Abstract
Purpose
This study aimed to examine the synergistic effect of Vitamin D (VD) Supplement and mindfulness on neuropathic pain severity, Pain-Related Disability and Neuropathy-Specific Quality of Life (QOL) dimensions in painful diabetic neuropathy.
Methods
In this randomized controlled trial, 225 patients with painful diabetic neuropathy were randomly allocated to five groups: (1) mindfulness and placebo, (2) placebo, (3) mindfulness, (4) VD, and (5) mindfulness and VD. Mindfulness training includes twelve sessions, and VD patients received a daily four thousand IU oral dosage (four capsules) with 28,000 IU vitamin D weekly for 12 weeks. Laboratory analyses, Sun exposure time, Vitamin D intake, BMI and physical activity were measured in pre-test and post-test. Pain-Related Disability measured with The Pain Disability Index (PDI). For other outcome variables, Neuropathy, a Specific QOL questionnaire and Neuropathic pain severity scale were utilized. Data were analyzed using one-way repeated-measures analysis of variance (ANOVA), Scheffe Post-hoc test and paired sample t-test.
Results
In baseline, measures were not different among the groups. At the end-of-treatment, results showed improvement in all groups except the “placebo only” group for outcome variables. There was no difference between VD and mindfulness groups (within and not combined with placebo) in posttest. However, “VD + mindfulness” has a greater improvement rather than VD and mindfulness groups (P < 0.05). Moreover, both protocols have no significant effects on FBS, BMI and energy intakes (P > 0.05).
Conclusion
Combining VD and mindfulness can reduce pain severity and pain-related disability, so with these changes, patients experience improve in their quality of life.
Keywords: Pain, Quality of life, Vitamin D, Mindfulness, Neuropathy
Background
Today’s, more than 500 million patients with type-2 diabetes live worldwide, and this prevalence steadily increases [1]. Based on the International Diabetes Federation (IDF) reports, more than five million of the Iranian population have diabetes, and it has been estimated that in the year 2030, this rate will become 9.2 million patients [2, 3]. Painful diabetic neuropathy (PDN) with 60% prevalence is the main complication of patients with diabetes, specified by feet cramps, burning, numbness and tingling. The major problems in diabetic neuropathy patients are pain severity and pain-related disability. Consequently, these problems reduce their quality of life (QOL) [4]. For pain relief and improving QOL, there are various advocated treatments. The therapeutic efficacy is, at best, approximately 50% and is restricted due to side effects [5]. So, we need novel treatment packages with higher efficacy. Also, as the bio-psycho-social nature of pain and related problems, these new treatments must include multi-dimensional effects [4, 5]. Recently, growing evidence showed that adjunctive treatments such as vitamin supplements, psychoeducation, and massage therapy could play a key role in a better outcome in improving diseases and disorders without adding any side effects [6, 7]. One of these adjunctive treatments is the Vitamin–D supplement. Vitamin D deficiency is more prevalent in the Middle East, with 40–80% prevalence. Vitamin D (VD) insufficiency happened in more than 80% of patients with painful diabetic neuropathy cases (8). VD or neurotrophic hormone has a neuroprotective effect and enhances sensory neural response and axonogenesis [8]. Results have shown that VD can improve QOL and relief pain in neuropathy [9]. However, the effect size of VD on pain is somewhat small, and results reported mixed results [10, 11]. Pain has bio-psycho-social aspects and consists of a constellation of affective, cognitive and sensory factors; we need to add psychological training to improve these problems [12, 13].On the other hand, mindfulness showed promising effects on pain reduction and enhanced QOL. Mindfulness with enhancing OFC, right anterior insula, Subgenual Anterior Cingulate Cortex, the prefrontal cortex (PFC), and PFC function can reduce the severity of experiencing pain. Therefore, short mental practice in mindfulness engages cortical–thalamic–cortical interactions to decrease pain by mechanisms such as reappraisal or inhibitory control to basically “close the gate” on rising nociceptive information [14]. Results showed that mindfulness could reduce pain severity and enhancing QOL in various physical conditions such as migraine [15], Chronic Pelvic Pain [16] and IBS [17]. For neuropathic pain, results are mixed. Some studies showed the efficacy of mindfulness on neuropathic pain [18], and others showed inefficacy [19]. Mindfulness can have implemented as a part of the comprehensive pain treatment program, just like VD. We also try to eliminate the limitations of previous studies, including the lack of placebo groups and small sample size [4]. As both VD and mindfulness effectively affect pain reduction and QOL, there is no study about the synergistic effects of combined vitamin D supplements and the mindfulness training in PDN. This study aims to examine the synergistic effects of Vitamin D and mindfulness training for neuropathic pain sym-ptoms, pain-related disability, and QOL in PDN.
Martial and methods
Study design and participants
This randomized placebo-controlled trial was conducted between September 2019 and January 2020 in the Beheshti hospital (section of Endocrinology) in Kermanshah, Iran. Participants include all patients with VD deficiency referred to Beheshti hospital, Kermanshah, Iran, between 1 September 2019 and 18 October 2019. A group diagnosed with type 2 diabetes mellitus and neuropathy include endocrinologist and neurologist physicians.
Inclusion and exclusion criteria
Inclusion criteria include (1) lack of major co-morbid disease such as coronary heart disease or psychotic disorder (2) age of 20 to 70 years, (3) willingness to participate in studying and (4) VD insufficiency or deficiency [between 10 (ng/mL) to 30 (ng/mL) serum vitamin D]. Exclusion criteria include (1) existence of psychiatric or neurological diseases, (2) took VD or any multi-vitamins during the last three months, (3) using any substance and drinking alcohol, (4) being pregnant, and (5) more than one absence in mindfulness sessions..
