Abstract
Purpose
Diabetic peripheral neuropathy (DPN) affects up to 50 % diabetic patients. Moreover, uncontrolled diabetes associated with impaired wound healing. The present study was aimed at exploring the effect of apple peel extract (APE) on type 2 diabetes (T2D)-induced DPN and delayed wound healing.
Methods
In adult male Sprague-Dawley rats on high-fat diet, a single low dose streptozotocin (STZ, 35 mg/kg) was administered via intraperitoneal route to induce T2D. Plantar test using Hargreaves apparatus was used to evaluate the DPN. Six different groups of rats were treated orally with saline (naïve control and DPN control), APE (100, 200 and 400 mg/kg) and gabapentin (30 mg/kg) daily for 7 consecutive days and thermal paw withdrawal latency (PWL) was measured. To elucidate the underlying antioxidant effect of APE, the catalase (CAT), glutathione (GSH) and malonaldehyde (MDA) levels were measured. To evaluate the wound healing potential of APE, excision ischemic open wound model was used. Six different groups of rats were applied with 2 % gum acacia (naïve control and diabetic control), 1 % silver sulfadiazine (SSD) cream and APE cream (5, 10 and 20 %) twice daily for 28 days. Dry connective tissue parameters like hydroxyproline and hexosamine were also measured to further confirm the wound healing activity.
Results
Diabetes produced thermal hyperalgesia in rats with a significant decrease in PWL as compared to naive controls indicating induction of DPN. APE and gabapentin significantly improved PWL in diabetic animals. Biochemical analysis revealed a significant improvement in oxidative stress parameters such as catalase, GSH and MDA. Wound closure was significantly more after day 15 of topical application of APE and SSD as compared to control group. APE significantly increased hydroxyproline and hexosamine levels as compared to standard cream. Moreover, histopathology revealed that, topical application of APE cream showed an enhanced healing process.
Conclusions
On the basis of the findings, we conclude that APE has a potential to be used as a therapeutic intervention for the management of DPN and delayed wound healing in the diabetic condition.
Keywords: Apple peel extract, Antioxidants, Type 2 diabetes, Peripheral neuropathy, Wound healing
Introduction
India is one of the 7 countries of the International Diabetes Federation South-East-Asia (SEA) Region. There are 463 million people have diabetes in the world and 88 million people in the SEA region; by 2045 this will rise to 153 million [1]. Diabetes is associated with various comorbid complications of which diabetic peripheral neuropathy (DPN) is a frequent and chronic complication that affects up to 50 % diabetic patients [2]. It is a major cause of morbidity and increased mortality, and is associated with duration of diabetes, hyperlipidemia, and poor glycemic control [2]. Diabetes causes DPN by promoting neuronal damage and inhibiting nerve regeneration, which leads to significantly abnormal neuronal sensations like tactile sensitivity, vibration sense, lower-limb proprioception, and kinesthesia [3]. The pathophysiology of DPN is very complex and it has been associated with peripheral demyelination, a decrease in peripheral nerve conduction and degeneration of myelinated and unmyelinated sensory fibers [4]. The DPN depends upon various causative factors including persistent hyperglycemia, microvascular insufficiency, oxidative stress, nitrosative stress, defective neurotrophism, and autoimmune-mediated nerve destruction [5, 6]. Glycemic control has been shown to be effective in slowing the progression of DPN [7], but patients with painful DPN often need other agents to palliate their symptoms.
Reduced or absence of sensation in the foot can increase the risk of injury and wounds that may develop into serious infections requiring amputations. Wound involves a disruption in the cellular, anatomical and functional epithelial integrity of the skin subsequent to chemical, physical, microbial, thermal or immunological insult; followed by disruption of the structure and function of underlying normal tissue [8–10]. The underlying mechanisms for delayed wound healing in diabetic patients are not fully understood but plausibly attributed to the delay in collagen synthesis and impairment in epithelial formation coupled with reduced angiogenesis [11].
According to the Centre for Disease Control, diabetic people experience complications caused by infected wounds. Diabetic foot ulcers (DFUs), a leading cause of amputations, affecting 15 % of population with diabetes. Delayed wound healing in the diabetic patients can increase the risk of infections and development of other complications which affects overall health and quality of life. Thus, over the years this complication may leads to increase in the morbidity and mortality in diabetes [12]. Approved growth factor and cell therapies for DFU and other diabetic-related wounds are rare to obtain and costly in the developing countries like India, and improper wound healing control could degenerate into DFU or possibly into amputation especially in poor resource settings. However, it is of fascination to note that nature has gifted us a platform for medicinal agents in the treatment of various ailments and diseases including diabetic wounds.
Currently, tricyclic antidepressants, anticonvulsants, serotonin-norepinephrine reuptake inhibitors (SSRIs), opiates, opiate-like substances and topical medications are being used for the management of DPN. However, these medications usually at best provide only partial pain relief and have severe side effects. Therefore, for an effective treatment of neuropathic pain, there is still a need to obtain suitable therapeutic agent, which possess higher efficacy and a greater level of tolerability and safety. Hence, apart from available marketed formulations, the quest of natural products to control hyperglycemia and its associated complications still continues.
Recent data suggest that natural polyphenols, due to their biological properties may be unique nutraceuticals and represent supplementary treatments for various aspects of diabetes mellitus [13]. Based on several in-vitro animal models and human studies, polyphenols may play a role in many metabolic processes. They can modulate carbohydrate and lipid metabolism, attenuate hyperglycemia, dyslipidemia and insulin resistance, improve adipose tissue metabolism, and alleviate oxidative stress, stress-sensitive signaling pathways and inflammatory processes. Some flavonoid compounds have found to have neuroprotective effect in experimental animal models in diabetic rats [13].
Apples, the most consumed fruits of temperate climate countries, are a considerable source of phenolic compounds in the human diet [14]. Several studies have shown beneficial effects of apple against cancer, cardiovascular diseases, diabetes, asthma, pulmonary dysfunction and Alzheimer’s disease [15]. A fresh apple contains 14–36 mg of flavonoids per 100 g [16]. Apple peel contain 3-6-fold more flavonoids than the flesh [17]. Several polyphenols are identified in apple peel extract (APE) such as phloridzin, rutin, hyperoside, quercitrin, chlorogenic acid, epicatechin, quercetin and caffeic acid [18]. APE has proven antidiabetic activity by virtue of its insulin sensitizing action [19]. Moreover, APE supplementation shown to have protective effects against deleterious complications of diabetes mellitus [20].
In this background, the present study was aimed at exploring the effects of APE in diabetes induced DPN and impaired wound healing. To explore the neuroprotective role of APE, experimental animal model of DPN was used and its antioxidant activity was explored by various biochemical assays.
Materials and methods
Animals, induction of diabetes, blood collection and tissue harvest
Animals
Adult male Sprague-Dawley rats weighing 160–180 g were housed for acclimatization in the institute animal house maintained at controlled room temperature (22 ± 2 °C) and humidity (55 ± 5 %) with 12:12 h light and dark cycle and provided with commercially available normal pellet diet (NPD, Amrut Diet, New Delhi, India) and water ad libitum before dietary manipulation. The experiments were approved by Institutional Animal Ethics Committee (IAEC), Faculty of Pharmacy, Pacific Academy of Higher Education and Research University, Udaipur, Rajasthan, India.
