Abstract
Objectives
This study aimed to investigate the effects of raw red beetroot consumption on metabolic markers and cognitive function in type 2 diabetes patients.
Methods
In a quasi-experimental study, 44 type 2 diabetes patients (57 ± 4.5 years) consumed raw red beetroot (100 g, daily), for 8 weeks. Metabolic markers including body weight, glucose and lipid profile parameters, inflammatory and oxidative stress markers, paraoxonase-1 activity, hepatic enzymes, blood pressure and cognitive function were measured at the beginning and end of 8 weeks.
Results
Raw red beetroot consumption resulted in a significant decrease in fasting blood sugar (FBS) levels (−13.53 mg/dL), glycosylated hemoglobin (HbA1c)(−0.34%), apolipoproteinB100 (ApoB100) (−8.25 mg/dl), aspartate aminotransferase (AST) (−1.75 U/L), alanine aminotransferase (ALT) (−3.7 U/L), homocysteine (−7.88 μmol/l), systolic (−0.73 mmHg) and diastolic blood pressure (−0.34 mmHg), anda significant increase in total antioxidant capacity (TAC) (105 μmol/L) and cognitive function tests (all P values <0.05). Other variables did not change significantly after the intervention.
Conclusions
Raw red beetroot consumption for 8 weeks in T2DM patients has beneficial impacts on cognitive function, glucose metabolism and other metabolic markers.
Keywords: Raw red beetroot, Type 2 diabetes, Metabolic markers, Cognitive function
Introduction
Type 2 diabetes mellitus (T2DM) represents a public health challenge all over the world. Approximately 425 million individuals had diabetes in 2017 and it has been projected that this figure will increase to 693 million by 2040 [1]. T2DM is characterized by high blood glucose levels and is one of the highest causes of death on a worldwide level [2]. It is also a potential risk factor for complications such as blindness, renal failure and the need for lower limb amputation, all of which contributes to decreased quality of life [3]. Moreover, patients with T2DM have measurable declines in cognitive performance when compared with age and sex matched individuals [4]. This latter effect may result from numerous potential causes including hyperglycemia, dyslipidaemia, microangiopathy and genetic factors [5].
While the causes of T2DM are still not fully known, it is generally recognized that T2DM is caused by both genetic and environmental factors. In recent years, there has been an increasing interest by scientific community in the potential benefits of phytochemicals in the treatment of this disease [6, 7]. Medicinal plants have been used throughout the world for many types of diseases including metabolic disorders for hundreds or even thousands of years. Such antidiabetic medicinal plants are more readily available than synthesized drugs and are likely to have fewer adverse side effects. These plants can contain numerous bioactive phytochemicals, including phenols, flavonoids and alkaloids, which have antidiabetic properties [8]. The available evidence also shows that some plant species contain substantial types of substances that exert antioxidant effects that can ameliorate T2DM [7].
Polyphenols and associated components have been reported to diminish postprandial hyperglycemia and prevent reactive hyper-insulinemia by decreasing the digestion, absorption and transport of glucose [9, 10]. Some studies over the last decade have shown that consumption of beetroot (Beta vulgaris L.) has physiological effects that may help to ameliorate pathologies including hypertension, atherosclerosis, T2DM, dementia and cognitive dysfunction [11–13]. Beetroot contains bioactive pigments termed betalains [14, 15]. These compounds have been shown to have antioxidant and anti-inflammatory effects in vitro and in some studies of animal models [15, 16]. Most investigations on beetroot supplementation have directed at understanding the capacity of the component inorganic nitrate (NO3) on these properties, particularly those regarding the hypotensive and ergogenic effects.
In addition, the plasma nitrite (NO2) level has been identified as a biomarker of endothelial nitric oxide (NO) generation and thus as an indicator of vascular health [17]. Hence, plasma nitrite may be an important marker for risk of cognitive deficits. There is some evidence demonstrating that dietary nitrate supplementation may enhance blood flow in cerebral white matter, considerably in the dorso-lateral prefrontal cortex and anterior cingulate cortex, which are areas related to executive performance in the brain [17]. Moreover, purified polyphenols and diets rich in polyphenols can block chronic complications in human diabetes such as neuropathy [18]. Due to the fact that high inflammation, oxidative stress, and effects on multiple other pathways are linked with diabetic neuropathy, it is expected that polyphenols possessing anti-inflammatory and antioxidant properties could have significant health benefits by targeting these in T2DM [18].
Here, we evaluated the effects of raw red beetroot consumption on metabolic markers and cognitive function in T2DM patients.
Methods and materials
The present research conducted as a quasi-experimental study. Enrollment took place in the Olympic Health Center, Tehran, Iran, between January and September in 2018. The study protocol was approved by the Medical Ethics Committee of Iran University of Medical Sciences, conforms with the declaration of Helsinki (approval number IR.IUMS.REC 1395.932132004) and registered at the Iranian Registry of Clinical Trials (IRCT registration number IRCT201612262365N16; available at:http://irct.ir/user/trial/20288/view). Informed written consent was obtained from all subjects. Volunteers were included in the study if they: 1) were diagnosed with T2DM within the previous 5 years; 2) had a fasting blood glucose (FBG) ≥ 126 mg/dL or a 2-h postprandial glucose ≥200 mg/dL before diagnosis and treatment; 3) were aged 30–65 years; 4) had a body mass index (BMI) < 35 kg/m2; 5) had a FBG < 180 mg/dL; 6) had 6.5% < HbA1c < 8.5%; 7) had a serum level of triacylglycerol (TAG) < 250 mg/dL; and 8) were taking oral hypoglycemic medications. They were excluded if they: 1) were suffering from hepatic, renal or thyroid diseases; 2) were smokers; or 3) taking vitamin-mineral supplements or hormone medications. We also excluded patients who had less than 80% compliance with the intervention or those with an altered treatment plan.
Sample size
Sample size was calculated using the homeostatic model assessment for insulin resistance (HOMA-IR) [19] with 80% power. To allow for attrition, 50 patients were enrolled.
Intervention
The 45 patients who met the inclusion criteria were assigned to consume 100 g raw red beetroot, daily for 8 weeks. The raw red beetroots were provided for the patients each week (7 × 100 g) and they were instructed to consume 100 g per day. They were required to follow a low-nitrate diet throughout the study period. Thus, they were asked to limit the consumption of foods with high nitrate content including cheese, processed or cured meats, and green leafy vegetables [20]. Moreover, throughout the study period, the patients were asked not to alter their physical activity level, use mouth wash and to limit caffeine intake.