Randomization and blinding
Participants were randomly allocated into groups using a random table. All patients were blinded to receive VD or placebo groups. Also as these treatments are adjunctive treatments, so, they received usual treatment based on neurologist prescribes.
Sample size
Concerning 0.2 as type 2 error (with 80%power) and 0.05 as type 1 error; and regarding pervious research, 35 participants are required for each group. Toward covering potential dropouts, the sample size increased 25% and reaches to 45 subjects for each group [20].
Grouping
The patients are randomly allocated into the following groups: 1) mindfulness training, 2) mindfulness training + VD supplement, 3) mindfulness training + Placebo, 4) VD supplement, and 5) placebo.
Interventions
Supplement
Between 1, November 2019 to 24, January 2020 (12 weeks), VD groups received a daily four thousand IU oral dosage (four capsules) with 28,000 IU vitamin D weekly. This supplement includes twenty-eight oily drops weekly (JALINOUS CO, Iran). Placebo (JALINOUS) groups receive totally similar drops in shape (without any VD) and duration.
Mindfulness
Patients allocated to the mindfulness groups received 12 weeks (90 min per session) of modified mindfulness manual based on pain relief protocols [21, 22]. A three trained psychotherapist has implemented the intervention in mindfulness with three assistances. They were blinded about study aims and other groups’ existence (VDs and other mindfulness groups). Sessions content, including:
Familiarity with participants. Discuss neuropathy and its nature. Giving a pamphlet about neuropathy for home reading. Free discussion about the psychological aspect of neuropathy and pain.
Introduce the content of the intervention. Teaching about the relationship between mind and body.
Learn about meditation and body scan, then practicing them.
Teaching relaxation skills (belly breathing, guided imagery and progressive muscle relaxation).
Teaching regarding chronic pain, relationships between physical, emotional and thought reactions. Also, explain pain coping strategies with brainstorming.
Learn to experience negative emotions and pay attention to them.
Meditation in sitting posture while concentrating on breathing and external sounds.
Meditation in sitting posture while concentrating on internal emotions and thoughts.
Measures
Laboratory analyses
Ten ML of fasting blood units from each participant was taken after ten hours of fasting at the first stage and at the end of the study. The blood samples stored at −80 °C (with 10 min centrifuging at 3000 Revolutions per minute) until future analysis with centrifuged to isolate serums. Fasting blood glucose (FBS) was measured by spectrophotometry utilizing the Pars Azmun kit in auto-analyzer equipment (BT3000, China).
Pain-related disability
The Pain Disability Index (PDI) is a seven-item scale to examine the extent of self-reported pain-related inability, independent from the region of pain or diagnosis. The items of the scale are evaluated on a 0–10 numeric evaluation scale in which 0 indicates no inability and 10 is the maximum disability. Higher scores indicating higher interference of the pain with regular activities. The PDI showed high test-retest reliability in many studies [23].
Neuropathic pain severity
The Neuropathic pain scale (NPS) is measured for severity of neuropathic pain. This scale calculated as the sum of ten pain descriptor items (each item rated on 0 to 10 with ten representing the most severe pain). Thus, this scale total score varies from 0 to 100. In many studies, psychometric properties were examined and results showed adequate properties [24].
Neuropathy specific quality of life
The Neuropathy Specific Quality of Life questionnaire (NeuroQol) with 28 items is a particular validated scale for neuropathic QOL. NeuroQol evaluates diabetic neuropathy-related emotional and physical difficulties affecting diurnal life and well-being. This scale consists of painful symptoms, paresthesia, unsteadiness while walking or standing, emotional distress, interpersonal problems, emotional and physical dependence on others, and restriction in daily activities in five subscales. Reliability of the subscales ranged from 0.86 to 0.95.As-four items repeated in more than one subscale, the total score is 31–155. Higher scores mean more impairments of QOL [25].
Sun exposure
Before implementing intervention and in the post-test by a validated questionnaire the sun exposure rate was assessed. The span of sun exposure was measured by total minutes exposing to the sun in the last two week and divided into two [26].
Vitamin-D intake
for assessing Vitamin D intake, the fourth version Modified Nutritionist software program was used by a trained nutritionist (three-day food recording include one weekend day and two weekdays [4, 7, 8].
Anthropometric assessments
BMI (Body mass index) by dividing weight (kg) to square height (m2) was calculated at the baseline and post-test [27].
Physical activity
The short version of the International Physical Activity Questionnaire (SF-IPAQ) was applied for determining the level of physical activity (MET-minutes/week) participants [28].
Statistical analysis
For assessing data, SPSS (version 26) software was employed. Data were analyzed by paired sample t-test, chi-squared test, the one-way ANOVA and, post-hoc test (Scheffe). P-values <0.05 were considered statistically significant.
