Abstract
For patients with relapsed or refractory classical Hodgkin lymphoma (cHL), salvage chemotherapy followed by consolidation with autologous stem cell transplant (ASCT) remains the standard of care. Even with this aggressive treatment strategy, 5-year progression-free survival is ≤ 50%, and there remains interest in maintenance strategies to improve long-term disease-free survival. Lenalidomide is an immunomodulatory agent with demonstrated activity in multiple subtypes of lymphoma including cHL, and has also been shown to improve both progression free and overall survival as maintenance therapy after ASCT in multiple myeloma. This multicenter study evaluated maintenance lenalidomide after ASCT for patients with cHL. Patients were enrolled 60–90 days post-transplant and received oral lenalidomide on days 1–28 of 28-day cycles for a maximum of 18 cycles. Lenalidomide was started at 15 mg daily and increased to maximum of 25 mg daily if tolerated. The primary objective of this study was to assess the feasibility of this regimen, with a goal < 30% rate of discontinuation at or before cycle 12 for drug-related reasons. Twenty-seven patients were enrolled and 26 received at least one dose of lenalidomide. With a median follow-up of 51.3 months (range 12.2 – 76.2 months), 23 of 26 patients were alive. Median event-free survival was 9.4 months and median progression free survival had not been reached, with 17 of 26 patients (65.4%) remaining in remission at last follow-up. Excluding four patients who discontinued therapy for progression and two who discontinued due to non-compliance, the discontinuation rate at or before cycle 12 was 52%. Treatment was complicated by a high frequency of hematologic adverse events, with 15 patients (58%) experiencing grade 3–4 hematologic toxicity and 5 (19%) experiencing grade 4 hematologic toxicity. We conclude that the regimen of maintenance lenalidomide explored in this study is not feasible for cHL patients immediately following ASCT. An alternative lenalidomide dose or schedule may be better tolerated following ASCT for patients with rel/ref cHL.
Keywords: Lenalidomide maintenance, autologous transplantation, relapsed Hodgkin lymphoma
Graphical Abstract
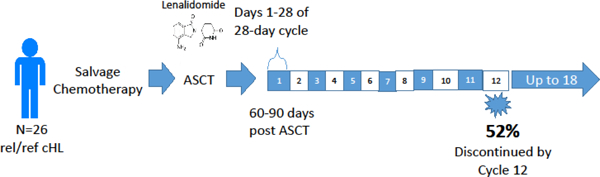
Introduction
Classical Hodgkin lymphoma (cHL) is an uncommon lymphoma that affects primarily younger individuals, with a median age at diagnosis of 39 years. Substantial improvements in therapy over the past several decades have resulted in a 5-year overall survival of approximately 90% for those diagnosed between ages 20 and 64 (1). For patients refractory to first-line chemotherapy or who relapse after initial treatment (rel/ref cHL), outcomes are considerably worse. Salvage high-dose chemotherapy followed by autologous stem cell transplant (ASCT) is the standard of care and retrospective analyses suggest a 5-year progression free survival (PFS) of approximately 48% with this treatment strategy (2). Two randomized trials have demonstrated improvements in PFS with high-dose chemotherapy followed by ASCT relative to chemotherapy alone in rel/ref cHL, although neither demonstrated improvement in overall survival (OS) (3, 4). Maintenance therapy with brentuximab vedotin (BV) post-transplant has likewise been shown to improve PFS but not OS in high-risk patients with rel/ref cHL (5).
Our current understanding of the pathophysiology of cHL supports the use of immunomodulatory treatment approaches in this disease. Malignant RS cells are situated within an array of reactive lymphocytes that may protect RS cells from apoptosis and immunosurveillance. In lymph nodes from cHL patients, CD4+ T-cells with a regulatory phenotype predominate, whereas CD4+ TH1 cells and CD8+ cytotoxic T-cells are under-represented. RS cells are thought to promote this dysregulated microenvironment by secretion of cytokines that promote TH2 responses and inhibit TH1, cytotoxic T-cell, and NK-cell responses (6). Constitutive signaling through tumor necrosis receptor (TNFR) family members, due to both autocrine production of TNFα by RS cells and production of other TNFR ligands by surrounding cells, leads to constitutive activation of nuclear factor kB (NF-kB) signaling and promotes RS cell survival (7). Increased vascularization and angiogenic signaling has also been described in cHL lymph node samples, suggesting a role for antagonism of vascular endothelial growth factor (VEGF) signaling in treatment of this disease (8, 9). Lenalidomide is a thalidomide derivate with powerful immunomodulatory properties. It is a potent inhibitor of TNF-alpha signaling (10) and has been shown to augment both CD8+ T-cell and NK cell activity in vitro (11–13). In addition, lenalidomide has anti-angiogenic properties that could help counteract the increased VEGF signaling that characterizes cHL lymph nodes (14, 15).
