Abstract
Background
Illustrating the temporal pattern of acute myocardial infarction (AMI), as a major cause of mortality, may help with disease prevention and better treatment. Therefore, the aim of our study was to investigate the circadian, daily, monthly and seasonal patterns of AMI occurrence in patients with diabetes mellitus, and other likely associated factors.
Methods
This cross-sectional study was performed on 142 diabetic patients admitted to the Imam Ali cardiovascular hospital, Kermanshah, Iran with a diagnosis of ST-segment elevation myocardial infarction (STEMI) from March 2018 to February 2019. Data were collected using a checklist developed based on the study goals. One-way Analysis of Variance (ANOVA) and Chi-Square test (or Fishers҆ Exact test) were used to assess the differences between subgroups. Multiple logistic regression model was constructed to evaluate independent predictors of the AMI occurrence.
Result
Out of the 142 diabetic patients, 90 (63.4%) were male. The mean age of the patients was (mean ± SD) 62.81 ± 10.21 years. Occurrence of STEMI was the most common during winter (p = 0.001). Prior angina, prior congestive heart failure (CHF), prior stroke, High-density lipoprotein (HDL), and Creatine Phosphokinase (CPK) were significantly associated with seasonal pattern of STEMI (p-value < 0.05). Angiotensin receptor blockers (ARBs) use was associated with an increased odds of the AMI occurrence in winter (OR = 8.32; P = 0.027).
Conclusion
The present study of Iranian patients with diabetes revealed that AMI occurred more frequently during the winter compared to the other seasons. ARBs use was associated with an increased odd of the AMI occurrence in winter.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-021-00813-3.
Keywords: Seasonal rhythm, Circadian rhythm, Diabetes mellitus, Myocardial infarction, Iran
Introduction
Acute myocardial infarction (AMI) is a leading cause of mortality across the world. ST-segment elevation myocardial infarction (STEMI), a deadly sub-class of AMI, accounting for more than 35% of AMI cases [1]. Alive creatures have a chronological mechanism that may automatically alter their condition by temporal changing of hour, day, month and seasonal pattern. Some physiological factors may trigger MI, and some of these factors are recognized to fluctuate with seasonal and circadian pattern [2]. In fact, seasonal and circadian patterns affect cardiovascular physiology by changing multiple biologic functions like blood pressure, heart rate, cardiac output, endothelial function, hormone production and release, and etc.[3].
Previous studies have well evidenced that the onset of an AMI significantly changes through the day, with a morning peak [4]. The reasons for morning peak of AMI onset have been partially known, and might be due to the morning blood pressure, activity levels, platelet agreeability, releasing asymmetric dimethyl arginine, emotional stress, coagulation parameters, lipoprotein levels, decreasing the fibrin lytic activity, sympathetic neurotransmission, and etc.[2, 5–7] Likewise, previous studies reported a winter peak for AMI onset, indicating more infarcts occurred on colder days [8, 9].
As AMI is the leading cause of death in both developed and less developed countries, primary prevention of AMI is an important healthcare issue [1]. Also, new therapeutic approaches based on circadian and seasonal pattern have led to the better timing of drugs and treatments to optimize outcomes and minimize adverse effects [10]. On the other hands, AMI in patients with diabetes is often associated with considerable attenuation.
Furthermore, previous evidences were conflicting in their findings based on geographical and climatic variations. However, the temporal pattern of MI is not fully shown in previous studies, especially in diabetic patients. The aim of our study was to investigate the hourly, daily, monthly and seasonal rhythm of MI occurrence in diabetic patients.
