Abstract
Purpose
Selenium (Se) is a trace element having significant effects on human metabolism. Recent studies suggest that Se supplementation have a pivotal effect on the inflammatory markers. Therefore, the aim of this study was to assess the effect of Se supplementation on plasma inflammatory markers including C-reactive protein (CRP) and high-sensitivity C-reactive protein (hs-CRP) and nitric oxide (NO) as a stress oxidative index, among patients with metabolic diseases.
Methods
To assess the effects of Se on the inflammatory markers, following the PRISMA-P guidelines, we systematically searched ISI/WOS, PubMed/MEDLINE, and Scopus for studies that assessed the effect of Se supplementation on the inflammatory markers. Data extraction was performed by two independent investigators. Using the random effects or fixed-effects model depending on the results of heterogeneity tests was used to estimate the pooled standardized mean difference (SMD). Heterogeneity between studies was assessed using Cochran's Q test and I2 index.
Results
The initial search revealed 3,320 papers. After screening process and considering inclusion criteria, 7 publications were eligible for inclusion in the meta-analysis. The meta-analysis results showed that Se supplementation did not significantly affect CRP and hs-CRP concentrations (mean difference (MD) = -0.15; 95% CI: -0.55- 0.23; P = 0.43). Subgroup analysis of CRP type showed that Se supplementation significantly decreased hs-CRP level (pooled SMD = -0.44; 95% CI: -0.67–0.21). Moreover, no significant change was observed in NO level by continuing to take Se supplementation, (pooled SMD: 0.003, 95%CI: -0.26, 0.26).
Conclusions
This study revealed that Se supplementation would have desirable effects on cardio-metabolic indicators through affecting the levels of inflammatory markers. Given the importance of concerns, more attention should be given to more prospective studies with longer follow-up.
Keywords: Selenium, Supplementation, Inflammatory markers, Inflammation, Systematic review
Background
The trace mineral selenium (Se) is an essential micronutrient and plays important biological roles in human body [1, 2]. This essential mineral must be obtained through nutritional diet. In this regard, evidence reveal that, low Se level may be related to increased risk of adverse health outcomes, such as cancer and cardiovascular disease (CVD) [3]. Evidence shows that Se is also essential for optimal functioning of the CVD system [4].
Selenium can affect the inflammatory processes; it is one of the most interesting research fields. Se also regulates the progression of many infectious diseases [4]. The antioxidant nature of some selenoproteins like glutathione peroxidase, is discussed as predisposing factors for many morbidities and mortalities such as carcinogenesis and heart diseases [5–7].
Previous studies have suggested that the Se supplementation through inhibiting the production of advanced glycation end products and decreasing the free radical production and lipid hydroperoxide could affect inflammatory mechanisms, glucose homeostasis, and oxidative stress [8]. In many cases, increasing serum levels of Se and the correction of required prescribed doses might be accompanied by interested results in reducing the risk of many diseases [6, 9, 10]. Metabolic diseases, such as diabetes, metabolic syndrome, obesity, and cardiovascular diseases are associated with increased inflammation in the body, leading to increasing inflammatory markers like C-reactive protein (CRP), nitric oxide (NO) and high-sensitivity C-reactive protein (hs-CRP). In this case, some evidence has shown that Se can reduce inflammation and decrease the rate of metabolic diseases [11–18].
Despite the importance of Se effects on related complications of metabolic disorders, such as the significant disability, increased dependency, reduced quality of life and increased economic burden of health care costs, there is still an evident gap among the related scientific literature, practical experiences and proposed plans [19–23]. Therefore, present paper provides the comprehensive systematic review on the probable effects of Se supplementation on inflammatory and stress oxidative markers among patients with metabolic diseases.
Methods
To assess the effect of Se on inflammatory markers, following the PRISMA-P guide-lines, we developed a systematic review which aggregated and analyzed all related evidence. The details of the study protocol have previously been published [16].
The review question
Considering the main question of the effect of Se on inflammatory and stress oxidative markers, this review investigated the effect of Se supplementation on the inflammatory and stress oxidative markers including CRP, NO, and hs-CRP.
PICOS: we considered adults and adolescents with metabolic diseases as population (P); Se supplementation as intervention (I); patients with metabolic diseases and taking placebo as comparator (C); plasma inflammatory markers including CRP and hs-CRP and NO as a stress oxidative index as outcome (O), and clinical trial as study design (S).
Inclusion and exclusion criteria
Studies that met the following criteria were included in the meta-analysis: [1] clinical trials in which control group received the placebo, [2] studies assessing the effect of se supplements or Se-enriched yeast on inflammatory markers in subjects with metabolic disorders; and [3] Se could be used as mono therapy or even in combination therapy. Duplicate publications and irrelevant papers considering other inflammatory markers or dietary Se instead of Se supplementation or studies conducted on other diseases were excluded from the study.
