Abstract
Purpose
Recent trials have demonstrated the possible improvements in lipid profile & anthropometric indices after probiotics supplementation. We aimed to reanalyze the related literature to explore the efficacy of probiotics in Diabetic Nephropathy (DN) patients.
Methods
PubMed, Embase, Web of science, google scholar, Scopus, and Cochrane Library databases were systematically searched to find the related data on diabetic nephropathy population. All Randomized controlled trials (RCTs) that investigated the effect of probiotics on serum lipid markers (High-Density Lipoprotein [HDL], Triglyceride, Total Cholesterol, TC-to-HDL ratio, Low-Density Lipoprotein, Very Low-Density Lipoprotein) and anthropometric indices (Body Weight, Body Mass Index, waist-to-hip ratio) were included (PROSPERO No.CRD42020186189). Meta-analysis was performed using the random-effect model.
Results
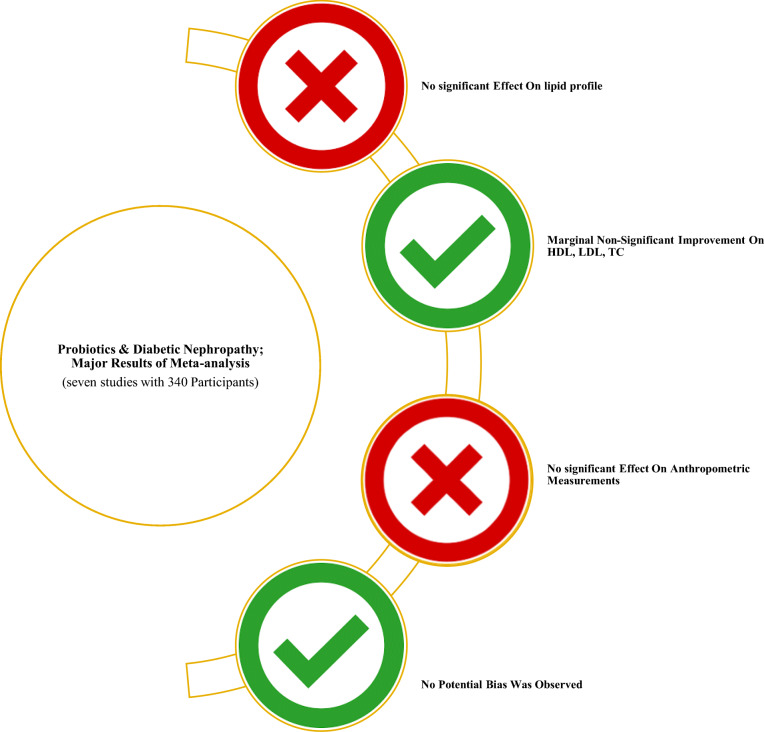
Of 156 studies, seven were eligible for inclusion. Lipid biomarkers had a marginal reduction (except for HDL; WMD = 2.59 mg/dl; 95% CI = -0.28, 5.47; P = 0.077); whereas anthropometric indices increased in a non-significant manner.
Conclusion
There is limited evidence to support the efficacy of probiotics for the modulation of lipid profile and anthropometric indices in DN patients.
Graphical abstract
Probiotics did not beneficial effect on lipid profile & anthropometric markers in Diabetic Nephropathy; anyway, more trials should be conducted.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-021-00765-8.
Keywords: Probiotics, Lipoproteins, Body weights and measures, Diabetic nephropathies, Meta-analysis, Systematic review
Introduction
Diabetic Nephropathy (DN; also called Diabetic kidney disease) occurs in approximately 30% of diabetic people as the leading cause of End-Stage Renal Disease (ESRD) [1]. Researchers have shown that Individuals with DN represent a particularly high-risk group for adverse Cardiovascular Disease (CVD) [2]. Dyslipidemia, defined by the presence of one or more abnormal serum lipid concentrations (Total Cholesterol [3], Triglyceride [TG], Low-Density Lipoprotein [LDL], and High-Density Lipoprotein [HDL]) and obesity (especially abdominal obesity), are of the main risk factors of DN and CVD [4–6]. Therefore, it is urgent to develop therapeutic strategies for DN and its comorbidities such as dyslipidemia and obesity; probiotic supplementation has high potential for achieving this goal [7–9].
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. Lactobacillus (L) spp., Bifidobacterium (B) spp., Streptococcus spp., Enterococcus spp., and Saccharomyces boulardii are the most common administered strains for supplementation [10, 11]. Human clinical studies using various probiotics have yielded inconsistent results. A randomized trial reported that probiotics supplementation improves glycemic control and anthropometric indices in adults with Type 2 Diabetes (T2D) [12]. Furthermore, probiotics supplement consisted of 7 viable strains Lactobacillus, Bifidobacterium and Streptococcus significantly reduced HDL without any significant effect on TC, TG and anthropometric measurements [13]. Results of two recent meta-analyses showed that supplementation with probiotics improves the metabolic variables such as TC, TG and HDL in T2D patients [14, 15]. In contrast, Two meta-analyses did not find any significant effect of probiotics on lipid profile [16, 17].
The effect of probiotic strains on Body Weight (BW) has also been investigated. Certain Lactobacillus species, such as L. fermentum, L. ingluviei and L. acidophilus induces weight gain, while supplementation with L. gasseri and L. plantarum resulted in weight loss in animal and human studies [18]. In practice, the limited number of studies in this regard have shown controversial results.
Recently, a systematic review by Vlachou et al. [19] indicated that probiotic supplementation has beneficial impact on systemic inflammation, oxidative stress and renal biomarkers in subjects with DN, although the authors did not discuss the possible effects on lipid profile and anthropometric measurements. Although several studies have reported the beneficial effect of probiotics in patients with T2D, a lot of their results were marginally significant and inconclusive. Therefore, the present study was planned to collect and reanalyze the available information and reach a conclusion on the effectiveness of probiotics on lipid profile and anthropometric indices in DN patients.