Outcome measurement
Anthropometric parameters, dietary intakes, and physical activity level were evaluated at baseline and at the end of the 8-week intervention. Height and body weight were measured using standard protocols to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated by the formula (weight (kg)/height squared (m2)). Dietary intake was monitored with a 24-h food recall for 3 days (2 week days and 1 weekend day) and energy, macronutrients, fiber, and micronutrient intakes were estimated using the Nutritionist 4 software. Physical activity level was assessed using the Persian form of the International Physical Activity Questionnaire (IPAQ) and was reported in MET-min/week [21].
Metabolic markers
Blood was collected after a 12 h overnight fast from each subject at baseline and post intervention, and serum obtained by centrifugation at 1000 x g for 10 min. Sera were frozen and stored at −80 °C. Systolic and diastolic blood pressure, nitric oxide (NO), FBG, fasting insulin, glycosylated hemoglobin (HbA1c), total cholesterol (TC), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), TAG, ApoB100, ApoA1, homocysteine (Hcy), total antioxidant capacity (TAC), high sensitivity C-reactive protein (hs-CRP), hepatic enzymes (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)), and paraoxonase-1 (PON1) activity, were measured before and after the intervention.
Serum levels of NO were measured by the GRIESS method, using the MATRIX kit. Enzymatic methods were used to assess lipid profiles (Pars Azmoon Co., Tehran, Iran). The Friedwald formula was used for LDL-c assessment [22]. The levels of FBG (Pars Azmoon Co., Tehran, Iran) and HbA1C (Pishtazteb Co., Tehran, Iran), were evaluated with enzymatic methods usingan auto-analyzer (SELECTRA-E). Insulin sensitivity and insulin resistance were assessed by quantitative insulin sensitivity check index (QUICKI) and HOMA-IR indices, respectively [23, 24], as follows:
A photometric method was used to analyze AST and ALT (Pars AzmoonCo.), Hcy (Biorex Fars Co., Tehran, Iran) using the SELECTRA-E auto analyzer Also, TAC (NAXIFER Co., Tehran, Iran) and PON1 (TALIGENE PARS Co., Tehran, Iran) were determined spectrophotometrically (CECIL). PNO1 activity was evaluated by adding serum to Tris buffer containing 5.5 mmol/L o,o-diethyl-o-p-nitrophenylphsphate and 2 mmol/L CaCl2. The generation rate of p-nitrophenol was estimated at 405 nm, 25 °C, as explained previously [25]. APOA1, APOB100 (Pars Azmoon Co.) and hs-CRP (Biorex Fars Co., Tehran, Iran) were evaluated with an imonotorbidometric method using the SELECTRA-E auto-analyzer.
Blood pressure (BP) was measured after each subject had rested 15 min in the sitting position by using an automated BP monitor (Omron M2 Basic, UK) at baseline and at the end of the intervention.
Cognitive function
Cognitive function scores for each patient were evaluated using the Toulouse-Pieron (TP) and Digit Learning (DL) tests [26]. The TP test is a psychometric instrument for evaluation of selective, sustained attention, processing speed, and fatigue resistance. This method provides three main indexes: 1) work efficiency (WE); 2) dispersion index (DI); and 3) a total result (TR) [26]. The DL test is a measure of rote learning that investigates how well an individual learns a series of digits [27].
Statistical analysis
Statistical analyses were carried out using SPSS v22.0 (SPSS Inc. Chicago, IL, USA). Normality of the data was investigated using the Kolmogorov-Smirnov test. All results were reported as mean ± SD or median (interquartile). Categorical data were presented as frequencies and percentages. Comparison of baseline and post-intervention values was performed by paired-samples t test and Wilcoxon-signed ranks for normally and non-normally distributed data, respectively. P < 0.05 was considered statistically significant.
Results
In the present research, 50 patients were recruited. Six subjects were excluded from the study because of discontinuation of the intervention, gastrointestinal adverse effects, and for private reasons. A flowchart demonstrating the enrollment process is detailed in Fig. 1. Finally, a total of 44 patients (10 men, 34 women) completed the study. The mean age of participants was 57 ± 4.5 years. Of these 44 patients, two were taking glibenclamide, 18 were taking metformin, and 24 reported using glibenclamide and metformin.
Fig. 1.
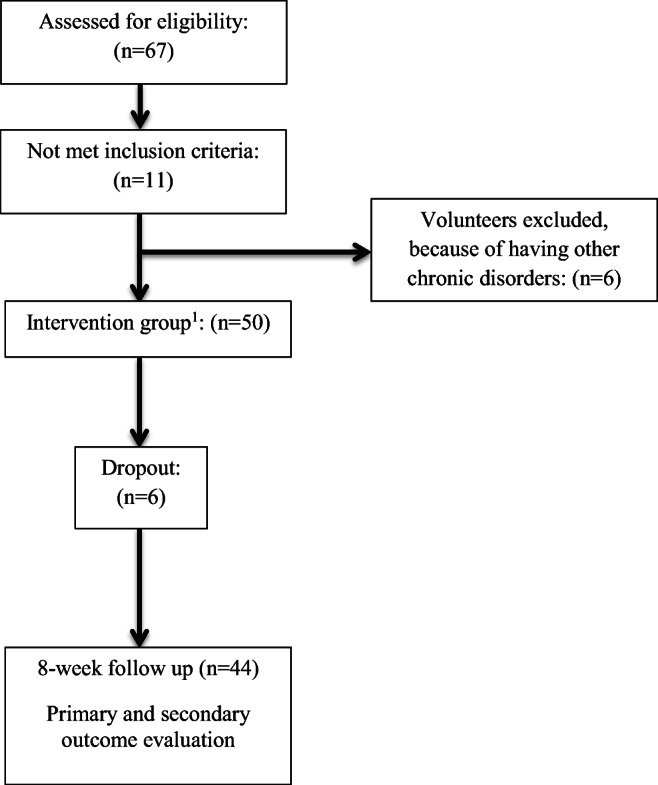
The consort flow chart. 1Patients consumed raw red beetroot (100 g/daily), for 8 weeks
There were no significant differences in total energy, carbohydrate, fiber, fat, protein and micronutrient intake (P > 0.05), except folate (vitamin B9) intake (P = 0.03) at the end of the intervention compared with baseline values (Table 1). The post-intervention values of folate intake was increased significantly (P = 0.03) (Table 1). In addition, there was no significant difference in physical activity level at the end of the intervention compared to the baseline (P > 0.05) (data not shown).
Table 1.
