Abstract
Objectives
Osteoporosis is characterized by slow deterioration in bone mass and disruption of its structure, leading to an increased risk of bone fractures. Gut microbiota plays an important role in the transport and absorption of nutrients needed for bone health. Akkermansia muciniphila is one of the gut microbiota members that its beneficial role in prevention of metabolic disorder was suggested. The aim of the current pilot study was the assessment of fecal A. muciniphila in patients with osteoporosis and osteopenia.
Methods
A total of 36 subjects including eight with osteoporosis (three men and five women), eight with osteopenia (two men and six women), and 20 normal controls (six men and 14 women) were selected. Microbial genome was extracted from fresh stool samples. The bacterial load was determined by quantitative real-time PCR using 16S rRNA specific primers.
Results
The participants’ mean age in the osteoporosis, osteopenia and control groups were 61.71, 45 and 45.05 years, respectively. The majority of osteoporosis patients were post-menopause women, while in osteopenia group was pre-menopause. There were significant differences in terms of age, T-score, Z-score, and menopause among groups (P value < 0.05). The presence of A. muciniphila was higher in the healthy group compared to osteopenia group; however, these differences were not statistically significant.
Conclusions
In conclusion, however, there was no statistically significant difference between the study groups; it seems that the load of A. muciniphila may be related to bone health. Further in vivo and in vitro studies are needed to investigate the immunological and biochemical pathways.
Keywords: Intestinal microbiota, Bone health, Akkermansia muciniphila
Introduction
Osteoporosis is characterized by reduced bone density and a higher risk of fragility of the bone. Bone mineral density (BMD) measurement is the most common technique for bone strength determination. Osteoporotic fractures are a public health concern and lead to high costs for health care systems [1]. The balance between osteoclasts and osteoblasts is crucial for bone health, on the other hand; inflammation plays an important role in disrupting this balance and directly linked to bone loss [2].
The human gut contains about 1013 different microorganisms, most of which are anaerobic bacteria, and collectively known as microbiota. In brief, the benefits of gut microbiota include immune homeostasis, digestion, absorption of non-digestible nutrients in our diet and intestinal pathogens colonization resistance. So, it makes sense to consider this as an opportunity to prevent, diagnose, and treat various diseases [3]. In addition, many pieces of evidence show that the gut microbiota can influence bone health, which represented the connection between gut microbiome and distant organs e.g. bone [4–6].
The mucosal layer that covers the gastrointestinal tract, besides its role in the host defense against pathogens, is the site of commensal bacteria capable of using the mucus as a nutrition source [7], one of which is Akkermansia muciniphila (A. muciniphila) [8]. A. muciniphila and other mucin-degrading bacteria make up about 1% of the microbiota population [9]. They also play a key role in glucose homeostasis, gut barrier integrity, alleviation of inflammation, and host metabolic status. It has been shown that obesity and other obesity-associated metabolic disorders could be ameliorated by A. muciniphila treatment [10–13].
According to recent studies, gut dysbiosis leads to dysfunction of nutrients, calcium, and vitamin D intestinal absorption and systemic inflammation, so there is a clear relationship between the gut microbes dysbiosis and decreased bone density in the patients with osteoporosis [14].
Considering that mucosal surfaces, microbiota, and mucus secretion are influenced by environmental factors e.g. aging, therefore the quality of mucus may modify the contact between intestinal microbiota and mucosal dendritic cells. Thus, it seems that the relationship between mucin and mucin-degrading bacteria such as A. muciniphila has a potential role in preventing some diseases [7].
Since inflammation plays a crucial role in bone health and A. muciniphila has a negative correlation with inflammation [15], It is possible that there be a correlation between the abundance of A. muciniphila and bone disease. Therefore, in the present pilot study, we assessed the fecal A. muciniphila load in patients with osteoporosis and osteopenia based on real-time PCR using 16S rRNA specific primer.
Material and methods
Subject recruitment
This was a pilot cross-sectional study performed at Pasteur Institute of Iran. Participants in this study were enrolled from Amir al-Momenin Hospital, Tehran, Iran. Participants met the following inclusion criteria: body mass index (BMI) range between 18.5 and 25 kg/m2 and willingness to participate in the study. Exclusion criteria were antibiotic use and history of acute or chronic diarrhea, for the past three months, having autoimmune diseases, chronic inflammatory bowel diseases, cardiovascular disease, diabetes, hyperlipidemia, cancer, kidney and liver disorders, and specific physiological conditions like pregnancy and lactation.