Drugs and preparation of APE
All the drugs used in treatment protocols were obtained from different sources. STZ (S0130) and gabapentin (G154) was obtained from Sigma-Aldrich, USA. Gum acacia (West-Coast Pharmaceutical Works Ltd., Ahmedabad, Gujarat) and 1 % silver sulfadiazine (SSD) cream (SSD™, Dr. Reddy’s Laboratory, Inc.) were obtained from the market.
For preparation of APE, Lal Ambri (Red Delicious) Apple (Maluspumila) which is widely grown in Jammu and Kashmir, Himachal Pradesh, Uttaranchal, Arunachal Pradesh and Nagaland states of India was used. Apples were purchased from a local retail shop. The APE was prepared using method reported previously [18]. Briefly, the apples were washed in deionized water, wiped with cloth and peeled using an autoclaved kitchen peeler then the peels were left to dry. The dried peels were separately milled into fine powders and stored overnight at 4 °C in the dark before extraction. Fine powder of apple peels was extracted with 75 % methanol using an ultrasonic cleaning bath for three times and each time for 20 min. The extract was centrifuged twice at 5000 rpm for 15 min and a third time at 10,000 rpm for 15 min. Ascorbic acid (2 %) was added in the extraction to inhibit polyphenol oxidases. The combined extract was filtered and condensed at 40 °C to remove traces of methanol. Then the extract was supplemented with 200 mL of deionized water and evaporated continuously to remove methanol completely. The residual aqueous solution rich in polyphenols was freeze-dried. Finally, the powdered extract of APE containing polyphenols was obtained.
Treatments
Rats were subjected to ad libitum high-fat diet (HFD; 58 % fat, 25 % protein and 17 % carbohydrate, as a percentage of total kcal) till the end of the experiments. After two weeks of HFD feeding, rats were injected intraperitoneally (i.p.) with a single low dose of STZ (35 mg/kg, freshly prepared in citrate buffer pH 4.4). One week after STZ administration, the rats with ≥ 300 mg/dl non-fasting blood glucose levels measured using hand-held glucometer were considered as type 2 diabetic. Further, to confirm the induction of type 2 diabetes (T2D), the oral glucose tolerance test was performed. The animals were fasted for 6 h and then administered orally with 2 g/kg of glucose solution. Blood glucose level was measured after 0 (before glucose dosing), 30, 60, 90 and 120 min of glucose administration to measure the areas under curve (AUC) of blood glucose. The calculation of AUC was based upon the polygonal lines joining glucose values for different time sections. The strategy to induce T2D was adopted from Srinivasan et al. [21], and the same has been already standardized in our previous study [22]. More than 90 % of patients with T2D are overweight or obese [23]. Moreover, the major factor that contributes to the high blood glucose levels during T2D is the resistance to the actions of insulin. As a compensatory response, the pancreatic beta cells hypersecrete insulin, and overtime, beta cell function is impaired. This progressively leads to impaired pancreatic secretion of insulin and loss of its action on the target cells [24]. Therefore, we induced T2D in rats by offering HFD following low-dose STZ to closely mimic the natural history of the disease condition. This strategy has been widely used in the experimental pharmacology [21, 22, 25].
Diabetic animals were used to evaluate DPN and wound healing activity of APE as described in following sections. In addition to diabetic animals, one set of rats was kept on NPD until the end of the study, and was subjected to the experiments described below in parallel with diabetic animals.
Evaluation of peripheral neuropathy: plantar test using Hargreaves apparatus
After two weeks of STZ administration, animals were divided into different groups (n = 7 per group) and subjected for daily oral treatments of saline (control; 1 ml/rat), APE (100, 200 and 400 mg/kg) and gabapentin (30 mg/kg) for 7 consecutive days. In parallel, one group of non-diabetic rats (NPD-fed) was also administered with saline. One hour after dosing, rats were subjected for the thermal paw withdrawal latency (PWL) test using Hargreaves apparatus [26]. In this test, the thermal stimuli response of the left hind paw was measured using a full-automatic plantar analgesia system. Rats were placed on a 3-mm-thick glass plate within a Plexiglas chamber. Then, the left hind paw was exposed to a heat stimulus directly from a fixed distance. The elapsed time for withdrawal of the paw from the heat was recorded. The average value of five tests performed at 5-min intervals was calculated. A maximum cut-off value of 30 seconds was used to prevent possible damage.
Evaluation of wound healing in diabetic rats using excision ischemic open wound model
The delayed excision wound-healing model was used as it mimics the physiological and molecular abnormalities of chronic wounds in humans as described earlier by Hofmann et al. [27]. We created one full thickness wound per T2D animal, 8 mm in diameter, within a narrow bipedicled flap raised on the dorsum of rat by incisions, under ketamine (50 mg/kg) and xylazine (5 mg/kg) anesthesia. In this model, the blood supply to the wounds is mostly impaired, resulting in ischemia and slower healing [28]. These animals with wound injury further divided into different groups (n = 7 per group) for the topical application of 0.2 ml of gum acacia in normal saline (control group), APE cream (5, 10 and 20 %), and 1 % SSD cream daily for 28 days. Wound healing was assessed by measuring wound area using tracing on days 1, 4, 8, 12, 16, 20, 24 and 28. Topical application was made twice a day for all groups in a thin film (approximately 1 mm thick) covering the full wound. Wounds were cleaned gently with saline prior to topical application. Gross observations were made on each wound daily. Wound area was measured by tracing twice onto clear plastic sheets every 2 days. Wound area was calculated from the tracing measurement. The initial (day 1) wound area following the creation of wound was used to calculate % wound closure for each wound on any given day.
Where, Area i is the initial area and Area n is the area at day n.
Estimation of dry connective tissue parameters
The method of collection of dry granulation tissues was adopted from previously used method by Kumari et al. [29]. Dry sterilized glass cylinder measuring (2.5 × 0.5 cm) was introduced into the wound pouch. The wounds were sutured and mopped with alcoholic swabs. Animals were placed in individual cages after recovery from anesthesia. The day of the wound creation was considered as day zero. On 5th and 15th post wounding day, the granulation tissue formed on the implanted tube was carefully dissected under anesthesia.
The dry granulation wound tissue (40 mg/mL) was collected in 6 N hydrochloric acid and placed in boiling water bath for 24 h for hydrolysis. The hydrolysate was cooled, and excess of acid was neutralized by 10 N sodium hydroxide using phenolphthalein as an indicator. The volume of neutral hydrolysate was diluted to a concentration of 20 mg/mL with distilled water. The final hydrolysate was used for estimation of hydroxyproline, hexosamine, MDA, catalase and GSH levels enzyme assay following the standard procedure [30].
Hydroxyproline
Aliquots of standard Hydroxyproline (2–20 µg/ml) were prepared from stock solution and volume made up by deionized water. Test samples were mixed gently with 50 µl sodium hydroxide (2N). The samples were hydrolyzed by autoclaving at 120° C for 20 min. 450 µl of chloramine-T was added to the hydrolysate, mixed gently, and the oxidation was allowed to proceed for 25 min at room temperature. 500 µl of Ehrlich’s reagent was added to each sample, mixed gently, and the chromophore was developed by incubating the samples at 65° C for 20 min. Absorbance of each sample was read at 550 nm using a spectrophotometer.