Lenalidomide has clinical activity in a variety of B-cell malignancies including both indolent and aggressive non-Hodgkin lymphoma (16–20). A phase II trial demonstrated single-agent efficacy for lenalidomide in patients with rel/ref cHL. In this multicenter study, 38 patients with rel/ref cHL were treated with lenalidomide 25 mg/day on days 1–21 of 28-day cycles, with an overall response rate (ORR) of 19% and a cytostatic ORR of 33%. Therapy was overall well-tolerated, with the most common grade 3–4 adverse events (AEs) being neutropenia (47%), anemia (29%), and thrombocytopenia (18%) (21). Lenalidomide was also evaluated in the rel/ref HL setting on a continuous (non-interrupted) schedule, resulting in a higher ORR but similar cytostatic ORR and tolerability (22). Furthermore, lenalidomide has been shown to be safe, well-tolerated, and effective as maintenance therapy in multiple myeloma, including in the post-ASCT setting (23–26). We hypothesized that post-ASCT lenalidomide maintenance might improve long-term outcomes in rel/ref cHL, if tolerable. Here, we performed a single-arm prospective study to examine the feasibility of maintenance lenalidomide after ASCT in rel / ref cHL.
Materials and Methods
Patient Eligibility
Patients age ≥ 18 years of age with pathologically confirmed cHL per World Health Organization (WHO) criteria (27) were eligible for this study. Patients must have had disease that was relapsed or refractory after at least one previous line of systemic therapy. Patients also must have undergone ASCT between 60 and 90 days prior to study registration. Patients with progressive disease during salvage chemotherapy or following ASCT were excluded. Additional inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2 and adequate renal (serum creatinine < 1.5X institutional upper limit of normal, ULN), hepatic (AST and ALT ≤ 3X ULN and total bilirubin ≤ 1.5 mg/dL), and hematologic (absolute neutrophil count ≥ 1000 / µL and platelets ≥ 30,000 / µL) function. Patients who were pregnant or breastfeeding were not eligible. The study was conducted in accordance with the Declaration of Helsinki, was approved by the institutional review board of all participating institutions, and all patients provided written informed consent.
Study Design and Treatment
This was an open-label multicenter pilot study of oral lenalidomide given on days 1–28 of 28-day cycles (ClinicalTrials.gov, identifier NCT01207921). Lenalidomide was provided by the manufacturer (Celgene). It was initiated at a dose of 15 mg daily (dose 0) and then escalated to 20 mg daily (dose +1) if dose 0 was tolerated during cycle 1. Escalation to a maximum dose of 25 mg daily (dose +2) was likewise permitted if dose +1 was tolerated during the previous cycle. Dose reductions were allowed in 5 mg increments to a minimum dose of 5 mg daily (dose −2). No dose re-escalation was permitted. Criteria for starting the next cycle of therapy were as follows: ANC ≥ 1000 / µL, platelet count ≥ 30,000 / µL, resolution of lenalidomide-related rash, hypersensitivity reaction, or cardiac arrhythmia to grade ≤ 1, and resolution of any other drug-related AE to grade ≤ 2. If these criteria were not met, therapy was held and patients were re-evaluated weekly. Once the above criteria were met, lenalidomide was resumed at the next lower dose level. If these criteria were not met at the 5 mg dose, lenalidomide therapy was discontinued. Patients were also removed from the study if any of the following occurred: desquamating rash of any grade, grade ≥ 3 cardiac toxicity, any other grade 4 toxicity, pregnancy, or disease progression. Patients were otherwise continued on lenalidomide for a maximum of 18 cycles. Growth factor support was not required but was allowed at the discretion of the treating physician. Aspirin (81 or 325 mg), or prophylactic Lovenox or heparin for those with contraindications to aspirin, was required for those receiving concomitant erythropoiesis-stimulating agents and suggested for those deemed by the treating physician to be at high risk of thrombosis. AEs were evaluated and coded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Response Criteria and Statistical Analysis
The primary endpoint of this study was feasibility of drug administration as defined by drug-related drop-out rate, or the proportion of patients who stopped lenalidomide for drug-related reasons at or before 12 months of therapy. A drop-out rate less than or equal to 30% was set as the threshold to consider this a feasible regimen for further study. Fleming’s one-stage design was used with a target enrollment of 28 patients based on an α of 0.10 and a power of 0.93 to test the null hypothesis that the drop-out rate is ≥ 0.55 vs the alternative that it is ≤ 0.3. Secondary outcomes included OS, event-free survival (EFS, defined as time from the first dose of study drug to treatment failure, recurrence, or death due to any cause), PFS, defined as time from the first dose of study drug to progression or death due to disease, and the nature and frequency of drug-related AEs. The rate of conversion from SD post-ASCT to PR or CR or from PR post-ASCT to CR was an additional secondary endpoint. Responses were classified based on the revised International Working Group Criteria (28). For the primary endpoint we included all enrolled patients, excluding one patient who was found to be ineligible due to receipt of mediastinal radiation (n = 27). For safety analysis, we included all patients who met enrollment criteria and received at least one dose of the study medication (n = 26). Though data on all AEs was collected, only those AEs determined by the investigators to be at least possibly related to the study medications were included in analysis. For secondary efficacy endpoints, we included all enrolled patients who met eligibility criteria and had adequate follow-up data, including one patient who did not receive any of the study medication due to being lost to follow-up (n = 26).