Search strategy and locate studies
We systematically searched the databases of PubMed, EMBASE, MEDLINE, Cochrane Library, ISI/WOS, and Scopus for relevant papers. The search terms developed based on the topics of research question. The search strategy included: "inflammation"[Mesh] OR "inflammatory factors"[Title/Abstract] OR “C-reactive protein”[Title/Abstract] OR CRP[Title/Abstract] OR “nitric oxide”[Title/Abstract] OR NO[Title/Abstract] OR “high-sensitivity C-reactive protein” [Title/Abstract] OR hs-CRP[Title/Abstract] combined with "Se"[Mesh] OR "se"[Title/Abstract] OR selenium[Title/Abstract] combined with “metabolic syndrome”[Title/Abstract] OR “cardiovascular diseases”[Title/Abstract] OR diabetes[Title/Abstract] OR GDM[Title/Abstract] OR PCOS[Title/Abstract] OR obesity[Title/Abstract], restricted to "human subject". The same process followed in other databases.
All of the relevant review articles and meta-analysis were checked for their references. If there were more than one paper from a specific study, the one with more complete data was considered. There was no limitation for language. Searches were done on March 2021. As other resources, all publications’ references were checked.
Select studies
To establish the inter-rater reliability (IRR), a comprehensive list of abstracts was retrieved and reviewed by two independent investigators. Possible disagreements and controversies were resolved by discussion and consensus or even third investigator. Relevant eligible papers obtained were reviewed in full.
Assess study quality
Using comprehensive recommended guidelines of the Consolidated Standards of Reporting Trials (CONSORT) 2010, 25-items checklist [24], study quality was assessed by two independent research experts.
Data extraction
Using a data extraction form, according to the study groups, the information was extracted from each study for citation, type of study, study subjects, publication year, sample size, dose of supplementation, intervention group, control group, mean age of participant, outcome, intervention duration, follow up duration, measurement interval, result, effect size.
Statistical analysis
The mean change (endpoint from baseline) and standard deviation (SD) in inflammatory markers for both intervention and placebo groups were used to calculate the standardized mean difference (SMD) as effect size. We calculated the SMD using the means and SD of the two groups using a previously described method [18].
Meta-analysis was performed to estimate the pooled SMD with the 95% confidence interval (CI) of the effect of Se supplementation on the inflammatory markers. The Cochran's Q test and the I2 statistic were used to assess the heterogeneity between studies. The result of Q test was considered as statistically significant at 0.1. Either a fixed-effects or, in the presence of heterogeneity, a random-effect model was used to calculate pooled SMD [25]. The I2 statistic also was used to quantify the degree of heterogeneity between studies, which I2 values of 25%, 50%, and 75% were considered to correspond to low, medium, and high levels of heterogeneity, respectively [26]. Subgroup analysis was performed based on CRP types and dose of Se supplementation. We performed sensitivity analysis to evaluate the extent to which the pooled SMD depending on excluding studies with low quality score. Publication bias was estimated visually and statistically using Begg's funnel plots and Egger’s test, respectively and results of Egger’s test was considered as statistically significant at 0.1 [27]. The statistical analysis was performed using STATA version 10.
Results
Search result & characteristics of included studies
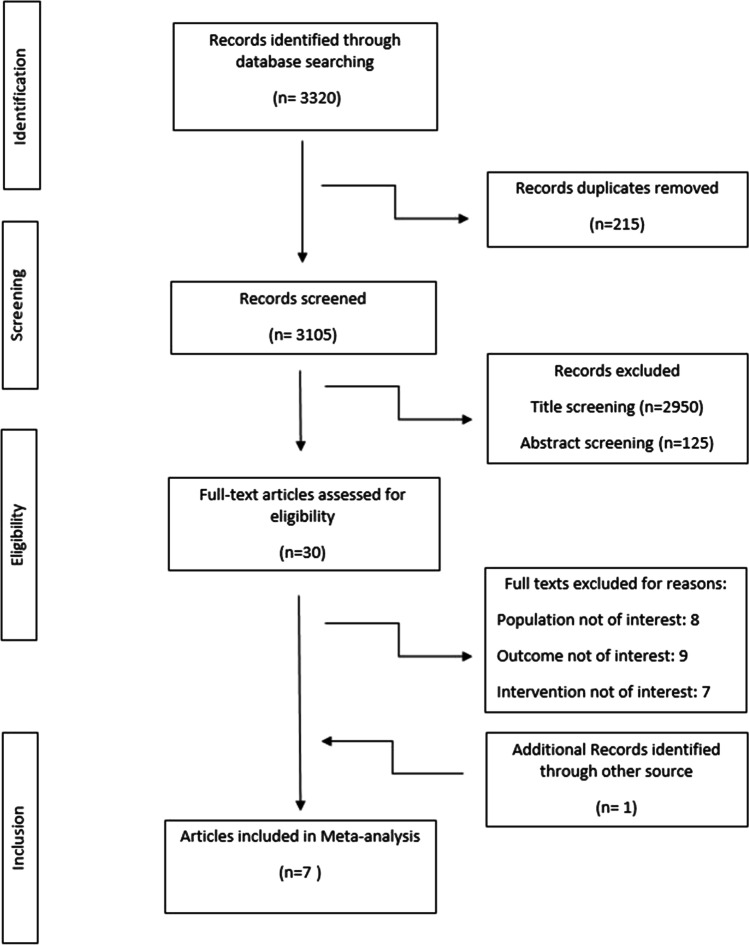
Figure 1 shows the detailed processes of the search and study selection. A total of 7 trials [6 randomized controlled trials and 1 crossover trial] were included in this study (Table 1). The initial search revealed 3,320 papers. After excluding the duplicate studies, 3105 publications were screened based on title and abstract. Totally, 30 publications were assessed for eligibility based on the study inclusion and exclusion criteria. Finally, 7 articles were included in the meta-analysis. Other studies were excluded based on different types of population, outcome, and intervention (Fig. 1).