Hexosamine (HXA)
0.05 ml of hydrolyzed fraction was diluted to 0.5 ml with distilled water. To this was added 0.5 ml of acetyl acetone reagent and heated in boiling water bath for 20 min then cooled under tap water. To this 1.5 ml of 95 % alcohol was added, followed by an addition of 0.5 ml of Ehrlich’s reagent. The reaction was allowed for 30 minutes to complete. Color intensity was measured at 530 nm against the blank and value expressed as mg of hexosamine/ mg of protein.
Evaluation of oxidative stress
On the day 5 and 15 post-wounding, the dry granulation wound tissues were collected and dried at 60 °C for 24 h. Tissue homogenate (10 %) was prepared with 0.15 mmol/L potassium chloride and centrifuged at 3000 × g for 10 min. The cell free supernatant was used for the antioxidative enzyme assay.
Measurement of reduced glutathione (GSH)
Glutathione levels in the tissue were measured by using method described by Ellman [31]. Briefly, to 0.5 ml of tissue homogenate (prepared in 0.1M sodium phosphate and 5 mM EDTA buffer, pH 8), 0.45 ml of phosphate EDTA buffer and 0.5 ml of metaphosphoric acid (25 %) were added and centrifuged at 10,000 rpm for 10 minutes at 40C. Then, 2.25 ml of phosphate EDTA buffer was added to 0.25 ml of supernatant, 50 ml of this was separated and 900 ml of phosphate EDTA buffer and 50 ml of pthalaldehyde (1 mg/ml in methanol) were added to this, mixed well and incubated at room temperature for 15 minutes. Fluorescence intensity was measured using spectrophotometer (Synergix MX, Biotek) and values expressed as mg of GSH/mg protein.
Determination of catalase (CAT) activity
To determine the activity of CAT enzyme 5 % homogenate was prepared in potassium phosphate buffer (100 mM, pH 7.0; 1 mM EDTA; 1 mM PMSF) and centrifuged at 10,000 r/min for 30 min at 40 C. To 5 mL of the above supernatant, 1875 mL assay buffer (50 mM Na2HPO4, 50 mM KH2PO4, pH 7.0) and 120 mL H2O2 (0.66 M) were added. Enzyme kinetics was recorded at 240 nm over a period of 1 min. An extinction coefficient of 43.6 M/cm was used to determine the enzyme activity and values were expressed as millimoles of hydrogen peroxide degraded per minute per milligram of protein [32].
Measurement of malonaldehyde (MDA)
Lipid peroxidation was quantified by MDA levels in the tissue which is in turn determined by employing a method described by Ohkawa et al. [33]. Briefly, 10 % tissue homogenate (in 1.15 % KCl) was prepared and centrifuged for 10 min at 6000 r/min. To 0.1 ml of the above supernatant, 0.2 ml of 8.1 % sodium dodecyl sulfate was added, followed by addition of 1.5 ml of 20 % acetic acid (pH 3.5) and 1.5 ml of 0.8 % thiobarbituric acid. This was followed by boiling at 950 C for 1 h and cooling and centrifugation at 6000 r/min for 15 min. The supernatant was separated, and optical density of the supernatant was taken at 532 nm. The values were expressed as mg of MDA/mg protein.
Histopathology
The animals were sacrificed on 15th day of treatment by thiopentone sodium (75 mg/kg, i.p.). The tissue samples (skin) were collected, pruned and fixed in 10 % neutral buffered formalin solution for 24 hours at room temperature. After proper fixation pieces of organs were processed by dehydration in graded series of alcohol and toluene in Auto-tissue processor (Leica TP 1020). The processed tissues were embedded in paraffin wax. Multiple sections of 4–5 µm thickness from each block were cut on rotatory microtome (Microm, Germany), mounted on slide with albumin coating and air dried overnight. The sections were deparaffinised and stained with hematoxylin and eosin [34] in auto-stainer and cover slipped by auto cover slipper (Leica, Germany). After drying sections were photographed in light microscope using DM 500 camera (Leica, Germany).
Statistical analysis
All the data from biochemical and behavioral studies are presented as means ± SEM. Statistical analysis was carried out using GraphPad Prism 5.0 software (GraphPad Software Inc., USA). Student’s t test and one-way or two-way analysis of variance (ANOVA) followed by post-hoc Bonferroni’s multiple comparison test was applied wherever suitable. The data on blood glucose and body weight were analyzed using two-way ANOVA for inter-group comparison, whereas intra-group comparison was processed with one-way ANOVA followed by Bonferroni’s multiple comparison test. The data of the AUC of blood glucose were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparison test. The differences were considered significant at p < 0.05.
Results
Evaluation of the features of HFD fed STZ treated rats
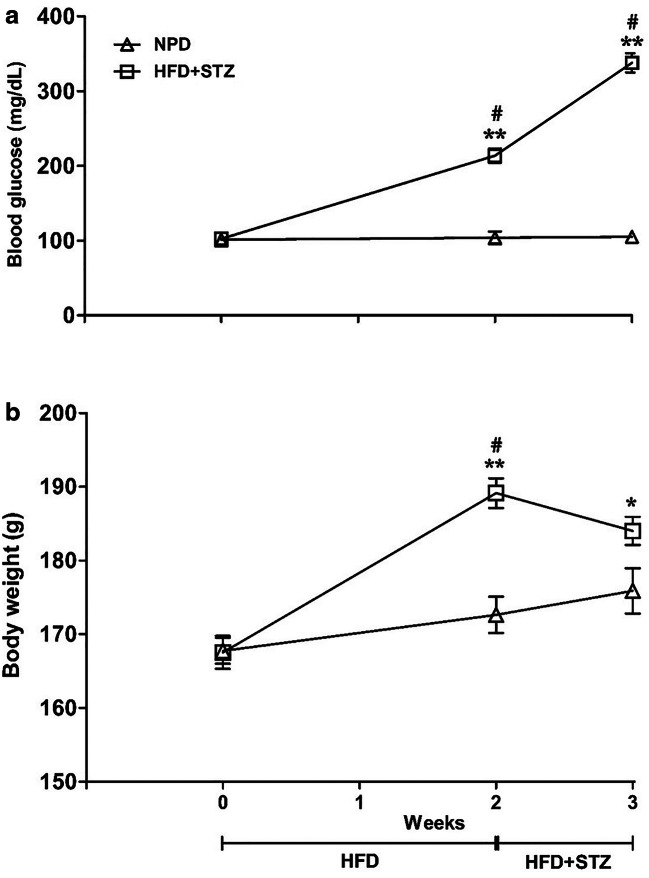
Two weeks after HFD feeding, the rats showed significant increase in blood glucose levels. The control animals showed normal blood glucose levels throughout the experiment. One week after the STZ injection (3 weeks after HFD feeding), the blood glucose level was further increased drastically (more than 300 mg/dL), thus confirming the development of frank hyperglycemia i.e. T2D (Fig. 1a). Animals on HFD for 2 weeks showed significant increase in blood glucose (P < 0.0001) as compared to the animals on NPD [Factor ‘HFD’ F(1,42) = 220.2, p < 0.0001] over the period of 3 weeks [Factor ‘weeks’ F(2,42) = 80.02, p < 0.001]. There was significant rise in blood glucose on 2nd and 3rd week consistently as compared to that on NPD (p < 0.001).