Results
Patient demographics and baseline attributes
From February 2013 to March 2016, 32 patients were consented and four were found to be ineligible prior to initiation of therapy, with reasons for screen failures as follows: elevated AST/ALT, insurance denial of study participation, biopsy-proven relapse prior to initiation of lenalidomide, and withdrawal of consent prior to initiation of therapy. A fifth patient was found to be ineligible due to receipt of mediastinal radiation, initiated with local providers concurrently with start of lenalidomide. Twenty-seven eligible patients were therefore enrolled and treated on this study. The median age at time of study enrollment was 35 years (range 20 – 70). Fourteen patients had primary refractory disease and 13 had relapsed disease. Of the relapsed patients, 6 of 13 relapsed less than 12 months after first remission and 5 had extra-nodal disease at relapse. The conditioning regimen for all 27 patients was BEAM (BCNU, etoposide, cytarabine and melphalan). Post-transplant and before initiation of lenalidomide, 24 patients (89%) achieved a CR per revised International Working Group Criteria (28), 2 achieved a PR, and 1 achieved SD. Of the patients who achieved less than CR after transplant, two had negative PET-CT prior to transplant and had residual masses on post-transplant CT. One had a positive PET before transplant and had near resolution of all FDG-avid lesions after transplant. Patient characteristics are listed in Table 1.
Table 1.
Patient demographics (n = 27).
| Sex, n (%) | |
| Male | 17 (63.0) |
| Female | 10 (37.0) |
| Age (years) | |
| Median | 35.0 |
| Range | 20–70 |
| Histology, n (%) | |
| Nodular sclerosis | 14 (51.9) |
| Mixed cellularity | 2 (7.4) |
| Classical, not-otherwise-specified | 11 (40.7) |
| Disease status, n (%) | |
| Relapsed | 13 (48.1) |
| Refractory | 14 (51.9) |
| ECOG Performance Status | |
| 0 | 20 (74.1) |
| 1 | 7 (25.9) |
| Relapse < 12 months after frontline therapy? | |
| Yes | 6 (22.2) |
| No | 7 (25.9) |
| Not applicable (refractory) | 14 (51.9) |
| Extra-nodal disease at relapse, n (%) | |
| Yes | 5 (18.5) |
| No | 7 (25.9) |
| Unknown | 1 (3.7) |
| Not applicable (refractory) | 14 (51.9) |
| B symptoms prior to ASCT, n (%) | |
| Yes | 5 (18.5) |
| No | 20 (74.1) |
| Unknown | 2 (7.4) |
| Result of Pre-transplant PET-CT, n (%) | |
| Positive | 9 (33.3) |
| Negative | 15 (55.6) |
| Not applicable (not performed) | 1 (3.7) |
| Unknown | 2 (7.4) |
| Response to Salvage Chemotherapy, n (%) | |
| PR | 8 (29.6) |
| CR | 19 (70.4) |
| Disease Status Following ASCT, n (%) | |
| SD | 1 (3.7) |
| PR | 2 (7.4) |
| CR | 24 (88.9) |
| Hematologic parameters at registration (median, range) | |
| ANC (x 103 cells/µL) | 3.3 (1.7 – 10.1) |
| Platelet count (cells/µL) | 166,000 (62,000 – 369,000) |
| Hemoglobin (g/dL) | 12.3 (7.7 – 14.7) |
Abbreviations: ANC – absolute neutrophil count; ASCT – autologous stem cell transplantation; CR – complete response; PET-CT – position emission tomography – computed tomography; PR – partial response. PET scans were classified as positive or negative per International Working Group Criteria (28, 33).
Adverse events and tolerability
The median number of cycles of lenalidomide administered was 7 (range 0 – 18). Of the 27 patients evaluated for the primary endpoint, 4 discontinued therapy due to progression and 2 for non-compliance. Of the remaining 21, 10 completed 12 months of lenalidomide. Reasons for discontinuation were hematologic toxicity (n = 7) and non-hematologic toxicity (n = 4). Specific non-hematologic toxicities resulting in drug discontinuation were pneumonia and atrial fibrillation (1 patient), venous thromboembolism and headache (1 patient), renal failure, hypokalemia and rash (1 patient) and pancreatitis (1 patient). Specific hematologic toxicities resulting in drug discontinuation were neutropenia (n = 5) and neutropenia + thrombocytopenia (n = 2). Though this study permitted the use of growth factors, treating providers opted for dose reduction as opposed to growth factor support for neutropenia. The drop-out rate before 12 months of therapy was 52% (11 of 21). Only 7 of 21 patients (33%) completed the full 18 cycles of therapy. For the 7 patients who completed 18 months, 2 tolerated lenalidomide at planned maximum dose (25 mg daily). The remaining patients required dose reductions to 10 mg (1 patient) or 5 mg (4 patients). Hematologic toxicities were common in this patient population. When considering only patients who received at least one dose of lenalidomide, 15 of 26 patients (58%) experienced grade 3–4 hematologic toxicity, with 5 (19%) experiencing grade 4 hematologic toxicity. Half of all patients experienced grade ≥ 3 neutropenia. The most frequent non-hematologic toxicities (any grade) were elevated transaminases (39%), fatigue (35%), hypokalemia (31%), infection (31%), and rash (27%). The only grade ≥ 3 non-hematologic toxicity seen in more than one patient was hypokalemia (4 patients, 15%). Adverse events are summarized in Table 2.