Total energy and nutrient intake of the patients at baseline and week 8 (n = 44)
| Variable | Baseline | Week 8 | P |
|---|---|---|---|
| Energy intake, kcal/day | 1738.01 ± 191.00 | 1729.00 ± 189.02 | .081a |
| Carbohydrate intake, g/day | 219.00 ± 26.00 | 214.00 ± 29.00 | 0.38a |
| Fat intake, g/day | 71.00 ± 18.00 | 72.01 ± 16.00 | 0.77a |
| Protein intake, g/day | 63.00 ± 13.00 | 63.00 ± 12.00 | 0.99a |
| Fiber intake, g/day | 15.00 (5.00) | 16.00 (6.00) | .021b |
| Vitamin A, RE/day | 554.00 (673.00) | 498.00 (489.00) | 0.96b |
| Vitamin B1, mg/day | 1.35 (1.00) | 1.29 (1.00) | 0.12b |
| Vitamin B3, mg/day | 15.00 (4.00) | 14.00 (4.00) | 0.34b |
| Vitamin B9, μ/day | 249.00 (128.00) | 306.00 (151.00) | 0.03b |
| Vitamin C, mg/day | 88.00 (55.00) | 82.00 (50.00) | 0.62b |
| Vitamin E, mg/day | 26.50 (14.00) | 28.00 (14.00) | 0.35b |
a Values are shown as mean ± SD; P value is based on Paired-Samples T test
b Values are shown as median (interquartile range); P value is based on Wilcoxon Signed Ranks test
No significant differences were observed in body weight (P = 0.36) and BMI (P = 0.39) at the end of the intervention compared with the baseline (Table 2). There was a significant decrease in FBG (P = 0.01) and serum levels of HbA1c (P < 0.01), ApoB100 (P = 0.03), AST (P = 0.04), ALT (P < 0.01), Hcy (P < 0.001), SBP (P < 0.001), and DBP (P = 0.02), at the end of the study compared to baseline values (Table 2). In addition, there was a significant increase in the ApoA1/ApoB100 ratio (P = 0.04), and serum level of TAC (P < 0.001) at week 8 compared to the beginning of the study (Table 2). However, no significant changes were observed for other variables including insulin, HOMA-IR, QUICKI, levels of TC, HDL-C, LDL-C, TAG, ApoA1, hs-CRP, NO, and PON1 activity (P > 0.05) (Table 2).
Table 2.
Weight status and metabolic markers of the patients at baseline and week 8 (n = 44)
| Variable | Baseline | Week 8 | P |
|---|---|---|---|
| Weight, kg | 69.09 ± 8.84 | 68.95 ± 8.95 | 0.36a |
| BMI, kg/m2 | 27.93 ± 3.34 | 27.88 ± 3.38 | 0.39a |
| NO, μM | 56.72 ± 25.67 | 64.84 ± 17.78 | 0.11a |
| FBG, mg/dl | 156.68 ± 39.26 | 143.15 ± 48.37 | 0.01a |
| HbA1c, % | 6.78 (0.96) | 6.44 (0.94) | < 0.01b |
| Insulin, micIU/ml | 7.95 ± 4.53 | 7.20 ± 4.18 | 0.59a |
| HOMA-IR, N | 2.91 (1.62) | 2.61 (1.92) | 0.26b |
| QUICKI, N | 0.34 (0.03) | 0.34 (0.04) | 0.44b |
| TAG, mg/dl | 140.40 ± 61.50 | 136.00 ± 55.00 | 0.56a |
| TC, mg/dl | 157.22 ± 36.92 | 150.20 ± 33.73 | 0.18a |
| LDL-c, mg/dl | 144.00 (40.31) | 136.20 (40.00) | 0.12b |
| HDL-c, mg/dl | 44.00 (7.46) | 44.00 (8.31) | 0.79b |
| ApoA1, mg/dl | 133.04 ± 19.52 | 128.06 ± 19.52 | 0.10a |
| ApoB100, mg/dl | 101.00 ± 32.28 | 92.75 ± 30.33 | 0.03a |
| ApoA1/ApoB100 | 1.43.00 (0.46) | 1.52 (0.51) | 0.04b |
| AST, U/L | 21.40 (8.18) | 19.56 (8.33) | 0.04b |
| ALT, U/L | 17.88 (9.77) | 14.18 (8.52) | < 0.01b |
| Hcy, μmol/L | 27.77 ± 9.26 | 19.89 ± 9.58 | < 0.001a |
| hs-CRP, mg/l | 2.50 (1.93) | 2.87 (1.76) | 0.17 b |
| TAC, μmol/L | 90.00 (28.00) | 195.00 (59.50) | < 0.001b |
| PON1, U/L | 200.57 (18.15) | 230.01 (14.96) | 0.57 b |
| SBP, mmHg | 12.21 ± 1.01 | 11.48 ± 1.59 | < 0.001a |
| DBP, mmHg | 8.02 (0.95) | 7.68 (0.47) | 0.02 b |
a Values are shown as mean ± SD; P value is based on Paired-Samples T test
b Values are shown as median (interquartile range); P value is based on Wilcoxon Signed Ranks test
BMI, body mass index; NO, nitric oxide; FBG, fasting blood glucose; TAG, triacylglycerol; TC, total cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; Hcy, homocysteine; hs-CRP, high-sensitive C reactive protein; TAC, total antioxidant capacity; PON1, paraoxonase-1; SBP, systolic blood pressure; DBP, diastolic blood pressure
A significant increase in the scores of TP (from 71.25 ± 22.61 to 77.70 ± 22.84; P < 0.01) and Digit Learning (from 5.27 ± 2.27 to 8.36 ± 2.89; P < 0.001) tests was observed at the end of the intervention compared to baseline scores (Table 3).
Table 3.
Cognitive function scores of the patients at baseline and week 8 (n = 44)
| Variable | Baseline | Week 8 | P |
|---|---|---|---|
| TP, total score | 71.25 ± 22.16 | 77.70 ± 22.84 | < 0.001 a |
| DL, total score | 5.27 ± 2.27 | 8.36 ± 2.89 | < 0.001 a |
a Values are shown as mean ± SD; P value is based on Paired-Samples T test
TP, Toulouse Pieron; DL, Digit Learning
Discussion
To our knowledge, this is the first study to investigate effects of raw red beetroot consumption on metabolic markers and cognitive function in T2DM patients. We found that consumption of 100 g raw red beetroot for 8 weeks, parallel to taking medications (metformin and/or glibenclamide) in T2DM patients had a significant beneficial impact on some metabolic markers (FBG, HbA1c, ApoB100, ApoA1/ApoB100 ratio, TAC, Hcy, AST, ALT, SBP, DBP) and cognitive function compared to the baseline values.
