Abstract
The aim of meta-analysis was to assess the effects of propolis on markers of oxidative stress, lipid profiles, inflammation and glycemic control, liver enzymes, and weight control. The heterogeneity between the included studies was indicated using the Cochrane’s Q test and I-square (I2) statistic. 14 trials were included in this meta-analysis. Our meta-analysis indicated a significant reduction in fating glucose (WMD: -17.00; 95% CI: −30.88, −3.11), HbA1C (WMD: -0.42; 95% CI: −0.75, −0.10), and insulin (WMD: -1.75; 95% CI: −3.24, −0.26) and a marginally significant reduction in insulin resistance (WMD: -0.60; 95% CI: −1.20, 0.00) following propolis supplementation in 10, 8, 6, and 5 studies, respectively. Pooling 5 effect sizes, a significant reduction was seen in ALT (WMD: -5.63; 95% CI: −10.59, −0.67) and aspartate aminotransferase (AST) (WMD: -3.09; 95% CI: −5.15, −1.03) following propolis. A significant beneficial effect was observed for CRP (WMD: -1.11; 95% CI: −1.92, −0.29), TNF-α (WMD: -6.71; 95% CI: −9.44, −3.98) and interleukin-6 (IL-6) (WMD: -17.99; 95% CI: −35.56, −0.42) concentrations after propolis supplementation. This study demonstrated the beneficial effects of propolis on FPG, HbA1c, insulin, CRP, TNF-α and liver enzymes levels.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-020-00696-w.
Keywords: Propolis, LDL-cholesterol, Insulin resistance, HDL-cholesterol, Oxidative stress, Meta-analysis
Introduction
Propolis is a natural material made with honey bees (workers) from gums, blossoms, and plants. After being made with saliva and enzymes secreted by the bee’s salivary glands, it produces a compound and a viscous substance used in the hive [1]. Because of the physical properties and waxy nature of this bee product, propolis is used as cementing substances in the manufacture and repair of beehives. It also acts as a protective barrier against animals and natural agents and antiseptic to preserve the larvae, honey stores, comb from microbial invaders, invaders corpse, hive temperature [2]. Propolis has been widely used by men in traditional medicine to treat wounds, injuries, cancer infection and maintenance of corpses [3]. In Persian Medicine propolis is described as a drug with efficacy against rheumatism, eczemas and myalgia [4].
Propolis has been reported to exhibit diverse roles including anti-inflammatory [5–7], antibacterial [8, 9], antioxidant [9–11], hepatoprotective [12, 13], anti-cancer [9, 14], neuroprotective [15], and immune activity [16, 17]. In limited studies, the effect of propolis on blood pressure has also been evaluated [18, 19]. Recently, Karimian et al. [20] conducted on six RCTs among diabetic patients and indicated that propolis supplementation improved the status of FPG and HbA1c, while did not affect insulin resistance, and insulin status.
Propolis compounds are highly affected by geographical area, environmental factors and beekeeper actions [1]. The main constituents of propolis responsible for biological activity are flavonoids (rutin, quercetin, galangin, CAPE, terpenes, phenols,β-steroids, sesquiterpenes and aromatic [21]. Recently, several human studies have investigated the effect of propolis from different geographical regions on metabolic parameters in different populations. However, existing studies have contradictory results. Discrepancies among evidence might related to difference in the duration of supplementation, source region and dosage of propolis, population characteristics and the sample size of trials. Therefore, with accumulating evidence, the present meta-analysis was conducted to determine the efficacy of propolis on inflammation, glycemic control, oxidative stress, and lipid profiles, liver enzymes, and weight control.
Methods
Current review was done according to the PRISMA directions, except the protocol has not already been registered in the PROSPERO. PRISMA checklist is provided in supplemental Table 1.
This meta-analysis was performed to determine the effects of propolis supplementation on 1) anthropometric measurements: Weight and BMI. 2) Markers of glycemic control: FPG, HOMA-IR, HbA1c. 3) Lipid profiles: total-, HDL, TG, LDL-, and VLDL- levels. 4) Inflammatory profiles: CRP, TNF-a, and IL-6. 5) Oxidative stress markers: TAC, and MDA. 6) Liver enzymes: ALT, and AST. In this study, primary outcomes were FPG and TG.
Search strategy
Search strategy was done using the following text keywords, and MeSH; intervention (“propolis”), and outcomes markers (“FPG” OR “fasting glucose” OR “fasting blood sugar (FBS)” OR “insulin” OR “HOMA-IR” OR “HbA1c” OR “high-density lipoprotein (HDL-cholesterol)” OR “TG” OR “total cholesterol (TC)” OR “LDL-C” OR “low-density lipoprotein (LDL-cholesterol)” OR “HDL-C” OR “VLDL” OR “very low-density lipoprotein” OR “tumor necrosis factor-α (TNF-α)” OR “oxidative stress” OR “C-reactive protein (CRP)” OR “malondialdehyde (MDA)” OR “total antioxidant capacity (TAC)” OR “interleukin-6 (IL-6)” OR “alanine aminotransferase (ALT)” OR “aspartate aminotransferase (AST)” OR “BMI” OR “weight”). In addition, we searched international prospective register of systematic reviews (PROSPERO) database to identify similar records.
Inclusion criteria
RCTs which fulfilled the following criteria were included: 1) Intervention: propolis supplementation. 2) Comparisons: control, including placebo, usual diet/treatment or no intervention. 3) Outcomes: body weight, BMI, glycemic control factors, lipoproteins, liver enzymes, inflammatory biomarkers and markers of oxidative stress. 4) Study design: parallel or cross-over.
Exclusion criteria
Following studies were excluded: in vitro studies, animal experiments, case reports, observational studies, and trials without control group.
Data extraction
Two independent reviewers (JH and EA) screened included articles and extracted relevant information. Any disagreements were resolved by discussion and judgment of the third author (SMM). The following data were extracted: the first authors’ name, publication year, study sample size and location, propolis dosage, study design, type of disease, study duration, mean and SD of markers of glycemic status, inflammation, serum lipoproteins and oxidative stress, liver enzymes and weight control in each groups at baseline and final or mean and SDs of changes in these variables throughout the study.