Materials & methods
Protocol registration
The review protocol has been published in the International Prospective Register of Systematic Reviews (www.crd.york.ac.uk/PROSPERO) (Registration ID. CRD42020186189; submission date: July 05, 2020; last update date: November 30, 2020) and developed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [20]. PRISMA checklist of the meta-analysis was presented in S1 Appendix.
Search strategy
The electronic search for the current systematic review and meta-analysis was performed on material published through May 10, 2020. The included databases were Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials (CENTRAL), Ovid Cochrane Database of Systematic Reviews, Google Scholar, PubMed, ScienceDirect, ISI Web of Science, and Scopus. One author searched the above electronic database. The gray literature including conference abstracts, presentations, proceedings; regulatory data; unpublished trial data; government publications; reports (such as white papers, working papers, internal documentation); dissertations/theses; patents; and policies & procedures were not entered.
Two authors independently searched the aforementioned databases to identify RCTs, using the following MeSH and text keywords: ((“Diabetic Nephropathy” OR “Diabetic kidney disease” OR “DKD”) AND (“High-Density Lipoprotein” OR “HDL” OR “Triglyceride” OR “TG” OR “Total Cholesterol” OR “TC” OR “TC-to-HDL ratio” OR “Low-Density Lipoprotein” OR “LDL” OR “Very Low-Density Lipoprotein” OR “VLDL” OR “Body Weight” OR “Body Mass Index” OR “BMI” OR “waist-to-hip ratio” OR “WHR”) AND (“Synbiotics” OR “Probiotics” OR “Prebiotics” OR “Probiotic”) AND (“Intervention Studies” OR “intervention” OR “controlled trial” OR “randomized” OR “randomised” OR “random” OR “randomly” OR “placebo” OR “assignment” OR “randomized controlled trial” OR “trial” OR “Clinical Trial” OR “RCT”)). Also, all references of previous relevant meta-analyses, systematic reviews, and selected RCTs were manually reviewed to find any additional trials that had not been confined via online database searches.
Study selection criteria
We included trials with English language and the following characteristics: (1) Type of study: RCT with either parallel or cross-over design, and at least two arms; (2) Participants: limited to DN patients aged ≥18 years and disease duration between 2 and 20 years; (3) Intervention: probiotic-contained food products or supplements, compared with placebo for more than two weeks; and (4) Outcomes: assessed blood parameters of lipid profiles (HDL, TG, TC, TC-to-HDL ratio, LDL, VLDL), and anthropometric indices (Body Weight [BW], BMI, Waist-to-Hip Ratio [WHR]).
We excluded trials that reported: (1) studies without clear inclusion and exclusion criteria; (2) outcomes that had not been clearly stated; (3) uncontrolled studies; and (4) preclinical studies with animal models.
The relevance of articles and abstracts for inclusion was judged by two independent authors. Then, one reviewer independently evaluated the full text of potentially relevant non-duplicated articles. Any disagreement between investigators was resolved by the third reviewer.
Data extraction
Two reviewers independently extracted data. Any discrepancies were resolved by a third author. The following details were extracted and tabulated: study first author publication year & country, study design and duration, samples’ characteristics such as gender, disease duration, mean BMI and age; composition, and dose of probiotics/placebo and outcome indicators. Corresponding authors of included studies, in which published data had some ambiguous results, were contacted to respond to the related questions.
Risk of bias (quality) assessment
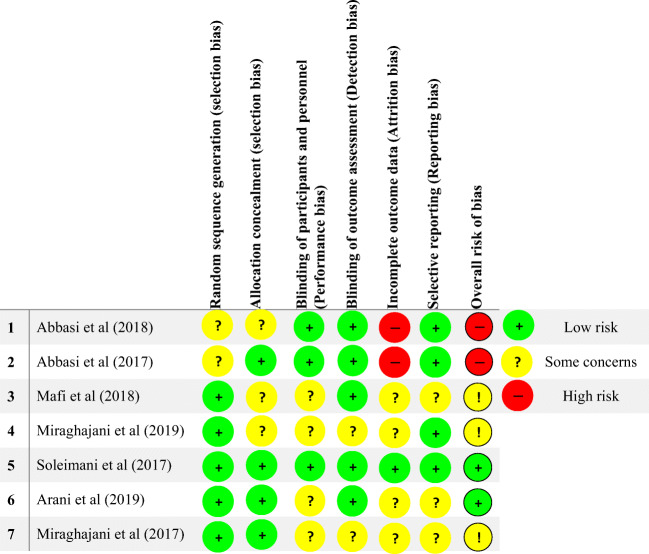
The included studies were evaluated for bias by using the Cochrane Risk-Of-Bias (RoB) tool (version 5.0) [21]. Each included study was evaluated for the following biases: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participant and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. The reviewers’ judgment was classified as “Low risk,” “High risk” or “Unclear risk” of bias.
Statistical analysis
Using STATA software version 14 (Stata Corp LP, College Station, TX, USA), statistical analysis was done. Data from RCTs were analyzed using mean difference with standard deviation (SD). Moreover, the random-effects model was used to compute Weighted Mean Differences (WMDs) with 95% confidence intervals (CIs) for all selected markers. The conversion of median/interquartile range (IQR) (or 95% Confidence Interval [CI]) to the mean ± SD values was performed according to Hozo et al. [22] method. In some states where only the standard error of the mean (SEM) was available, the following formula was used to calculate SD [23]:
Therefore, the mean changes (difference of values before and after the intervention) and SD were used to pool effect sizes. Results were visualized using forest plots. The potential for publication bias was measured through the Egger weighted regression method. A P value of 0.05 was considered to be statistically significant.