Some researchers have provided convincing evidence that beetroot consumption exerts advantageous physiological impacts associated with ameliorated clinical outcomes for a number of pathologies, including T2DM [11, 28]. These effects have been ascribed to the presence of betalains [14, 15, 29], with proven antioxidant and anti-inflammatory effects in vitro and in vivo [15, 16, 30–34]. This led to the hypotheses that beetroot consumption might serve as an advantageous strategy to strengthen endogenous antioxidant defense and protect cellular components against oxidative damage [34].
Our finding indicated no significant differences in NO levels at the end of the intervention compared to the baseline levels. In potential contrast with our findings, a study of healthy young men found that plasma levels of nitrite were elevated 2.5 h after ingestion of concentrated beetroot [35]. Potential justification for the differences in the plasma nitrite responses between our research and the previous study include inter-individual differences (T2DM patients versus healthy young participants), and the dosage of supplementation. Smaller impacts of dietary nitrate on plasma level of nitrite might be observed in older individuals because of age-associated alterations in oral microbiota and a weakened enterosalivary circuit [36]. In addition, NO levels may have been unaffected in the present study due to the larger time interval between the last dose of red beetroot and blood sampling (12-24 h). According to the evidence, the duration of blood sampling is really important because of the short half-life of NOs [37, 38].
We found that raw red beetroot consumption led to a significant decrease in FBG and HbA1c levels in the present study group. This is consistent with the findings of a cross-over study which showed that administration of 270 mL beetroot juice to healthy adults delayed the postprandial glycemic response and reduced the sustainability and peak of blood glucose level compared to the effects of a sugar-matched control drink [37]. Other studies on the blood glucose-lowering impact of beetroot, have proposed several potential mechanisms of action for these effects. Some studies focused on the crucial role of its bioactive components [38, 39].Bioactive molecules in beetroot include polyphenols, flavonoids, and betalains, as well as some enzymes, ascorbic acid and dehydroascorbic acid (DHAA), which regulate glucose absorption glycolysis, glycogenesis and gluconeogenesis [40–42]. Nitric oxide can trigger GLUT4 signaling and thus increase the cellular response to insulin [43]. Effects on the nitrite-nitrate pathway, which occurs in the oral cavity by the commensal bacteria, have also been proposed [44]. Other potential mechanisms include modulation of intracellular signal transduction as an important mechanism of decreasing blood glucose by foods and hormonal activities, and suppression of α-amylase and α-glucosidase [45]. In addition, the reported increase in serum cortisol with decreased glucose concentrations after beetroot intake may be a compensatory mechanism or associated with increased adrenocorticotropic hormone release from the pituitary [46].However, contrary to expectations, insulin, insulin sensitivity (QUICKI) and insulin resistance (HOMA-IR) were not significantly changed at the end of the intervention compared with the baseline values in the present study. This could be due to the fact that the nitrate levels found in the red beetroot in our study were too low to improve insulin sensitivity and insulin resistance.
Our findings indicated significant differences in the ratio of serum ApoB100 and ApoA1/ApoB100 before and after the intervention. However, there were no differences observed in ApoA1 levels and the lipid profile. However, other studies have found that red beetroot consumption exerts beneficial effects on lipid profile markers (TG, TC, LDL-C, HDL-C), which are directly associated with the incidence of T2DM. For example, a randomized double-blind clinical trial found that consumption of beetroot juice led to reduced levels of circulating lipids compared to the control group, apart from HDL-C [39]. The lack of effect on HDL-C may be the result of inactivation by glycation in diabetic persons, as described in a separate study [47]. This may have occurred in the present study and the lack of effect of beetroot on other lipids in our study was potentially due to the normal concentrations of TC and TG at baseline. We also found no significant difference in PON1 levels after the intervention, which may also have resulted from glycation [48, 49]. PON1 is an HDL-related protein that has the ability to hydrolyze oxidized LDL-C, with potential atheroprotective effects [50].
In our study, there was a significant difference in TAC levels after intervention compared to the baseline. Some studies have shown that TAC is reduced in patients with T2DM [51]. Such an effect has been linked with the pathophysiology of more than 200 diseases or clinical conditions [52]. It has been hypothesized that the betalain pigments in beetroot may help to protect cellular components from oxidative harm in some of these conditions [53, 54]. Kanner et al. [55] showed that two betalain metabolites (betanin and betanidin) reduced the injury to linoleate caused by cytochrome C oxidase, as well as the lipid membrane oxidation induced by H2O2. The high antioxidant activity in betanin appears to be associated with a high electron-donating capacity, rendering it capable of removing highly reactive radicals that target cell membranes [55]. However, beetroot also contains numerous other antioxidant compounds including rutin, epicatechin, and caffeic acid [56–58]. Moreover, nitrite and other NO donors related to beetroot have been reported to inhibit radical formation and directly scavenge reactive oxygen and nitrogen species, indicating that nitrate may also have antioxidant influences [59, 60]. Several studies have shown that beetroot juice protects against oxidative injury to DNA, lipid, and protein structures in vitro [61–63]. One study [15] found that rats given beetroot extract for 7 days had significantly lower levels of lipid peroxidation after exposure to carbon-tetrachloride (CCl4). The same study found that the beetroot extract seemed to help maintain endogenous antioxidant activity at normal cellular concentrations following the oxidative injury. Based on in vitro data, such impacts might be associated partly with betanin and its impact on signaling pathways mediating transcription of antioxidant genes. It has been shown that betanin extracted from beetroot enhanced the activity of nuclear factor (erythroid-derived 2)-like 2 (NRF2) in a dose-dependent manner. This transcription factor causes activation of the antioxidant response element (ARE), which regulates the transcription of numerous antioxidant enzymes [64, 65].
Metformin that is widely used in patients with type 2 diabetes may lead to reduced serum levels of vitamin B12 and folate, and increased Hcy, increasing the risk of small blood vessel diseases [66]. In our study, after an analysis of dietary intake, it was observed that the average intake of folate was significantly increased due to the addition of 100 g raw red beetroot to participant diets. Red beetroot appears to improve Hcy levels through its antioxidant and folate content [67]. As expected, our study results also indicated a significant decrease in Hcy levels.