Results
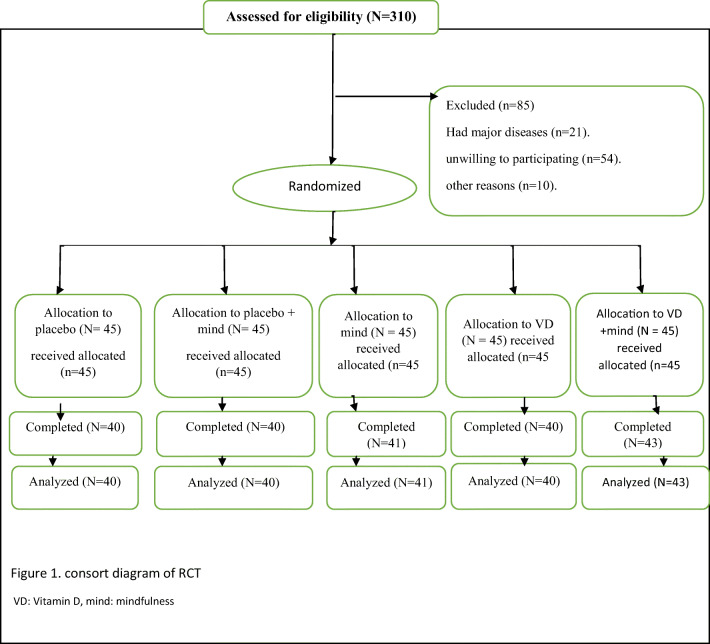
225 TDM2 with neuropathy diagnosis were involved in our investigation. Ultimately, 204 patients completed the study [Fig. 1]. In the administration of Vitamin D and placebo for neuropathic diabetic patients, no side effects were reported.
Fig. 1.
Consort diagram of RCT. VD: Vitamin D, mind: mindfulness.
Demographic measures
Demographic variables were measured in the pre-test and at the end of invention. In the pre-test phase, participants were not statistically different among groups. (P > 0.05). In the post-test, there is only statically differences for physical activity among groups (P < 0.05) .Table 1 presented demographic variables results in pre-test and post-test.
Table 1.
Scores of the participants by groups
| Characteristic | placebo | Placebo + mindfulness | mindfulness | Vitamin | Vitamin + Mindfulness | P value |
|---|---|---|---|---|---|---|
| Age (years) a | 56.25 ± 9.89 | 53.3 ± 8.94 | 54.8 ± 9.44 | 54.5 ± 9 | 56.6 ± 9.8 | 0.49 |
| sex (female)b (N) | 16 (40%) | 17(42.5%) | 16(39.2%) | 22(55%) | 21(48.8%) | 0.56 |
|
base line Vitamin D level (ng/mL) |
24.6 ± 5.5 | 25.4 ± 5.4 | 25.3 ± 5.2 | 27.4 ± 5.07 | 26.2 ± 5.7 | 0.1 |
| Duration of diabetes (years) | 14.7 ± 5.3 | 15.8 ± 4.8 | 14.8 ± 6.6 | 14.6 ± 4.6 | 15.3 ± 5.06 | 0.82 |
| FBS (pre-test) | 178.9 ± 29.2 | 172.5 ± 33.8 | 172.2 ± 28.9 | 170.37 ± 27.28 | 175.13 ± 28.19 | .73 |
| FBS (post-test) | 178.1 ± 27.2 | 169 ± 27.14 | 165.31 ± 28.09 | 163.7 ± 27.92 | 167.51 ± 30.92 | 0.18 |
| P valuec | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | |
| sun (pre-test) | 38.7 ± 14.1 | 41.7 ± 14.4 | 40.1 ± 15.8 | 39.5 ± 13.9 | 42.7 ± 14.02 | 0.7 |
| sun (post-test) | 42.5 ± 15.14 | 41 ± 14.8 | 39.2 ± 15.3 | 45.5 ± 13.7 | 43.4 ± 15.01 | 0.38 |
| P VALUEc | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | |
| VD(pre) | 3.7 ± 2.08 | 4.6 ± 1.9 | 3.7 ± 1.9 | 4.2 ± 2.01 | 4.09 ± 2.03 | 0.194 |
| VD(post) | 3.9 ± 2.1 | 4.1 ± 1.8 | 3.9 ± 2.05 | 3.5 ± 1.7 | 4.1 ± 2.1 | 0.71 |
| P VALUEc | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | |
| Energy (pre) | 2390.1 ± 279.2 | 2338.45 ± 269.8 | 2395.51 ± 276.35 | 2462.12 ± 293.2 | 2404.48 ± 313.87 | 0.44 |
| Energy (post) | 2377.15 ± 306.01 | 2452.17 ± 275.54 | 2374.46 ± 302.01 | 2446.05 ± 328.78 | 2357.46 ± 278.26 | 0.46 |
| P VALUEc | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | |
| PHY (pre) | 251.8 ± 81.8 | 260.75 ± 93.4 | 256.87 ± 91.46 | 255.22 ± 89.04 | 273.48 ± 95.25 | 0.247 |
| PHY (post) | 253.32 ± 79.4 | 378.8 ± 84.7 | 397.19 ± 96.82 | 403.67 ± 91.45 | 496.03 ± 76.92 | 0.00 |
| P VALUEc | P > 0.05 | P < 0.05* | P < 0.05* | P < 0.05* | P < 0.05* |
a = one way anova, B = chi-square test, c = paired sample t-test, FBS=Fasting blood sugar(mg/dL), Sun: Sunlight exposure (minutes/week), Energy: Energy intake (k Cal/day), PHY: Physical activity (MET/min*week), VD = Vitamin D intake (mcg/day).
For physical activity, all groups except “placebo” showed statically improvement in post-intervention rather than pre-test (p < 0.5). Besides, both Vitamin D and mindfulness training can improve physical activity. A comparison among groups done with post-hoc (Scheffe) analysis. Table 2 demonstrated that “Vitamin+Mindfulness” significantly greater than others could improve physical activity. Also, there is not any significant difference between “mindfulness” and “Vitamin D” in physical activity (with/without combination with placebo) (P < 0.05).
Table 2.