Results
Literature search
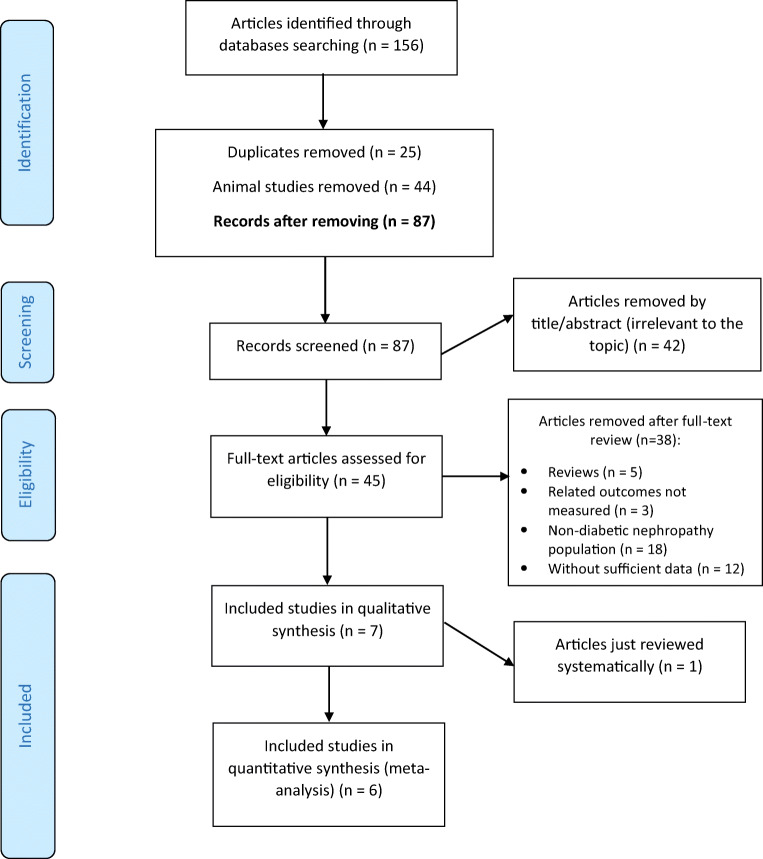
The study inclusion process was illustrated in Fig. 1. In total, 156 records were identified in a combined search of electronic databases; of the 131 unduplicated papers, 124 were excluded because they were animal studies (n = 44), not relevant to the topic (n = 42), review articles (n = 5), irrelevant outcomes (n = 3), non-diabetic nephropathy population (n = 18), or insufficient data (n = 12). Finally, six eligible articles with publication range of 2017–2019 were entered in the data synthesis (300 participants) [24–29] (Table 1). Miraghajani et al. [30] study did not enter into quantitative analysis, because the measured variables in Miraghajani et al. study (2017) were different from the other included trials. Therefore, it was just systematically reviewed.
Fig. 1.
Flow diagram of the included and excluded studies
Table 1.
Characteristic of randomized controlled trials that included for analysis; effects of probiotics on clinical manifestations of Diabetic Nephropathy
| First author (publication year) | Country | Analyzed Sample sizeIn/Co Male/Female | Target population | Disease duration M (SD) |
BMI at base (M) |
Age (M) |
Study design Duration |
Intervention, Dose |
Control, Dose |
Probiotic content and numbers | Investigated markers |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbasi (2018) | Iran |
20/20 19/21 |
Diabetic Nephropathy | 7.8 (3.5) | 26.6 | 55.2 |
R, DB, PC Parallel 8 wks |
Probiotics soy milk 200 ml QD |
soy milk 200 ml QD |
Lactobacillus plantarum A7 2 × 107 CFU/ml Totally 4 × 109 CFU |
TG, TC, LDL-C, HDL-C, non HDL-C, Serum creatinine, Serum phosphorus, Serum genistein, eGFR |
| Abbasi (2017) | Iran |
20/20 19/21 |
Diabetic Nephropathy | 7.8 (3.5) | 26.6 | 55.2 |
R, DB, PC Parallel 8 wks |
Probiotics soy milk 200 ml QD |
soy milk 200 ml QD |
lactobacillus plantarum A7 2 × 107 CFU/ml Totally 4 × 109 CFU |
Body Weight, BMI, WHR, IL-18, Serum sialic acid, Serum creatinine, Serum genistein, eGFR, Urinary albumin/creatinine ratio |
| Mafi (2018) | Iran |
30/30 NR |
Diabetic Nephropathy | 18.1 (5.4) | 25.8 | 59.9 |
R, DB, PC Parallel 12 wks |
Probiotic capsule QD |
Placebo capsule (contained only starch) QD |
Lactobacillus acidophilus strain ZT-L1, Bifidobacterium bifidum strain ZT-B1, Lactobacillus reuteri strain ZT-Lre, and Lactobacillus fermentum strain ZT-L3 (each 2 × 109 CFU/g) Totally 8 × 109 CFU per capsule |
Body Weight, BMI, TAC, MDA, hs-CRP, FPG, Insulin, GSH, HOMA-IR, NO, QUICKI, TC, TG, LDL-C, HDL-C, VLDL-C, Hb A1C, TC/HDL ratio, AGEs, BUN, Serum creatinine, GFR, Urine protein, gene expression (IL-1, TNF-α, TGF-ß, PPAR-γ and LDLR) |
| Miraghajani (2019) | Iran |
20/20 19/21 |
Diabetic Nephropathy | 7.8 (3.5) | 26.6 | 55.2 |
R, DB, PC Parallel 8 wks |
Probiotics soy milk 200 ml QD |
soy milk 200 ml QD |
Lactobacillus plantarum A7 (KC355240, LA7) 2 × 107 CFU/ml Totally 4 × 109 CFU |
Calorie, Protein, Fat, Carbohydrate, Fiber, Calcium, Magnesium, Potassium, NGAL, sTNFR1, Cys-C, PGRN, Weight, WHR, BMI |
| Soleimani (2017) | Iran |
30/30 40/20 |
Diabetic Hemodialysis | 18.1 (5.4) | 26.2 | 56.7 |
R, DB, PC Parallel 12 wks |
Probiotic capsule QD |
Placebo capsule (contained only starch) QD |
Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum (each 2 × 109 CFU/g) Totally 6 × 109 CFU per capsule |
Body Weight, BMI, MET, TAC, MDA, hs-CRP, FPG, Insulin, GSH, HOMA-IR, HOMA-B, NO, QUICKI, TC, TG, LDL-C, HDL-C, VLDL-C, Hb A1C, TC/HDL ratio, BUN, Serum creatinine, GFR, SGA score, Albumin, TIBC, Na, K |
| Arani (2019) | Iran |
30/30 NR |
Diabetic Nephropathy | 18.1 (5.4) | 26.2 | 56.7 |
R, DB, PC Parallel 12 wks |
Probiotic honey 25 g QD |
Control honey 25 g QD |
Bacillus coagulans T4 (IBRC-N10791) (108 CFU/g) Totally 2.5 × 109 CFU |
Body Weight, BMI, TAC, MDA, hs-CRP, FPG, Insulin, GSH, HOMA-IR, NO, QUICKI, TC, TG, LDL-C, HDL-C, VLDL-C, TC/HDL ratio, BUN, Serum creatinine |
| Miraghajani (2017)† | Iran |
20/20 NR |
Diabetic kidney disease | 7.8 (3.5) | 26.6 | 55.2 |
R, DB, PC Parallel 8 wks |
Probiotics soy milk 200 ml QD |
soy milk 200 ml QD |
Lactobacillus plantarum A7 (KC355240, LA7) 2 × 107 CFU/ml Totally 4 × 109 CFU |