Chronic inflammation is associated with the onset and progression of numerous clinical disorders [68] and the anti-inflammatory impacts of betalains and beetroot extracts appear to target pro-inflammatory signaling cascades. The most important of these is the NF-kB cascade, since this directly activates and leads to transcription of most gene targets that stimulate inflammatory response such as cytokines, chemokines, apoptotic and phagocytic cells [69]. In a preclinical study [70], NF-kB DNA-binding activity was found to be weakened in a dose-dependent manner in nephrotoxic rats supplemented with beetroot extract for 28 days. Kidney homogenates from the beetroot supplemented rats had lower levels of TNF-α, IL-6, and MDA, which could be directly associated with inhibition of the NF-kB pathway. These effects may be mediated by the betalains in beetroot. Another study [61] demonstrated that administration of beetroot extracts rich in betalin led to reduced inflammation and pain in osteoarthritic patients. As mentioned earlier, red beetroot also contains several bioactive phenolic compounds, such as epicatechin and caffeic acid, that are known to have antioxidant activity [71]. In addition, nitrite and other NO donors in red beetroot suppress free radicals and directly eliminate the free radicals of oxygen and nitrogen, which are the main cause of inflammatory diseases [72]. Nonetheless, our results showed no significant differences in hs-CRP levels. Some studies are in line [73] and some are inconsistent with these results [74, 75]. The lack of effect found here may be due to the relatively normal levelsof hs-CRP at baseline and potentially due to the fact that the nitric oxide of beetroot used in our study was lower than expected.
We observed a significant decrease in ALT and AST levels at week 8 compared to the beginning of the study. According to the results of previous studies, T2DM patients, compared with non-diabetics, are at increased risk of non-alcoholic fatty liver disease (NAFLD), fibrosis and cirrhosis [76]. In T2DM, the over-production of free fatty acids, inflammatory factors and oxidative stress can lead to fat accumulation in liver [77]. Red beetroot consumption can ameliorate this through its antioxidant, fiber and nitrate content, which can decrease oxidative stress, lower blood lipids and improve insulin action, respectively. In this regard, Krajka-Kuznika et al. [78] showed that beetroot juice had significant hepatic protection in a rat model against different inflammatory parameters induced by NDEA administration, including ALT, AST, gamma glutamyltransferase, and lactate dehydrogenase. Moreover, a study conducted by Rahimi et al. [79] showed betalains from red beetroot exert anti-dyslipidemic impact.
Our results are consistent with those of other studies which demonstrated a significant decrease in systolic and diastolic blood pressure after red beetroot consumption [80]. The nitrate in beetroot source is metabolized to nitrite, which can be further converted to NO [81]. Since NO mediates many endothelial functions, a decreased in NO availability has been implicated as a cause of endothelial dysfunction [82], a risk factor for some cardiovascular diseases, hypertension and atherosclerosis [83]. A meta-analysis of 12 RCTs (randomized clinical trials) highlighted the cardio-protective influences of beetroot juice supplementation in accordance with significant effect size on SBP [84]. In addition, the consumption of white and red beetroot bread, containing the same dose of nitrate, is reported to diminish BP to the same extent, indicating a positive relationship between nitrate content and the blood pressure lowering influence of beetroot [85]. Beetroot juice intake was also demonstrated to diminish BP, ameliorate endothelial function, and notably promote NO production [86].
Furthermore, patients with T2DM demonstrate slower reaction times than aged matched healthy individuals to different stimuli [87]. Previous studies have found that the nitrate content, and the antioxidant and anti-inflammatory properties of beetroot are associated with decreased FBG, Hcy, ApoB100, SBP, DBP, and an increased ApoA1/ApoB100 ratio and TAC levels, which can help to ameliorate cognitive problems and impairments in brain functions in patients with T2DM [43, 88]. As expected, our results also demonstrated a significant increase in Toulouse Pieron and Digit Learning test scores following beetroot consumption. One of the important risk factors for cerebral hypo-perfusion is an impairment in neurovascular performance caused by disrupted NO activity [89]. Decreased NO production can cause impairments in cerebral energy metabolism, glucose delivery, and neuronal activity which, in turn, could lead to neurodegeneration and impaired cognition [89]. Presley et al. [13] evaluated cerebral perfusion using magnetic resonance imaging (MRI) after administration of a high nitrate diet to elderly subjects for 24 h and found an enhancement in the frontal cortex area responsible for cognitive functions, compared to the effects of a nitrate-depleted diet. Another study by Bond et al. [90] supported these findings via demonstrating a reduction in cerebrovascular arterial resistance following a serving of nitrate rich beetroot juice. Another study [12] of older T2DM patients supplemented with 250 mL beetroot juice for 14 days, reported a significant amelioration in reaction time compared to a non-supplemented control group. The same authors [91] evaluated the impacts of supplementation with beetroot juice (140 mL/day) for 3 days on cognitive performance in healthy older participants, although this revealed no difference was found in cognitive function for memory, concentration, attention, and information processing ability between the supplemented and control groups. The inconsistent findings between these two investigations maybe partly due to the differences in subjects (T2DM versus healthy older adults), cognitive tests applied, dosage, and duration of the study.
Taken together, this study has shown that consumption of raw red beetroot (100 g, daily), for 8 weeks, parallel to taking antidiabetic medications in T2DM patients had a significant beneficial impact on some metabolic markers (FBG, HbA1c, ApoB100, ApoA1/ApoB100 ratio, TAC, Hcy, AST, ALT, SBP, DBP). However, our trial had some limitations. At first, the present research included a relatively short treatment period (8 weeks). Secondly, we had no control group in this trial due to budget and financial limitations. Third, no identification process was used on the plant species.
Conclusion
Raw red beetroot consumption, for 8 weeks, in T2DM patients, has beneficial impacts on cognitive function, glucose metabolism, and other metabolic markers. Therefore, red beetroot could be considered as a part of healthy diet for diabetic patients. However, more clinical studies are needed to confirm these effects. We recommended that future studies assess the effect of raw red beetroot on metabolic markers and hormonal changes in diabetes patients, using a cross-over design or with a control group in a larger sample size and longer follow-up periods.
Acknowledgements
We thank the participants for their cooperation and participation in this study. This research is a part of the M.Sc. thesis in nutrition sciences, which was financially supported by the Vice Chancellor of Research, Iran University of Medical Sciences (Tehran, Iran).
Funding sources
This research was funded by Iran University of Medical Sciences.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Abbreviations
- FBS
fasting blood sugar
- HbA1c
Glycosylated Hemoglobin
- ApoB100
Apolipoprotein B100
- AST
Aspartate Aminotransferase
- ALT
Alanine Aminotransferase
- TAC
total antioxidant capacity
- T2DM
Type 2 diabetes mellitus
- NO
nitric oxide
- BMI
body mass index
- TAG
triacylglycerol
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- hs-CRP
high sensitive C-reactive protein
- PON1
Paraoxonase-1
- Hcy
homocysteine
- BP
Blood pressure
- IPAQ
International Physical Activity Questionnaire
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- MRI
Magnetic resonance imaging
Author contributions
MA and MV designed this study. MA, LSB, and FA participated in the conduction of the study. AFH analyzed the data. BA drafted the manuscript. All authors read and approved the final manuscript.