Scheffe post-hoc analysis for physical activity in post-test
| Group I | Group J | Mean difference | P value | Group I | Group J | Mean difference | P value |
|---|---|---|---|---|---|---|---|
| placebo | Placebo+mindfulness | −134.5 | 0.00* | Placebo+mindfulness | mindfulness | −9.3 | 0.98 |
| mindfulness | −143.8 | 0.00* | Vitamin | −15.85 | 0.95 | ||
| Vitamin | −150.35 | 0.00* | Vitamin+Mindfulness | −108.2 | 0.00* | ||
| Vitamin+Mindfulness | −242.7 | 0.00* | |||||
| mindfulness | Vitamin | −6.47 | 0.9 | ||||
| Vitamin+Mindfulness | −98.8 | 0.00* | Vitamin | Vitamin+Mindfulness | −92.35 | 0.00* |
Outcome measures
For QOL (as the total measure), results showed that there is not a significant difference among groups in baseline (P > 0.05). However, at posttest, the result showed significant improvement in all groups except “placebo”. Moreover, there is not any difference between mindfulness and Vitamin D groups to enhance QOL. Nevertheless, “vitamin D + mindfulness” showed the most improvement regarding other groups. These results repeated in all QOL subscale except “reduced feeling” and “diffuse sensory motor” [Tables 3].
Table 3.
outcome measures from baseline to end of treatment
| variable | Characteristic | placebo | Placebo + mind | mind | Vitamin | Vitamin + Mind | P |
|---|---|---|---|---|---|---|---|
| QOL | pre pain A | 21.07 ± 7.03 | 19.6 ± 5.9 | 20.4 ± 5.8 | 18.9 ± 7.1 | 19.9 ± 5.6 | 0.6 |
| post pain A | 20.3 ± 5. 4 | 15.2 ± 5.4 | 16.3 ± 6.7 | 14.8 ± 5.8 | 10.3 ± 4.7 | 0.00* | |
| p value B | P > 0.05 | P < 0.05* | P < 0.05* | P < 0.05* | P < 0.05* | ||
| pre feeling A | 4.4 ± 1.7 | 5.2 ± 1.5 | 4.8 ± 1.3 | 4.6 ± 1.4 | 4.4 ± 1.5 | 0.1 | |
| POST feeling A | 4.2 ± 1.4 | 5.1 ± 1.49 | 5.17 ± 1.44 | 4.4 ± 1.3 | 5.1 ± 2.1 | 0.1 | |
| P VALUEB | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | ||
| pre sensory A | 5.6 ± 1.9 | 6.02 ± 1.8 | 6.1 ± 2.2 | 6.02 ± 1.09 | 6.1 ± 2.08 | 0.86 | |
| POST sensory A | 5.8 ± 1.9 | 5.9 ± 2.3 | 6.6 ± 1.7 | 5.8 ± 2.06 | 6.8 ± 1.9 | 0.08 | |
| P VALUEB | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | ||
| PRE inter | 32.49 ± 9.3 | 32.7 ± 10.8 | 33.5 ± 10.4 | 34.8 ± 11.1 | 31.9 ± 11.3 | 0.7 | |
| POST inter | 32.5 ± 10.2 | 26.1 ± 9.4 | 25.6 ± 7.9 | 25.6 ± 8.3 | 17.8 ± 6.5 | 0.00* | |
| P VALUEB | P > 0.05 | P < 0.05* | P < 0.05* | P < 0.05* | P < 0.05* | ||
| PRE ACTIVITYA | 24.7 ± 5.5 | 23.3 ± 6.7 | 23.8 ± 4.7 | 23.7 ± 6.1 | 22.8 ± 5.6 | 0.6 | |
| POST ACTIVITYA | 24.2 ± 7.02 | 17.9 ± 5.5 | 19.4 ± 5.6 | 18.4 ± 4.5 | 13.02 ± 4.6 | 0.00* | |
| P VALUEB | P > 0.05 | P < 0.05* | P < 0.05* | P < 0.05* | P < 0.05* | ||
| PRE TOTLAA | 88.3 ± 12.5 | 87.05 ± 15.05 | 88.8 ± 12.4 | 88.5 ± 17.5 | 86.2 ± 15.2 | 0.9 | |
| POST TOTALA | 87.09 ± 14.9 | 69.6 ± 12.7 | 73.2 ± 11.2 | 69.3 ± 13.02 | 53.16 ± 11.5 | 0.00* | |
| P VALUEB | P > 0.05 | P < 0.05* | P < 0.05* | P < 0.05* | P < 0.05* | ||
| Pain Severity | PRE TEST | 47.02 ± 13.7 | 45.6 ± 13.5 | 50.8 ± 13.5 | 49.4 ± 14.7 | 46.02 ± 14.5 | 0.3 |
| POST TEST | 45.6 ± 13.4 | 30.6 ± 14.6 | 33.7 ± 12.8 | 31.03 ± 14..8 | 21.02 ± 9.09 | 000*. | |
| P VALUE | P > 0.05 | P < 0.05* | P < 0.05* | P < 0.05* | P < 0.05* | ||
| Disability | PRE TEST | 42.6 ± 14.2 | 42.5 ± 14.1 | 41.5 ± 13.5 | 38.08 ± 13.05 | 42.2 ± 13.2 | 0.7 |
| POST TEST | 41.8 ± 12.5 | 31.6 ± 11.7 | 31.2 ± 12.3 | 29.7 ± 11.7 | 21.5 ± 9.4 | 0.00* | |
| P VALUE | P > 0.05 | P < 0.05* | P < 0.05* | P < 0.05* | P < 0.05* |
a = one way anova, b = paired sample t-test, feeling: reduced feeling, sensory: diffuse sensory motor, inter: interpersonal and emotional burden, ACTIVITY: activity limitations, Inter: interpersonal emotional burden, ACTIVITY: activity limitations, MIND: mindfulness.