Statistical analysis
Propolis supplementation on changes in outcomes of interest was calculated using random-effect model and weighted mean difference (WMD) with 95% CI were obtained. Mean/median with SDs of study outcomes were used in our analyses. Therefore, other reports including 95% confidence intervals or SE or interquartile range (IQR) were converted to SDs. Among-study heterogeneity was evaluated using Cochrane’s Q test (P value <0.1 considered as statistically significant) by the I-square statistic [22]. The funnel plot, also the Egger’s regression test were used to evaluate publication bias [23].
Results
Study characteristics
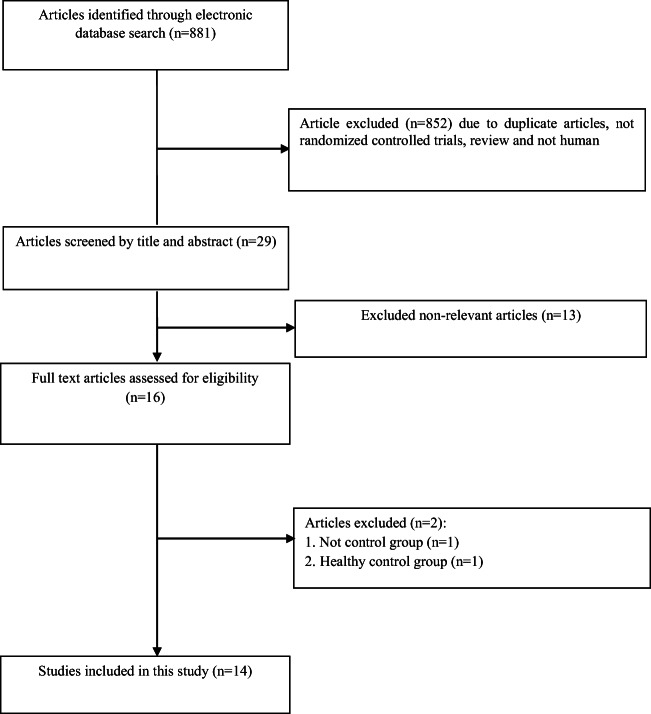
Fourteen studies were included in the current review. Flow-diagram of study selection is presented in Fig. 1. This evidence was published between 2003 and 2019. A total of 812 subjects were participated. General characteristics of included are summarized in Table 1. Evidence was done in Japan, Egypt, China, Iran, Chile, Brazil, and South Korea. Participants were healthy or had type 2 diabetes, chronic periodontitis, chronic kidney disease, athma, and asthenozoospermia. Study duration varied from 4 weeks to 24 months. Body weight, BMI, FPG, insulin,, HbA1C, TC, LDL,TG, HDL, HOMA-IR, CRP, IL-6, TNF-α, MDA, TAC, ALT, and AST were measured in the included studies.
Fig. 1.
Literature search and review flowchart for selection of studies
Table 1.
Characteristics of included studies
| Authors (Ref) | Publication year | Sample size (control/intervention) | Country/Population | Intervention (name and daily dose) | Duration | Age (y) (control, intervention) |
Presented data |
|---|---|---|---|---|---|---|---|
| Khayyal et al. [48] | 2003 | 23/22 | Egypt/ Mild to moderate asthma | 2 mL/d of 13% aqueous extract of propolis solution (260 mg/d) | 8 weeks | 19–52 | IL-6, TNF-α |
| Fukuda et al. [49] | 2015 | 39/41 | Japan/T2DM | 226.8 mg/d Brazilian green propolis | 8 weeks |
62.9 ± 7.8, 63.7 ± 9.3 |
FPG, Ins, HOMA-IR, HbA1c, TC, HDL-C, LDL-C, IL-6, TNF-α |
| El-Sharkawy et al. [50] | 2016 | 26/24 | Egypt/T2DM + chronic periodontitis | 400 mg/d propolis + scaling and root planning | 6 months |
51.2 ± 6.5, 48.9 ± 8.3 |
FPG, HbA1c |
| Zhao et al. [31] | 2016 | 32/33 | China/T2DM | 900 mg/d Brazilian green propolis | 18 weeks |
60.8 ± 8.6 59.5 ± 8.0 |
FPG, Ins, HbA1c, TAC, MDA |
| Afsharpour et al. [51] | 2017 | 30/30 | Iran/T2DM | 1500 mg/d Iranian propolis | 8 weeks |
49.05 ± 8.2, 51.81 ± 6.35 |
Weight, BMI, FPG, Ins, HOMA-IR, HbA1c, CRP, TNF-α, AST, ALT |