Fig. 1.
Flow chart of the articles selected for mete-analysis
Table 1.
Characteristics of the included studies in the systematic review
| Author, year | Country | Type of study | Study subject | Sample size | Dose of supplementation | Intervention group | Control group | Mean age or age range(Y) | Outcome | Intervention duration | Measurement interval | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Asemi, 2015 | Iran | RCT | GDM Women |
I = 35 C = 35 |
200 µg/d | MT | placebo | 28.6 ± 4.6 | hs-CRP (ng/mL)NO (mmol/L) | 6 weeks from weeks 24 to 28 of gestation | Baseline and end of study |
| 2 | Shargorodsky, 2010 | Israel | RCT | Patient with > 2 CVD RFs |
I = 36 C = 34 |
100 µg/d | CT | placebo | 62.17 ± 6.21 |
hs-CRP(mg/dl) Homocysteine(μmol/l) Ur cathacholamines(mg/24 h) |
6 months | Base line and end of study |
| 3 | Ravn-Haren, 2007 | Denmark | R cross over |
Diabetic Men |
I = 20 C = 20 |
300 µg/d | CT | placebo | 26·8 | CRP | 1 week | Baseline and after 1 week and end of study |
| 4 | Alizadeh, 2012 | Iran | RCT | Premenopausal women with central obesity |
I = 17 C = 17 |
200 µg/d | CT | placebo | 33.9 ± 8.5 | hs-CRP, mg/l NOx, _mol/l |
2 weeks run-in period 6 weeks intervention period |
Baseline and after 3, and 6 weeks |
| 5 | Murer, 2014 | Switzerland | RCT | Obese children and adolescents |
I = 23 C = 21 |
50 µg/d | CT | placebo | 12.7 ± 1.5 | CRP,2 mg/L serum | 4 months | Baseline and after 4 months |
| 6 | Razavi, 2016 | Iran | RCT | PCOS patients |
I = 32 C = 32 |
200 µg/d | MT | placebo | 18–40 |
hs-CRP (ng/ml) NO (μmol/l) |
8 weeks | Baseline and after 2 months |
| 7 | Farrokhian, 2016 | Iran | RCT | Patients with type 2 diabetes & CVD | I = 30 C = 30 | 200 µg/d | MT | placebo | 40–85 | hs-CRP (ng/ml) NO (μmol/l) | 8 weeks | Baseline and after 2 months |
Overall, 193 participants were included in the Se supplementation group and 189 participants in the placebo group. The age of the patients ranged from 10 to 85 years. Four trials recruited both men and women and in other three studies only female subjects were enrolled [10, 28, 29]. Four trials used Se combined with other vitamins or minerals [3, 9, 10, 30] and 3 trials used Se alone as oral supplement. The daily dose of Se were 200 mg/day in four trials [10, 12, 28, 29], one trial used 300 mg/day [3], one RCT used 50 mg/day [12] and one study used 100 mg/day [9]. Four trials were conducted in Iran and 3 in Europe. All RCTs were placebo-controlled, and all were double-blinded. Duration of treatment ranged from 42 [28] to 168 days [9]. The effect of se supplementation on the inflammatory markers in included studies is presented in Table 2. The effect of Se supplementation on inflammatory markers was statistically significant in four studies.
Table 2.