Declarations
Ethics approval and consent to participate
The protocol of this study was approved by the Medical Ethics Committee of Iran University of Medical Sciences, is in conformity with the declaration of Helsinki (approval number IR.IUMS.REC 1395.932132004) and was registered at the Iranian Registry of Clinical Trials (IRCT registration number IRCT201612262365N16) which is available at:http://irct.ir/user/trial/20288/view. Written informed consent was obtained from all subjects.
Conflict of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Salas-Salvadó J, Becerra-Tomás N, Papandreou C, Bulló M. Dietary Patterns Emphasizing the Consumption of Plant Foods in the Management of Type 2 Diabetes: A Narrative Review. Adv Nutr. 2019;10(Suppl_4):S320–S331. doi: 10.1093/advances/nmy102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, editor. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: WHO; 2009. [Google Scholar]
- 3.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 4.Euser S, Sattar N, Witteman J, Bollen E, Sijbrands E, Hofman A, et al. A prospective analysis of elevated fasting glucose levels and cognitive function in older people: results from PROSPER and the Rotterdam study. Diabetes. 2010;59:1601–7. [DOI] [PMC free article] [PubMed]
- 5.Reijmer YD, van den Berg E, Ruis C, JaapKappelle L, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010;26:507–519. doi: 10.1002/dmrr.1112. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, Yang C, Wai STC, Zhang Y, Portillo MP, Paoli P, et al. Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Crit Rev Food Sci Nutr. 2018;59:830–47. 10.1080/10408398.2018.1501658. [DOI] [PubMed]
- 7.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59(7):365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Rezaeiamiri E, Bahramsoltani R, Rahimi R. Plant-derived natural agents as dietary supplements for the regulation of glycosylated hemoglobin: a review of clinical trials. Clin Nutr. 2020;39(2):331–342. doi: 10.1016/j.clnu.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Wootton-Beard PC, Brandt K, Fell D, Warner S, Ryan L. Effects of a beetroot juice with high neobetanin content on the early-phase insulin response in healthy volunteers. J Nutr Sci. 2014;3:e9. doi: 10.1017/jns.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naseri R, Farzaei F, Haratipour P, Nabavi SF, Habtemariam S, Farzaei MH, et al. Anthocyanins in the Management of Metabolic Syndrome: A Pharmacological and Biopharmaceutical Review. Front Pharmacol. 2018 Dec 4;9:1310. 10.3389/fphar.2018.01310 PMID: 30564116; PMCID: PMC6288909. [DOI] [PMC free article] [PubMed]
- 11.Ninfali P, Angelino D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia. 2013;89:188–199. doi: 10.1016/j.fitote.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Gilchrist M, Winyard PG, Fulford J, Anning C, Shore AC, Benjamin N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide. 2014;40:67–74. doi: 10.1016/j.niox.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24:34–42. [DOI] [PMC free article] [PubMed]
- 14.Lee CH, Wettasinghe M, Bolling BW, Ji LL, Parkin KL. Betalains phase II enzyme-inducing components from red beetroot (Beta vulgaris L.) extracts. Nutr Cancer. 2005;53:91–103. doi: 10.1207/s15327914nc5301_11. [DOI] [PubMed] [Google Scholar]
- 15.Vulić JJ, Ćebović TN, Čanadanović-Brunet JM, Ćetković GS, Čanadanović VM, Djilas SM, et al. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J Funct Foods. 2014;6:168–75.
- 16.Zielińska-Przyjemska M, Olejnik A, Dobrowolska-Zachwieja A, Grajek W. In vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytophera Res. 2009;23:49–55. doi: 10.1002/ptr.2535. [DOI] [PubMed] [Google Scholar]
- 17.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. [DOI] [PubMed]
- 18.Naseri R, Farzaei F, Fakhri S, El-Senduny FF, Altouhamy M, Bahramsoltani R, et al. Polyphenols for diabetes associated neuropathy: Pharmacological targets and clinical perspective. Daru. 2019 Dec;27(2):781–98. 10.1007/s40199-019-00289-w E PMID: 31352568; PMCID: PMC6895369. [DOI] [PMC free article] [PubMed]
- 19.Gilchrist M, Winyard P, Aizawa K, Anning A, Shore A, Nigel B. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radica lBiology and Medicine. 2013:2792–3. [DOI] [PubMed]
- 20.Jajja A, Sutyarjoko A, Lara J, Rennie K, Brandt K, Qadir O, et al. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr Res. 2014;34(10):868–75. [DOI] [PubMed]
- 21.Abiri B, Vafa MR, Dehghani M, Moslehi N, Sarrafzadeh J. Effect of vitamin D supplement consumption on muscle strength, muscle function and body composition in vitamin D-deficient middle-aged women: a randomized clinical trial. Nutr Food Sci Res. 2016;3(3):17–24. [Google Scholar]
- 22.Bairaktari E, Hatzidimou K, Tzallas C, Vini M, Katsaraki A, Tselepis A, et al. Estimation of LDL cholesterol based on the Friedewald formula and on apo B levels. Clin Biochem. 2000;33(7):549–555. doi: 10.1016/s0009-9120(00)00162-4. [DOI] [PubMed] [Google Scholar]
- 23.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endo crinol Metab. 2000;85(7):2402–10. [DOI] [PubMed]
- 25.Mackness B, Davies GK, Turkie W, et al. Paraoxonase status in coronary heart disease. Are activity and concentration more important than genotype? ArteriosclerThromb Vasc Biol. 2001;21:1451–1457. doi: 10.1161/hq0901.094247. [DOI] [PubMed] [Google Scholar]
- 26.Lima M, Duro D, Freitas S, Simes MR, Santana I. Validation study of the Toulouse-Piéron cancellation test for Portuguese patients with mild cognitive impairment and alzheimer’s disease. Sinapse. 2019;19(1–2):26–35.