| Mujica et al. [44] | 2017 | 32/35 | Chile/Healthy individuals | 13 drops/d propolis | 90 days |
44.5 ± 13.7, 48 ± 12.1 |
Weight, BMI, FPG, HOMA-IR, HbA1c, TG, TC, HDL-C, LDL-C, CRP, AST, ALT |
| Samadi et al. [29] | 2017 | 27/30 | Iran/T2DM | 900 mg/d propolis | 12 weeks |
56.07 ± 9.02 51.30 ± 6.57 |
Weight, BMI, FPG, Ins, HOMA-IR, HbA1c, TG, TC, HDL-C, LDL-C |
| Zhu et al. [42] | 2018 | 30/30 | China/Elderly people living at high altitude | 830 mg/d Brazilian green propolis | 24 months |
73.23 ± 7.32, 72.28 ± 7.23 |
IL-6, TNF-α, AST, ALT |
| Gao et al. [32] | 2018 | 30/25 | China/T2DM | 900 mg/d Chinese propolis | 18 weeks |
60.6 ± 8.4, 57.7 ± 7.5 |
FPG, Ins, HbA1c, TAC, MDA |
| Afsharpour et al. [45] | 2019 | 30/30 | Iran/T2DM | 1500 mg/d Iranian propolis | 8 weeks |
49.05 ± 8.2, 51.81 ± 6.35 |
TAC |
| Zakerkish et al. [52] | 2019 | 44/50 | Iran/T2DM | 1000 mg/d Iranian propolis | 90 days |
54.86 ± 8.89, 55.40 ± 9.09 |
Weight, BMI, FPG, Ins, HOMA-IR, HbA1c, TG, TC, HDL-C, LDL-C, CRP, IL-6, TNF-α, AST, ALT |
| Silveira et al. [43] | 2019 | 14/18 | Brazil/Chronic kidney disease | 500 mg/d Brazilian green propolis | 12 months |
61.50 ± 10.77, 61.39 ± 10.47 |
AST, ALT |
| Gholaminejad et al. [28] | 2019 | 28/29 | Iran/Infertile Men with Asthenozoospermia | 1500 mg/d Iranian propolis | 10 weeks |
30 ± 3.96 31.61 ± 4.18 |
Weight, BMI, CRP, TNF-α, TAC, MDA |
| Koo et al. [53] (a) | 2019 | 5/10 | South Korea/Smokers | 600 mg/d propolis | 4 weeks | 20–28 | FPG |
| Koo et al. [53] (b) | 2019 | 5/10 | South Korea/Smokers | 180 mg/d propolis +420 mg/d aloe polysaccharide | 4 weeks | 20–28 | FPG |
BMI, Body Mass Index; FPG, Fasting Plasma Glucose; HbA1C, Hemoglobin A1C; IR, Insulin Resistance; TC, Total Cholesterol; TG, Triglyceride; LDL, Low Density Lipoprotein; HDL, High Density Lipoprotein; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; CRP, C - reactive protein; IL-6, Interleukin-6; TNF-α, Tumor Necrosis Factor-α; MDA, Malondialdehyde; TAC, Total Antioxidant Capacity
Findings for the effects of propolis on anthropometric measures
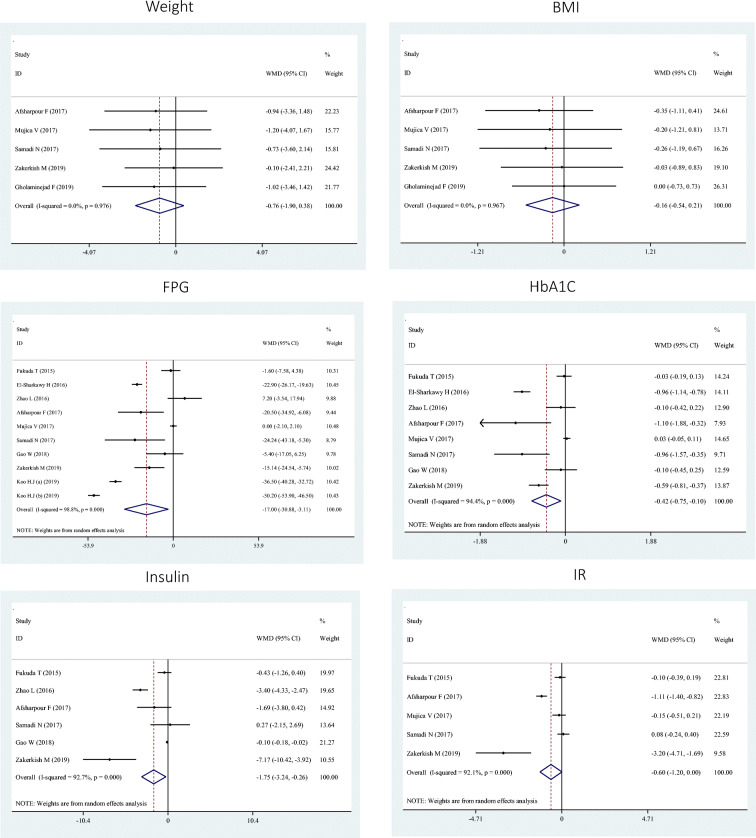
Combining 5 effect sizes, we shown no significant effect of propolis on WMD: -0.76; 95% Confidence Interval (CI): −1.90, 0.38] and BMI (WMD: -0.16; 95% CI: −0.54, 0.21) (Fig. 2). Subgroup analyses did not change overall findings (Table 3).
Fig. 2.
Meta-analysis markers of glycemic control, lipid profiles, inflammation and oxidative stress, liver enzymes, and weight control weighted mean difference estimates in the Propolis and control groups (CI = 95%)
Table 3.