Materials and methods
Study population and design
This cross-sectional study was conducted in Imam Ali Cardiovascular Center, Kermanshah University of Medical Sciences (KUMS), Kermanshah, Iran. We assessed all patients with diabetes who admitted to this hospital with a definite diagnosis of STEMI between March 1st, 2018 and February 30th, 2019. We included all eligible patients based on inclusion criteria. Inclusion criteria comprised of age ≥ 18 years old, having diabetes mellitus type 2, and the definite diagnosis of STEMI. Criteria for the diagnosis of STEMI were according to third universal definition of myocardial infarction defined by the ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction [11]. The diagnosis of STEMI was made according to the following elements: 1) significant chest pain 2) electrocardiographic (ECG) changes in accordance with new ST-segment elevations or left bundle branch block (LBBB), and 3) elevated markers of AMI (Troponins and etc.). Diagnosis of diabetes type 2 was defined based on (at least) one of the following criteria: a fasting plasma glucose ≥ 126 mg/dl, a casual plasma glucose ≥ 200 mg/dl in the presence of symptoms, 2-h plasma glucose during the 75-g oral glucose tolerance test ≥ 200 mg/dl, and/or an HbA1c ≥ 6.5% [12]. Patients excluded if they had diabetes mellitus type 1, and also those with incomplete personal or medical information.
Ethical considerations
The Research Ethics Committee of KUMS approved the study protocol (IR.KUMS.REC.1398.702). In addition, individual personal information was kept confidentially.
Instrument and data collection
Data gathering was done by an assistant researcher using a checklist developed based on the study's objectives. The assistant researcher was trained for data gathering. A researcher-developed questionnaire was used to collect the required data on demographic information (e.g. gender), medical history (e.g. previous angina), medication (e.g. aspirin), cardiac enzymes (e.g. CPK), and the time of onset of AMI. The validity of the questionnaire had been assessed and approved by obtaining experts' opinions, including two cardiologists and a statistician. The reliability of questionnaire was also measured using a pilot study with 15 STEMI patients. We obtained the data from both paper medical records and electronic medical records. Moreover, the results were evaluated through checking hospital managerial information.
Statistical methods
Data analysis was performed using statistical package for social sciences statistical software (Version 23.0; IBM Corporation, Chicago, USA). Quantitative variables (e.g., BMI or age) were described using mean ± standard deviation (SD) and qualitative/categorical expressed as frequencies and percentages. Differences between subgroups were assessed using ANOVA followed by Tukey post‐hoc test for continuous and normally distributed variables and chi-square (or Fisher exact tests) for categorical variables. Chi-squared goodness-of-fit test was performed to test uniformity of distribution of the patients among the seasons, months, days and hours. The univariate regression analysis was performed followed by multivariate regression analysis to identify the independent predictors of the AMI occurrence in winter. The univariate regression analysis was conducted for independent variables with p < 0.20 on bivariate analysis, whereby three univariate significant predictors were then selected for multiple regression. Odds ratios (ORs) and 95% confidence intervals were calculated for covariates. In the univariate model, prior MI, prior angina, prior CHF, prior coronary artery bypass grafting (CABG), hypercholesterolemia, total cholesterol, HDL, triglycerides, beta-blocker user, ARB user, and statin user were used as independent variables, whereas the AMI occurrence in winter was assumed as the dependent variable. A probability value (p-value) of less than 0.05 was considered statistically significant.
Result
During 12 months, a total of 142 diabetic patients with STEMI, 90 (63.4%) men, and 52 (36.6%) women met the inclusion criteria for the present study. The mean age of the patients was 62.81 ± 10.21 years, ranged from 18 to 91 years old. 142 (100%) patients reported previous chest pain, 31.7% were current smoker, 58.5% were hypertensive, 47.9% had hypercholesterolemia, 31.7% had history of angina, about 20% had history of MI, 38.7% was aspirin user, 28.9% took beta-Blockers, and 16.9% was taking angiotensin converting enzyme inhibitors.
The mean systolic blood pressure was 132.38 ± 26.94 mmHg. The mean heart rate was 85.71 ± 22.05 bpm. The mean ejection fraction was 35.10 ± 11.14 percent. Regarding the lipid profile, the mean low-density lipoprotein was 105.71 ± 36.21 mg/dl, the mean total cholesterol was 174.15 ± 46.01 mg/dl, the mean triglyceride was 140.76 ± 96.22 mg/dl. All of the patients’ demographic and clinical characteristics are shown in Table 1.