Calorie, Protein, Fat, Carbohydrate, Fiber, Cholesterol, MUFA, PUFA, Saturated fatty acid, Selenium, Vitamin E, and C, MDA, TAC, GSH, 8-iso-PGF2a, Oxidized glutathione, Glutathione peroxidase, Glutathione reductase |
†One selected study was just systematically reviewed and not included in analysis due to irrelevant outcome markers
Functional abbreviations: In, intervention group; Co, control group; M, mean; SD, standard deviation; QD, once a day; CFU, colony forming unit; RDBPC, randomized double blind placebo control trial; wks, weeks; NR, not reported
Study outcome abbreviations: hs-CRP, high sensitive C-reactive protein; MDA, malondialdehyde; TAC, total antioxidant capacity; IL interleukin; FPG, fasting plasma glucose; GHQ, general health questionnaire; GSH, total glutathione; HOMA-IR, homeostasis model of assessment-estimated insulin resistance; HOMA-B, homeostasis model of assessment estimated B cell function; NO, nitric oxide; QUICKI, quantitative insulin sensitivity check index; TG, Triglycerides; TC, Total cholesterol; LDL, low density lipoprotein; VLDL, very low density lipoprotein; HDL, high density lipoprotein; TG, triacylglycerol; AGEs, advanced glycation end products; GFR, glomerular filtration rate; BUN, blood urea nitrogen; LDLR, low-density lipoprotein receptor; PPAR-γ, peroxisome proliferator-activated receptor gamma; TNF-α, tumor necrosis factor alpha; TGF-ß, transforming growth factor beta; HbA1c, hemoglobin A1c; NGAL, neutrophil gelatinase-associated lipocalin; sTNFR1, soluble tumor necrosis factor receptor 1; PGRN, Progranulin; Cys-C, cystatin C; METs, metabolic equivalents; SGA, subjective global assessment; TIBC, total iron binding capacity; Na, sodium; K, potassium; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; 8-iso-PGF2a, 8-iso-prostaglandin F2a
Study characteristics
The characteristics of seven studies were summarized in Table 1. A total of 340 participants (170 as intervention group/ 170 as controls) were included. The mean age of participants ranged from 55 to 60 years old with a mean disease duration of 8–18 years for all trials. Mean baseline BMI was under 30 kg/m2, representing an overweight state. Three papers did not report sexuality [26, 27, 30], however, others were conducted on both sexes [24, 25, 28, 29].
All studies were performed in Iran, had parallel design, and were related to nephropathy secondary to T2D. The study duration varied between 8 and 12 weeks.
In four papers, probiotics were administrated with soy milk [24, 25, 28, 30]. One study used probiotic honey [26], and other trials prescribed probiotics capsules [27, 29]. Lactobacillus plantarum A7 [24, 25, 28, 30], L. acidophilus [27, 29], Bifidobacterium bifidum [27, 29], L. reuteri [27], L. fermentum [27], L. casei [29], and Bacillus coagulans T4 [26] were considered for intervention. Besides, the daily dose of probiotics ranged from 2.5 × 109 to 8 × 109 CFU.
Risk of bias (quality) assessment
Risk of bias was low in the following domains: selection bias (random sequence generation and allocation concealment, five and four studies, respectively), performance bias (three studies), detection bias (five studies), attrition bias (one study), and reporting bias (four studies).
Two studies seemed to be at a high risk of attrition bias (through high loss to follow-up and no explanation of how data were addressed), and in overall state, two were rated as having a high risk of bias. However, Soleimani et al. [29] and Arani et al. [26] had a low overall risk.
Unclear risk of bias was recognized for selection bias (random sequence generation and allocation concealment, two and three studies, respectively), performance bias (four studies), detection bias (two studies), attrition bias (four studies), and reporting bias (three studies). Overall, two studies had unclear risk of bias. More details were presented in Fig. 2.
Fig. 2.
Risk of bias summary across the included studies
Results of meta-analysis for serum lipid profiles
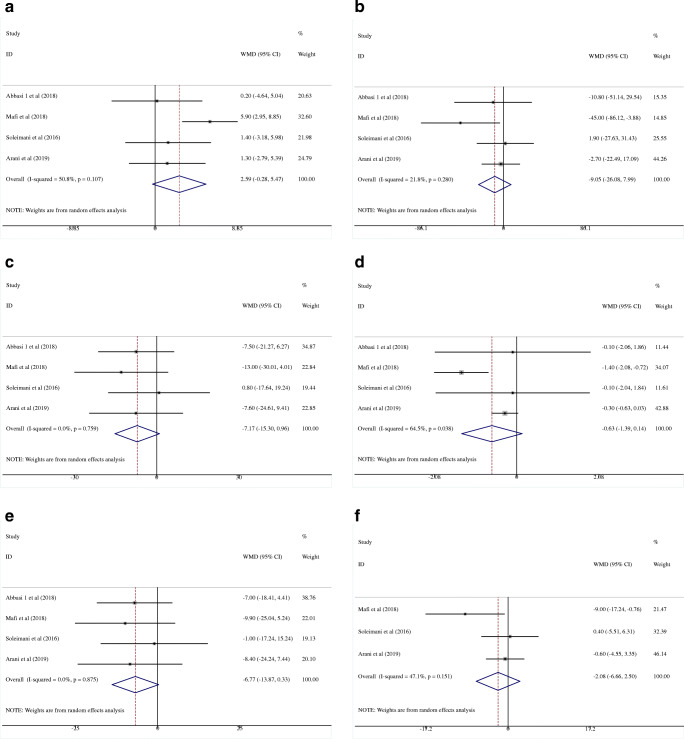
The effects of probiotics on HDL
The efficacy of probiotics on HDL was reported by four studies with 220 participants (intervention, 110; control, 110) [25–27, 29]. No significant improvement was observed in patients who received treatment (WMD = 2.59 mg/dL; 95% CI = −0.28, 5.47, P = 0.077) with no between-study heterogeneity (I2 = 50.8%, P = 0.107) (Fig. 3a).