In post-test, there are significant differences among groups in QOL as a whole scale, and also in most QOL subscales include pain, interpersonal, and emotional burden. About total scores of QOL scale-, all groups except “placebo” showed a significant reduction in QOL scores (enhancement). We used the Scheffe post hoc test for assessing differentiation among groups. There were no significant differences between mindfulness groups and vitamin groups, but “Vitamin + Mindfulness” showed the most significant pain subscale reduction rather than vitamin, mindfulness, and mindfulness plus placebo. These results repeated for all subscales of QOL, except “REDUCED FEELING.” These results are presented in Table 4.
Table 4.
Scheffe Post-hoc analysis for QOL subscales measures
| PAIN | |||||||
|---|---|---|---|---|---|---|---|
| Group I | Group J | Mean difference | P value | Group I | Group J | Mean difference | P value |
| placebo | Placebo+mindfulness | 5.1 | 0.003* | Placebo+mindfulness | mindfulness | −1.04 | 0.95 |
| mindfulness | 4.07 | 0.03* | Vitamin | 0.45 | 0.99 | ||
| Vitamin | 5.5 | 0.001* | Vitamin+Mindfulness | 4.8 | 0.005* | ||
| Vitamin+Mindfulness | 9.9 | 0.00* | |||||
| mindfulness | Vitamin | 1.4 | 0.84 | ||||
| Vitamin+Mindfulness | 5.9 | 0.001* | Vitamin | Vitamin+Mindfulness | 4.5 | 0.005* | |
| Interpersonal Emotional Burden | |||||||
| placebo | Placebo+mindfulness | 6.3 | 0.02* | Placebo+mindfulness | mindfulness | 0.49 | 0.9 |
| mindfulness | 6.8 | 0.01* | Vitamin | 0.45 | 0.9 | ||
| Vitamin | 6.84 | 0.01* | Vitamin+Mindfulness | 8.3 | 0.001* | ||
| Vitamin+Mindfulness | 14.7 | 0.001* | |||||
| mindfulness | Vitamin | −0.03 | 0.9 | ||||
| Vitamin+Mindfulness | 7.8 | 0.002* | Vitamin | Vitamin+Mindfulness | 7.87 | 0.001* | |
| Activity Limitations | |||||||
| placebo | Placebo+mindfulness | 6.3 | 0.00* | Placebo+mindfulness | mindfulness | −1.5 | 0.81 |
| mindfulness | 4.8 | 0.005* | Vitamin | −0.5 | 0.9 | ||
| Vitamin | 5.8 | 0.00* | Vitamin+Mindfulness | 4.9 | 0.005* | ||
| Vitamin+Mindfulness | 11.2 | 0.00* | |||||
| mindfulness | Vitamin | 0.97 | 0.9 | ||||
| Vitamin+Mindfulness | 6.4 | 0.00* | Vitamin | Vitamin+Mindfulness | 5.4 | 0.001 | |
| Total | |||||||
| placebo | Placebo+mindfulness | 17.4 | 0.001* | Placebo+mindfulness | mindfulness | −3.5 | 0.8 |
| mindfulness | 13.8 | 0.001* | Vitamin | 0.34 | 0.9 | ||
| Vitamin | 17.7 | 0.001* | Vitamin+Mindfulness | 16.5 | 0.001* | ||
| Vitamin+Mindfulness | 33.9 | 0.001* | |||||
| mindfulness | Vitamin | 3.9 | 0.75 | ||||
| Vitamin+Mindfulness | 20.08 | 0.001* | Vitamin | Vitamin+Mindfulness | 16.17 | 0.001* | |
For pain-related disability, and pain severity results showed that there were not significant difference among groups in base-line (P > 0.05). But, at posttest, result showed significant reduction in all groups except “placebo”. Moreover, there is not any difference between mindfulness and Vitamin D groups to reduce pain disability and pain severity. Yet, the “vitamin D + mindfulness” group showed the most improvement rather than other groups [Tables 3 and 5].
Table 5.
Posthoc analysis for pain severity and pain-related disability measures
| Pain Severity | |||||||
|---|---|---|---|---|---|---|---|
| Group I | Group J | Mean difference | P value | Group I | Group J | Mean difference | P value |
| placebo | Placebo+mindfulness | 15 | *0.00 | Placebo+mindfulness | mindfulness | −3.3 | 0.9 |
| mindfulness | 11.9 | *0.006 | Vitamin | −0.6 | 0.9 | ||
| Vitamin | 14.3 | *0.00 | Vitamin+Mindfulness | 9.6 | 0.02* | ||
| Vitamin+Mindfulness | 24.6 | *0.00 | |||||
| mindfulness | Vitamin | 2.3 | 0.9 | ||||
| Vitamin+Mindfulness | 12.6 | 0.001* | Vitamin | Vitamin+Mindfulness | 10.3 | 0.026* | |
| Pain Disability | |||||||
| Group I | Group J | Mean difference | P value | Group I | Group J | Mean difference | P value |
| placebo | Placebo+mindfulness | 10.2 | 0.003* | Placebo+mindfulness | mindfulness | 0.3 | 0.9 |
| mindfulness | 10.5 | 0.001* | Vitamin | 1.8 | 9.7 | ||
| Vitamin | 12.05 | 0.001* | Vitamin+Mindfulness | 10.08 | 0.004* | ||
| Vitamin+Mindfulness | 20.2 | 0.001* | |||||
| mindfulness | Vitamin | 1.4 | 0.9 | ||||
| Vitamin+Mindfulness | 8.4 | 0.02* | Vitamin | Vitamin+Mindfulness | 8.2 | 0.037* | |
Discussion
The present study demonstrated that VD supplement and mindfulness training separately reduce pain severity and pain-related disability and then improved QOL in painful diabetic neuropathy. Moreover, our research provides substantial evidence confirming that VD, combined with mindfulness, has a synergistic effect on pain severity, pain-related disability, and QOL in painful diabetic neuropathy. However, in two of the QOL subscales, there was no significant difference between baseline and post-test for reduced feeling and diffuse sensory-motor. As these subscales were low in baseline (without a destructive role in QOL), they did not need improvement, so they remained steady. This is the first study that combining VD and mindfulness on diabetic neuropathy. Nevertheless, previous results showed that VD and mindfulness as a separate