Table 1.
The demographic, clinical and lab parameters of patients (N = 142)
| Characteristics | N (%) | |
|---|---|---|
| Age¶ (year) | 62.81 ± 10.21 | |
| Body mass index¶ (kg/m2) | 26.56 ± 3.60 | |
| Male | 90 (63.4) | |
| Prior chest pain | 142 (100.0) | |
| Prior myocardial infraction | 29 (20.4) | |
| Prior angina | 45 (31.7) | |
| Prior congestive heart failure | 8 (5.6) | |
| Prior stroke | 13 (9.2) | |
| Prior percutaneous coronary intervention | 18 (12.7) | |
| Prior coronary artery bypass grafting | 6 (4.2) | |
| Current smoker | 45 (31.7) | |
| Hypertension | 83 (58.5) | |
| Hypercholesterolemia | 68 (47.9) | |
| Aspirin user | 55 (38.7) | |
| Clopidogrel user | 10 (7.0) | |
| Angiotensin receptor blockers user | 41 (28.9) | |
| Beta -Blocker user | 41 (28.9) | |
| Angiotensin converting enzyme inhibitors user | 24 (16.9) | |
| Statin user | 40 (28.2) | |
| Low-density lipoprotein¶ (mg/dl) | 105.71 ± 36.21 | |
| High-density lipoprotein¶ (mg/dl) | 41.54 ± 11.76 | |
| Triglycerides¶ (mg/dl) | 140.76 ± 96.22 | |
| Total cholesterol¶ (mg/dl) | 174.15 ± 46.01 | |
| Creatine Kinase Myocardial Band¶ (U/l) | 128.68 ± 122.42 | |
| Creatine Phosphokinase¶(U/l) | 1489.35 ± 1241.73 | |
| Troponin¶(ng/ml) | 9.05 ± 10.97 | |
| Creatinine¶ (mg/dl) | 1.15 ± 0.18 | |
| Erythrocyte sedimentation rate¶(mm/hour) | 8.56 ± 6.70 | |
| Heart rate¶ bpm | 85.71 ± 22.05 | |
| Systolic blood pressure¶(mm Hg) | 132.38 ± 26.94 | |
| Ejection Fraction¶ (%) | 35.10 ± 11.14 | |
Continuous variables expressed as mean ± SD, otherwise n (%)
We classified the 365 days of the year into the four seasons: spring (March 21 – June 21), summer (June 22 – September 22), autumn (September 23 – December 21), and winter (December 22 – March 20). Also, we classified the day into the four, six-hour intervals from 00:00 to 06:00, 6:01 to 12:00, 12:01 to 18:00, and 18:01 to 24:00.
Seasonal variation of MI occurrence is shown in Fig. 1. The occurrence of STEMI in patients with diabetes was more prevalent during winter (35.2%), followed by spring (29.6%), autumn (25.4%), and summer (9.9%), respectively (p = 0.001). Regarding the month variation, the results showed that there was no statistical difference between months (p = 0.153) (Fig. 2). The results demonstrated that there was no statistical difference between days (p = 0.414) (Fig. 3). The occurrence of STEMI in patients with diabetes was not statistically different between the four six-hour intervals (p = 0.190) (Fig. 4).
Fig. 1.