Fig. 3.
Forest plot of randomized controlled trials investigating the effect of probiotics on serum lipid profile “HDL (a), TG (b), TC (c), TC-to-HDL ratio (d), LDL (e), and VLDL (f)” in Diabetic Nephropathy patients. The area of each square is proportional to the inverse of the variance of the WMD. Horizontal lines represent 95% CIs. HDL, high-density lipoprotein; TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein
The effects of probiotics on TG
In the pooled analysis of four studies with 220 participants (intervention, 110; control, 110) [25–27, 29], effect of probiotics on serum TG level (WMD = −9.05 mg/dL; 95% CI = −26.08, 7.99, P = 0.298) was not statistically significant with no study heterogeneity (I2 = 21.8%; P = 0.280) (Fig. 3b).
The effects of probiotics on TC
There was no significant effect of probiotics on TC (WMD = −7.17 mg/dL; 95% CI = −15.30, 0.96, P = 0.084) after analyzing four studies with 220 participants (intervention, 110; control, 110) [25–27, 29] with no heterogeneity (I2 = 0.0%, P = 0.759) (Fig. 3c).
The effects of probiotics on TC-to-HDL ratio
The pooled estimate did not demonstrate any significant reduction in TC-to-HDL ratio as a result of probiotics intervention in 220 DN patients (WMD = −0.63; 95% CI = −1.39, 0.14, P = 0.107) [25–27, 29](Fig. 3d). Results showed a significant heterogeneity (I2 = 64.5%, P = 0.038).
The effects of probiotics on LDL
Four clinical trials examined the effect of probiotics on LDL (total subjects = 220; intervention, 110; control, 110) [25–27, 29]. In the pooled analysis of selected studies, a marginal non-significant reduction was observed (WMD = −6.77 mg/dL; 95% CI = −13.87, 0.33, P = 0.062) without any study heterogeneity (I2 = 0.0%, P = 0.875) (Fig. 3e).
The effects of probiotics on VLDL
Probiotics supplementation could not decrease serum VLDL levels in overall results of the three clinical trials (subjects = 180; intervention, 90; control, 90) (WMD = −2.08 mg/dL; 95% CI = −6.66, 2.50, P = 0.374; I2 = 47.1%, P = 0.151) [26, 27, 29].
Results of meta-analysis for anthropometric indices
The effects of probiotics on BW
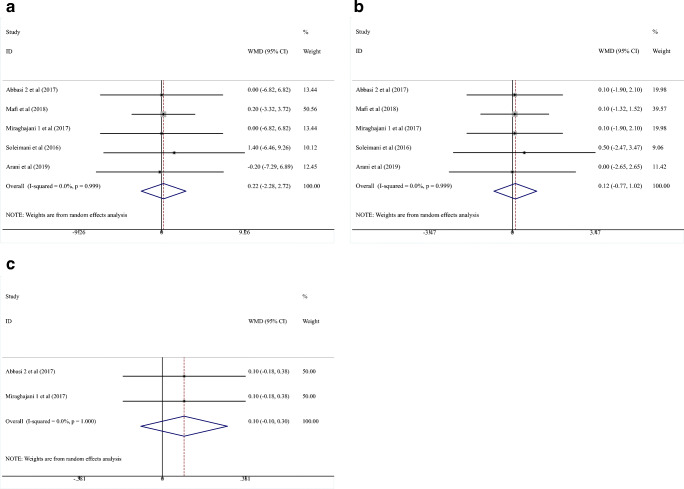
Five RCTs [24, 26–29] investigated the impact of probiotics administration on BW (subjects = 260; intervention, 130; control, 130). Overall, probiotics could not make significant reduction in BW (WMD = 0.22 kg; 95% CI = −2.28, 2.72, P = 0.864) (Fig. 4a). No heterogeneity was recognized (I2 = 0.0%, P = 0.999).
Fig. 4.
Forest plot of randomized controlled trials investigating the effect of probiotics on anthropometric indices “Body Weight (a), BMI (b), and WHR (c)” in Diabetic Nephropathy patients. The area of each square is proportional to the inverse of the variance of the WMD. Horizontal lines represent 95% CIs. BMI, body mass index; WHR, waist-to-hip ratio
The effects of probiotics on BMI
Results of the meta-analysis indicated that probiotics could not significantly improve BMI levels (WMD = 0.12 kg/m2; 95% CI = −0.77, 1.02; P = 0.784). After evaluation of five studies with 260 participants (intervention, 130; control, 130) [24, 26–29], no heterogeneity was seen (I2 = 0.0%, P = 0.999) (Fig. 4b).
The effects of probiotics on WHR
Of the two RCTs investigating the impact of probiotics vs. placebo on WHR in 80 patients (intervention, 40; control, 40), no one reported significant favorable effects [24, 28].
Sensitivity analysis and publication bias
Sensitivity analysis showed that the effect of probiotics on the level of selected markers was not significant except for TC-to-HDL ratio (WMD = −0.29; 95% CI, −0.61- 0.03; conducted by Mafi et al. [27]).