intervention have promising effects. For example, Basit et al. (2015) demonstrated that intramuscular injection of vitamin D reduced pain scores in painful diabetic neuropathy [4]. Also, recently, one research conducted by Izgu (2020) showed that mindfulness meditation had a significant impact on pain severity in patients with type 2 diabetes [29]. This result repeated about the efficacy of mindfulness on quality of life in diabetic patients [30]. Also, previous researches showed that mindfulness training increased physical function and decreased pain-related disability and pain intensity in patients with chronic pain [31, 32]. Also, Nathan et al. (2017) showed that MBSR training reduced pain severity, perceived stress, pain catastrophizing, and, with these reductions, improved QOL [19]. About the efficacy of mindfulness on pain severity, the organization and modulation of pain are mediated by cognitive, affective, and sensory factors. Mindfulness training with significant activation of thalamus and insula as the sensory processing–related brain areas and decreased activation in brain regions that process the appraisement of pain (orbitofrontal cortex and medial PFC). Also, there was a significant association among lower pain reports with meditative experience and higher deactivation of the orbitofrontal cortex and medial PFC. By reducing the pain’s severity, patients are empowered to do things they were previously unable to do. For example, their social activity increases, and sexual behavior problems decrease. Also, with mindful abilities, patients learn to reduce catastrophizing of pain and accept some grades of pain as part of their life to find recreation and interpersonal activities despite pain. With these improvements, their pain-related disability was reduced, and improved their QOL [16, 30, 33]. On the other hand, in animal models, several genes such as Oxt, Penk, Pomc Payne, Pth, and Tgfb1-encoding for peptides are top candidates to describe the remedial advantage vitamin D supplementation on neuropathic pain [34]. Potential mechanisms for VD effects in pain reduction are the anti-inflammatory consequences mediated by effects on T cell responses, reduced cytokines, and prostaglandins (PG) release [35]. So with a reduction in pain severity and pain disability, their QOL was improved. As stated in former sentences, pain is a bio-psycho-social problem, and with targeting behavior (with mindfulness) and physical aspects (VD supplement), the pain severity can reduce in great amount, and patients can managing pain with mulita-dimensional benefits. So combining VD and mindfulness enhancing patients’ QOL without increasing side effects. Moreover, both protocols have no significant effects on FBS, BMI, and energy intakes. As these variables are highly associated with the life-style, patients need extensive changes in habits, attitudes, and behaviors about energy intake and BMI [36–38]. Also, VD supplementation was not efficient in fat mass. However, with a reduction in pain severity and pain-related disability, patients abler to self-care behaviors and find recreation in life -situations, so their physical activity improved [36, 37]. However, there is no comparable investigation for FBS’s mindfulness, so we need more mindfulness studies in FBS.
Limitations and future directions
Besides promising results of current research, this study has some limitations. First, we were unable to examine the seasonal changes that could influence the result. Second, we used self-rating sales. Future researches must eliminate these limitations. Future studies can also assess the efficacy of other adjunctive supplements such as omega-3 and other vitamins. Another suggestion could be evaluating other mindfulness treatments such as acceptance and commitment therapy or dialectical behavior therapy. Also, these combinations can be evaluated in other forms of painful neuropathy.
Conclusion
Combining Vitamin D and mindfulness training could have reduced pain severity, Pain-Related Disability and then improved Neuropathy-Specific Quality of Life dimensions in painful diabetic neuropathy.
Abbreviations
- VD
Vitamin D
- PDN
Painful Diabetic Neuropathy
- QOL
Quality of Life
Compliance with ethical standards
Competing interests
The authors certify that they have no competing interests.
Ethical consideration
Before administering the investigation, five-sessions were held to justify all participants. In these sessions, ethical matters and explanations about the investigation were given to the individuals. Then, a consent form was achieved for all patients. Participants were convinced that their data and identities would be kept private. Also, their medical information will not be given to the third party. The present research was evaluated before implementing the Research Ethics Committee of Kermanshah University of Medical Sciences (Ir.kums.rce.1399.0403). Also, this study with the code (TCTR20200629004) has been registered in the Thailand Registry of Clinical Trials (TRCT).at the end of the study, all groups received VD and mindfulness training.