Effect of selenium supplementation on inflammatory markers in included studies
| Author, year | Outcome | Results | ||||
|---|---|---|---|---|---|---|
| Group | Mean change ± SD | significance | SMD | |||
| 1 | Asemi, 2015 | hs-CRP (ng/mL) |
I: -791.88 ± 2271.84 P: 500.55 ± 2563.34 |
Yes | -0.53 | |
| NO (mmol/L) |
I: 20.91 ± 65.32 P: 5.03 ± 51.68 |
No | 0.26 | |||
| 2 | Shargorodsky, 2010 | hs-CRP(mg/dl) |
I: -1.32 ± 2.8 P: -0.49 ± 1.4 |
No | -0.37 | |
| Homocysteine(μmol/l) |
I: -0.05 ± 2.83 P: 0.36 ± 3.2 |
No | -0.13 | |||
| Ur cathacholamines (mg/24 h) |
I: 3.76 ± 20 P: 2.46 ± 18 |
No | 0.07 | |||
| 3 | Ravn-Haren, 2007 | CRP |
I: 0.08 ± 1.15 P: -0.08 ± 0.65 |
No | 0.17 | |
| 4 | Alizadeh, 2012 | hs-CRP, mg/l |
I: 0 ± 1.45 P: -0.3 ± 1.64 |
No | 0.19 | |
| NOx, _mol/l |
I: -8 ± 58.6 P: 3.7 ± 32.5 |
Yes | -0.24 | |||
| 5 | Murer, 2014 | CRP,2 mg/L serum |
I: 5.52 ± 8.3 P: -1.9 ± 4.5 |
No | 1.11 | |
| 6 | Razavi, 2016 | hs-CRP (ng/ml) |
I: − 711.3 ± 1959.3 P: 193.5 ± 1117.4 |
Yes | -0.56 | |
| NO (μmol/l) |
I: 3.85 ± 13.37 P: 0.07 ± 23.38 |
No | 0.19 | |||
| 7 | Farrokhian, 2016 | hs-CRP (ng/ml) |
I: − 1 372.3 ± 2 318.8 P: − 99.8 ± 1 453.6 |
Yes | -0.76 | |
| NO (μmol/l) |
I: − 8.9 ± 16.7 P: − 4.3 ± 9.3 |
No | -0.34 | |||
I Intervention group, C Control group, hs-CRP, high-sensitivity C-reactive protein, CRP C-reactive protein, NO nitric oxide, SMD standardized mean difference
Meta-analysis and sub group analysis
Overall, five trials involving 298 participants in Se or placebo groups reported the effect of Se supplementation on hs-CRP level and 2 studies assessed the effect of Se supplementation on the CRP. Four studies reported level of NO as an outcome at baseline and follow-up (Table 3).
Table 3.
Meta-analysis of effect of selenium supplementation on inflammatory profile
| Inflammatory variables | Group (Number)a | Number of study | Pooled SMD ( 95% CI) | Model | Heterogeneity assessment | ||
|---|---|---|---|---|---|---|---|
| I2 | Q test | P-value | |||||
| NO |
I = 114 P = 114 |
4 | 0.003 (-0.26, 0.26) | Fixed | 28.4% | 4.19 | 0.24 |
| hs-CRP |
I = 150 P = 148 |
5 | -0.44 (-0.67,-0.21)* | Fixed | 12.6% | 4.58 | 0.33 |
| CRP |
I = 43 P = 41 |
2 | 0.6(0.16, 1.04)* | Random | 73.2% | 3.73 | < 0.001 |
I Intervention group, C Control group, hs-CRP, high-sensitivity C-reactive protein, NO, nitric oxide, I2 I-square index, Q Cochran's Q test
*Statistically significant
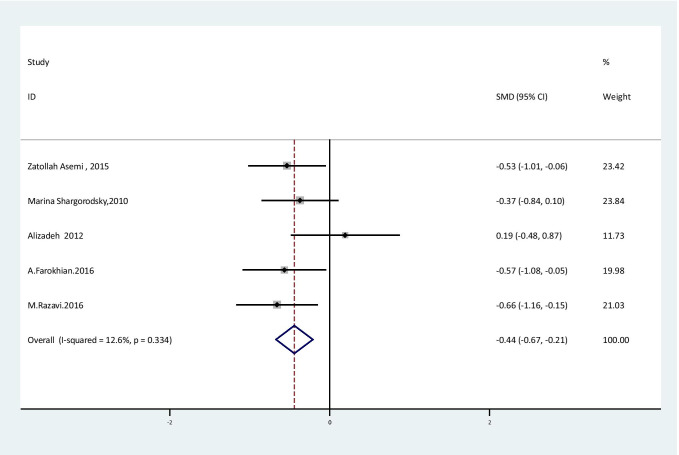
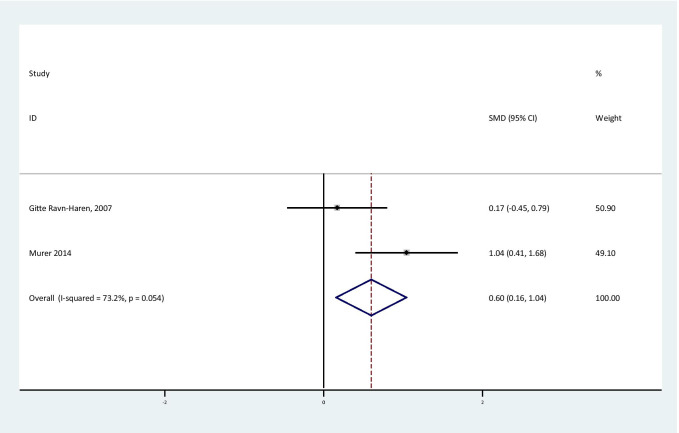
The overall effect of Se supplementation on the level of inflammatory markers is shown in Table 3. The results of the random effect meta-analysis showed that Se supplementation led to a significant reduction in hs-CRP levels [SMD = -0.44; 95% CI: (-0.67,-0.21); P < 0.001] in subjects with metabolic diseases with no obvious heterogeneity between studies [I2:12.6%, Q test: 4.58; P: 0.33] (Fig. 2). CRP level was significantly increased, but it should be considered that combining two effect size was not reliable [SMD = 0.6; 95% CI: (0.16, 1.04); P < 0.001] (Fig. 3).