- 27.Blackburn H, Benton A. Revised administration and scoring of the digit spantest. J Consult Psychol. 1957;21(2):139–143. doi: 10.1037/h0047235. [DOI] [PubMed] [Google Scholar]
- 28.Wootton-Beard PC, Brandt K, Fell D, Warner S, Ryan L. Effects of a beetroot juice with high neobetanin content on the early-phase insulin response in healthy volunteers. J Nutr Sci. 2011;3:1–9. doi: 10.1017/jns.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadipour E, Taleghani A, Tayarani-Najaran N, Tayarani-Najaran Z. Biological effects of red beetroot and betalains: a review. Phytother Res. 2020;34(8):1847–1867. doi: 10.1002/ptr.6653. [DOI] [PubMed] [Google Scholar]
- 30.Pavlov A, Georgiev V, Ilieva M. Betalain biosynthesis by red beet (Beta vulgaris L.) hairy root culture. Process.Biochem. 2005;40:1531–1533. [Google Scholar]
- 31.Tesoriere L, Allegra M, Butera D, Livrea MA. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: potential health effects of betalains in humans. Am J Clin Nutr. 2004;80:941–945. doi: 10.1093/ajcn/80.4.941. [DOI] [PubMed] [Google Scholar]
- 32.Vidal PJ, López-Nicolás JM, Gandía-Herrero F, García-Carmona F. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food Chem. 2014;154:246–254. doi: 10.1016/j.foodchem.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Mirmiran P, Houshialsadat Z, Gaeini Z, Bahadoran Z, Azizi F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. NutrMetab (Lond). 2020 Jan 7;17:3. 10.1186/s12986-019-0421-0 PMID: 31921325; PMCID: PMC6947971. [DOI] [PMC free article] [PubMed]
- 34.Ali MMA. Protective Effects of Beetroot on Streptozotocin Induced Diabetes in Adult Male Albino Rats. Bull Egypt Soc Physiol Sci. 2021;41(2):270–282. [Google Scholar]
- 35.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, et al. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J Appl Physiol. 1985;2013(115):325–36. [DOI] [PubMed]
- 36.Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, et al. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol. 2013;304:R73–83. [DOI] [PubMed]
- 37.Chang P, Hafiz M, Boesch C. Beetroot juice attenuates glycaemic response in healthy volunteers. Proc Nutr Soc P Nutr Soc. 2018;77(OCE4):E165. 10.1017/S0029665118001714.
- 38.Wootton-Beard PC, Brandt K, Fell D, Warner S, Ryan L. Effects of a beetrootjuice with high neobetanin content on the early-phase insulin response in healthy volunteers. J Nutr Sci. 2014;3:e9. doi: 10.1017/jns.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown H, Natuanya IN, Briggs O. Post-prandial effect of beetroot (Beta vulgaris) juice on glucose and lipids levels of apparently healthy subjects; 2018. p. 60–2.
- 40.Murthy, K.N.C. and S. Manchali, Anti-diabetic potentials of red beet pigments and other constituents, in Red Beet Biotechnology. 2013, Springer. p. 155–174.
- 41.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metabolic Disorders. 2013;12(1):43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwai K, Kim MY, Onodera A, Matsue H. α-Glucosidase inhibitory and Antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. J Agric Food Chem. 2006;54(13):4588–4592. doi: 10.1021/jf0606353. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H, Torregrossa AC, Potts A, Pierini D, Aranke M, Garg HK, et al. Dietary nitrite improves insulin signaling through GLUT4 translocation. Free Radic Biol Med. 2014;67:51–7. [DOI] [PubMed]
- 44.Beals JW, Binns SE, Davis JL, Giordano GR, Klochak AL, Paris HL, et al. Concurrent beet juice and carbohydrate ingestion: influence on glucose tolerance in obese and nonobese adults. J NutrMetab. 2017;2017:73946. doi: 10.1155/2017/6436783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida-Maruki H, Inagaki H, Ito R, Kurita I, Sai M, Ito T. Piceatannol lowers the blood glucose level in diabetic mice. Biol Pharm Bull. 2015;38(4):629–633. doi: 10.1248/bpb.b15-00009. [DOI] [PubMed] [Google Scholar]
- 46.Olumese F, Oboh H. Effects of Daily Intake of Beetroot Juice on Blood Glucose and Hormones in Young Healthy Subjects. Nigerian Quarterly J Hospital Med. 2016;26:455–462. [Google Scholar]
- 47.Sutherland WH, et al. Hormone-replacement therapy increases serum paraoxonase arylesterase activity in diabetic postmenopausal women. Metabolism-Clin Exp. 2001;50(3):319–324. doi: 10.1053/meta.2001.20201. [DOI] [PubMed] [Google Scholar]
- 48.Radi S, et al. Evaluation of salivary level of Paraoxonase and Total antioxidant capacity in type II diabetic subjects. Avicenna J Clin Med. 2015;22(2):114–121. [Google Scholar]
- 49.Esatbeyoglu T, Wagner AE, Motafakkerazad R, Nakajima Y, Matsugo S, Rimbach G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem Toxicol. 2014;73:119–126. doi: 10.1016/j.fct.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Getz GS, Reardon CA. Paraoxonase, a cardioprotective enzyme: continuing issues. Curr Opin Lipidol. 2004;15(3):261–267. doi: 10.1097/00041433-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Rifai N, Bachorik PS, Albers JJ. Lipids, lipoproteins and apolipoproteins. Tietz textbook of clinical chemistry. 3rd ed. Philadelphia: WB Saunders Company; 1999. pp. 809–861. [Google Scholar]
- 52.Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 53.Reddy MK, Alexander-Lindo RL, Nair MG. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J Agric Food Chem. 2005;53:9268–9273. doi: 10.1021/jf051399j. [DOI] [PubMed] [Google Scholar]
- 54.Tesoriere L, Fazzari M, Angileri F, Gentile C, Livrea MA. In vitro digestion of betalainicfoods.Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J Agric Food Chem. 2008;56:10487–10492. doi: 10.1021/jf8017172. [DOI] [PubMed] [Google Scholar]
- 55.Kanner J, Harel S, Granit R. Betalains a new class of dietary Cationized antioxidants. J Agric Food Chem. 2001;49:5178–5185. doi: 10.1021/jf010456f. [DOI] [PubMed] [Google Scholar]
- 56.Georgiev VG, Weber J, Kneschke EM, Denev PN, Bley T, Pavlov AI. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods HumNutr. 2010;65:105–111. doi: 10.1007/s11130-010-0156-6. [DOI] [PubMed] [Google Scholar]
- 57.Frank T, Stintzing FC, Carle R, Bitsch I, Quaas D, Strass G, et al. Urinary pharmacokinetics of betalains following consumption of red beet juice in healthy humans. Pharmacol Res. 2005;52:290–7. [DOI] [PubMed]
- 58.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230–242. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 59.Lundberg JO, Carlström M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011;89:525–532. doi: 10.1093/cvr/cvq325. [DOI] [PubMed] [Google Scholar]
- 60.Wink DA, Hines HB, Cheng RYS, Switzer CH, Flores-Santana W, Vitek MP, et al. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89:873–91. [DOI] [PMC free article] [PubMed]
- 61.Pietrzkowski Z, Nemzer B, Spórna A, Stalica P, Tresher W, Keller R, et al. Influence of betalin-rich extracts on reduction of discomfort associated with osteoarthritis. New Med. 2010;1:12–7.