Consent for publication
Consent letter for publishing data was also received from participants.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, Suzuki K, Tam CHT, Tabara Y, Kwak SH, Takeuchi F, Long J, Lim VJY, Chai JF, Chen CH, Nakatochi M, Yao J, Choi HS, Iyengar AK, Perrin HJ, Brotman SM, van de Bunt M, Gloyn AL, Below JE, Boehnke M, Bowden DW, Chambers JC, Mahajan A, McCarthy MI, Ng MCY, Petty LE, Zhang W, Morris AP, Adair LS, Akiyama M, Bian Z, Chan JCN, Chang LC, Chee ML, Chen YDI, Chen YT, Chen Z, Chuang LM, du S, Gordon-Larsen P, Gross M, Guo X, Guo Y, Han S, Howard AG, Huang W, Hung YJ, Hwang MY, Hwu CM, Ichihara S, Isono M, Jang HM, Jiang G, Jonas JB, Kamatani Y, Katsuya T, Kawaguchi T, Khor CC, Kohara K, Lee MS, Lee NR, Li L, Liu J, Luk AO, Lv J, Okada Y, Pereira MA, Sabanayagam C, Shi J, Shin DM, So WY, Takahashi A, Tomlinson B, Tsai FJ, van Dam RM, Xiang YB, Yamamoto K, Yamauchi T, Yoon K, Yu C, Yuan JM, Zhang L, Zheng W, Igase M, Cho YS, Rotter JI, Wang YX, Sheu WHH, Yokota M, Wu JY, Cheng CY, Wong TY, Shu XO, Kato N, Park KS, Tai ES, Matsuda F, Koh WP, Ma RCW, Maeda S, Millwood IY, Lee J, Kadowaki T, Walters RG, Kim BJ, Mohlke KL, Sim X. Identification of type 2 diabetes loci in 433,540 east Asian individuals. Nature. 2020;582:240–245. doi: 10.1038/s41586-020-2263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteghamati A, Larijani B, Aghajani MH, Ghaemi F, Kermanchi J, Shahrami A, et al. Diabetes in Iran: prospective analysis from first nationwide diabetes report of National Program for prevention and control of diabetes (NPPCD-2016) Sci Rep. 2017;7(1):1–10. doi: 10.1038/s41598-017-13379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirzaei M, Rahmaninan M, Mirzaei M, Nadjarzadeh A, Dehghani Tafti AA. Epidemiology of diabetes mellitus, pre-diabetes, undiagnosed and uncontrolled diabetes in Central Iran: results from Yazd health study. BMC Public Health. 2020;20(1):166. doi: 10.1186/s12889-020-8267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basit A, Basit KA, Fawwad A, Shaheen F, Fatima N, Petropoulos IN, Alam U, Malik RA. Vitamin D for the treatment of painful diabetic neuropathy. BMJ Open Diabetes Res Care. 2016;4(1):e000148. doi: 10.1136/bmjdrc-2015-000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, Vinik AI, Boulton AJM, on behalf of The Toronto Expert Panel on Diabetic Neuropathy Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. 2011;27(7):629–638. doi: 10.1002/dmrr.1225. [DOI] [PubMed] [Google Scholar]
- 6.Dinoff A, Lynch ST, Sekhri N, Klepacz L. A meta-analysis of the potential antidepressant effects of buprenorphine versus placebo as an adjunctive pharmacotherapy for treatment-resistant depression. J Affect Disord. 2020;271:91–99. doi: 10.1016/j.jad.2020.03.089. [DOI] [PubMed] [Google Scholar]
- 7.Kruse AB, Kowalski CD, Leuthold S, Vach K, Ratka-Krüger P, Woelber JP. What is the impact of the adjunctive use of omega-3 fatty acids in the treatment of periodontitis? A systematic review and meta-analysis. Lipids Health Dis. 2020;19(1):100. doi: 10.1186/s12944-020-01267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesfaye S, Boulton A (2009) Diabetic neuropathy: Oxford diabetes library
- 9.Hoffmann MR, Senior PA, Mager DR. Vitamin D supplementation and health-related quality of life: a systematic review of the literature. J Acad Nutr Diet. 2015;115(3):406–418. doi: 10.1016/j.jand.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Alam U, Fawwad A, Shaheen F, Tahir B, Basit A, Malik RA. Improvement in neuropathy specific quality of life in patients with diabetes after vitamin D supplementation. Journal of diabetes research. 2017;2017:7928083. doi: 10.1155/2017/7928083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sari A, Akdoğan Altun Z, Arifoglu Karaman C, Bilir Kaya B, Durmus B. Does vitamin D affect diabetic neuropathic pain and balance? J Pain Res. 2020;13:171–179. doi: 10.2147/JPR.S203176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okifuji A, Ackerlind S. Behavioral medicine approaches to pain. The Medical clinics of North America. 2007;91(1):45–55. doi: 10.1016/j.mcna.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Day MA (2017) Mindfulness-based cognitive therapy for chronic pain: a clinical manual and guide: John Wiley & Sons
- 14.Zeidan F, Vago DR. Mindfulness meditation-based pain relief: a mechanistic account. Ann N Y Acad Sci. 2016;1373(1):114–127. doi: 10.1111/nyas.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells RE, Burch R, Paulsen RH, Wayne PM, Houle TT, Loder E. Meditation for migraines: a pilot randomized controlled trial. Headache. 2014;54(9):1484–1495. doi: 10.1111/head.12420. [DOI] [PubMed] [Google Scholar]
- 16.Fox SD, Flynn E, Allen RH. Mindfulness meditation for women with chronic pelvic pain: a pilot study. The Journal of reproductive medicine. 2011;56(3–4):158–162. [PubMed] [Google Scholar]
- 17.Garland EL, Gaylord SA, Palsson O, Faurot K, Douglas Mann J, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med. 2012;35(6):591–602. doi: 10.1007/s10865-011-9391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teixeira E. The effect of mindfulness meditation on painful diabetic peripheral neuropathy in adults older than 50 years. Holist Nurs Pract. 2010;24(5):277–283. doi: 10.1097/HNP.0b013e3181f1add2. [DOI] [PubMed] [Google Scholar]