Table 2.
Treatment-related AEs in 26 relapsed / refractory HL patients receiving lenalidomide maintenance after ASCT, n (%)
| Characteristic | Grade 1–2 | Grade 3 | Grade 4 | Total Grade 3 and 4 |
|---|---|---|---|---|
| Hematologic | ||||
| Anemia | 12 (46.2) | 2 (7.7) | 0 (0) | 2 (7.7) |
| Neutropenia | 8 (30.8) | 9 (34.6) | 4 (15.4) | 13 (50) |
| Lymphopenia | 9 (34.6) | 6 (23.1) | 1 (3.8) | 7 (26.9) |
| Thrombocytopenia | 10 (38.5) | 4 (15.4) | 1 (3.8) | 5 (19.2) |
| Non-hematologic | ||||
| Fatigue | 9 (34.6) | 0 (0) | 0 (0) | 0 (0) |
| Elevated AST / ALT | 9 (34.6) | 1 (3.8) | 0 (0) | 1 (3.8) |
| Infection | 7 (26.9) | 1 (3.8) | 0 (0) | 1 (3.8) |
| Rash | 6 (23.1) | 1 (3.8) | 0 (0) | 1 (3.8) |
| Musculoskeletal pain | 5 (19.2) | 0 (0) | 0 (0) | 0 (0) |
| Creatinine elevation | 4 (15.4) | 0 (0) | 0 (0) | 0 (0) |
| Cough | 4 (15.4) | 0 (0) | 0 (0) | 0 (0) |
| Constipation | 4 (15.4) | 0 (0) | 0 (0) | 0 (0) |
| Psychiatric disturbance | 3 (11.5) | 0 (0) | 0 (0) | 0 (0) |
| Pulmonary embolism | 0 (0) | 1 (3.8) | 0 (0) | 1 (3.8) |
| Pancreatitis | 0 (0) | 1 (3.8) | 0 (0) | 1 (3.8) |
| Laboratory | ||||
| Low calcium | 6 (23.1) | 0 (0) | 0 (0) | 0 (0) |
| Low phosphorous | 0 (0) | 1 (3.8) | 0 (0) | 1 (3.8) |
| Low potassium | 4 (15.4) | 2 (7.7) | 2 (7.7) | 4 (15.4) |
| Low sodium | 3 (11.6) | 0 (0) | 0 (0) | 0 (0) |
Table includes all grade 1–2 AEs occurring in at least 10% of patients and all grade ≥3 AEs that occurred in one or more patients. Only AEs determined by the investigators to be at least possibly related to the study drug are displayed.
Patient response and secondary outcomes
Of the 27 patients enrolled in this study, adequate follow-up data was available to assess treatment response in 26 patients. With a median follow-up of 51.3 months (range 12.2 – 76.2 months), 23 of 26 patients were alive. Median event-free survival (defined as time from first dose of study drug to treatment failure, progression, or death, with treatment failure including discontinuation of the study drug for any reason) was 9.4 months and median PFS had not been reached, with 17 of 26 patients (65.4%) remaining in remission at last follow-up (Figure 3). When patients who discontinued lenalidomide prior to cycle 12 for progression were excluded, there was no difference in PFS between those who did and did not complete 12 cycles of lenalidomide, though the number of events in both groups was small and not powered for this analysis (Figure S2).
Figure 3.