Fig. 1.
Represents effect of high fat diet (HFD) and streptozotocin (STZ) treatment on blood glucose levels (a) and body weight (b). Different groups of rats were offered with HFD and normal pellet diet (NPD). Two weeks after HFD feeding, single dose of STZ (35 mg/kg, i.p.) was administered. Non-fasting blood glucose level (mg/dL) and body weight (g) were monitored 2 weeks after dietary manipulations and 1 week after STZ injection. Blood glucose level and body weight of rats fed with NPD were concomitantly measured. The data between the groups were analyzed by two-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. Moreover, the values of different time points within a group were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparison test. Each line represents the mean±SEM. *p<0.05 **p<0.001 vs NPD; #p<0.001 vs preceding time points.
In case of body weight measurements (Fig. 1b), animals on HFD for 2 weeks showed significant increase in body weight (p < 0.0001) as compared to that on NPD [Factor ‘HFD’ F(1,42) = 19.04, p < 0.0001 and factor ‘weeks’ F(2,42) = 20.88, p < 0.001]. Body weight of HFD treated group was higher on 2nd week (p < 0.001) as compared to NPD group. Although weight gain was slightly attenuated following administration of STZ (35 mg/kg, ip), it was still considerably higher (p < 0.05) than body weight of NPD-fed group.
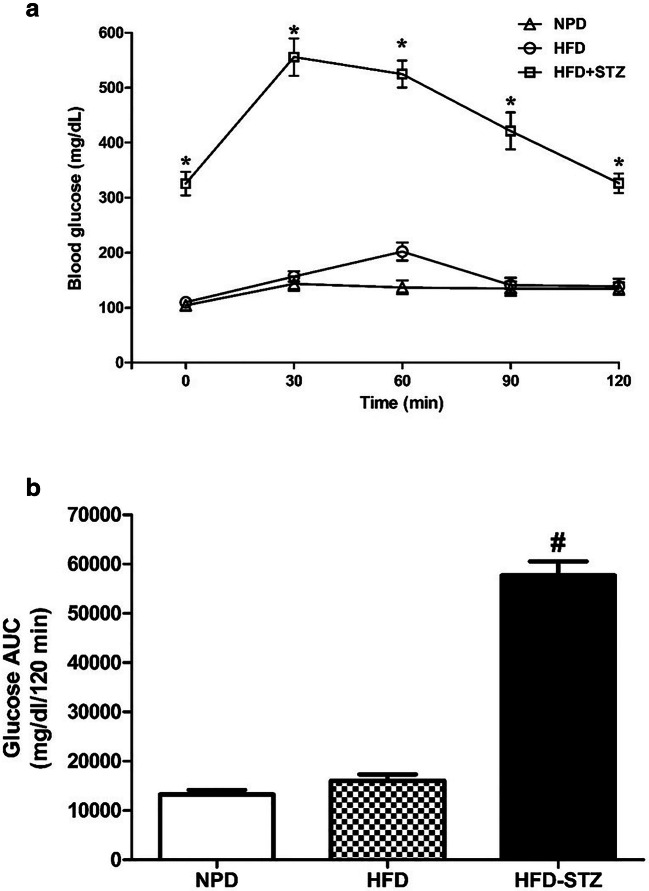
In oral glucose tolerance test (OGTT), significantly high blood glucose level was detected [Factor ‘HFD-STZ’ F(2,105) = 410, p < 0.0001 and factor ‘time’ F(4,105) = 20.91, p < 0.0001]. The high glucose levels were observed in the NPD-fed and HFD-fed rats at 30 min time point. However, in HFD-fed STZ treated rats, significantly high glucose level was noticed at 30, 60, 90 and 120 min time points (p < 0.001) (Fig. 2a). Blood glucose level was significantly high at all the time points after oral glucose administration in HFD-STZ treated group as compared to NPD or HFD only group (p < 0.001). The area under the curve (AUC) of glucose in the HFD-fed STZ treated group was significantly higher than that in the NPD fed and HFD-fed groups (p < 0.001) (Fig. 2b). This delayed glucose disappearance confirms the development of insulin resistance i.e. T2D in HFD-fed STZ treated rats.
Fig. 2.
Represents blood glucose level during oral glucose tolerance test (a) and area under the curve (AUC) of the blood glucose (b). Different groups of rats were fed for 2 weeks with normal pellet diet (NPD), high fat diet (HFD) and HFD with single dose of streptozotocin (STZ) (35 mg/kg, i.p.). The animals were fasted for 6 h and then administered orally with 2 g/kg of glucose solution. Blood glucose level was measured after 0 (before glucose dosing), 30, 60, 90 and 120 min of glucose administration. The area under the curve (AUC) of blood glucose was measured. The data were analyzed with one-way analysis of variance (ANOVA) followed by post-hoc Bonferroni's multiple comparison test. Each line represents the mean of blood glucose level ± SEM, whereas each bar represents the mean of AUC of blood glucose level ± SEM of each group. *p<0.001 vs respective time points of NPD; #p<0.001 vs HFD and NPD
APE produced analgesic effect in diabetic neuropathic rats subjected to thermal hyperalgesia
Diabetes produced thermal hyperalgesia in rats. As shown in Fig. 3, diabetic rats showed statistically significant decrease in PWL as compared to naïve control (p < 0.001) rats indicating induction of DPN. One-way ANOVA showed significant effect of APE (100, 200 and 400 mg/kg) and gabapentin (30 mg/kg) on increasing PWL in rats with diabetic neuropathy [F(4,34) = 28.81, p < 0.001]. Post-hoc Bonferroni’s multiple comparison test reveals that APE at the dose of 200 and 400 mg/kg and gabapentine (30 mg/kg) significantly increased PWL (p < 0.001) as compared to vehicle treatment in diabetic rats.
Fig. 3.