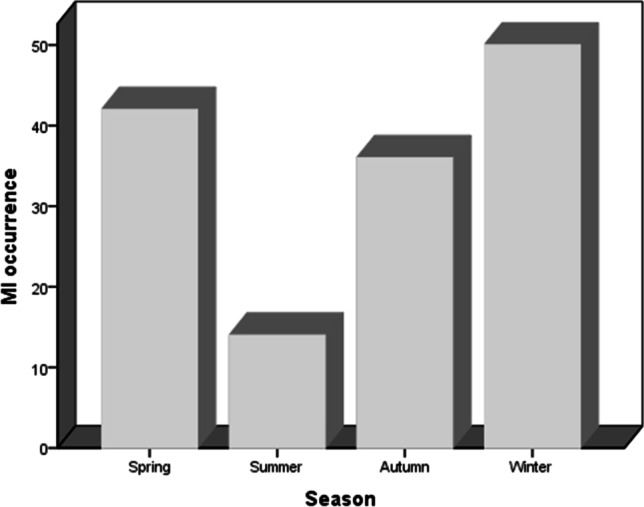
seasonal variation of MI occurrence; the incidence of MI in winter was significantly higher when compared with the other seasons and incidence MI in summer was significantly lower when compared with the other three seasons (p = 0.001)
Fig. 2.
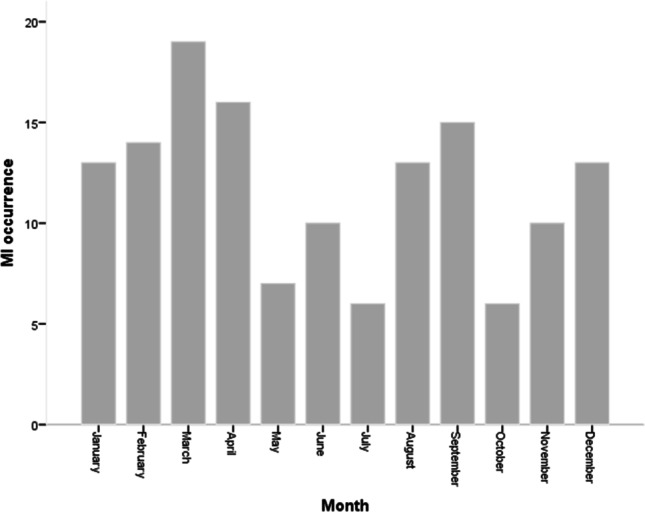
monthly variation of MI occurrence (p = 0.153)
Fig. 3.
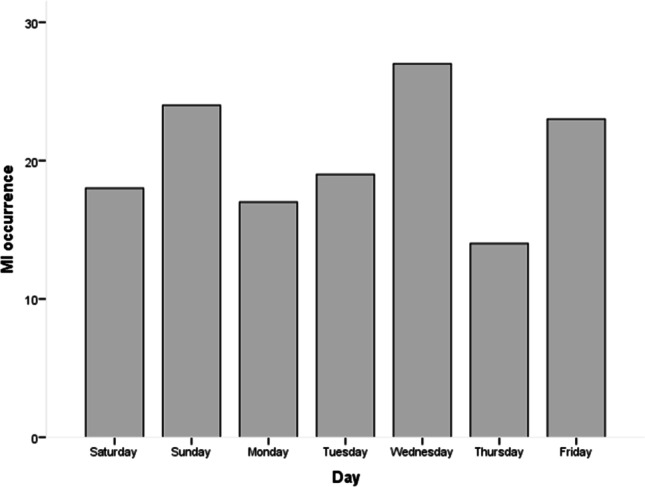
daily variation of MI occurrence (p = 0.414)
Fig. 4.