Finally, a total of 36 subjects including eight with osteoporosis (OP), eight with osteopenia (ON), and twenty normal controls (NC) were selected for further analysis. Fasting blood samples were taken; and fasting blood sugar (FBS), triglyceride (TG), total cholesterol (TC), High-density lipoprotein (HDL), low-density lipoprotein (LDL), very-low-density lipoprotein (VLDL), and high-sensitivity C-reactive protein (hsCRP) concentrations were measured by Roche kits using an auto-analyzer instrument (Hitachi, Cobas C 311, Roche Diagnostics GmbH) and vitamin D level in serum was assessed with the use of the VIDAS® 25-OH Vitamin D Total (bioMerieux, France). Dual X-ray absorptiometry (DXA) was performed for all participants to detect the bone mineral density (BMD) of total hip and lumbar vertebrae of subjects. A BMD T score less than −2·5 defined as osteoporosis and between −1 and − 2·5 defined as osteopenia [16]. The study was approved by the Biomedical Research Ethics Committee of Pasteur Institute of Iran. Each participant provided written informed consent.
Fecal sample collection and DNA extraction
Fresh stool samples were collected in sterile boxes, then frozen and stored immediately at −80 °C for further use. The microbial genome was extracted using QIAamp DNA Stool Mini Kit (Qiagen Germany) according to the manufacturer’s instructions. Sample DNA purity and concentration were tested using a Nanodrop (NanoDrop™ 1000 Spectrophotometer, Thermo Scientific Co. USA).
Real time PCR and bacterial load
The bacterial load in the fecal samples was determined by absolute quantitative real-time PCR using 16S rRNA gene specific primers, A. muciniphila: F: 5’-CAGCACGTGAAGGTGGGGAC-3′, R: 5′- CCTTGCGGTTGGCTTCAGAT-3′, and E. coli: F: 5’-CATTGACGTTACCCGCAGAAGAAGC-3′, R: 5’-CTCTACGAGACTCAAGCTTGC-3′ [15]. The primers specificity was checked using the online primer BLAST [17]. All reactions were run three times and positive controls were run for each reaction, the final volume of each reaction was 20 μl and the reaction mixture containing 1 μl of DNA, 10 μl of Super SYBR Premix Ex Taq II (Takara), and 1 μl of each primer and 7 μl distilled water. The real-time program: Initial DNA denaturation at 95 °C for 1 min, 40 cycles of denaturation at 95 °C for 5 s; primer annealing at 55 °C for 30 s; extension at 72 °C for 30 s. Melting curve analysis for confirming the specificity of the amplification products by slowly cooling the PCRs from 95 °C to 60 °C. Finally, the bacterial load of A. muciniphila was calculated using standard curves at concentrations of 2, 20, and 200 μg/ml from E. coli standard strain (ATCC 25922). The data were expressed as Log bacteria/g of feces according to previous studies [18].
Statistical analysis
Statistical analysis was performed by GraphPad Prism 8.3.0 (GraphPad Software Inc., CA, United States) (SPSS Inc. Chicago, IL, USA). A P value < 0.05 was considered as the level of significance. Data were expressed as mean ± standard error of mean (SEM). The normality distribution for different variables was tested by the D’Agostino & Pearson test (K2). Chi-square test was used to determine associations between categorical variables. Comparison of quantitative variables among three groups was done using one-way ANOVA or Kruskal-Wallis test for variables with normal and non-normal distribution, respectively. Following one-way ANOVA, Tukey’s post hoc test was run for pairwise multiple comparisons.
Results
Demographic characteristics in osteopenia and osteoporosis patients
Patient characteristics and clinical data are presented in Table 1. The participants’ mean age in the osteoporosis, osteopenia and control groups were 61.71, 45 and 45.05 years, respectively. The majority of osteoporosis and osteopenia patients were women; (6 vs. 2) and (7 vs. 1) respectively. All women with osteoporosis were post-menopause while in osteopenia group was pre-menopause. There were significant differences in terms of age, T-score, Z-score, and menopause among groups (P value < 0.05).