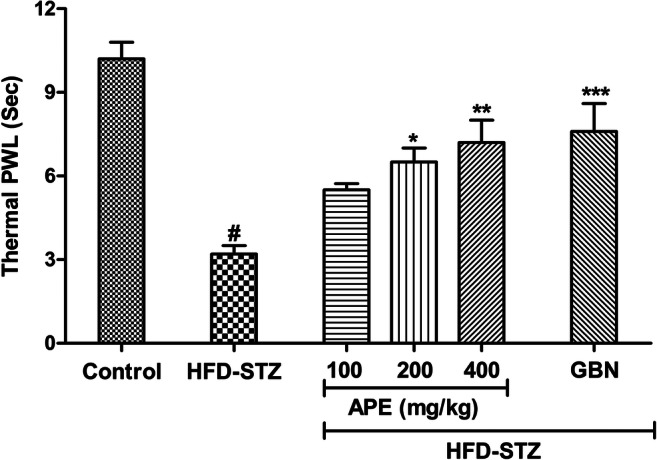
Effect of apple peel extract (APE) on the thermal paw withdrawal latency (PWL) in high-fat diet (HFD) + streptozotocin (STZ)-induced diabetic rats subjected to Hargreave’s test. Different group of rats were treated with APE (100, 200 and 400 mg/kg, p.o.) or gabapentin (30 mg/kg, p.o.) for 7 consecutive days after two weeks of STZ injection. Values are expressed as mean±S.E.M. (n=7 per group). The data were analyzed with one-way analysis of variance (ANOVA) followed by post-hoc Bonferroni's multiple comparison test. #P<0.001 vs naïve control group; *P<0.05, **p<0.01, ***p<0.01 vs diabetic control (HFD-STZ) group
APE topical application promotes wound closure in diabetic rats
We analyzed wound closure over a period of 28 days following APE or standard SSD cream applied topically. Wound area was calculated on day one upon wounding and was used for calculating the percentage of wound closure for each respective wound on any given day. The observations revealed that wounds treated with APE 5, 10 and 20 % or with the SSD cream revealed some signs of dermal healing and healed relatively faster in comparison with the group that was given the placebo control treatment (gum acacia in normal saline). Two-way ANOVA revealed that wound closure was significantly more after day 15 (APE 10 % p < 0.05, APE 20 % p < 0.001) as compared to control group. In SSD group, healing was significant as early as day 12 and last till day 28. Gum acacia-treated wounds showed swelling and exudate formation at early time points (Day 1 and 3). APE-treated wounds did not show swelling but appeared more vascular. Wound healing effect of APE was found to be compared with standard cream. APE accelerates closure of ischemic wounds in diabetic rats (Fig. 4).
Fig. 4.
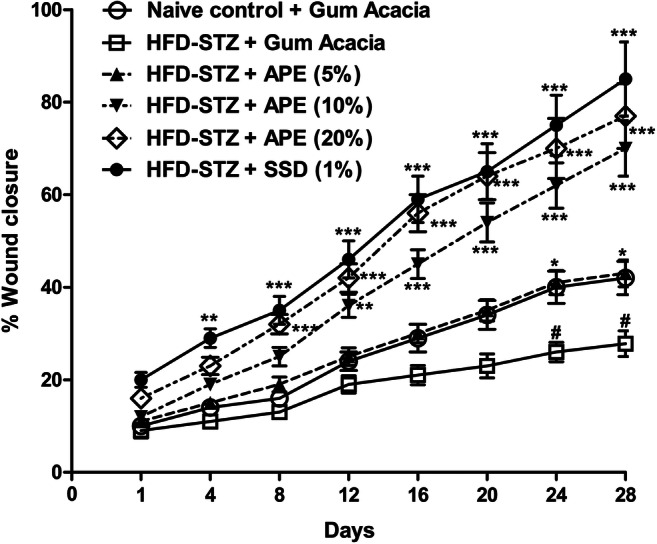
Topical apple peel extract (APE) accelerates healing of ischemic wounds in high-fat diet and streptozotocin (HFD-STZ)-induced diabetic rats. Excision wound was produced in normal and HFD-STZ-induced diabetic rats. Wound on normal (naïve control) and diabetic rats were applied with gum acacia, APE (5, 10 or 20%) or silver sulfadiazine (SSD) creams daily for 28 days. Wound healing was assessed by measuring wound area using tracing on days 1, 4, 8, 12, 16, 20, 24 and 28. Each point shows the mean ± SEM of % wound area healed relative to day 1. The data were analyzed with two-way analysis of variance (ANOVA) followed by post-hoc Bonferroni's multiple comparison test. *P<0.05, **P<0.01, ***p<0.001 vs HFD-STZ (gum acacia); #p<0.05 vs naïve control (gum acacia)
Effect of drug treatment on wound connective tissue
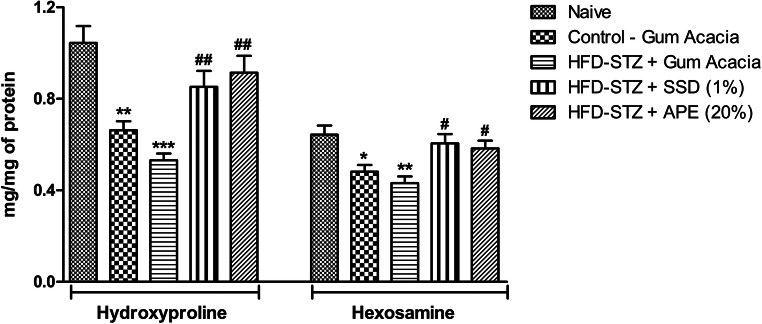
Hydroxyproline and hexosamine was found to be increased by APE in excision (p < 0.001) on day 15 (Fig. 5). This result is also comparable to that of SSD treatment which showed significant increase in the excision (p < 0.001) wound model as compared to control. Post-hoc Bonferroni’s multiple comparison test revealed significant decreased in hydroxyproline (0 < 0.001) and hexosamine (p < 0.01) levels in diabetic control when compared to naïve control animals. However, treatment of SSD and APE cream significantly reversed hydroxyproline and hexosamine levels in diabetic animals (p < 0.05). On day 5, test or standard treatment did not induce changes in the level of hydroxyproline and hexosamine (data not shown).
Fig. 5.
The effects of apple peel extract (APE) on hydroxyproline and hexosamine in tissue homogenates of dermal wounds in naïve rats or high-fat diet and streptozotocin (HFD-STZ)-induced diabetic rats treated with either base formulation, standard silver sulfadiazine (SSD) cream or APE (20%). Dry tissue from wound was collected on day 15 of wound creation. Statistical Analysis of the data were carried out using one way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test for average comparison between groups. Data were presented as mean ± SEM. *p< 0.05, **p<0.01, ***p<0.001 vs tissue from naïve rats. #p<0.05, ##p<0.01 vs diabetic group
Effect of APE cream application on wound tissue
The level of lipid peroxidation was assessed by determining the level of MDA. A notable reduction in the GSH, CAT and MDA levels were observed in the tissue obtained from animals treated with standard cream and test formulation compared with levels in tissue from the control group (Table 1).
Table 1.