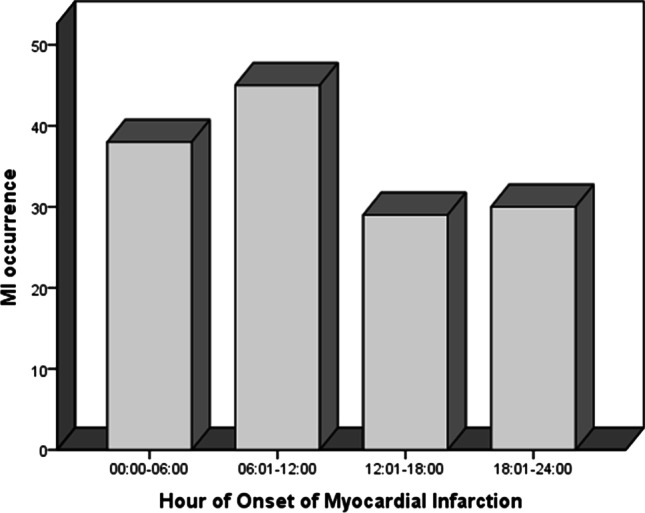
distribution of MI occurrence by the four, six-hour intervals (p = 0.190)
Table 2; prior angina, prior CHF, prior stroke, HDL, and CPK were significantly associated with seasonal pattern of STEMI (p-value < 0.05). At winter season, the frequency of patients who had prior angina (p = 0.027) at the time of presentation were significantly higher than other season. A statistically significant seasonal pattern of MI symptom onset with a peak in the autumn was observed in patients with prior CHF (P = 0.009). A significant summer peak in MI symptom onset was seen among patients with prior stroke (P = 0.004).
Table 2.
Association of various characteristics and seasonal pattern of STEMI (N = 142)
| Characteristics | Spring | Summer | Autumn | Winter | p-value |
|---|---|---|---|---|---|
| Age¶ (year) | 61.39 ± 10.65 | 64.05 ± 7.94 | 64.47 ± 11.63 | 62.42 ± 9.36 | 0.573* |
| Body mass index¶ (kg/m2) | 26.58 ± 3.01 | 25.67 ± 3.33 | 26.64 ± 3.75 | 26.75 ± 4.03 | 0.801* |
| Male | 27 (64.3) | 10 (71.4) | 24 (66.7) | 29 (58.0) | 0.754** |
| Prior myocardial infraction | 9 (21.4) | 4 (28.6) | 3 (8.3) | 13 (26.0) | 0.107*** |
| Prior angina | 13 (31.0) | 3 (21.4) | 6 (16.7) | 23 (46.0) | 0.027** |
| Prior congestive heart failure | 1 (2.4) | 1 (7.1) | 6 (16.7) | 0 (0) | 0.001*** |
| Prior stroke | 3 (7.1) | 5 (35.7) | 2 (5.6) | 3 (6.0) | 0.004** |
| Prior percutaneous coronary intervention | 6 (14.3) | 2 (14.3) | 3 (8.3) | 7 (14.0) | 0.844** |
| Prior coronary artery bypass grafting | 1 (0.7) | 0 (0) | 1 (0.7) | 4 (2.8) | 0.405** |
| Current smoker | 13 (31.0) | 7 (50.0) | 12 (33.3) | 13 (26.0) | 0.396** |
| Hypertension | 23 (54.8) | 6 (42.9) | 21 (58.3) | 33 (66.0) | 0.443** |
| Hypercholesterolemia | 22 (52.4) | 4 (28.6) | 15 (41.7) | 27 (54.0) | 0.176** |
| Aspirin user | 18 (42.9) | 7 (50.0) | 10 (27.8) | 20 (40.0) | 0.407** |
| Clopidogrel user | 3 (7.1) | 1 (7.1) | 2 (5.6) | 4 (8.0) | 0.979** |
| Angiotensin receptor blockers user | 10 (23.8) | 4 (28.6) | 8 (22.2) | 19 (38.0) | 0.344** |
| Beta -Blocker user | 12 (28.6) | 2 (14.3) | 9 (25.0) | 18 (36.0) | 0.399** |
| Angiotensin converting enzyme inhibitors user | 11(26.2) | 0 (0) | 4 (11.1) | 9 (18.0) | 0.097** |
| Statin user | 9 (21.4) | 4 (28.6) | 8 (22.2) | 19 (38.0) | 0.342** |
| Low-density lipoprotein¶ (mg/dl) | 108.42 ± 38.59 | 125.85 ± 49.55 | 95.13 ± 31.29 | 105.42 ± 31.18 | 0.052* |
| High-density lipoprotein¶(mg/dl) | 39.19 ± 7.97 | 39.99 ± 8.42 | 38.69 ± 8.45 | 46.23 ± 15.75 | 0.009* |
| Triglycerides¶ (mg/dl) | 206.83 ± 162.38 | 153.0 ± 135.29 | 124.0 ± 62.43 | 124.30 ± 60.72 | 0.290* |
| Total cholesterol¶ (mg/dl) | 172.19 ± 47.36 | 183.07 ± 58.13 | 161.86 ± 42.10 | 182.16 ± 43.0 | 0.197* |
| Creatine Kinase-Myocardial Band¶ (U/l) | 113.46 ± 104.93 | 137.30 ± 124.31 | 131.50 ± 118.63 | 136.82 ± 138.86 | 0.831* |