Table 1.
Compare with NC group: Results are presented as mean ± SEM
| Healthy control | Osteopenia | Osteoporosis | P value | ||
|---|---|---|---|---|---|
| Age | 45.05 ± 7.4 | 45 ± 15.5 | 61.71 ± 13.6 | 0.024 | |
| Sex (n) | Men | 6 | 1 | 2 | 0.68 |
| Women | 14 | 7 | 6 | 0.55 | |
| BMI | 27.41 ± 4.1 | 25.27 ± 3.20 | 24.63 ± 3.9 | 0.35 | |
| T-score | −0.072 ± 0.7 | −1.62 ± 0.24 | −3.021 ± 0.6 | 0.00 | |
| Z-score | 0.049 ± 0.7 | −1.39 ± 0.23 | −2.38 ± 0.9 | 0.00 | |
Blood sample analysis of subjects with osteoporosis, osteopenia, and healthy individuals was shown in Table 2. The osteopenia group had the lowest level of FBS, TG, VLDL, and vitamin D and the highest level of cholesterol and LDL among other groups, although it was not statistically significant. The osteoporosis patients had the highest serum level of vitamin D, according to its supplement consumption. Although the serum level of HDL was low in healthy individuals, serum biochemical parameters of the patient’s groups did not show any significant differences with the healthy controls.
Table 2.
Serum biochemical parameters of subjects with osteopenia, osteoporosis, and healthy controls (Results are presented as Mean ± SEM
| Healthy control (N = 20) |
Osteopenia (N = 8) |
Osteoporosis (N = 8) |
P value | |
|---|---|---|---|---|
| FBS (mg/dl) | 86.5 ± 23.6 | 80.2 ± 9.0 | 84.00 ± 9.28 | 0.64 |
| TG (mg/dl) | 110 ± 41.6 | 89.1 ± 33.7 | 102 ± 31.7 | 0.46 |
| Cholesterol (mg/dl) | 172 ± 38.3 | 196 ± 15.4 | 180 ± 29.6 | 0.21 |
| HDL(mg/dl) | 47.09 ± 8.86 | 50.50 ± 7.0 | 65.50 ± 39.0 | 0.19 |
| LDL (mg/dl) | 93.04 ± 36.0 | 125 ± 15.7 | 115 ± 22.7 | 0.21 |
| VLDL (mg/dl) | 22.90 ± 90.9 | 19.00 ± 8.0 | 22.62 ± 9.1 | 0.58 |
| Vitamin D (ng/mL) | 31.01 ± 14.5 | 25.90 ± 11.4 | 45.91 ± 21.5 | 0.06 |
Bacterial load of A. muciniphila in osteopenia and osteoporosis patients
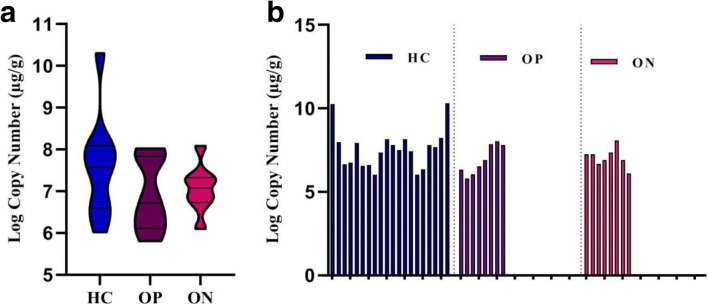
The results showed the load of A. muciniphila was higher in the healthy group (7.176 ± 0.1742 μg/g) and the lowest in the osteopenia group (6.891 ± 0.3028 μg/g) but this difference was not significant (P value = 0.40, 0.94) (Fig. 1).