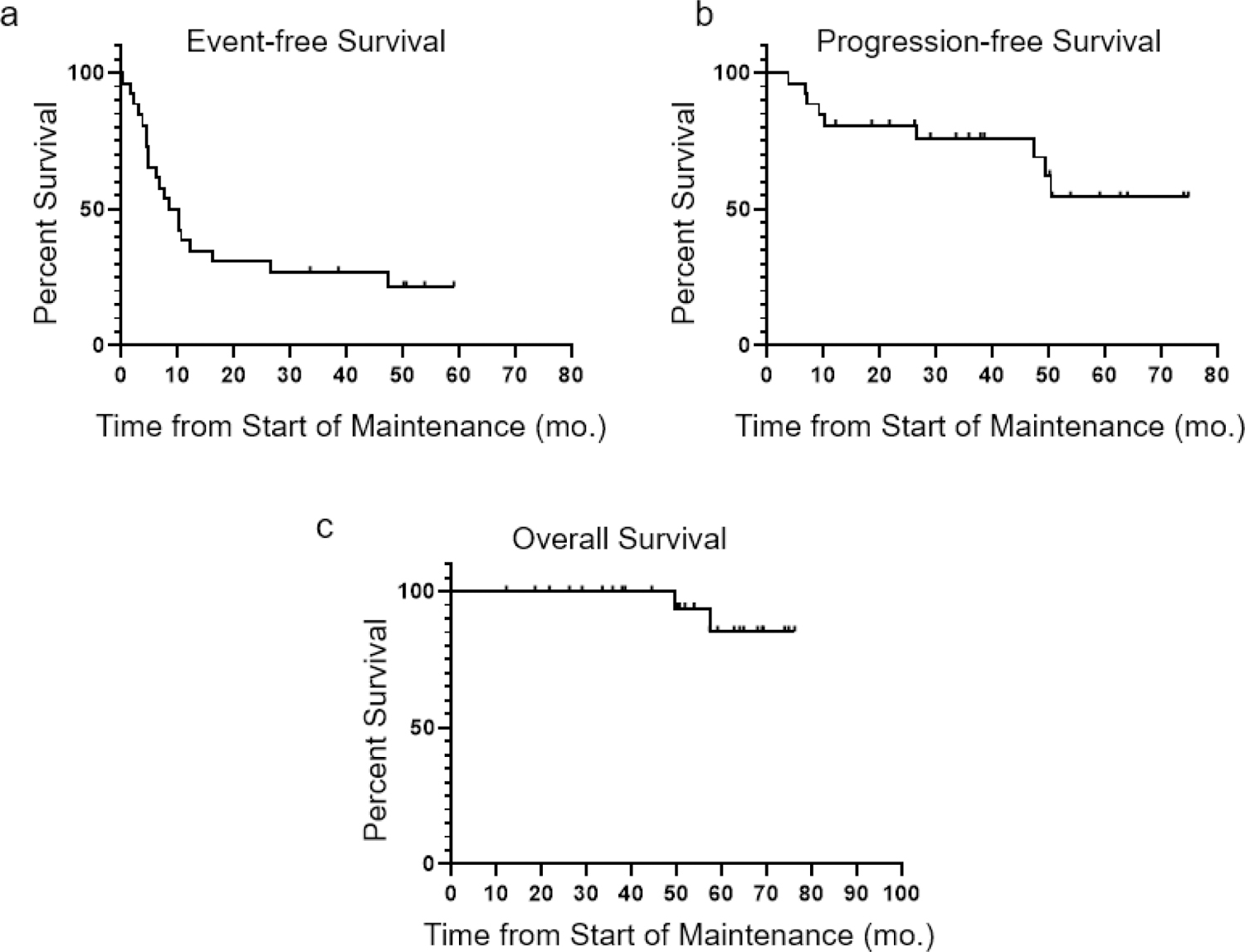
Kaplan-Meier plots demonstrating a) event-free survival (EFS, defined as time from the first dose of lenalidomide to treatment failure, recurrence, or death due to any cause, with treatment failure including discontinuation of the study drug for any reason) b) progression-free survival (PFS, defined as time from the first dose of lenalidomide to progression or death due to disease) and c) overall survival (OS), for all patients evaluable for these endpoints (n = 26). Patients in remission at the time of last follow-up were censored at this time point. With a median follow-up of 51.3 months (range 12.2 – 76.2 months), median EFS was 9.4 months and median PFS and OS had not been reached. Twenty-three of 26 patients (88.5%) were alive at last follow-up, including 6 who experienced progression after ASCT.
Discussion
In this multicenter study of maintenance lenalidomide after ASCT for rel/ref cHL, we observed a high drop-out rate prior to completion of 12 cycles of lenalidomide. Excluding the four patients who discontinued lenalidomide maintenance due to progression and the two who discontinued due to non-compliance, 10 of 21 patients (47.6%) completed 12 cycles of therapy. Furthermore, only 3 of these 10 patients were able to continue at the maximum dose of 25 mg daily, with the others requiring dose reductions. Our data is in contrast to the collective experience with post-transplant lenalidomide maintenance in multiple myeloma, where single-agent lenalidomide is relatively well-tolerated. In a meta-analysis compiling data from three major trials of post-transplant lenalidomide maintenance in multiple myeloma, the median duration of treatment was 28 months with lenalidomide vs 22 months with placebo or observation (26). 29.1% of lenalidomide-treated patients discontinued lenalidomide due to treatment-related AEs vs 12.2% of patients randomized to placebo or observation. As in our study, the most common class of AEs leading to treatment discontinuation was hematologic, specifically neutropenia and thrombocytopenia. The superior tolerability of post-transplant maintenance in the multiple myeloma setting may be in part explained by dosing. In three major trials of post-transplant lenalidomide in multiple myeloma, patients were treated with 10 mg daily on days 1–21 of 28 day cycles (29), 10 mg daily continuously with maximum target dose 15 mg daily (23), or 25 mg daily on days 1–21 of 28 day cycles for two cycles as consolidation, followed by maintenance with 10 mg daily continuously with option to escalate to maximum target dose of 15 mg daily (24). In cHL, an alternative dosing strategy such as interrupted treatment on days 1–21 of 28-day cycles, or possibly the addition of growth factor support, might improve tolerability relative to the dosing regimen employed in the current study.