Moreover, there was no evidence of publication bias (assessed by Egger’s test) for studies examining the probiotics on HDL (P = 0.056), TG (P = 0.337), TC (P = 0.684), TC-to-HDL (P = 0.803), LDL (P = 0.795), VLDL (P = 0.464), BW (P = 0.767), and BMI (P = 0.424).
Discussion
The present study systematically reviewed and quantitatively synthesized six RCTs to evaluate the effects of probiotics on lipid profile and anthropometric measurements among patients with DN. The pooled analysis showed that supplementation with probiotics did not significantly affect the selected biomarkers.
To date, there were no studies performing point-to-point comparison in DN patients for clarification and these findings should be interpreted with caution. One meta-analysis has shown the effects of probiotics on other clinical markers. Jia et al. [31] included eight studies with 261 Chronic Kidney Disease (CKD) patients (stage 3 to 5)- with and without dialysis - and found a decrease of P-Cresyl Sulfate (PCS) and an increase of IL-6 in the probiotics group. In addition, no significant changes were obtained for serum Cr, BUN, and C-reactive protein (CRP) (P = 0.55). Thongprayoon et al. [3] also reported that there were no significant differences in serum Cr and GFR after probiotic therapy (Five RCTs with 161 CKD patients had been enrolled).
Based on our knowledge, this study is the first meta-analysis investigated the potential effects of microbial therapy on lipid profile and anthropometric indices in DN patients. Recently, a meta-analysis was also conducted by AbdelQadir et al. [32] in T2D and DN patients. They included three trials to evaluate the effect of probiotics on lipid profile and anthropometric indices (BW, BMI). Similar to our results, the overall effect size for lipid profile (except for LDL) were not significant. Unlike AbdelQadir et al. meta-analysis, we did not find any significant results for BMI, perhaps due to the inclusion of five studies in the analysis.
Resent meta-analysis on T2D patients showed that probiotics significantly decreased the serum level of TC (SMD = −0.57 mg/dL, 95% CI = −0.92 to 0.21), TG (SMD = −0.66 mg/dL, 95% CI = −0.93 to 0.39) and LDL (SMD = −0.40 mg/dL, 95% CI = −0.79 to 0.01) compared with the placebo group. Apart from this, probiotics could significantly improve HDL concentration (SMD = 0.38 mg/dL, 95% CI = 0.03 to 0.73) (11). Kocsis et al. (26) also showed a significant effect of probiotics on reducing TC (−10.06 mg/dL, 95% CI = −15.94 to −4.18; P = 0.001) and TG (−17.18 mg/dL, 95% CI = −26.17 to −8.19; P < 0.001) in T2D subjects. However, similar to our results, they did not observe a significant effect on BMI or LDL levels. Another similarity was found by Yao et al. [17]; they did not detect any significant changes for lipid profile after pooled analysis; 12 studies with 684 T2D patients were included.
Anthropometric measurements did not positively change in the current meta-analysis, however, Koutnikova et al. [33] reported positive results in overweight but not obese subjects. Probiotics - mostly bifidobacteria (Bifidobacterium breve, B. longum), Streptococcus salivarius subsp. thermophilus and lactobacilli (L. acidophilus, L. casei, L. delbrueckii - induced improvements in BW (WMD = −0.94 kg, 95% CI = −1.17 to −0.70; P < 0.05), BMI (WMD = −0.55 kg/m2, 95% CI = −0.86 to −0.23; P < 0.05), waist circumference (WMD = −1.31 cm, 95% CI = −1.79 to −0.83; P < 0.05), body fat mass (WMD = −0.96 kg, 95% CI = −1.21 to −0.71; P < 0.05) and visceral adipose tissue mass (WMD = −6.30 cm2, 95% CI = −9.05 to −3.56; P < 0.05).
Although we were unable to show the anti-dyslipidemic and BW-modulatory effects of probiotics in DN patients, some mechanisms were defined to describe these positive effects. It seems that careful targeting of Toll-Like Receptor 2 (TLR2), TLR4, and NOD-Like Receptor & Pyrin Domain-Containing Protein 3 (NLRP3) signaling pathways can be beneficial for the treatment of T2D and DN [34]. Probiotics may reduce TLR4 activation which may explain their beneficial impact on serum lipid profile [35, 36]. TLR4 is a transmembrane protein which is capable to produce inflammatory cytokines [37]. In recent reports, probiotics were also considered as cholesterol-lowering agents [38].
We attempted to determine whether the observed heterogeneity in our outcomes was due to the differences in the study/disease duration, baseline BMI or in the dose of probiotics used. However, according to our subgroup analyses high heterogeneity still remained unknown.
Limitations
There are substantial limitations in our study. The number of analyzed studies was small and the included trials had been performed in small sample sizes. Many of the analyzed studies used probiotic mixtures or food-based probiotic products (e.g. dairy products, honey); the food-supplement interactions might decrease the consistency of results. Usual dietary intakes were not assessed in terms of possible prebiotics and probiotics consumption through the normal dietary patterns of participants. The most probiotic interventions were limited to one strain in our data, for example L. plantarum A7 was administered in four included RCTs [24, 25, 28, 30]. Therefore, our evidence is not applicable to all probiotic strains.
The included studies had follow-up periods ranging from 8 to 12 weeks, which were relatively short-term. The current meta-analysis was performed in age group between 50 and 60 years, so it is unclear how probiotics affect lipid profile and anthropometric measurements in children. The most important limitation was the heterogeneity between studies, which might stem from the intra-individual strain differences, duration of intervention, optimal dose of the probiotics, and genotype of individuals, among other factors. The responses to probiotic administration may also have been influenced by a number of within-study factors, such as antibiotic use [39] and corticosteroid therapy [40].
The subgroup analysis also had some limitations. The restricted number of included studies resulted in the tiny subgroups, which suggested that the evidence was not generalizable.
Strengths
We performed an exclusive investigation for DN; this is the main strength of the current study. The prevalence of DN is now growing worldwide and the treatments are very limited, hence the probiotics-related studies can allow clinicians to use these compounds as adjunct therapy.