Subgroup analyses for the effects of propolis supplementation on study variables
| Variables | Subgroups | Number of effect sizes | Pooled WMD | 95% CI | I2 (%) | Between-study I2 (%) |
|
|---|---|---|---|---|---|---|---|
| Weight | Participants’ age | <50 year | 2 | −1.10 | −2.96, 0.77 | 00.0 | 0.65 |
| ≥50 year | 3 | −0.56 | −2.00, 0.88 | 00.0 | |||
| Chronic disease | Yes | 3 | −0.56 | −2.00, 0.88 | 00.0 | 0.65 | |
| No | 2 | −1.10 | −2.96, 0.77 | 00.0 | |||
| Study duration | <12 weeks | 2 | −0.98 | −2.70, 0.74 | 00.0 | 0.73 | |
| ≥12 weeks | 3 | −0.59 | −2.11, 0.94 | 00.0 | |||
| Study sample size | <60 | 2 | −0.90 | −2.76, 0.96 | 00.0 | 0.85 | |
| ≥60 | 3 | −0.68 | −2.12, 0.77 | 00.0 | |||
| BMI | Participants’ age | <50 year | 2 | −0.07 | −0.66, 0.52 | 00.0 | 0.69 |
| ≥50 year | 3 | −0.22 | −0.71, 0.26 | 00.0 | |||
| Chronic disease | Yes | 3 | −0.22 | −0.71, 0.26 | 00.0 | 0.69 | |
| No | 2 | −0.07 | −0.66, 0.52 | 00.0 | |||
| Study duration | <12 weeks | 2 | −0.17 | −0.69, 0.36 | 00.0 | 0.96 | |
| ≥12 weeks | 3 | −0.15 | −0.69, 0.38 | 00.0 | |||
| Study sample size | <60 | 2 | −0.10 | −0.67, 0.48 | 00.0 | 0.77 | |
| ≥60 | 3 | −0.21 | −0.70, 0.29 | 00.0 | |||
| FPG | Participants’ age | <50 year | 4 | −18.07 | −19.54, −16.60 | 99.6 | <0.001 |
| ≥50 year | 6 | −5.71 | −9.70, −1.73 | 73.6 | |||
| Chronic disease | Yes | 7 | −16.00 | −18.52, −13.47 | 90.3 | 0.58 | |
| No | 3 | −16.85 | −18.49, −15.20 | 99.7 | |||
| Study duration | <12 weeks | 4 | −36.21 | −38.60, −33.83 | 98.4 | <0.001 | |
| ≥12 weeks | 6 | −6.74 | −8.43, −5.05 | 96.6 | |||
| Study sample size | <60 | 6 | −32.92 | −34.90, −30.94 | 97.5 | <0.001 | |
| ≥60 | 4 | −1.16 | −3.09, 0.76 | 81.9 | |||
| HbA1C | Participants’ age | <50 year | 2 | −0.12 | −0.19, −0.05 | 98.9 | 0.06 |
| ≥50 year | 6 | −0.25 | −0.36, −0.14 | 82.0 | |||
| Study duration | <12 weeks | 2 | −0.07 | −0.23, 0.09 | 85.4 | 0.27 | |
| ≥12 weeks | 6 | −0.17 | −0.24, −0.11 | 95.7 | |||
| Study sample size | <60 | 4 | −0.65 | −0.80, −0.51 | 90.7 | <0.001 | |
| ≥60 | 4 | −0.05 | −0.11, 0.02 | 91.5 | |||
| Insulin | Study duration | <12 weeks | 2 | −0.60 | −1.37, 0.17 | 16.0 | 0.23 |
| ≥12 weeks | 4 | −0.12 | −0.20, −0.05 | 95.5 | |||
| Study sample size | <60 | 3 | −0.12 | −0.20, −0.05 | 95.9 | 0.03 | |
| ≥60 | 3 | −0.95 | −1.70, −0.20 | 87.5 | |||
| IR | Chronic disease | Yes | 2 | −0.61 | −0.82, −0.40 | 95.6 | <0.01 |
| No | 3 | −0.10 | −0.34, 0.14 | 88.5 | |||
| ALT | Chronic disease | Yes | 3 | −2.91 | −5.76, −0.06 | 75.3 | 0.99 |
| No | 2 | −2.91 | −4.52, −1.29 | 96.0 | |||
| AST | Chronic disease | Yes | 3 | −3.82 | −5.84, −1.80 | 9.9 | 0.04 |
| No | 2 | −1.67 | −2.30, −1.04 | 91.9 | |||
| CRP | Participants’ age | <50 year | 2 | −0.64 | −0.87, −0.41 | 95.9 | <0.001 |
| ≥50 year | 2 | −0.002 | −0.001, −0.003 | 98.1 | |||
| Chronic disease | Yes | 2 | −0.002 | −0.001, −0.003 | 98.1 | <0.001 | |
| No | 2 | −0.64 | −0.87, −0.41 | 95.9 | |||
| Study duration | <12 weeks | 2 | −0.002 | −0.001, −0.003 | 97.9 | <0.001 | |
| ≥12 weeks | 2 | −0.60 | −0.84, −0.35 | 97.2 | |||
| TNF-α | Chronic disease | Yes | 4 | −0.16 | −0.39, 0.06 | 95.6 | <0.001 |
| No | 2 | −2.32 | −2.75, −1.89 | 97.8 | |||
| Study duration | <12 weeks | 4 | −0.62 | −0.82, −0.42 | 97.8 | <0.001 | |
| ≥12 weeks | 2 | −63.05 | −75.82, −50.27 | 00.0 | |||
| TAC | Study duration | <12 weeks | 2 | 0.71 | 0.69, 0.73 | 95.4 | <0.001 |
| ≥12 weeks | 2 | −0.01 | −0.03, 0.02 | 00.0 | |||
BMI, Body Mass Index; FPG, Fasting Plasma Glucose; HbA1C, Hemoglobin A1C; IR, Insulin Resistance; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; CRP, C - reactive protein; TNF-α, Tumor Necrosis Factor-α; TAC, Total Antioxidant Capacity
Findings for the effects of propolis on glycemic control
We showed a significant reduction in serum FPG (WMD: -17.00; 95% CI: −30.88, −3.11), HbA1C (WMD: -0.42; 95% CI: −0.75, −0.10), and insulin (WMD: -1.75; 95% CI: −3.24, −0.26) and a marginally significant reduction in HOMA-IR (WMD: -0.60; 95% CI: −1.20, 0.00) following propolis supplementation in 10, 8, 6, and 5 studies, respectively (Fig. 2). These findings remained significant after subgroup analysis in most cases. However, it disappeared in studies with a sample size of ≥60 for FPG, evidence with a duration of <12 weeks or a sample size of ≥60 for HbA1C, evidence with a duration of <12 weeks for insulin, and studies done on individuals without chronic diseases for HOMA-IR (Table 3).