| Creatine Phosphokinase¶ (U/l) | 1118.92 ± 1081.35 | 2383.77 ± 1551.47 | 1440.02 ± 1215.22 | 1656.82 ± 1240.41 | 0.028* |
| Troponin¶ (ng/ml) | 8.22 ± 7.12 | 10.49 ± 7.31 | 6.59 ± 6.28 | 11.22 ± 15.66 | 0.259* |
| Creatinine¶ (mg/dl) | 1.11 ± 0.24 | 1.20 ± 0.15 | 1.11 ± 0.17 | 1.19 ± 0.18 | 0.606* |
| Erythrocyte sedimentation rate¶ (mm/hour) | 12.40 ± 9.93 | 8.42 ± 6.39 | 6.53 ± 6.93 | 9.42 ± 5.10 | 0.366* |
| Heart rate¶ bpm | 84.92 ± 24.37 | 82.50 ± 22.18 | 88.63 ± 20.27 | 85.16 ± 21.63 | 0.801* |
| Systolic blood pressure¶ (mm Hg) | 135.23 ± 23.47 | 132.85 ± 21.27 | 132.72 ± 29.80 | 129.60 ± 29.29 | 0.801* |
| Ejection Fraction¶ (%) | 37.50 ± 10.19 | 36.42 ± 12.46 | 32.50 ± 10.65 | 34.60 ± 11.72 | 0.241* |
Continuous variables expressed as mean ± SD, otherwise n (%)
* t-test; ** chi-square; *** Fisher exact test
P-value < 0.05 was considered statistically significant
We used ANOVA followed by Tukey post‐hoc test to show the difference in following variables: the HDL and CPK between the four seasons. According to Tukey’s test, the mean level of CPK in summer was significantly higher than that in spring (p = 0.029). Likewise, the mean level of HDL in winter was significantly higher than that in spring (p = 0.022) and autumn (p = 0.022).
A binary logistic regression model was constructed to identify the predictors of the AMI occurrence in winter. ARBs use was associated with an increased odds of the AMI occurrence in winter (OR = 8.32; P = 0.027) (Table 3).
Table 3.
Predictors for the AMI occurrence in winter, all patients (n = 142)
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Risk factor | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Prior myocardial infraction | 2.23 (1.15–4.33) | 0.018 | 6.11 (0.98–38.17) | 0.053 |
| Prior angina | 2.71 (1.30–5.64) | 0.080 | ||
| Prior congestive heart failure | 2.15 (1.32–3.52) | 0.002 | 1.08 (0.98–1.19) | 0.100 |
| Prior coronary artery bypass grafting | 3.91 (0.69–22.19) | 0.123 | ||
| Hypercholesterolemia | 1.76 (0.99–3.14) | 0.054 | ||
| Total cholesterol | 1.00 (0.99–1.01) | 0.128 | ||
| High-density lipoprotein | 1.05 (1.02–1.09) | 0.079 | ||
| Triglycerides | 0.99 (0.98–1.00) | 0.463 | ||
| Beta -Blocker user | 1.68 (0.80–3.55) | 0.169 | ||
| Angiotensin receptor blockers user | 1.95 (0.92–4.10) | 0.002 | 8.32 (1.27–54.61) | 0.027 |
| Statin user | 1.58 (0.80–3.10) | 0.185 | ||
OR, Odd Ratio; CI, Confidence Interval
P-value < 0.05 was considered statistically significant
Discussion
This cross-sectional study aimed to assess the hourly, daily, monthly and seasonal rhythm of AMI occurrence in patients with diabetes admitted with a definite diagnosis of STEMI in Imam Ali cardiovascular center, Kermanshah, Iran between March 2018 to February 2019.