Fig. 2.
Forest plot of randomized controlled trials to investigate the effect of Selenium supplementation on levels of hs-CRP
Fig. 3.
Forest plot of randomized controlled trials to investigate the effect of Selenium supplementation on levels of CR
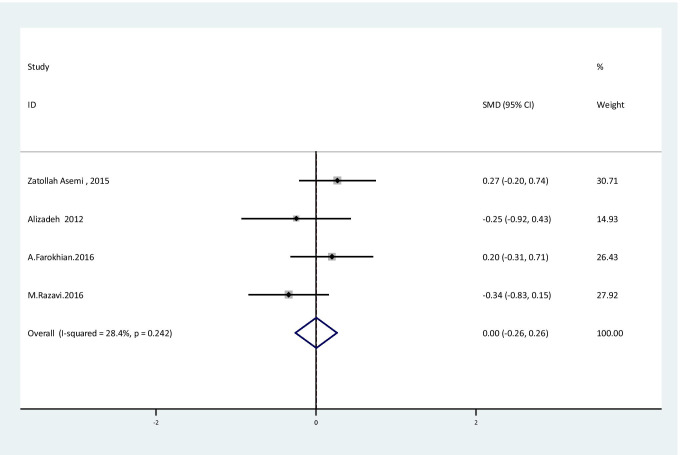
The results of fixed effect meta-analysis showed that there were no significant improvement in NO levels [SMD: 0.003, 95%CI: (-0.26, 0.26); P = 0.98] with no obvious heterogeneity between studies [I2:28.4%, Q test: 4.19; P: 0.24] following Se supplementation (Fig. 4).
Fig. 4.
Forest plot of randomized controlled trials to investigate the effect of Selenium supplementation on levels of NO
In subgroup analysis, according to dose of Se supplementation (< 200 µg/d and > = 200 µg/d), the effect of Se supplementation on all inflammatory markers (CRP, hs-CRP and NO) did not change significantly. Unfortunately, due to both the lack of studies in this field and a lack of the available data, it was not possible for us to perform subgroup analysis based on other effective factors.
Quality assessment
Table 4 shows the quality of included studies. Three studies were classified as high quality, with CONSORT score higher than 30 [10, 12, 28], 3 as medium quality, with CONSORT score in range of 25–29 [9, 29, 30], and 1 as low quality, with CONSORT score lower than 25 [3]. Randomization as a prerequisite for inclusion in this meta-analysis was conducted in 7 studies. All 6 RCTs were double-blind, but only two studies was described as blinding [12, 29].
Table 4.
Quality assessment of included studies according to the CONSORT checklist
| Zatollah Asemi, 2015 | Marina Shargorodsky, 2010 | lizadeh 2012 | Gitte Ravn-Haren, 2007 | Murer 2014 | M. Razavi, 2016 | A. Farrokhian, 2016 | |
|---|---|---|---|---|---|---|---|
| 1a | Yes | No | Yes | Not applicable | Yes | No | Yes |
| 1b | Yes | Yes | Yes | Yes | No | Yes | Yes |
| 2a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2b | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3b | No | No | No | No | No | No | No |
| 4a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4b | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6b | No | No | No | No | No | No | No |
| 7a | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 7b | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 8a | Yes | No | Yes | Not applicable | Yes | Yes | Yes |
| 8b | No | No | Yes | Not applicable | Yes | Yes | Yes |
| 9 | Yes | No | Yes | Not applicable | Yes | Yes | Yes |
| 10 | Yes | No | No | Not applicable | No | Yes | Yes |
| 11a | No | No | No | Not applicable | No | Yes | Yes |
| 11b | No | No | No | No | No | No | No |
| 12a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12b | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 13a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 13b | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 14a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 14b | No | No | No | No | No | No | No |
| 15 | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 16 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 17a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 17b | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 18 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 19 | Yes | Yes | Yes | No | Yes | No | Yes |
| 20 | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 21 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 22 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 23 | Yes | No | Yes | Not applicable | Yes | No | Yes |
| 24 | No | No | No | No | No | No | No |
| 25 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total | 30 | 25 | 30 | 20 | 29 | 28 | 32 |
|
(1a) Identification as a randomized trial in the title (1b) Structured summary of trial design, methods, results, and conclusions (2a) Scientific background and explanation of rationale (2b) Specific objectives or hypotheses (3a) Description of trial design (such as parallel, factorial) including allocation ratio (3b) Important changes to methods after trial commencement (such as eligibility criteria), with reasons (4a) Eligibility criteria for participants (4b) Settings and locations where the data were collected (5) The interventions for each group with sufficient details to allow replication, including how and when they were actually administered (6a) Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed (6b) Any changes to trial outcomes after the trial