Findings for the effects of propolis on lipid profiles
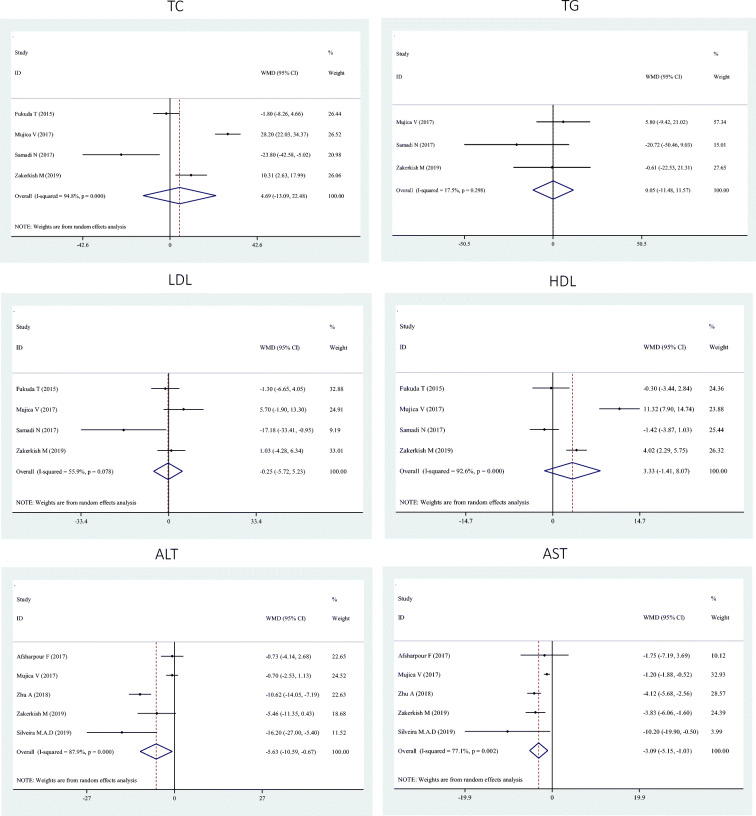
Our indicated no significant effects of propolis on serum TC (WMD: 4.69; 95% CI: −13.09, 22.48), TG (WMD: 0.05; 95% CI: −11.48, 11.57), LDL (WMD: -0.25; 95% CI: −5.72, 5.23), and HDL (WMD: 3.33; 95% CI: −1.41, 8.07) concentrations (Fig. 2).
Findings for the effects of propolis on liver enzymes
Pooling 5 effect sizes, a significant reduction was seen in ALT (WMD: -5.63; 95% CI: −10.59, −0.67) and AST (WMD: -3.09; 95% CI: −5.15, −1.03) following propolis supplementation (Fig. 2). These findings remained unchanged after subgroup analysis (Table 2).
Table 2.
The effects of propolis supplementation on study variables
| Variables | Number of effect sizes | Weighted mean difference | CI 95% | Heterogeneity | |
|---|---|---|---|---|---|
| I2 (%) | P- value heterogeneity | ||||
| Weight | 5 | -0.76 | −1.90, 0.38 | 00.0 | 0.97 |
| BMI | 5 | −0.16 | −0.54, 0.21 | 00.0 | 0.96 |
| FPG | 10 | −17.00 | −30.88, −3.11 | 98.8 | <0.001 |
| HbA1C | 8 | −0.42 | −0.75, −0.10 | 94.4 | <0.001 |
| Insulin | 6 | −1.75 | −3.24, −0.26 | 92.7 | <0.001 |
| IR | 5 | −0.60 | −1.20, 0.00 | 92.1 | <0.001 |
| TC | 4 | 4.69 | −13.09, 22.48 | 94.8 | <0.001 |
| TG | 3 | 0.05 | −11.48, 11.57 | 17.5 | 0.29 |
| LDL | 4 | −0.25 | −5.72, 5.23 | 55.9 | 0.07 |
| HDL | 4 | 3.33 | −1.41, 8.07 | 92.6 | <0.001 |
| ALT | 5 | −5.63 | −10.59, −0.67 | 87.9 | <0.001 |
| AST | 5 | −3.09 | −5.15, −1.03 | 77.1 | <0.01 |
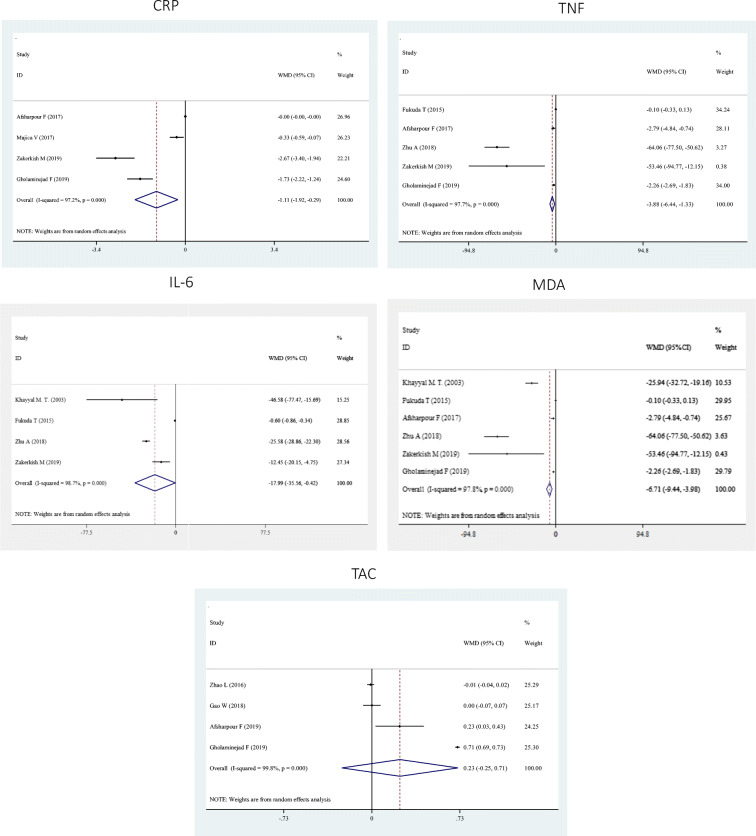
| CRP | 4 | −1.11 | −1.92, −0.29 | 97.2 | <0.001 |
| IL-6 | 4 | −17.99 | −35.56, −0.42 | 98.7 | <0.001 |
| TNF-α | 6 | −6.71 | −9.44, −3.98 | 97.8 | <0.001 |
| MDA | 3 | 0.15 | −1.51, 1.82 | 54.4 | 0.11 |
| TAC | 4 | 0.23 | −0.25, 0.71 | 99.8 | <0.001 |
BMI, Body Mass Index; FPG, Fasting Plasma Glucose; HbA1C, Hemoglobin A1C; IR, Insulin Resistance; TC, Total Cholesterol; TG, Triglyceride; LDL, Low Density Lipoprotein; HDL, High Density Lipoprotein; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; CRP, C - reactive protein; IL-6, Interleukin-6; TNF-α, Tumor Necrosis Factor-α; MDA, Malondialdehyde; TAC, Total Antioxidant Capacity
Findings for the effects of propolis on oxidative stress, and inflammation
A significant reduction was found in CRP (WMD: -1.11; 95% CI: −1.92, −0.29), TNF-α (WMD: -6.71; 95% CI: −9.44, −3.98) and IL-6 (WMD: -17.99; 95% CI: −35.56, −0.42) concentrations after propolis supplementation, whereas, we failed to find significant changes in serum status of MDA (WMD: 0.15; 95% CI: −1.51, 1.82) as well as in TAC levels (WMD: 0.23; 95% CI: −0.25, 0.71) by the propolis supplementation (Fig. 2). Subgroup analysis did not change overall findings in most cases, however, propolis had no significant effect on TNF-α concentrations among evidence done on individuals with chronic diseases and it increased TAC levels among studies with a duration of <12 weeks (Table 3).