Effect of apple peel extract (APE) on oxidative stress related biomarkers in wound tissues
| Days | Treatment | GSH (mg/mg of protein) | CAT (mM of H2O2 degraded /min/mg protein) | MDA (mg/mg of protein) |
|---|---|---|---|---|
| Day 5 | Naïve Control | 47.95 ± 5.12 | 0.060 ± 0.21 | 834.7 ± 80.29 |
| Wound control | 27.9 ± 2.09* | 0.251 ± 0.01** | 1279.0 ± 81.48* | |
| Wound + SSD (Std.) | 43.83 ± 5.65# | 0.038 ± 0.06## | 535.5 ± 54.22# | |
| Wound + APE (20 %, test) | 43.81 ± 5.12# | 0.130 ± 0.09# | 663.2 ± 62.25# | |
| Day 15 | Naïve Control | 44.28 ± 3.19 | 0.065 ± 0.02 | 820.8 ± 64.74 |
| Wound control | 34.80 ± 4.57* | 0.281 ± 0.03** | 1013.2 ± 95.91* | |
| Wound + SSD (Std.) | 47.11 ± 5.05# | 0.063 ± 0.03## | 487.1 ± 52.51## | |
| Wound + APE (20 %, test) | 45.40 ± 5.13# | 0.070 ± 0.04## | 589.3 ± 61.16## |
The effects of APE cream formulation on glutathione (GSH), catalase (CAT) and malonaldehyde (MDA) in tissue homogenates of dermal wounds in diabetic rats. Group 1 rats were naïve normal-control with no wound, Group 2 (diabetic wound-control) rats were applied with gum acacia, group 3 (standard group) rats were applied with standard 1 % silver sulfadiazine (SSD) and group 4 (test group) rats were applied with test formulation APE cream (20 %) for 15 days daily. Statistical Analysis of the data were carried out using one-way analysis of variance (ANOVA) and Bonferroni’s post hoc test for average comparison on day 5 and 15. Data were presented as mean ± SEM. *p < 0.05, **p < 0.01 vs. naïve control group; #p < 0.05, ##p < 0.01 vs. wound control group
Histology
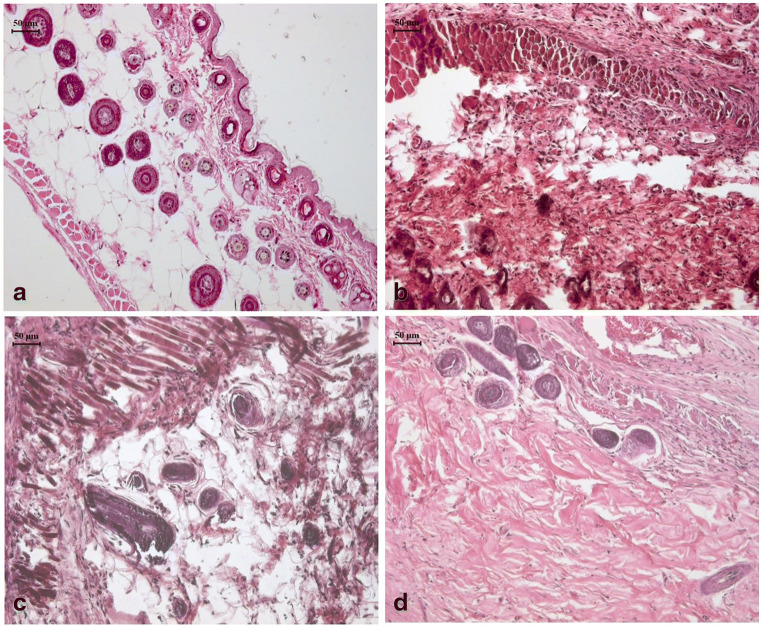
Photomicrograph are representing the rat skin section after wound created and 15 days topical treatment following H and E staining (Fig. 6). Control rat skin without wound showed normal histology with intact epidermis and dermis and normal architecture of adenexa. In diabetic rats, skin section from the wound area showed severe necrosis in epithelial cells, blood congestion, hair follicle degeneration and vacuolation in keratinocytes, edema and severe infiltration in the dermis. The initial fibrosis also visible. Topical application with standard (SSD) cream on wound in diabetic rats showed decreased level of infiltration in dermis as compare to that in vehicle application. Adenexal atrophy, hyperplasia of basal epidermis with initial proliferation of collagen tissue appearance of hair follicles are clearly visible. Similar effects were observed in the skin sections of rats treated with topical application of APE cream. It revealed application of APE cream accelerates fibrous tissue proliferation and hyperplasia in epidermis of basal layer which are the symbol of quick healing process.
Fig. 6.
Photomicrographs of skin section from the wound area of rats. Control rat skin without wound showed normal histology with intact epidermis and dermis and normal architecture of adenexa (a). In diabetic rats, skin section from the wound area showed severe necrosis in epithelial cells, blood congestion, hair follicle degeneration and vacuolation in keratinocytes, edema and severe infiltration in the dermis (b). Topical application with standard (silver sulfadiazine) cream on wound in diabetic rats showed decreased level of infiltration in dermis as compare to that in vehicle application (c). Similar effects were observed in the skin sections of diabetic rats treated with topical application of apple peel extract (APE) cream with maximum level of fibrous tissue proliferation and hyperplasia in epidermis of basal layer (d)
Discussion
Wound healing is a natural process that involves a series of complex cellular and biomolecular processes that restore damaged wound tissue into its original state when injury occurred. Altered wound healing is one of the most common complications in diabetes. The wound healing process in patients with diabetes is deteriorated due to hyperglycaemic conditions that lead to DFUs, a major chronic complications [6]. Abnormal wound healing often leads to chronic ulcer formation, which is a major reason of morbidity due to various clinical and socioeconomic issues. According to Brem and Canic [35], in diabetic subjects, delayed wound healing is a 15 % risk of getting foot ulcers, of which 85 % will have to undergo lower extremity amputations.
APE is the richest source of bioflavonoids having antioxidant and anti-inflammatory properties. There are several studies on the efficiency of apple and bioflavonoids in a wide variety of pharmacological activities, including antioxidant, anti-inflammation and wound healing [36]. Pang et al. [37] supported that the expression of biomarkers such as VEGF and TGF-β accelerated wound healing, which is attributed to the presence of flavonoids. Considering the therapeutic properties of APE, in vivo tests were employed to observe the efficacy of APE on wound excision in diabetic rats to reveal the underlying mechanism of APE on wound healing.
Topical administration of hydrocolloid films containing APE (5–20 %) on diabetic wounds gradually decreased wound surface area. Mechanism for this is that the action of APE, which was released from the hydrocolloid film dressing into the wound and then into the bloodstream, can increase host immunity and reduced production of the inflammatory markers. Previous studies have also reported similar observations, as cashew apple juice isolated from Anacardium occidentale was able to improve the immunological mechanisms as well as an optimal balance between ROS and antioxidants leading to a better wound healing process [36].
Following the topical application of APE-containing hydrocolloid film, the gross wound size of diabetic rats was found to be significantly reduced and was accompanied by dose-dependent wound contraction. This suggests that APE is effective in promoting wound healing in diabetic rats. Similar findings were reported in our previous studies, where topical application of flavonoid, quercetin comprised of APE enhanced the healing of excision wounds in diabetic rats through improvements in wound contraction and reductions in wound size [38].
Increased hexosamine content reflects the stabilization of collagen molecules via enhanced electrostatic and ionic interactions [39]. Hence, enhanced hydroxyproline and hexosamine synthesis provides strength to repaired tissue and stimulates healing. In present study, APE significantly increased hydroxyproline and hexosamine levels as compared to standard cream. The results are in consistent with the previous reports, where flavonoid extracts have shown to improve wound contraction and increased tensile strength by improving hexosamine and hydroxyproline expressions [40].