This study had a relatively small sample size with feasibility as the primary outcome, and there were too few patients with less than CR post-transplant to evaluate the effect of lenalidomide maintenance on improving the best response achieved after ASCT. It would be interesting to study a larger cohort of cHL patients post-ASCT to see if lenalidomide might improve the best response achieved, as has been suggested in several other lymphoid malignancies (19, 20, 30), or to employ newer strategies for disease follow-up including circulating tumor DNA that might be more sensitive in detecting deepening of response (31).
One notable finding from this study is the excellent long-term survival outcomes in a high-risk cohort of rel/ref cHL patients. With a median follow-up of 51.3 months (range 12.2 – 76.2 months), median PFS had not been reached, with 17 of 26 patients (65.4%) remaining in remission and 23 of 26 patients (88.5%) alive at last follow-up, including 6 who experienced relapse after ASCT. In comparison, a retrospective study of 414 patients with cHL who underwent autologous transplant between 1989 and 1995 revealed three-year OS rates of 58% and 75% for those transplanted in first relapse and in second CR, respectively (32). In our study, prolonged OS despite progression post-ASCT likely speaks to the availability of highly effective salvage therapies including BV and anti-PD1 monoclonal antibodies that have transformed contemporary treatment of rel/ref cHL.
The role of maintenance lenalidomide in the current treatment landscape for cHL is unclear. After this study was opened, results of the AETHERA trial have led to widespread adoption of consolidation with BV after ASCT in high-risk patients with cHL. In AETHERA, BV was overall well-tolerated, and although 67% of patients experienced peripheral neuropathy (any grade), neuropathy improved with long-term follow-up in 90% and resolved in 73% of affected patients (5). The risk of secondary primary malignancies (SPMs) in lenalidomide-treated patients remains a significant concern as well. In a meta-analysis of randomized trials comparing lenalidomide maintenance with observation or placebo after ASCT in multiple myeloma, the incidence of SPMs was higher in lenalidomide-treated patients, though the risk of developing progressive disease remained higher than that of developing an invasive SPM in both the lenalidomide and observation / placebo groups, and an overall survival benefit was still seen with lenalidomide maintenance (26). In cHL, which affects an overall younger patient population, it is unclear if the same risk-benefit balance would apply.
In summary, in a multicenter study of maintenance lenalidomide after ASCT for patients with rel/ref cHL, 52.4% of patients discontinued therapy for drug-related reasons before completing twelve cycles of maintenance therapy. Therapy in this patient population was complicated by relatively high rates of ≥ Gr 3 hematologic toxicity as well as non-hematologic toxicities. Lenalidomide at the dose and schedule employed in this study (5 to 25 mg daily given continuously for up to 18 28-day cycles) is not a feasible post-ASCT maintenance regimen in rel/ref cHL. It is possible that other schedules of administration (lower target doses, dosing on days 1–21 of 28-day cycles) might prove more tolerable.
Supplementary Material
Figure 1.
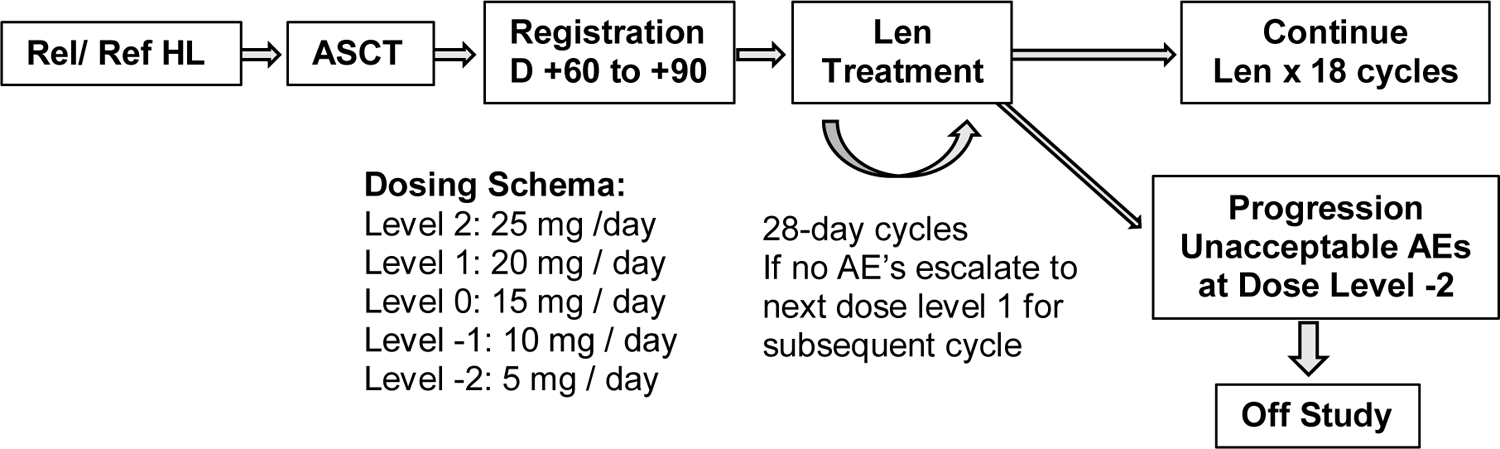
Study schema.