Discussion
This study was conducted to summarize the results of previous RCTs evaluated the effects of propolis consumption on glycemic and lipid control, inflammatory indicators, parameters of oxidative stress, liver enzymes and some anthropometric variables. This meta-analysis demonstrated the beneficial effects of propolis on FPG, HbA1c, insulin, CRP, TNF-α and liver enzymes levels.
Effects of propolis on weight control
In the present study, after pooling data for propolis administration, no significant effect was found on weight and BMI. Also, in subgroup analyses based on participants’ age, disease, duration and sample size the results did not differ. In accordance with the results of our study, Salehi-Sahlabadimet al. [24] conducted a meta-analysis on five studies and reported that propolis supplementation was not linked to a significant improvement in anthropometric indices. To date, several human RCT studies have indicated that propolis had no effect on weight and BMI [25–29]. However, an experimental investigations has proposed that the administration of propolis could reduce body weight gain in part through the inhibition of repository of visceral adipose tissue via controlling transcription factors like SREBP- 1, and SREBP-2 which are involved in fatty acid [30].
Effects of propolis on glycemic parameters and lipid control
This meta-analysis revealed that the consumption of propolis supplements decreased indices of glycemic control including FPG, HbA1c and insulin levels while did not improve HOMA-IR and parameters of lipid control. Also in subgroup analysis based on the presence of chronic diseases, direction and significance of results did not differ for FPG, while a significant reduction of HOMA-IR was found in individuals with chronic diseases. Similar with our findings, a previous Meta by Karimian et al. [20] conducted on six RCTs among diabetic patients demonstrated that propolis supplementation improved FPG and HbA1c. Another meta-analysis which conducted on five clinical trials revealed that propolis administration led to decreased TG and increased HDL-cholesterol while had no significant effect on other lipid profiles [24]. On the other hand, a trial by Samadi et al. [29], reported that the intake of 900 mg/d propolis during 12 weeks in patients with T2DM significantly decreased FPG, HbA1c, TG, total and LDL-cholesterol, but did not change HDL-cholesterol and insulin resistance indices. However, supplementation with 900 mg/day Brazilian green propolis during 18 weeks significantly did not change insulin, FPG, HbA1C [31]. Also, an 18-week supplementation with 900 mg/day Chinese propolis did not improve glycemic status [32]. It is suggested that possesses the potential to exert glucose-lowering effects due to the excitation of pancreatic β-cells for production of more insulin and increased sensitivity of cells to insulin via increasing insulin receptor number and phosphorylation [25, 33], increased glucose uptake and increased translocation of insulin-sensitive GLUT 4 [34], and down-regulation of genes involved in gluconeogenesis pathway [35]. One of the possible mechanisms for the blood lipid-lowering effect of propolis is the up-regulation of the ATP-binding cassette transporters (ABC) A1 and G1 expression in the liver’s cells that can lead to increase cholesterol efflux, and HDL [36]. On the other hand, propolis could increase the up-regulation of the ABCA1 pathway via stimulation of PPAR gamma, and liver X receptor expression that can lead to reduced cholesterol accumulation in macrophage foam cells [37]. Also, propolis can lower blood TG levels through the improvement of insulin sensitivity and insulin-mediated lipoprotein lipase activity [38].
Effects of propolis on liver enzymes, inflammation and oxidative stress
For control of oxidative stress, inflammation and liver enzymes, diverse approaches have been suggested including the use of various supplements [39, 40].
In this meta, it was indicated that propolis resulted in reduced the status of inflammatory indicators and liver enzymes, but it did not change oxidative stress indices. In line with our findings, a recent study by Shang et al. [41] indicated that propolis supplementation decreased TNF-a, and CRP concentrations. The effects of propolis on parameters of oxidative stress and liver functions have been investigated in several trials. Zhu et al. [42] reported that two years supplementation with 830 mg/d Brazilian green propolis to elderly individuals living at high altitude decreased the levels of liver enzymes. In another study, it was indicated that 12 months supplementation with 500 mg/d Brazilian green propolis prevented the increase in ALT concentrations in CKD patients [43]. In contrast, Mujica et al. [44] found that the administration of 13 drops/d propolis to healthy individuals did not reduce liver enzymes values. On the other hand, two 18-week clinical trials have reported that after the supplementation with 900 mg/d Brazilian [31] and Chines [45] propolis there were no significant changes in TAC, and MDA in diabetic patients. Although, a significant improvement was indicated in the status of oxidative stress indicators in infertile men with asthenozoospermia who received 1500 mg/d Iranian propolis for 10 weeks [28]. Evidence have proposed that propolis administration could alleviate inflammation through the downregulation of NF-κB, and c-Jun-N-terminal kinase and cyclooxygenase 2 expression [21]. In addition, hepatoprotective effects of propolis mainly could be mediated by CAPE which is one of its active components [46]. Although we did not observe any significant effects on TAC and MDA concentrations, propolis is proposed to act antioxidant roles due to high polyphenols content which are capable to inhibiting the formation of ROS, scavenging free radicals, chelating metal ions and exerting synergistic roles with other antioxidants [47].