Conclusion
Our meta-analysis did not find any beneficial effect of probiotics on lipid profile and anthropometric measurements; however, there were marginal improvements in some lipid markers such as TC (P = 0.084), LDL (P = 0.062) and HDL (P = 0.077). Subgroup analyses also failed to show any significant subset across the selected confounders (study duration, disease duration, probiotics dose, baseline BMI). More trials are needed to characterize specific alterations of the intestinal microbiota in diabetic nephropathy and to assess possible effects of probiotic, prebiotic, and synbiotic treatments in this setting. The authors suggest researchers to conduct more special interventions (single strains of probiotics; prebiotics alone; synbiotics) in DN patients.
Supplementary Information
(WMF 8 kb)
(WMF 8 kb)
(WMF 8 kb)
(WMF 8 kb)
(WMF 8 kb)
(WMF 8 kb)
(WMF 9 kb)
(WMF 9 kb)
(WMF 7 kb)
Acknowledgements
This research was supported by Isfahan University of Medical Sciences, Isfahan, Iran.
PROSPERO Registration ID. CRD42020186189.
Authors’ contributions (CRediT author statement)
Amir Reza Moravejolahkami: Conceptualization, Investigation, Methodology, Software, Validation, Formal analysis, Writing - Original Draft.
Mohammad Ali Hojjati Kermani: Visualization, Project administration, Writing - Review & Editing.
Zakiyeh Balouch Zehi: Data Curation, Resources, Writing - Review & Editing.
Seyed Mohammad Sadegh Mirenayat: Writing - Review & Editing.
Marjan Mansourian: Supervision, Funding acquisition.
Declaration
Conflict of interest
The authors declare that they have no conflict of interest to the publication of this article.
Footnotes
This study has not been duplicate publication or submission elsewhere.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amir Reza Moravejolahkami, Email: a.moravej@mail.mui.ac.ir, Email: amimohs@gmail.com.
Mohammad Ali Hojjati Kermani, Email: Imhojjati@gmail.com.
Zakiyeh Balouch Zehi, Email: zakiyezb@gmail.com.
Seyed Mohammad Sadegh Mirenayat, Email: Sadegh.mir1374@gmail.com.
Marjan Mansourian, Email: j_mansourian@hlth.mui.ac.ir.
References
- 1.Kikuchi K, Saigusa D, Kanemitsu Y, Matsumoto Y, Thanai P, Suzuki N, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. 2019;10(1):1–17. doi: 10.1038/s41467-019-09735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morimoto K, Matsui M, Samejima K, Kanki T, Nishimoto M, Tanabe K, Murashima M, Eriguchi M, Akai Y, Iwano M, Shiiki H, Yamada H, Kanauchi M, Dohi K, Tsuruya K, Saito Y. Renal arteriolar hyalinosis, not intimal thickening in large arteries, is associated with cardiovascular events in people with biopsy-proven diabetic nephropathy. Diabet Med. 2020;37:2143–2152. doi: 10.1111/dme.14301. [DOI] [PubMed] [Google Scholar]
- 3.Thongprayoon C, Hatch ST, Kaewput W, Sharma K, Ungprasert P, Wijarnpreecha K, et al. The effects of probiotics on renal function and uremic toxins in patients with chronic kidney disease; a meta-analysis of randomized controlled trials. Journal of Nephropathology. 2018;7(3).
- 4.Maric C, Hall JE. Obesity, metabolic syndrome and diabetic nephropathy. Diabetes and the Kidney. 2011;170:28–35. doi: 10.1159/000324941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabapathy V, Stremska ME, Mohammad S, Corey RL, Sharma PR, Sharma R. Novel immunomodulatory cytokine regulates inflammation, diabetes, and obesity to protect from diabetic nephropathy. Front Pharmacol. 2019;10:572. doi: 10.3389/fphar.2019.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo G, Piscitelli P, Giandalia A, Viazzi F, Pontremoli R, Fioretto P, et al. Atherogenic dyslipidemia and diabetic nephropathy. Journal of Nephrology. 2020:1–8. [DOI] [PubMed]
- 7.Fang B, Zhang M, Dong L, Zhou X, Ren F, Ge S. Probiotic camel milk powder improves glycemic control, dyslipidemia, adipose tissue and skeletal muscle function in T2DM patients: a randomized trial. 2020. [Google Scholar]
- 8.Asad F, Anwar H, Yassine HM, Ullah MI, Kamran Z, Sohail MU. White button mushroom, agaricus bisporus (Agaricomycetes), and a probiotics mixture supplementation correct dyslipidemia without influencing the colon microbiome profile in hypercholesterolemic rats. International Journal of Medicinal Mushrooms. 2020;22(3). [DOI] [PubMed]
- 9.López-Moreno A, Suárez A, Avanzi C, Monteoliva-Sánchez M, Aguilera M. Probiotic strains and intervention Total doses for modulating obesity-related microbiota Dysbiosis: a systematic review and meta-analysis. Nutrients. 2020;12(7):1921. doi: 10.3390/nu12071921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahreini-Esfahani N, Moravejolahkami AR. Can synbiotic dietary pattern predict Lactobacillales strains in breast Milk? Breastfeeding Medicine. 2020. [DOI] [PubMed] [Google Scholar]
- 11.Moravejolahkami A, Chitsaz A. Mediterranean-style diet co-supplemented with synbiotics improved quality of life, fatigue and disease activity in five secondary progressive multiple sclerosis patients. Ann Med & Surg Case Rep: AMSCR. 2019;2019(02).