- 19.Nathan HJ, Poulin P, Wozny D, Taljaard M, Smyth C, Gilron I, Sorisky A, Lochnan H, Shergill Y. Randomized trial of the effect of mindfulness-based stress reduction on pain-related disability, pain intensity, health-related quality of life, and A1C in patients with painful diabetic peripheral neuropathy. Clinical diabetes : a publication of the American Diabetes Association. 2017;35(5):294–304. doi: 10.2337/cd17-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamilian M, Foroozanfard F, Rahmani E, Talebi M, Bahmani F, Asemi Z (2017) Effect of Two Different Doses of Vitamin D Supplementation on Metabolic Profiles of Insulin-Resistant Patients with Polycystic Ovary Syndrome. Nutrients. 9(12) [DOI] [PMC free article] [PubMed]
- 21.Liu H, Gao X, Hou Y (2019) Effects of mindfulness-based stress reduction combined with music therapy on pain, anxiety, and sleep quality in patients with osteosarcoma. Revista brasileira de psiquiatria (Sao Paulo, Brazil : 1999). 41(6):540–5 [DOI] [PMC free article] [PubMed]
- 22.Turk DC, Winter F (2006) The pain survival guide: how to reclaim your life: American Psychological Association
- 23.Marbouti L, Jafari H, Noorizadeh-Dehkordi S, Behtash H. Pain-related disability measurement: the cultural adaptation and validation of “pain disability index (PDI)” and “pain disability questionnaire (PDQ)” among Iranian low back pain patients. Med J Islam Repub Iran. 2011;25(1):27–34. [Google Scholar]
- 24.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the neuropathic pain scale. Neurology. 1997;48(2):332–338. doi: 10.1212/WNL.48.2.332. [DOI] [PubMed] [Google Scholar]
- 25.Vileikyte L, Peyrot M, Bundy C, Rubin RR, Leventhal H, Mora P, Shaw JE, Baker P, Boulton AJM. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care. 2003;26(9):2549–2555. doi: 10.2337/diacare.26.9.2549. [DOI] [PubMed] [Google Scholar]
- 26.Webb AR. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006;92(1):17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Newby PK, Muller D, Hallfrisch J, Andres R, Tucker KL. Food patterns measured by factor analysis and anthropometric changes in adults. Am J Clin Nutr. 2004;80(2):504–513. doi: 10.1093/ajcn/80.2.504. [DOI] [PubMed] [Google Scholar]
- 28.Fogelholm M, Malmberg J, Suni J, Santtila M, Kyröläinen H, Mäntysaari M, et al. International physical activity questionnaire: validity against fitness. Med Sci Sports Exerc. 2006;38(4):753–760. doi: 10.1249/01.mss.0000194075.16960.20. [DOI] [PubMed] [Google Scholar]
- 29.Izgu N, Gok Metin Z, Karadas C, Ozdemir L, Metinarikan N, Corapcıoglu D. Progressive muscle relaxation and mindfulness meditation on neuropathic pain, fatigue, and quality of life in patients with type 2 diabetes: a randomized clinical trial. Journal of nursing scholarship : an official publication of Sigma Theta Tau International Honor Society of Nursing. 2020;52:476–487. doi: 10.1111/jnu.12580. [DOI] [PubMed] [Google Scholar]
- 30.Bogusch LM, O'Brien WH (2019) The Effects of Mindfulness-Based Interventions on Diabetes-Related Distress, Quality of Life, and Metabolic Control Among Persons with Diabetes: A Meta-Analytic Review. Behavioral medicine (Washington, DC). 45(1):19–29 [DOI] [PubMed]
- 31.Cassidy EL, Atherton RJ, Robertson N, Walsh DA, Gillett R. Mindfulness, functioning and catastrophizing after multidisciplinary pain management for chronic low back pain. Pain. 2012;153(3):644–650. doi: 10.1016/j.pain.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Bogosian A, Chadwick P, Windgassen S, Norton S, McCrone P, Mosweu I, et al. (2015) Distress improves after mindfulness training for progressive MS: A pilot randomised trial. Multiple sclerosis (Houndmills, Basingstoke, England). 21(9):1184–94 [DOI] [PubMed]
- 33.Zeidan F, Salomons T, Farris SR, Emerson NM, Adler-Neal A, Jung Y, Coghill RC. Neural mechanisms supporting the relationship between dispositional mindfulness and pain. Pain. 2018;159(12):2477–2485. doi: 10.1097/j.pain.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poisbeau P, Aouad M, Gazzo G, Lacaud A, Kemmel V, Landel V, Lelievre V, Feron F. Cholecalciferol (vitamin D-3) reduces rat neuropathic pain by modulating opioid signaling. Mol Neurobiol. 2019;56(10):7208–7221. doi: 10.1007/s12035-019-1582-6. [DOI] [PubMed] [Google Scholar]
- 35.Helde-Frankling M, Björkhem-Bergman L. Vitamin D in Pain Management. Int J Mol Sci. 2017;18(10):21–70. doi: 10.3390/ijms18102170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fazelian S, Amani R, Paknahad Z, Kheiri S, Khajehali L. Effect of vitamin D supplement on mood status and inflammation in vitamin D deficient type 2 diabetic women with anxiety: a randomized clinical trial. Int J Prev Med. 2019;10:17. doi: 10.4103/ijpvm.IJPVM_174_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golzarand M, Hollis BW, Mirmiran P, Wagner CL, Shab-Bidar S. Vitamin D supplementation and body fat mass: a systematic review and meta-analysis. Eur J Clin Nutr. 2018;72(10):1345–1357. doi: 10.1038/s41430-018-0132-z. [DOI] [PubMed] [Google Scholar]
- 38.Lv WS, Zhao WJ, Gong SL, Fang DD, Wang B, Fu ZJ, Yan SL, Wang YG. Serum 25-hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: a systematic review and meta-analysis. J Endocrinol Investig. 2015;38(5):513–518. doi: 10.1007/s40618-014-0210-6. [DOI] [PubMed] [Google Scholar]