Strength and limitations
One of the strength of present study is that all included trials were designed as RCTs. In addition, in the present study we conducted certain subgroup analyses to achieve more complete results. First limitations, in this study some of the included trials were conducted on healthy individuals and some others were performed among patients with chronic diseases like diabetes mellitus, which could be a source of heterogeneity. In addition, the number of participants and included studies were middle and intervention period was limited in most of the included studies. Also, due to the other possible sources of heterogeneity including difference in propolis composition in different regions and various doses the results of present study should be interpreted with cautious.
Conclusions
In conclusion, propolis supplementation lowered serum FPG, insulin, HbA1C and also decreased TNF-α, AST, IL-6, CRP and ALT, but did not changed HOMA-IR, stress oxidative biomarkers(MDA,TAC), lipid profiles (TG,HDL-C,LDL-C,TC) and weight parameters (weight, BMI).
Supplementary Information
(DOCX 16 kb)
Abbreviations
- LDL
Low Density Lipoprotein
- AST
Aspartate Aminotransferase
- FPG
Fasting Plasma Glucose
- BMI
Body Mass Index
- HbA1C
Hemoglobin A1C
- IR
Insulin Resistance
- TG
Triglyceride
- TC
Total Cholesterol
- ALT
Alanine Aminotransferase
- HDL
High Density Lipoprotein
- IL-6
Interleukin-6
- CRP
C-reactive protein
- TAC
Total Antioxidant Capacity
- MDA
Malondialdehyde
- TNF-α
Tumor Necrosis Factor-α
Author contributions
JH and SMM contributed in design, statistical analysis, conception and drafting of the manuscript. AM, VEA, EA and HM contributed in manuscript drafting and data collection.
Funding
IR.QUMS.REC.1398.306.
Data availability
Not applicable.
Compliance with ethical standards
Ethics approval and consent to participate
Not applicable.
Competing interests
No.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jamal Hallajzadeh, Email: jamal.hallaj@yahoo.com.
Alireza Milajerdi, Email: amkhv@yahoo.com.
Elaheh Amirani, Email: e.amirani74@gmail.com.
Vahideh Ebrahimzadeh Attari, Email: ebrahimzadeh.va@gmail.com.
Hossein Maghsoudi, Email: Hosseinm2002@gmail.com.
Seyyed Mehdi Mirhashemi, Email: mirhashemismm@gmail.com.
References
- 1.Toreti VC, Sato HH, Pastore GM, Park YK (2013) Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evidence-based complementary and alternative medicine 2013.
- 2.Mizrahi A, Lensky Y (2013) Bee products: properties, applications, and apitherapy. Springer Science & Business Media,
- 3.Hausen B, Wollenweber E, Senff H, Post B. Propolis allergy.(II). The sensitizing properties of 1, 1-dimethylallyl caffeic acid ester. Contact Dermatitis. 1987;17(3):171–177. doi: 10.1111/j.1600-0536.1987.tb02700.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuropatnicki AK, Szliszka E, Krol W. Historical aspects of propolis research in modern times. Evidence-based complementary and alternative medicine : eCAM. 2013;2013:964149–964111. doi: 10.1155/2013/964149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bueno-Silva B, Alencar SM, Koo H, Ikegaki M, Silva GV, Napimoga MH, Rosalen PL. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J Agric Food Chem. 2013;61(19):4546–4550. doi: 10.1021/jf305468f. [DOI] [PubMed] [Google Scholar]
- 6.Martin LFT, Rocha EM, Garcia SB, Paula JS. Topical Brazilian propolis improves corneal wound healing and inflammation in rats following alkali burns. BMC Complement Altern Med. 2013;13(1):337. doi: 10.1186/1472-6882-13-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asemi Z, Akbari M, Lankarani KB, Tabrizi R, Ghayour-Mobarhan M, Ferns G, Ghaderi A. The effects of curcumin on weight loss among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2019;10:649. doi: 10.3389/fphar.2019.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma MK, Pandey RK, Khanna R, Agarwal J. The antimicrobial effectiveness of 25% propolis extract in root canal irrigation of primary teeth. Journal of Indian Society of Pedodontics and Preventive Dentistry. 2014;32(2):120. doi: 10.4103/0970-4388.130786. [DOI] [PubMed] [Google Scholar]
- 9.Campos JF, dos Santos UP, Macorini LFB, de Melo AMMF, Balestieri JBP, Paredes-Gamero EJ, Cardoso CAL, de Picoli SK, dos Santos EL. Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae) Food Chem Toxicol. 2014;65:374–380. doi: 10.1016/j.fct.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Lopes AA, Ferreira TS, Nesi RT, Lanzetti M, Pires KMP, Silva AM, Borges RM, Silva AJR, Valença SS, Porto LC. Antioxidant action of propolis on mouse lungs exposed to short-term cigarette smoke. Bioorg Med Chem. 2013;21(24):7570–7577. doi: 10.1016/j.bmc.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 11.Fuliang H, Hepburn H, Xuan H, Chen M, Daya S, Radloff S. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol Res. 2005;51(2):147–152. doi: 10.1016/j.phrs.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Shukla S, Bhadauria M, Jadon A. Evaluation of hepatoprotective potential of propolis extract in carbon tetrachloride induced liver injury in rats. Indian J Biochem Biophys. 2005;42(5):321–325. [PubMed] [Google Scholar]
- 13.Babatunde IR, Abdulbasit A, Oladayo MI, Olasile OI, Olamide FR, Gbolahan BW. Hepatoprotective and pancreatoprotective properties of the ethanolic extract of Nigerian propolis. Journal of intercultural ethnopharmacology. 2015;4(2):102–108. doi: 10.5455/jice.20150202023615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omene C, Kalac M, Wu J, Marchi E, Frenkel K, O’Connor OA. Propolis and its active component, caffeic acid phenethyl ester (CAPE), modulate breast cancer therapeutic targets via an epigenetically mediated mechanism of action. Journal of cancer science & therapy. 2013;5(10):334–342. [PMC free article] [PubMed] [Google Scholar]