- 12.Khalili L, Alipour B, Jafarabadi MA, Hassanalilou T, Abbasi MM, Faraji I. Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetology & metabolic syndrome. 2019;11(1):5. doi: 10.1186/s13098-019-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019;13(1):175–182. doi: 10.1016/j.dsx.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Bock PM, Telo GH, Ramalho R, Sbaraini M, Leivas G, Martins AF, et al. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2020:1–16. [DOI] [PubMed]
- 15.Kocsis T, Molnár B, Németh D, Hegyi P, Szakács Z, Bálint A, et al. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Sci Rep. 2020;10(1):1–14. doi: 10.1038/s41598-020-68440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasińska MA, Drzewoski J. Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn. 2015;125(11):803–813. doi: 10.20452/pamw.3156. [DOI] [PubMed] [Google Scholar]
- 17.Yao K, Zeng L, He Q, Wang W, Lei J, Zou X. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials. Medical science monitor: international medical journal of experimental and clinical research. 2017;23:3044–3053. doi: 10.12659/MSM.902600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53(2):100–108. doi: 10.1016/j.micpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Vlachou E, Ntikoudi A, Govina O, Lavdaniti M, Kotsalas N, Tsartsalis A, Dimitriadis G. Effects of probiotics on diabetic nephropathy: a systematic review. Curr Clin Pharmacol. 2020;15:234–242. doi: 10.2174/1574884715666200303112753. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151(4):W-65–W-94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 21.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. bmj. 2019;366. [DOI] [PubMed]
- 22.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng W, Mao P, Liu L, Chen K, Zhong Y, Xia W, et al. Effect of carnosine supplementation on lipid profile, fasting blood glucose, HbA1C and insulin resistance: a systematic review and meta-analysis of long-term randomized controlled trials. Complementary Therapies in Medicine. 2019;102241. [DOI] [PubMed]
- 24.Abbasi B, Ghiasvand R, Mirlohi M. Kidney function improvement by soy milk containing Lactobacillus plantarum A7 in type 2 diabetic patients with nephropathy: a double-blinded randomized controlled trial. Iran J Kidney Dis. 2017;11(1):36–43. [PubMed] [Google Scholar]
- 25.Abbasi B, Mirlohi M, Daniali M, Ghiasvand R. Effects of probiotic soymilk on lipid panel in type 2 diabetic patients with nephropathy: a double-blind randomized clinical trial. Prog Nutr. 2018;20:70–78. [Google Scholar]
- 26.Arani NM, Emam-Djomeh Z, Tavakolipour H, Sharafati-Chaleshtori R, Soleimani A, Asemi Z. The effects of probiotic honey consumption on metabolic status in patients with diabetic nephropathy: a randomized, double-blind, controlled trial. Probiotics and antimicrobial proteins. 2019;11(4):1195–1201. doi: 10.1007/s12602-018-9468-x. [DOI] [PubMed] [Google Scholar]
- 27.Mafi A, Namazi G, Soleimani A, Bahmani F, Aghadavod E, Asemi Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct. 2018;9(9):4763–4770. doi: 10.1039/C8FO00888D. [DOI] [PubMed] [Google Scholar]
- 28.Miraghajani M, Zaghian N, Mirlohi M, Ghiasvand R. Probiotic soy milk consumption and renal function among type 2 diabetic patients with nephropathy: a randomized controlled clinical trial. Probiotics and antimicrobial proteins. 2019;11(1):124–132. doi: 10.1007/s12602-017-9325-3. [DOI] [PubMed] [Google Scholar]
- 29.Soleimani A, Mojarrad MZ, Bahmani F, Taghizadeh M, Ramezani M, Tajabadi-Ebrahimi M, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017;91(2):435–442. doi: 10.1016/j.kint.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 30.Miraghajani M, Zaghian N, Mirlohi M, Feizi A, Ghiasvand R. The impact of probiotic soy milk consumption on oxidative stress among type 2 diabetic kidney disease patients: a randomized controlled clinical trial. J Ren Nutr. 2017;27(5):317–324. doi: 10.1053/j.jrn.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Jia L, Jia Q, Yang J, Jia R, Zhang H. Efficacy of probiotics supplementation on chronic kidney disease: a systematic review and meta-analysis. Kidney Blood Press Res. 2018;43(5):1623–1635. doi: 10.1159/000494677. [DOI] [PubMed] [Google Scholar]
- 32.AbdelQadir YH, Hamdallah A, Sibaey EA, Hussein AS, Abdelaziz M, AbdelAzim A, et al. Efficacy of probiotic supplementation in patients with diabetic nephropathy: a systematic review and meta-analysis. Clinical Nutrition ESPEN. 2020;40:57–67. doi: 10.1016/j.clnesp.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Koutnikova H, Genser B, Monteiro-Sepulveda M, Faurie J-M, Rizkalla S, Schrezenmeir J, Clément K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2019;9(3):e017995. doi: 10.1136/bmjopen-2017-017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol. 2016;12(1):13–26. doi: 10.1038/nrneph.2015.175. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed SK, Magdy YM, El-Waseef DA, Nabih ES, Hamouda MA, El-kharashi OA. Modulation of hippocampal TLR4/BDNF signal pathway using probiotics is a step closer towards treating cognitive impairment in NASH model. Physiol Behav. 2020;214:112762. doi: 10.1016/j.physbeh.2019.112762. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed LA, Salem MB, El-Din SHS, El-Lakkany NM, Ahmed HO, Nasr SM, et al. Gut microbiota modulation as a promising therapy with metformin in rats with non-alcoholic steatohepatitis: role of LPS/TLR4 and autophagy pathways. Eur J Pharmacol. 2020;887:173461. doi: 10.1016/j.ejphar.2020.173461. [DOI] [PubMed] [Google Scholar]
- 37.Tuoheti A, Gu X, Cheng X, Zhang H. Silencing Nrf2 attenuates chronic suppurative otitis media by inhibiting pro-inflammatory cytokine secretion through up-regulating TLR4. Innate Immunity. 2020;1753425920933661. [DOI] [PMC free article] [PubMed]
- 38.Bhat B, Bajaj BK. Multifarious cholesterol lowering potential of lactic acid bacteria equipped with desired probiotic functional attributes. 3 Biotech. 2020;10:1–16. doi: 10.3390/biotech10010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, Versalovic J, Young V, Finlay BB. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12(5):611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Kaur L, Gordon M, Baines PA, Iheozor-Ejiofor Z, Sinopoulou V, Akobeng AK. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020;3. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(WMF 8 kb)
(WMF 8 kb)
(WMF 8 kb)
(WMF 8 kb)
(WMF 8 kb)
(WMF 8 kb)
(WMF 9 kb)
(WMF 9 kb)
(WMF 7 kb)