Figure 2.
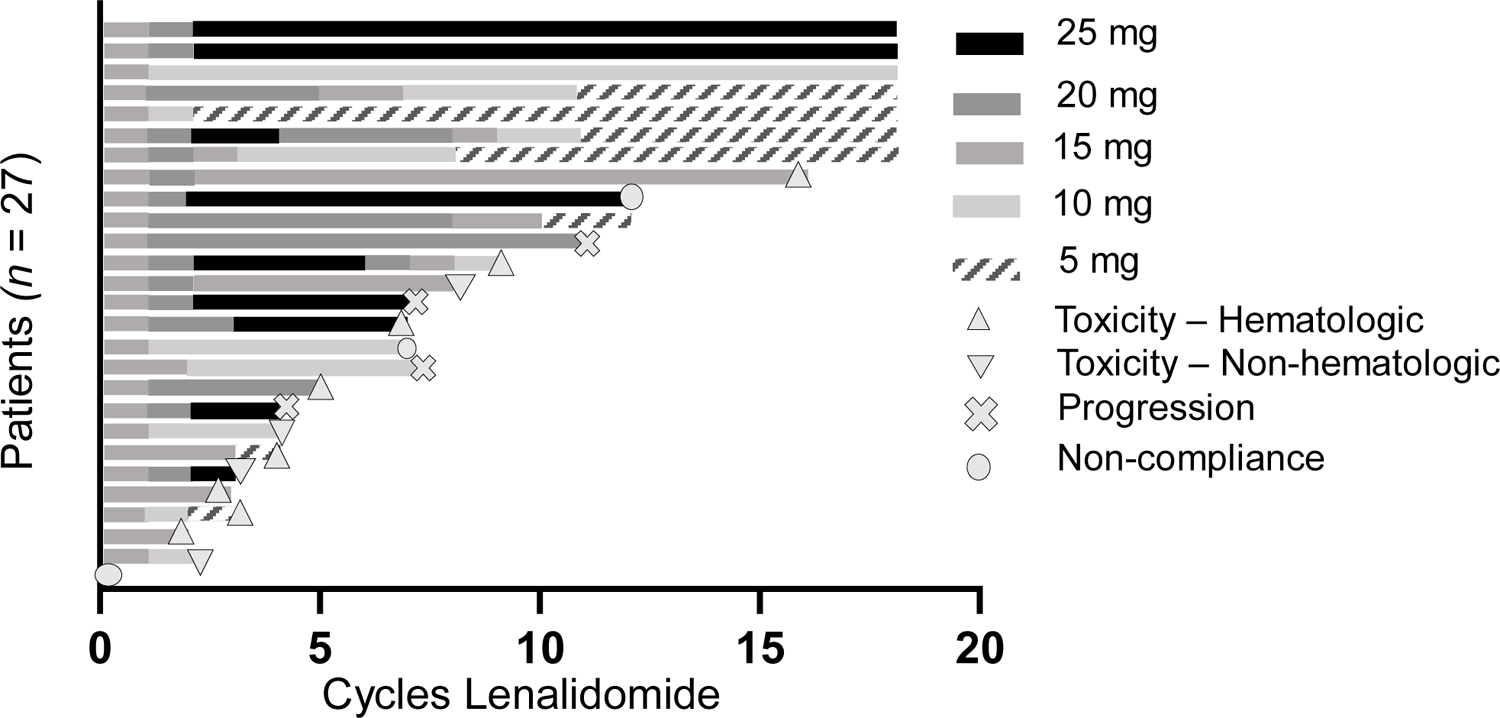
Swimmer’s plot demonstrating the duration and doses of lenalidomide (given continuously on 28-day cycles) for all patients evaluable for the primary endpoint (n = 27). Reasons for discontinuation of therapy are indicated for those who completed less than 18 cycles.
Highlights.
We performed a pilot clinical trial to determine the feasibility of maintenance lenalidomide post ASCT in relapsed/refractory cHL
An unexpectedly high frequency of hematological toxicities resulting in early lenalidomide discontinuation was observed
The lenalidomide maintenance dose and schedule used in this study are not feasible in rel/ref cHL
Acknowledgments:
The authors are indebted to the nursing and research staff involved in these studies and to all the participants and their families. This work was supported by the Siteman Cancer Center Investment Program Team Science Award (P30 CA091842). The authors recognize the support of the Biostatistics Core, Clinical Trials Core, and Imaging Response Assessment Team funded via the NCI CCC support grant P30 CA091842. The REDCap database used for managing clinical data was supported by Clinical and Translational Science Award (CTSA) Grant [UL1 TR000448] and P30 CA091842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registered at www.ClinicalTrials.gov as NCT01207921.