Fig. 1.
a) The load of A. muciniphila among osteopenia, osteoporosis, and control groups (Mean ± SEM), b) the load of A. muciniphila per participant (μg/g). The values calculated based on Log10 CFU/g stool
Discussion
Osteoporosis is characterized by low deterioration in the bone mass and disruption of its structure and metabolism, leading to an increased risk of bone fractures. It is the most common bone disease and an important economic and public health issue [19]. Our results showed that abundance of A. muciniphila was higher in healthy subjects compared to osteopenia and osteoporosis groups; supporting the view that bone health may influenced by intestinal microbiota [20]. In the first study conducted on the diversity analysis of gut microbiota composition in the osteopenia and osteoporosis groups, it was shown that abundance of Verrucomicrobia, which is the phylum of A. muciniphila, was higher in the osteopenia and osteoporosis groups than in the control group, while the genus and species of A. muciniphila were not reported [21]. On the other hand, Das et al. (2019) showed a decrease in A. muciniphila abundance in the osteoporosis group, whereas it was higher in the osteopenia than the healthy groups [22]. Given the different sample size and inclusion criteria of these studies and regarding that the phylum level could not represent the bacteria at the genus and species level, the inconsistency observed in the report of these studies could be partly explained.
The gut microbiota plays an important role in the transport and absorption of nutrients, which are required for bone growth, regeneration and health [23]. Vitamin D stimulates calcium uptake in the intestine, while 1, 25-dihydroxyvitamin D3 can regulate calcium homeostasis and eventually release calcium into the cells. Intestinal resistance to 1, 25 (OH) 2D3 increases and calcium absorption decreases in aging, which is directly correlated with dysbiosis. Consequently, intestinal dysbiosis can affect calcium and vitamin D uptake and cause osteoporosis [24]. Osteoclasts perform bone breakdown and regulated by several pathways, including vitamin D, estrogen and inflammation. Pro-inflammatory cytokines, especially TNF-α, IL6 and IL1 induced by intestinal dysbiosis, which can play an important role in activating osteoclasts and causing osteoporosis [25]. The serum biochemical analysis showed that patients with osteoporosis had higher vitamin D level among other groups. This discrepancy was due to the fact that all osteoporosis patients had previously been diagnosed and they were being treated with vitamin D. However, all osteopenia patients were diagnosed during the study and none of them were aware of their bone health status. Also, according to the results, the mean age of patients with osteoporosis and osteopenia was significantly different. Our findings were in consistent with Salehi et al. suggesting incidence of low bone density in young people and it its timely detection, management and prevention should be considered [26]. Various studies have shown a direct link between menopause and higher risk of osteoporosis around the world; however, the exact mechanisms underlying yet to be fully elucidated [27–29]. The present data also revealed that all women with osteoporosis were post-menopause while osteopenia group was pre-menopause. Although this result was consistent with previous studies, due to the different age range between the two groups, no definitive conclusion can be made.
Studies on mouse models showed that the association between intestinal dysbiosis and bone mineral density. The mechanism of this association is based on the induction of inflammation in the intestine, thus suggested that the immune system and inflammation mediate the association between intestinal microbiota and bone metabolism [30].
New advances in how the intestinal microbiota help and improve the host health physiologically have attracted much attention with the probable effect of probiotics consumption. When probiotics, as “living microorganisms”, used in proper amounts can provide specific health benefits to the host [31]. Probiotics modify the microbial pattern, the function of the gut barrier, and the immune system, resulting in systemic benefits of bone health (growth, density, and structure), improved intestinal permeability, and reduced inflammation [32]. In this regard, Pazzini et al. study showed that the use of Bacillus subtilis in mice caused a decrease in the number of osteoclasts and an increase in osteoblasts, compared to the control group [33]. The use of probiotics also prevents osteoporosis caused by steroids depletion by improving the function of the intestinal barrier and inhibiting inflammation [34]. A recent study showed that treatment with A. muciniphila accelerated fracture healing and improving bone quality, in the way treatment with A. muciniphila caused a reduction in local inflammation, an increase in H-vessels (bone density biomarker) and bone marrow-forming cells (35). Therefore, having more information about the amount of beneficial bacteria such as A. muciniphila in the intestines of patients in comparison with healthy controls can have useful insight for future planning in management of osteoporosis and osteopenia.