Evidence suggests that lipid peroxidation inhibition restores wound healing to nearly normal levels in experimental diabetes-impaired wounds and normalizes the defect in vascular endothelial growth factor (VEGF) regulation associated with diabetes-induced wound injuries. APE application significantly reduced MDA level in the wound tissues. Previous study reported that, apple extracts exhibited stronger phospholipid protective capacity [40] revealing a potential application of apple extracts to enhance the wound healing.
According to our data, APE has a beneficial effect in the prevention of experimental neuropathy in diabetic rats. In fact, we found a positive effect of APE on experimental model of diabetic neuropathy in Plantar test. Patterns of diabetes-related peripheral nerve abnormalities is the most common pattern of symmetric neuropathy that involves the injury to distal sensory and motor nerves; small sensory fiber neuropathy is often prominently affected in the form of peripheral neuropathy in diabetes mellitus. With progression of the disease, diabetic subjects developed decreased sensations in the distal extremities that will result later in the loss of pain sensation. Treatment of diabetic neuropathy is a major goal, but despite multiple attempts, no satisfactory management is yet available. Moreover, clinical data have shown that diabetic patients suffering from peripheral neuropathy manifest electrophysiological conduction abnormalities [41, 42]. The pathology of DPN also involves oxidative stress, accumulation of advanced glycation end products, and microvascular injuries. Oxidative stress is the cause as well as sequel of almost all major pathophysiological pathways in DPN. Growing attention has been paid to the pathogenic roles of oxidative stress in diabetic neurological complications. ROSs are generated by nonenzymatic protein glycation through a complex series of chemical and cellular intermediates [43]. Reduced GSH has been demonstrated to be an important marker for oxidative stress [44]. Decrease in GSH levels have been positively correlated with increased oxidative stress, which may result in neurodegeneration or neuronal damage leading to diabetic neuropathy. In accordance with the previous findings, we observed decreased GSH level in HFD-STZ animals as compared to the controlled animals suggesting T2D-associated oxidative stress. However, APE treated T2D animals showed an increase in GSH levels, which was comparable to control animals with T2D. These results suggest the antioxidant potential of APE in relieving oxidative stress to prevent neuronal damage in T2D. Oxidative stress and neuroinflammatory changes may be responsible for neuronal damage leading to loss of peripheral sensation. Thus, free radical scavengers and antioxidants have been shown to prevent neurodegeneration. Our results were largely consistent with previous evidences from in-vitro [45–47], in-vivo [48] and clinical studies [49] which has shown that polyphenols from apple juices possess a stronger neuroprotection than antioxidant vitamins. It also reduced oxidative stress and ameliorated neuro-inflammatory condition by varying TNF-α, IL-1β and nitrite levels as impaired in diabetic animals [50].
Collectively, our study shows that treatment with APE can ameliorate behavioral, neurophysiologic and pathological alterations induced by diabetes in the peripheral nerves of rats and an antioxidant-related mechanism contributed to the improvement of diabetic neuropathy. We found that APE-diet intake in diabetic rats provided protection against deterioration of the peripheral nerves and prevents wound infections. We conclude that antioxidant property is one of the major profiles that is implicated in neuron protection. Thus, use of APE may be considered as a potential preventative approach for peripheral diabetic neuropathy and diabetic foot injuries.
Conclusions
As the prevalence of diabetes and its complications continue to increase rapidly, there is an increasing need for the development of safe and effective functional bioactive compounds with antidiabetic effects. Our findings suggest that APE has a potential to be used as a therapeutic intervention for the clinical management of DPN and delayed wound healing in the subjects with diabetes. Nevertheless, further molecular and genetic studies are required to elucidate interactions between diabetes and DPN, and the separate, simultaneous effects of APE in diabetes induced-DPN and delayed wound healing would be performed in the future studies.
Acknowledgements
Authors are thankful to the Director, Faculty of Pharmacy, Pacific Academy of Higher Education and Research University, Udaipur, Rajasthan, India for providing necessary facilities to carry out the research activities. We are grateful to Dr. Ashish P. Bharne for his input and assistance during the research work.
Author contributions
SPK designed and conducted the research; SPK, AR and KTN analyzed the data and wrote the paper; and SPK had primary responsibility for the final content. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
There are no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed]
- 2.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(1):8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 3.Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006;82(964):95–100. doi: 10.1136/pgmj.2005.036137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyck PJ. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve. 1988;1(1):21–32. doi: 10.1002/mus.880110106. [DOI] [PubMed] [Google Scholar]
- 5.Vinik AI, Emley MS, Megerian JT, Gozani SN. Median and ulnar nerve conduction measurements in patients with symptoms of diabetic peripheral neuropathy using the NC-stat system. Diabetes Technol Ther. 2004;6(6):816–24. doi: 10.1089/dia.2004.6.816. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–62. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 7.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27(6):1458–86. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 8.Oso BJ, Abey N, Oyeleke MO, Olowookere B. Comparative study of the in vitro antioxidant properties of methanolic extracts of Chromolaena odorata and Ageratum conyzoides use in wound healings. Int Ann Sci. 2019;6(1):8–12. [Google Scholar]
- 9.Mekonnem W, Sidamo T, Asres K, Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petiana. J Ethnophramacol. 2013;145(2):638–46. doi: 10.1016/j.jep.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evid Based Complement Alternat Med. 2011;2011:1–17. doi: 10.1155/2011/438056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caskey RC, Zgheib C, Morris M, Allukiahj M, Dorsett-Martin W, Xu J, et al. Dysregulation of collagen production in diabetes following recurrent skin injury:Contribution to the development of chronic wound. Wound Repair Regen. 2014;22(4):515–20. doi: 10.1111/wrr.12199. [DOI] [PubMed] [Google Scholar]
- 12.Agyare C, Boakye YD, Bekoe EO, Hensel A, Oteng S, Appiah T. Review: African medicinal plants with wound healing properties. J Ethnopharmacol. 2016;177(4):85–100. doi: 10.1016/j.jep.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord. 2013;12(1):43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oszmiansk J, Wolniak M, Wojdyto A, Wawer I. Influence of apple purée preparation and storage on polyphenol contents and antioxidant activity. Food Chem. 2008;107(4):1473–84. [Google Scholar]