commenced, with reasons (7a) How sample size was determined (7b) When applicable, explanation of any interim analyses and stopping guidelines (8a) Method used to generate the random allocation sequence (8b) Type of randomization; details of any restriction (such as blocking and block size) (9) Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned (10) Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions (11a) If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how (11b) If relevant, description of the similarity of interventions (12a) Statistical methods used to compare groups for primary and secondary outcomes (12b) Methods for additional analyses, such as subgroup analyses and adjusted analyses (13a) For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome (13b) For each group, losses and exclusions after randomization, together with reasons (14a) Dates defining the periods of recruitment and follow-up (14b) Why the trial ended or was stopped (15) A table showing baseline demographic and clinical characteristics for each group (16) For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups 17a) For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) 17b) For binary outcomes, presentation of both absolute and relative effect sizes is recommended (18) Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory (19) All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) (20) Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses (21) Generalizability (external validity, applicability) of the trial findings (22) Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence 23) Registration number and name of trial registry (24) Where the full trial protocol can be accessed, if available (25) Sources of funding and other support (such as supply of drugs), role of funders | |||||||
Sensitivity analysis
Sensitivity analysis was performed according to the quality assessment score. Based on this analysis, the effect of Se supplementation on the level of inflammatory factors was evaluated by excluding low quality score studies including the Ravn-Haren et al. [3]. Results showed that the effect of Se supplementation on hs-CRP levels did not change significantly without this study in metabolic diseases.
Publication bias
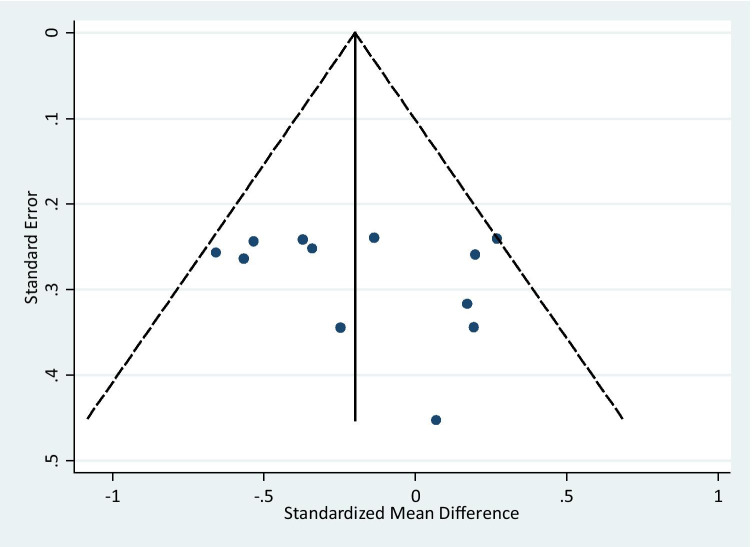
Publication bias was estimated by the Egger’s test. No evidence of publication bias was observed in the inflammatory markers after Se supplementation (coefficient = 4.9, P = 0.18). Figure 5 shows the funnel plot of the effect of Se supplementation on the level of inflammatory markers confirming that no publication bias exist between studies.
Fig. 5.
Funnel plot of randomized controlled trials to investigate the effect of Selenium supplementation on inflammatory markers
Discussion
Despite of studies conducted, the probable effects of Se supplementation on inflammatory markers remained a question. This is the first systematic review to date to assess association between Se supplementation and the inflammatory markers. Of total 3320 searched papers after screening, 7 studies were eligible for final analysis. The findings provide evidence-based document for health practitioners and policy makers.
Based on the included data of Se supplementation, it is revealed that the Se supplementation significantly decreased the hs-CRP levels. On the other hand, there was no significant improvement in level of NO as an oxidative stress marker with obvious heterogeneity by Se supplementation.
Considering the related literature, another trial showed the effect of the Se supplementation on reproductive outcomes, biomarkers of inflammation, and oxidative stress among women with polycystic ovary syndrome (PCOS) [29].
In a study, supplementation of Se in daily dietary programs of pregnant women with Gestational Diabetes (GDM) resulted in better glucose homeostasis, reduced inflammation, and improved oxidative stress [28]. Considering the probable mechanisms, these beneficial effects on improvement of glucose homeostasis may be rooted from its effect on the inhibition of inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1 [31]. In patients with type 2 Diabetes mellitus (T2DM) and coronary heart disease (CHD), effects of Se supplementation on metabolic status were evaluated through prescription of a 200 μg/day Se supplementation. This approach resulted in a significant decrease in insulin, HOMA-IR, HOMA-B, serum hs-CRP, QUICKI score and TAC concentrations [12].