Conclusion
In conclusion, the results of the present study showed that the abundance of A. muciniphila is probably related to bone density and bone health. Therefore, A. muciniphila could be considered as an agent for the prevention or treatment of osteoporosis and bone diseases. This was a pilot study with low statistical power. The individuals with osteopenia were diagnosed during the study while most of the osteoporosis patients had already been diagnosed and were undergoing treatment. The nutritional state was another limitation that was not investigated during the study; it is suggested that this be considered in future studies. The study population was not matched in terms of gender, age, and BMI. A bigger-scale study with a greater statistical power is recommended. So far, not too many human studies have been performed on the association of microbiota, especially A. muciniphila, with osteoporosis and osteopenia and there was also a narrow sample size for most other studies. Due to the role of various environmental factors such as ethnicity, geography and lifestyle in gut microbiota composition as well as the occurrence of diseases, the conduct of this pilot study in Iran is of great importance.
Further studies are needed to determine whether there is a link between changes in the frequency of A. muciniphila bacterium and osteoporosis, and investigate the exact mechanisms of intestinal beneficial bacteria in gut-bone interaction.
Acknowledgments
We thank all the lab members of Microbiology Research Center (MRC) & Department of Biochemistry, Pasteur Institute of Iran, and Amir al-Momenin Hospital for their assistance in this project.
Author contributions
SK performed the experiments, sampling, DNA extraction, and real-time PCR, analyzed the data, and wrote the paper, prepared figures and Tables. MA, ZHT, and AK; sampling, sample preparation and DNA extraction. SS; serum preparation and biochemical test. SDS, SK, and MZ; conceived and designed the experiments, reviewed drafts of the paper. HE, FA and ZHT; reviewed and edited the drafts of paper.
Funding
This project was funded by the Research Committee of Pasteur Institute of Iran (No. 1058/1061). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
No relevant conflict of interest has been declared by the authors.
Ethics approval
The following information was supplied relating to ethical approvals (i.e., approving body reference numbers (No. 1284) Pasteur Institute of Iran, Biomedical research ethics committee.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shahrbanoo Keshavarz Azizi Raftar, Email: Sh64_keshavarz@yahoo.com.
Zahra Hoseini Tavassol, Email: zhtavassol@gmail.com.
Meysam Amiri, Email: Meysamamiri21@yahoo.com.
Hanieh-Sadat Ejtahed, Email: haniejtahed@yahoo.com.
Mehrangiz Zangeneh, Email: zangeneh@iauTmu.ac.ir.
Sedigheh Sadeghi, Email: sedisadeghi@hotmail.com.
Fatemeh Ashrafian, Email: fatemeh.ashrafian24@gmail.com.
Arian Kariman, Email: ariankariman@yahoo.com.
Shohreh Khatami, Email: sh-khatami@pasteur.ac.ir.
Seyed Davar Siadat, Email: d.siadat@gmail.com.
References
- 1.Ohlsson C, Sjögren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2015;26(2):69–74. doi: 10.1016/j.tem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Kim B-J, Koh J-M. Coupling factors involved in preserving bone balance. Cell Mol Life Sci. 2019;76(7):1243–1253. doi: 10.1007/s00018-018-2981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behera J, Ison J, Tyagi SC, Tyagi N. The role of gut microbiota in bone homeostasis. Bone. 2020;135:115317. 10.1016/j.bone.2020.115317. [DOI] [PMC free article] [PubMed]
- 5.Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020;113:2019–40. 10.1007/s10482-020-01474-7. [DOI] [PubMed]
- 6.Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015;13(2):125–130. doi: 10.1007/s11914-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73(23):7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. Nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(5):1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 9.Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract Res Clin Gastroenterol. 2017;31(6):637–642. doi: 10.1016/j.bpg.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Tu P, Bian X, Chi L, Gao B, Ru H, Knobloch TJ, Weghorst CM, Lu K. Characterization of the functional changes in mouse gut microbiome associated with increased Akkermansia muciniphila population modulated by dietary black raspberries. ACS omega. 