- 15.Hyson DA. A comprehensive review of apples and apple components and their relationship to human health. Adv Nutr. 2011;2(5):408–20. doi: 10.3945/an.111.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arabbi PR, Genovese MI, Lajolo FM. Flavonoids in vegetable foods commonly consumed in Brazil and estimated ingestion by the Brazilian population. J Agric Food Chem. 2004;52(5):1124–31. doi: 10.1021/jf0499525. [DOI] [PubMed] [Google Scholar]
- 17.Balasuriya N, Rupasinghe V, Sweeney M, McCarron S, Gottschall-Pass K. Antihypertensive effects of apple peel extract on spontaneously hypertensive rats. Pharmacologia. 2015;6(8):371–376. [Google Scholar]
- 18.Tian J, Wu X, Zhang M, Zhou Z, Liu Y. Comparative study on the effects of apple peel polyphenols and apple flesh polyphenols on cardiovascular risk factors in mice. Clin Exp Hypertens. 2018;40(1):65–72. doi: 10.1080/10641963.2017.1313851. [DOI] [PubMed] [Google Scholar]
- 19.Manzano M, Giron MD, Vilchez JD, Sevillano N, El-Azem N, Rueda R, et al. Apple polyphenol extract improves insulin sensitivity in vitro and in vivo in animal models of insulin resistance. Nutr Metab (Lond) 2016;13:32. doi: 10.1186/s12986-016-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fathy SM, Drees EA. Protective effects of Egyptian cloudy apple juice and apple peel extract on lipid peroxidation, antioxidant enzymes and inflammatory status in diabetic rat pancreas. BMC Complement Altern Med. 2016;16:8. doi: 10.1186/s12906-015-0957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–20. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Nakhate KT, Yedke SU, Bharne AP, Subhedar NK, Kokare DM. Evidence for the involvement of neuropeptide Y in the antidepressant effect of imipramine in type 2 diabetes. Brain Res. 2006;1646:1–11. doi: 10.1016/j.brainres.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 23.Goyal SN, Reddy NM, Patil KR, Nakhate KT, Ojha S, Patil CR, Agrawal YO. Challenges and issues with streptozotocin-induced diabetes - A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem Biol Interact. 2016;244:49–63. doi: 10.1016/j.cbi.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Bramante CT, Lee CJ, Gudzune KA. Treatment of obesity in patients with diabetes. Diabetes Spectr. 2017;30(4):237–43. doi: 10.2337/ds17-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang JY, Lin CH, Huang TH, Chuang SY. In vivo rodent models of type 2 diabetes and their usefulness for evaluating flavonoid bioactivity. Nutrients. 2019;28(3):530. doi: 10.3390/nu11030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hargreaves, et al.,A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. [DOI] [PubMed]
- 27.Hofmann AT, Neumann S, Ferguson J, Red H, Mittermayr R. A rodent excision model for ischemia-impaired wound healing. Tissue Eng Part C Methods. 2017;23(12):995–1002. doi: 10.1089/ten.TEC.2017.0212. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Kirar V, Keshri GK, Gola S, Yadav A, Negi PS, et al. Wound healing activity of an aqueous extract of the Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (higher Basidiomycetes) Int J Med Mushrooms. 2014;16(4):345–54. doi: 10.1615/intjmedmushrooms.v16.i4.50. [DOI] [PubMed] [Google Scholar]
- 29.Kumari M, Eesha BR, Amberkar M, Rajshekar SB, Kumar N. Wound healing activity of aqueous extract of Crotalaria verrucosa in Wistar albino rats. Asian Pac J Trop Med. 2010;3(10):783–7. doi: 10.1016/S1995-7645(10)60187-3. [DOI] [Google Scholar]
- 30.Murthy S, Gautam MK, Goel S, Purohit V, Sharma H, Goel RK. Evaluation of in vivo wound healing activity of Bacopa monniera on different wound model in rats. Biomed Res Int. 2013;2013:972028. doi: 10.1155/2013/972028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 32.Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 33.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 34.McManus JFA, Mowry RW. Stainning methods, histologic and histochemical. New York: Harper Raw; 1965. [Google Scholar]
- 35.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–22. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Silveira Vasconcelos M, Gomes-Rochette NF, de Oliveira ML, Nunes-Pinheiro DC, Tome AR, Maia de Sousa FY, et al. Anti-inflammatory and wound healing potential of cashew apple juice (Anacardium occidentale L.) in mice. Exp Biol Med (Maywood) 2015;240(12):1648–55. doi: 10.1177/1535370215576299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang Y, Zhang Y, Huang L, Xu L, Wang K, Wang D, et al. Effects and mechanisms of total flavonoids from Blumea balsamifera (L.) DC. on skin wound in rats. Int J Mol Sci. 2017;18(12):2766. doi: 10.3390/ijms18122766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin G, Wang Z, Wang Z, Wang X. Topical application of quercetin improves wound healing in pressure ulcer lesions. Exp Dermatol. 2018;27(7):779–86. doi: 10.1111/exd.13679. [DOI] [PubMed] [Google Scholar]
- 39.Dwivedi D, Dwivedi M, Malviya S, Singh V. Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in wistar rats. J Tradit Complement Med. 2017;7(1):79–85. doi: 10.1016/j.jtcme.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu H, Qin C, Zhang P, Ge Q, Wu M, Wu J, et al. Antioxidant effect of apple phenolic on lipid peroxidation in Chinese-style sausage. J Food Sci Technol. 2015;52(2):1032–9. doi: 10.1007/s13197-013-1127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zangiabadi N. The effect of omega-3 fatty acids on nerve conduction velocity (NCV) and F-wave latency in patients with diabetic polyneuropathy. Am J Pharmacol Toxicol. 2007;2(1):1–3. [Google Scholar]
- 42.Coste TC, Gerbi A, Vague P, Maixent JM, Pieroni G, Raccah D. Peripheral diabetic neuropathy and polyunsaturated fatty acid supplementations: natural sources or biotechnological needs? Cell Mol Biol (Noisy-le-grand). 2004;50(7):845–53. [PubMed]
- 43.Greene DA, Stevens MJ, Obrosova I, Feldman EL. Glucose-induced oxidative stress and programmed cell death in diabetic neuropathy. Eur J Pharmacol. 1999;375(1–3):217–23. doi: 10.1016/s0014-2999(99)00356-8. [DOI] [PubMed] [Google Scholar]
- 44.Pocernich CB, Butterfield DA. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochi Biophys Acta. 2012;1822(5):625–30. doi: 10.1016/j.bbadis.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heo HJ, Lee CY. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J Agric Food Chem. 2004;52(25):7514–17. doi: 10.1021/jf049243r. [DOI] [PubMed] [Google Scholar]
- 46.Heo HJ, Kim DO, Shin SC, Kim MJ, Kim BG, Shin DH. Effect of antioxidant flavanone, naringenin, from Citrus junosonneuroprotection. J Agric Food Chem. 2004;52(6):1520–5. doi: 10.1021/jf035079g. [DOI] [PubMed] [Google Scholar]
- 47.Nakayama T, Yamada M, Osawa T, Kawakishi S. Suppression of active oxygen-induced cytotoxicity by flavonoids. Biochem Pharmacol. 1993;45(1):265–7. doi: 10.1016/0006-2952(93)90402-i. [DOI] [PubMed] [Google Scholar]
- 48.Patil CS, Singh VP, Satyanarayan PS, Jain NK, Singh A, Kulkarni SK. Protective effect of flavonoids against aging- and lipopolysaccharide-induced cognitive impairment in mice. Pharmacology. 2003;69(2):59–67. doi: 10.1159/000072357. [DOI] [PubMed] [Google Scholar]
- 49.O’Byrne DJ, Devaraj S, Grundy SM, Jialal I. Comparison of the antioxidant effects of Concord grape juice flavonoids alpha-tocopherol on markers of oxidative stress in healthy adults. Am J Clin Nutr. 2002;76(6):1367–74. doi: 10.1093/ajcn/76.6.1367. [DOI] [PubMed] [Google Scholar]
- 50.Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB. Fruit and vegetable juices and Alzheimer’s disease: the Kame Project. Am J Med. 2006;119(9):751–9. doi: 10.1016/j.amjmed.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]