The findings of studies conducted provide desirable effects of Se supplementation on cardiovascular risk factors, such as oxidative stress and insulin resistance [10].
Researcher confirmed that, regular administration of antioxidant supplements including vitamin C, vitamin E, coenzyme and Se could significantly increase the large and small artery elasticity in patients with multiple cardiovascular risk factors. Glucose and lipid metabolism and hypertension were also improved by these supplementations [9].
As a practical point for achieving the desired responses, the duration of intervention was discussed in many studies [3]. A group of researchers reported that according to their studies, short-term Se supplementation could not seem to be effective in blood lipid markers. This uses also in expression and activity of selected enzymes and a transcription factor involved in processes of glutathione-mediated detoxification and anti-oxidation [3]. In a practical trial, antioxidant supplementation with antioxidant and Se for four months significantly led to improvement of liver function tests as well as antioxidant-oxidant balance; however, it had no significant effects on the systemic inflammatory markers [30].
As the main strength, the present study is the first comprehensive systematic review of the effects of Se supplementation on the inflammatory markers. Using standard comprehensive systematic search approaches, we found and assessed all available related sources of data. The limitation of this study was the limited measured outcome variables conducted in scattered studies. Therefore, we faced with challenges for the appropriate comparison of available data. Also, it should be considered that different methodological designs (randomized controlled trials and cross over) of included studies can influence our findings.
Conclusions
The results of present study demonstrated that Se supplementation significantly decreased hs-CRP levels, but there was no significant improvement in the level of NO. As a practical application in the field of policy-related research, further studies on different mechanisms, different patterns of Se supplementation and various biochemical outcomes are recommended.
Acknowledgements
The authors are thankful of the team working on this study and all participants who made this experience.
Abbreviations
- SMD
Standardized mean difference
- CI
Confidence interval
- Se
Mineral selenium
- CRP
C-reactive protein
- IL
Interleukins
- TNFα
Tumor necrosis factor-α
- RCTs
Randomized control trials
- PCOS
Polycystic ovary syndrome
- GDM
Gestational Diabetes
- CHD
Coronary heart disease
Authors' contributions
The concept of this study was proposed by S.D., M.Q., This study was designed S.D., M.Q., M.H., Data extraction was done by H.A., H.-S.E., A.K., M.Z., F.B., H.R., A.M.G., Analysis or interpretation was performed by M.Q., S.D, Literature search was done by S.D. This study was written by S.D., M.Q. All authors read and approved the final manuscript.
Funding
This study was funded by the Alborz University of Medical Sciences.
Data availability
The dataset supporting the conclusions of this article is included within the article.
Declarations
Conflict of Interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rayman MP. The importance of selenium to human health. The lancet. 2000;356(9225):233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 2.Faghihi T, Radfar M, Barmal M, Amini P, Qorbani M, Abdollahi M, et al. A randomized, placebo-controlled trial of selenium supplementation in patients with type 2 diabetes: effects on glucose homeostasis, oxidative stress, and lipid profile. Am J Ther. 2014;21(6):491–495. doi: 10.1097/MJT.0b013e318269175f. [DOI] [PubMed] [Google Scholar]
- 3.Ravn-Haren G, Bügel S, Krath B, Hoac T, Stagsted J, Jørgensen K, et al. A short-term intervention trial with selenate, selenium-enriched yeast and selenium-enriched milk: effects on oxidative defence regulation. Br J Nutr. 2008;99(4):883. doi: 10.1017/S0007114507825153. [DOI] [PubMed] [Google Scholar]
- 4.Sun W, Wang Q, Guo Y, Zhao Y, Wang X, Zhang Z, et al. Selenium suppresses inflammation by inducing microRNA-146a in Staphylococcus aureus-infected mouse mastitis model. Oncotarget. 2017;8(67):110949. doi: 10.18632/oncotarget.20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Bayoumy K, Sinha R, Richie JP., Jr Forms of selenium in cancer prevention. Diversity of Selenium Functions in Health and Disease. 2015;38:137. [Google Scholar]
- 6.Nicastro HL, Dunn BK. The Selenium and Vitamin E Cancer Prevention Trial (SELECT): prevention of prostate cancer using selenium and/or vitamin E in the SELECT cancer prevention trial. Selenium2015. p. 428–57.