2018;3(9):10927–10937. doi: 10.1021/acsomega.8b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T, Li Q, Cheng L, Buch H, Zhang F. Akkermansia muciniphila is a promising probiotic. Microb Biotechnol. 2019;12(6):1109–1125. doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashrafian F, Shahryari A, Behrouzi A, Moradi HR, Lari A, Hadifar S, et al. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front Microbiol. 2019;10:2155. doi: 10.3389/fmicb.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David Yatsonsky I, Pan K, Shendge VB, Liu J, Ebraheim NA. Linkage of microbiota and osteoporosis: a mini literature review. World J Orthop. 2019;10(3):123. doi: 10.5312/wjo.v10.i3.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364–376. doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 17.Ejtahed H-S, Hoseini-Tavassol Z, Khatami S, Zangeneh M, Behrouzi A, Badi SA, et al. Main gut bacterial composition differs between patients with type 1 and type 2 diabetes and non-diabetic adults. J Diabetes Metab Disord. 2020;19(1):265–71. [DOI] [PMC free article] [PubMed]
- 18.De Martinis M, Sirufo MM, Ginaldi L. Osteoporosis: Current and emerging therapies targeted to immunological checkpoints. Curr Med Chem. 2020;27(37):6356–72. [DOI] [PMC free article] [PubMed]
- 19.Locantore P, Del Gatto V, Gelli S, Paragliola RM, Pontecorvi A. The interplay between immune system and microbiota in osteoporosis. Mediat Inflamm. 2020;2020:1–8. doi: 10.1155/2020/3686749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Wang Y, Gao W, Wang B, Zhao H, Zeng Y, Ji Y, Hao D. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ. 2017;5:e3450. doi: 10.7717/peerj.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das M, Cronin O, Keohane DM, Cormac EM, Nugent H, Nugent M, Molloy C, O’Toole PW, Shanahan F, Molloy MG, Jeffery IB. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology. 2019;58(12):2295–2304. doi: 10.1093/rheumatology/kez302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzoli, R. Nutritional influence on bone: role of gut microbiota. Aging Clin Exp Res. 2019;31:743–51. 10.1007/s40520-019-01131-8. [DOI] [PubMed]
- 23.McCabe LR, Parameswaran N. Understanding the gut-bone signaling Axis: mechanisms and therapeutic implications. Berlin: Springer; 2017. [Google Scholar]
- 24.Quach D, Britton RA. Understanding the Gut-Bone Signaling Axis. Berlin: Springer; 2017. Gut microbiota and bone health; pp. 47–58. [DOI] [PubMed] [Google Scholar]
- 25.Salehi I, Khazaeli S, Najafizadeh SR, Ashraf H, Malekpour M. High prevalence of low bone density in young Iranian healthy individuals. Clin Rheumatol. 2009;28(2):173–177. doi: 10.1007/s10067-008-1008-8. [DOI] [PubMed] [Google Scholar]
- 26.Khan A, Fortier M, Reid R, Abramson BL, Blake J, Desindes S, et al. Osteoporosis in menopause. J Obstet Gynaecol Can. 2014;36(9):839–840. doi: 10.1016/S1701-2163(15)30489-8. [DOI] [PubMed] [Google Scholar]
- 27.Hsu T-L, Tantoh DM, Chou Y-H, Hsu S-Y, Ho C-C, Lung C-C, et al. Association between osteoporosis and menopause in relation to SOX6 rs297325 variant in Taiwanese women. Menopause. 2020;27(8):887. doi: 10.1097/GME.0000000000001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Society NAM. Management of osteoporosis in postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause. 2006;13(3):340. doi: 10.1097/01.gme.0000222475.93345.b3. [DOI] [PubMed] [Google Scholar]
- 29.Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCabe L, Britton RA, Parameswaran N. Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep. 2015;13(6):363–371. doi: 10.1007/s11914-015-0292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibáñez L, Rouleau M, Wakkach A, Blin-Wakkach C. Gut microbiome and bone. Joint Bone Spine. 2019;86(1):43–47. doi: 10.1016/j.jbspin.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Pazzini CA, Pereira LJ, da Silva TA, Montalvany-Antonucci CC, Macari S, Marques LS, de Paiva SM. Probiotic consumption decreases the number of osteoclasts during orthodontic movement in mice. Arch Oral Biol. 2017;79:30–34. doi: 10.1016/j.archoralbio.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Li J-Y, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J-H, Yue T, Luo Z-W, Cao J, Yan Z-Q, Jin L, et al. Akkermansia Muciniphila promotes bone fracture healing by enhancing Preosteoclast-associated type H vessel formation. 2019. 10.2139/ssrn.3405551.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.