The results of this study illustrated that patients with diabetes showed a seasonal pattern of AMI occurrence with a winter peak. Our results concur with the findings of previous studies. For example, a study by Nagarajan et al. from US performed on 82,971 AMI patients found that AMI admissions were higher in winter in 2017 [8]. Keller et al. from Germany showed that AMI admissions and in-hospital mortality caused by AMI were higher in the winter in 2019 [13]. Conversely, Jalali et al. conducted a study on 1174 Iranian patients, showed that most of the MI occurred in autumn [14]. By contrast, Akioka and colleagues performed a study on 274 MI patients and illustrated that AMI onset was most frequently reported in summer in 2019 [15]. We didn't find similar studies regarding the temporal pattern of AMI occurrence in patients with diabetes.
The mechanism of a winter peak in AMI onset is partially known. For example, influenza and respiratory tract infections, as diseases that usually occurs in winter, are well recognized as risk factors for AMI [16]. A study done by Jeffrey and colleague in 2018 showed that a sixfold increase of AMI occurrence 7 days after an influenza infection [17]. Seasonal behavioral patterns, such as seasonal depression, dietary changes, decreased physical activity, and reduced sunshine duration and vitamin D uptake, may also lead to an increase of AMI occurrence in winter [18].
We observed some potentially interesting results in the subgroup analyses. Winter predominance was shown in the patients who had a prior angina, while patients with prior CHF had more admissions in autumn. Evaluating the patients who had a prior stroke, a significant seasonal variation with summer predominance was observed in AMI admissions. The mean level of CPK in summer was significantly higher than that in spring. Likewise, the mean level of HDL in winter was significantly higher than that in spring and autumn.
We observed that ARBs use was associated with an increased odd of the AMI occurrence in winter. Further studies are needed to determine mechanisms of action of the ARBs use as the predictors of the AMI occurrence in winter.
Our study had several limitations. First, its design is cross-sectional; thus, no associations over time can be inferred. Second, our data were obtained from Kermanshah province; therefore, our participants may not be representative of the whole patients with STEMI. As mentioned, the high prevalence of influenza and respiratory tract infections, seasonal depression, dietary changes, decreased physical activity, reduced sunshine duration, and vitamin D uptake, and etc. in the winter could play an important role in higher AMI rates. In this regard, it is highly recommended that future studies adjust these variables as confounding variables. Finally, the present study provides a foundation for future studies that should be performed in the other ethnic groups residing in different parts of Iran.
Conclusion
The present study of Iranian patients with diabetes revealed that AMI occurred more frequently during the winter compared to the other seasons. Moreover, prior angina, prior CHF, prior stroke, HDL, and CPK were significantly associated with seasonal pattern of AMI. ARBs use was associated with an increased odd of the AMI occurrence in winter. Further studies are needed to determine mechanisms of action of the ARBs use as the predictors of the AMI occurrence in winter.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Clinical Research Development Center of Taleghani and Imam Ali Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran, for the support, cooperation and assistance throughout the study. And also, we would like to thank Hooman Tadbiri and Mina Sharbati for proofreading and editing this manuscript.