- 62.Kujawska M, Ignatowicz E, Murias M, Ewertowska M, Mikołajczyk K, Jodynis-Liebert J. Protective effect of red beetroot against carbon tetrachloride- and N-nitrosodiethylamine-induced oxidative stress in rats. J Agric Food Chem. 2009;57:2570–2575. doi: 10.1021/jf803315d. [DOI] [PubMed] [Google Scholar]
- 63.Wootton-Beard PC, Moran A, Ryan L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res Int. 2011;44:217–224. [Google Scholar]
- 64.Esatbeyoglu T, Wagner AE, Motafakkerazad R, Nakajima Y, Matsugo S, Rimbach G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem Toxicol. 2014;73:119–126. doi: 10.1016/j.fct.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Satoh T, McKercher SR, Lipton SA. Reprint of: Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med. 2014;66:45–57. doi: 10.1016/j.freeradbiomed.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Ting Y, Jiang Y, Ho CT, Huang Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J Funct Foods. 2014;7:112–128. [Google Scholar]
- 67.Van Velzen AG, Sips AJAM, Schothorst RC, Lambers AC, Meulenbelt J. The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicol.Lett. 2008;181:177–181. doi: 10.1016/j.toxlet.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 68.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediat.Inflamm. 2010;2010:1–10. doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2001;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El Gamal AA, AlSaid MS, Raish M, Al-Sohaibani M, Al-Massarani SM, Ahmad A, et al. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediat Inflamm. 2014;2014:983–52. [DOI] [PMC free article] [PubMed]
- 71.Al-aboud NM. Effect of red beetroot (Beta vulgaris L.) intake on the level of some hematological tests in a group of female volunteers. ISABB J Food Agric Sci. 2018;8(2):10–17. [Google Scholar]
- 72.Vidal PJ, López-Nicolás JM, Gandía-Herrero F, García-Carmona F. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food Chem. 2014;154:246–254. doi: 10.1016/j.foodchem.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 73.Faldetta MC, O. L, G. D, M. B, Luca OD, M. M, et al. L-arginine infusion decreases plasma total homocysteine concentrations through increased nitric oxide production and decreased oxidative status in type II diabetic patients. Diabetologia. 2002;45(8):1120–7. [DOI] [PubMed]
- 74.Asgary S, et al. Improvement of hypertension, endothelial function and systemic inflammation following short-term supplementation with red beet (Beta vulgaris L.) juice: a randomized crossover pilot study. J Human Hypertension. 2016;30(10):627. doi: 10.1038/jhh.2016.34. [DOI] [PubMed] [Google Scholar]
- 75.El-Masry S, El-Rhman AA, The protective effect of BEETROOT and carrot juices on doxorubicin-induced cardiac injury and associated inflammatory response. 2017.
- 76.Basu S, Sussman JB, Berkowitz SA, Hayward RA, Bertoni AG, Correa A, et al. Validation of risk equations for complications of type 2 diabetes (RECODe) using individual participant data from diverse longitudinal cohorts in the US. Diabetes Care. 2018;41(3):586–95. [DOI] [PMC free article] [PubMed]
- 77.Eddowes P, Allison M, Tsochatzis E, Anstee Q, Sheridan D, Guha IN, et al. Algorithm to identify patients with an activity grade> 2 in type 2 diabetic patients with non-alcoholic fatty liver disease (NAFLD)-development in a large prospective multicenter UK study. J Hepatol. 2018;68:S552–3.
- 78.Krajka-Kuźniak V, Szaefer H, Ignatowicz E, Adamska T, Baer-Dubowska W. Beetroot juice protects against N-nitrosodiethylamine-induced liver injury in rats. Food Chem Toxicol. 2012;50:2027–2033. doi: 10.1016/j.fct.2012.03.062. [DOI] [PubMed] [Google Scholar]
- 79.Rahimi P, Abedimanesh S, MesbahNamin SA, Ostadrahimi A. Betalains, the nature-inspired pigments, in health and diseases. Crit Rev Food SciNutr. 2018;59:1–30. doi: 10.1080/10408398.2018.1479830. [DOI] [PubMed] [Google Scholar]
- 80.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regulat Integrative Comparative Physiol. 2010;299(4):R1121–31. [DOI] [PubMed]
- 81.Hobbs DA, Kaffa N, George TW, Methven L, Lovegrove JA. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Brit J Nutr. 2012;108:2066–2074. doi: 10.1017/S0007114512000190. [DOI] [PubMed] [Google Scholar]
- 82.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 83.Lidder S, Webb AJ. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br J Clin Pharmacol. 2013;75:677–696. doi: 10.1111/j.1365-2125.2012.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J Nutr. 2013;143(6):818–826. doi: 10.3945/jn.112.170233. [DOI] [PubMed] [Google Scholar]
- 85.Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A. Etal.Dietary nitrate improves vascular function in patients withhypercholesterolemia: a randomized, double-blind, placebo-controlledstudy. Am J ClinNutr. 2015;103(1):25–38. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56(2):274–81. [DOI] [PubMed]
- 87.Gilchrist M, Winyard PG, Fulford J, Anning C, Shore AC, Benjamin N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide. 2014;40:67–74. doi: 10.1016/j.niox.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 88.Wightman EL, Haskell-Ramsay CF, Thompson KG, Blackwell JR, Winyard PG, Forster J, et al. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Physiol Behav. 2015;149:149–58. [DOI] [PubMed]
- 89.De la Torre JC, Stefano GB. Evidence that Alzheimer’s disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res Rev. 2000;34:119–136. doi: 10.1016/s0165-0173(00)00043-6. [DOI] [PubMed] [Google Scholar]
- 90.Bond V Jr, Curry BH, Adams RG, Asadi MS, Millis RM, Haddad GE. Effects of dietary nitrates on systemic and cerebrovascular hemodynamics. Cardiol Res Pract. 2013:435–629. [DOI] [PMC free article] [PubMed]
- 91.Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, et al. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol -Reg I. 2013;304:73–83. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