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: Celgene provided research funding to Washington University to perform the investigator-initiated clinical trial.
References
- 1.Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majhail NS, Weisdorf DJ, Defor TE, Miller JS, McGlave PB, Slungaard A, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12(10):1065–72. [DOI] [PubMed] [Google Scholar]
- 3.Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–4. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–71. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz CH, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132(25):2639–42. [DOI] [PubMed] [Google Scholar]
- 6.Re D, Kuppers R, Diehl V. Molecular pathogenesis of Hodgkin’s lymphoma. J Clin Oncol. 2005;23(26):6379–86. [DOI] [PubMed] [Google Scholar]
- 7.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99(12):4283–97. [DOI] [PubMed] [Google Scholar]
- 8.Mainou-Fowler T, Angus B, Miller S, Proctor SJ, Taylor PR, Wood KM. Micro-vessel density and the expression of vascular endothelial growth factor (VEGF) and platelet-derived endothelial cell growth factor (PdEGF) in classical Hodgkin lymphoma (HL). Leuk Lymphoma. 2006;47(2):223–30. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen LM, Klausen TW, Davidsen UH, Johnsen HE. Early changes in serum IL-6 and VEGF levels predict clinical outcome following first-line therapy in aggressive non-Hodgkin’s lymphoma. Ann Hematol. 2005;84(8):510–6. [DOI] [PubMed] [Google Scholar]
- 10.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–6. [PubMed] [Google Scholar]
- 11.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210–6. [DOI] [PubMed] [Google Scholar]
- 12.LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103(5):1787–90. [DOI] [PubMed] [Google Scholar]
- 13.Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dredge K, Marriott JB, Macdonald CD, Man HW, Chen R, Muller GW, et al. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer. 2002;87(10):1166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69(1–2):56–63. [DOI] [PubMed] [Google Scholar]
- 16.Arora M, Gowda S, Tuscano J. A comprehensive review of lenalidomide in B-cell non-Hodgkin lymphoma. Ther Adv Hematol. 2016;7(4):209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard JP, Trneny M, Izutsu K, Fowler NH, Hong X, Zhu J, et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol. 2019;37(14):1188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morschhauser F, Fowler NH, Feugier P, Bouabdallah R, Tilly H, Palomba ML, et al. Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N Engl J Med. 2018;379(10):934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink AM, Bahlo J, Robrecht S, Al-Sawaf O, Aldaoud A, Hebart H, et al. Lenalidomide maintenance after first-line therapy for high-risk chronic lymphocytic leukaemia (CLLM1): final results from a randomised, double-blind, phase 3 study. Lancet Haematol. 2017;4(10):e475–e86. [DOI] [PubMed] [Google Scholar]
- 20.Thieblemont C, Tilly H, Gomes da Silva M, Casasnovas RO, Fruchart C, Morschhauser F, et al. Lenalidomide Maintenance Compared With Placebo in Responding Elderly Patients With Diffuse Large B-Cell Lymphoma Treated With First-Line Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J Clin Oncol. 2017;35(22):2473–81. [DOI] [PubMed] [Google Scholar]
- 21.Fehniger TA, Larson S, Trinkaus K, Siegel MJ, Cashen AF, Blum KA, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2011;118(19):5119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehniger TA, Larson S, Trinkaus K, Siegel MJ, Hurd DD, Blum KA, et al. A Phase 2 Multicenter Study of Continuous Dose Lenalidomide in Relapsed or Refractory Classical Hodgkin Lymphoma. Blood. 2012;120(21):1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782–91. [DOI] [PubMed] [Google Scholar]
- 25.Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895–905. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J Clin Oncol. 2017;35(29):3279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86. [DOI] [PubMed] [Google Scholar]
- 29.Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliva S, Bruinink DHo, ŘÍhová L, Spada S, Holt Bvd, Troia R, et al. Minimal residual disease (MRD) monitoring by multiparameter flow cytometry (MFC) in newly diagnosed transplant eligible multiple myeloma (MM) patients: Results from the EMN02/HO95 phase 3 trial. Journal of Clinical Oncology. 2017;35:abstract 8011. [Google Scholar]
- 31.Spina V, Bruscaggin A, Cuccaro A, Martini M, Di Trani M, Forestieri G, et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood. 2018;131(22):2413–25. [DOI] [PubMed] [Google Scholar]
- 32.Lazarus HM, Loberiza FR Jr., Zhang MJ, Armitage JO, Ballen KK, Bashey A, et al. Autotransplants for Hodgkin’s disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR). Bone Marrow Transplant. 2001;27(4):387–96. [DOI] [PubMed] [Google Scholar]
- 33.Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25(5):571–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