- 7.Benstoem C, Goetzenich A, Kraemer S, Borosch S, Manzanares W, Hardy G, et al. Selenium and Its Supplementation in Cardiovascular Disease—What do We Know? Nutrients. 2015;7(5):3094. doi: 10.3390/nu7053094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alissa E, Bahijri S, Ferns G. The controversy surrounding selenium and cardiovascular disease: a review of the evidence. Medical science monitor: international medical journal of experimental and clinical research. 2003;9(1):RA9. [PubMed]
- 9.Shargorodsky M, Debby O, Matas Z, Zimlichman R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr Metab. 2010;7:55. doi: 10.1186/1743-7075-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alizadeh M, Safaeiyan A, Ostadrahimi A, Estakhri R, Daneghian S, Ghaffari A, et al. Effect of L-arginine and selenium added to a hypocaloric diet enriched with legumes on cardiovascular disease risk factors in women with central obesity: a randomized, double-blind, placebo-controlled trial. Ann Nutr Metab. 2012;60(2):157. doi: 10.1159/000335470. [DOI] [PubMed] [Google Scholar]
- 11.Abramson SB. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res Ther. 2008;10(2):S2. doi: 10.1186/ar2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrokhian A, Bahmani F, Taghizadeh M, Mirhashemi S, Aarabi M, Raygan F, et al. Selenium supplementation affects insulin resistance and serum hs-CRP in patients with type 2 diabetes and coronary heart disease. Hormone and metabolic research= Hormon-und Stoffwechselforschung= Hormones et metabolisme. 2016;48(4):263. [DOI] [PubMed]
- 13.Kamath DY, Xavier D, Sigamani A, Pais P. High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: An Indian perspective. Indian J Med Res. 2015;142(3):261–268. doi: 10.4103/0971-5916.166582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luan Y-y, Yao Y-m. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Frontiers in Immunology. 2018;9(1302). [DOI] [PMC free article] [PubMed]
- 15.Morrow DA, Ridker PM. C-reactive protein, inflammation, and coronary risk. The Medical clinics of North America. 2000;84(1):149–61, ix. [DOI] [PubMed]
- 16.Djalalinia S, Khosravi M, Hasani M, Moghaddam SS, Atoofi MK, Mahdavi-Gorabi A, Noroozi M, Qorbani M, Asayesh H, Soleimani A. Effects of selenium supplementation on cardiometabolic risk factors, inflammatory, and antioxidant markers: A systematic review and meta-analysis protocol. International Journal of Preventive Medicine. 2019;10. [DOI] [PMC free article] [PubMed]
- 17.Razban MM, Eslami M, Bagherzadeh A. The relationship between serum levels of hs-CRP and coronary lesion severity. Clujul Med. 2016;89(3):322–326. doi: 10.15386/cjmed-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Frontiers in immunology. 2018;9:754-. [DOI] [PMC free article] [PubMed]
- 19.Wei J, Zeng C, Gong Q-y, Yang H-b, Li X-x, Lei G-h, et al. The association between dietary selenium intake and diabetes: a cross-sectional study among middle-aged and older adults. Nutrition Journal. 2015;14. [DOI] [PMC free article] [PubMed]
- 20.Lu C-W, Chang H-H, Yang K-C, Kuo C-S, Lee L-T, Huang K-C. High serum selenium levels are associated with increased risk for diabetes mellitus independent of central obesity and insulin resistance. BMJ Open Diabetes Research & Care. 2016;4(1). [DOI] [PMC free article] [PubMed]
- 21.Heinrich CJ. Evidence-based policy and performance management: Challenges and prospects in two parallel movements. The American Review of Public Administration. 2007;37(3):255–277. doi: 10.1177/0275074007301957. [DOI] [Google Scholar]
- 22.Djalalinia S, Ramezani-Tehrani F, Malekafzali H, Hejazi F, Peykari N. Development and evaluation of a nutritional health program for adolescents. Iran J Nurs Midwifery Res. 2013;18(5):425. [PMC free article] [PubMed] [Google Scholar]
- 23.Peykari N, Tehrani F, Eftekhari M, Malekafzali H, Dejman M, Neot R, et al. A peer-based study on adolescence nutritional health: a lesson learned from Iran. JPMA The Journal of the Pakistan Medical Association. 2011;61(6):549. [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557. [DOI] [PMC free article] [PubMed]
- 27.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asemi Z, Jamilian M, Mesdaghinia E, Esmaillzadeh A. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Nutrition (Burbank, Los Angeles County, Calif) 2015;31(10):1235. doi: 10.1016/j.nut.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Razavi M, Jamilian M, Kashan Z, Heidar Z, Mohseni M, Ghandi Y, et al. Selenium supplementation and the effects on reproductive outcomes, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome. Hormone and metabolic research= Hormon-und Stoffwechselforschung= Hormones et metabolisme. 2016;48(3):185. [DOI] [PubMed]
- 30.Murer S, Aeberli I, Braegger C, Gittermann M, Hersberger M, Leonard S, et al. Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J Nutr. 2014;144(2):193. doi: 10.3945/jn.113.185561. [DOI] [PubMed] [Google Scholar]
- 31.Brigelius-Flohe R, Banning A, Kny M, Böl G-F. Redox events in interleukin-1 signaling. Archives of biochemistry and biophysics. 2004;423(1):66–73. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article.