr. 2016; 39(12):1790–9. doi:10.3746/jkfn.2010.39.12.1790.
- 90.Ebrahimi-Mameghani M, Saghafi-Asl M, Niafar M, Asghari-Jafarabadi M, Mesgari-Abbasi M. Raw red onion intake and insulin resistance markers in overweight or obese patients with polycystic ovary syndrome: A randomized controlled-clinical trial. Prog. Nutr. 2018; 20(1-S):199–08. https://doi.org/10.23751/pn.v20i1-S.6109.
- 91.Ülger T.G, F. Pinar C. Effect of Allium cepa on Paraoxonase 1 Activity and Oxidative Stress in Streptozotocin Induced Diabetic Rats. J food Nutr Res. 2018; 6(11): 689–93.
- 92.Cheng A, Chen X, Jin Q, Wang W, Shi J, Liu Y. Comparison of phenolic content and antioxidant capacity of red and yellow onions. Czech J Food Sci. 2013; 31(5): 501–08.
- 93.Lee KA, Kim KT, Kim HJ, Chung MS, Chang PS, Park H, Hyun-Dong P. Antioxidant activities of onion (Allium cepa L.) peel extracts produced by ethanol, hot water, and subcritical water extraction. Food Sci Biotechnol. 2014; 23(2):615–21. https://doi.org/10.1007/s10068-014-0084-6.
- 94.Kim J, Kim JS, Park E. Cytotoxic and anti-inflammatory effects of onion peel extract on lipopolysaccharide stimulated human colon carcinoma cells. Food Chem Toxicol. 2013; 62:199–204. https://doi.org/10.1016/j.fct.2013.08.045. [DOI] [PubMed]
- 95.Abdel-Aal ESM, Rabalski I. Effect of baking on free and bound phenolic acids in wholegrain bakery products. J Cereal Sci. 2013; 57(3):312–18. 10.1016/j.jcs.2012.12.001.
- 96.Ou J, Wang M, Zheng J, Ou S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019; 284:90–9. 10.1016/j.foodchem.2019.01.096. [DOI] [PubMed]