Declarations
Competing interests
The authors declare that there they have no conflicts of interest. In addition, the authors have no financial interest related to any aspect of the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart. 2005;91(2):229–230. doi: 10.1136/hrt.2003.027599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller JE, Tofler G, Stone P. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79(4):733–743. doi: 10.1161/01.CIR.79.4.733. [DOI] [PubMed] [Google Scholar]
- 3.Suárez-Barrientos A, López-Romero P, Vivas D, Castro-Ferreira F, Núñez-Gil I, Franco E, et al. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011;97(12):970–976. doi: 10.1136/hrt.2010.212621. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Manson J, Buring JE, Muller JE, Hennekens CH. Circadian variation of acute myocardial infarction and the effect of low-dose aspirin in a randomized trial of physicians. Circulation. 1990;82(3):897–902. doi: 10.1161/01.CIR.82.3.897. [DOI] [PubMed] [Google Scholar]
- 5.Bergheanu SC, van der Laarse A, van der Bom JG, van der Hoeven BL, le Cessie S, de Jong MG, et al. Asymmetric dimethylarginine (ADMA) levels display a morning peak in patients with acute myocardial infarction. Dis Markers. 2011;30(5):245–252. doi: 10.1155/2011/941895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammoudeh AJ, Alhaddad IA. Triggers and the onset of acute myocardial infarction. Cardiol Rev. 2009;17(6):270–274. doi: 10.1097/CRD.0b013e3181bdba75. [DOI] [PubMed] [Google Scholar]
- 7.Singh RB, Pella D, Neki NS, Chandel J, Rastogi S, Mori H, et al. Mechanisms of acute myocardial infarction study (MAMIS) Biomed Pharmacother. 2004;58:S111–S115. doi: 10.1016/S0753-3322(04)80018-0. [DOI] [PubMed] [Google Scholar]
- 8.Nagarajan V, Fonarow GC, Ju C, Pencina M, Laskey WK, Maddox TM, et al. Seasonal and circadian variations of acute myocardial infarction: Findings from the Get With The Guidelines-Coronary Artery Disease (GWTG-CAD) program. Am Heart J. 2017;189:85–93. doi: 10.1016/j.ahj.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Keller K, Hobohm L, Munzel T, Ostad M. P2653 Seasonal variations of myocardial infarction and sex-specific differences in Germany. European Heart Journal. 2019;40(Supplement_1):ehz748. 0974. [DOI] [PubMed]
- 10.Willich SN, Linderer T, Wegscheider K, Leizorovicz A, Alamercery I, Schröder R. Increased morning incidence of myocardial infarction in the ISAM Study: absence with prior beta-adrenergic blockade. ISAM Study Group Circulation. 1989;80(4):853–858. doi: 10.1161/01.cir.80.4.853. [DOI] [PubMed] [Google Scholar]
- 11.Bax JJ, Baumgartner H, Ceconi C, Dean V, Fagard R, Funck-Brentano C, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Buysschaert M, Medina J-L, Buysschaert B, Bergman M. Definitions (and Current Controversies) of Diabetes and Prediabetes. Curr Diabetes Rev. 2016;12(1):8–13. doi: 10.2174/1573399811666150122150233. [DOI] [PubMed] [Google Scholar]
- 13.Keller K, Hobohm L, Munzel T, Ostad MA. P2653Seasonal variations of myocardial infarction and sex-specific differences in Germany. European Heart Journal. 2019;40(Supplement_1).
- 14.Jalali F, KO HT. Day of week, monthly and seasonal variation of acute myocardial infarction. Acta Medica Iranica. 2002:230–5.
- 15.Akioka H, Yufu K, Teshima Y, Kawano K, Ishii Y, Abe I, et al. Seasonal variations of weather conditions on acute myocardial infarction onset: Oita AMI Registry. Heart Vessels. 2019;34(1):9–18. doi: 10.1007/s00380-018-1213-6. [DOI] [PubMed] [Google Scholar]
- 16.Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR. Acute myocardial infarction and influenza: a meta-analysis of case–control studies. Heart. 2015;101(21):1738–1747. doi: 10.1136/heartjnl-2015-307691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 18.Rosengren A, Hawken S, Ôunpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case-control study. The Lancet. 2004;364(9438):953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