- 15.Nakajima Y, Shimazawa M, Mishima S, Hara H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sci. 2007;80(4):370–377. doi: 10.1016/j.lfs.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Fan Y, Ma L, Zhang W, Wang J, Chen Y, Gao Y, Feng W, Zhong L, Song X. The design of propolis flavone microemulsion and its effect on enhancing the immunity and antioxidant activity in mice. Int J Biol Macromol. 2014;65:200–207. doi: 10.1016/j.ijbiomac.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Orsatti C, Missima F, Pagliarone A, Bachiega TF, Búfalo M, Araújo J, Jr, Sforcin J. Propolis immunomodulatory action in vivo on toll-like receptors 2 and 4 expression and on pro-inflammatory cytokines production in mice. Phytother Res. 2010;24(8):1141–1146. doi: 10.1002/ptr.3086. [DOI] [PubMed] [Google Scholar]
- 18.Maruyama H, Sumitou Y, Sakamoto T, Araki Y, Hara H. Antihypertensive effects of flavonoids isolated from brazilian green propolis in spontaneously hypertensive rats. Biol Pharm Bull. 2009;32(7):1244–1250. doi: 10.1248/bpb.32.1244. [DOI] [PubMed] [Google Scholar]
- 19.Mishima S, Yoshida C, Akino S, Sakamoto T. Antihypertensive effects of Brazilian propolis: identification of caffeoylquinic acids as constituents involved in the hypotension in spontaneously hypertensive rats. Biol Pharm Bull. 2005;28(10):1909–1914. doi: 10.1248/bpb.28.1909. [DOI] [PubMed] [Google Scholar]
- 20.Karimian J, Hadi A, Pourmasoumi M, Najafgholizadeh A, Ghavami A. The efficacy of propolis on markers of glycemic control in adults with type 2 diabetes mellitus: a systematic review and meta-analysis. Phytotherapy research : PTR. 2019;33(6):1616–1626. doi: 10.1002/ptr.6356. [DOI] [PubMed] [Google Scholar]
- 21.Silva-Carvalho R, Baltazar F, Almeida-Aguiar C. Propolis: a complex natural product with a plethora of biological activities that can be explored for drug development. Evidence-based complementary and alternative medicine : eCAM. 2015;2015:206439–206429. doi: 10.1155/2015/206439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane database of systematic reviews. Int J Epidemiol. 2012;41(3):818–827. doi: 10.1093/ije/dys041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne JA, Egger M. Regression methods to detect publication and other bias in meta-analysis. Publication bias in meta-analysis: Prevention, assessment and adjustments. 2005:99–110.
- 24.Salehi-Sahlabadi A, Chhabra M, Rahmani J, Momeni A, Karam G, Nattagh-Eshtivani E, Nouri M, Clark C, Salehi P, Hekmatdoost A (2020) The effect of propolis on anthropometric indices and lipid profile: a systematic review and meta-analysis of randomized controlled trials. Journal of Diabetes & Metabolic Disorders:1–9. [DOI] [PMC free article] [PubMed]
- 25.Mujica V, Orrego R, Pérez J, Romero P, Ovalle P, Zúñiga-Hernández J, Arredondo M, Leiva E (2017) The role of propolis in oxidative stress and lipid metabolism: a randomized controlled trial. Evidence-Based Complementary and Alternative Medicine 2017. [DOI] [PMC free article] [PubMed]
- 26.Zakerkish M, Jenabi M, Zaeemzadeh N, Hemmati AA, Neisi N. The effect of Iranian propolis on glucose metabolism, lipid profile, insulin resistance, renal function and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-43838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afsharpour F, Hashemipour S, Khadem-Haghighian H, Koushan Y. Effects of Iranian propolis on glycemic status, inflammatory factors, and liver enzyme levels in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. Journal of Nutritional Sciences and Dietetics. 2017:9–14.
- 28.Gholaminejad F, Javadi M, Karami AA, Alizadeh F, Kavianpour M, Khadem Haghighian H. Propolis supplementation effects on semen parameters, oxidative stress, inflammatory biomarkers and reproductive hormones in infertile men with Asthenozoospermia; a randomized clinical trial. International Journal of Medical Laboratory. 2019;6(1):21–32. [Google Scholar]
- 29.Samadi N, Mozaffari-Khosravi H, Rahmanian M, Askarishahi M. Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: a randomized, double-blind clinical trial. Journal of integrative medicine. 2017;15(2):124–134. doi: 10.1016/S2095-4964(17)60315-7. [DOI] [PubMed] [Google Scholar]
- 30.Koya-Miyata S, Arai N, Mizote A, Taniguchi Y, Ushio S, Iwaki K, Fukuda S. Propolis prevents diet-induced hyperlipidemia and mitigates weight gain in diet-induced obesity in mice. Biol Pharm Bull. 2009;32(12):2022–2028. doi: 10.1248/bpb.32.2022. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L, Pu L, Wei J, Li J, Wu J, Xin Z, et al. Brazilian green Propolis improves antioxidant function in patients with type 2 diabetes mellitus. Int J Environ Res Public Health. 2016;13(5). 10.3390/ijerph13050498. [DOI] [PMC free article] [PubMed]
- 32.Gao W, Pu L, Wei J, Yao Z, Wang Y, Shi T, Zhao L, Jiao C, Guo C. Serum antioxidant parameters are significantly increased in patients with type 2 diabetes mellitus after consumption of Chinese Propolis: a randomized controlled trial based on fasting serum glucose level. Diabetes therapy : research, treatment and education of diabetes and related disorders. 2018;9(1):101–111. doi: 10.1007/s13300-017-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson RA. Chromium, glucose intolerance and diabetes. J Am Coll Nutr. 1998;17(6):548–555. doi: 10.1080/07315724.1998.10718802. [DOI] [PubMed] [Google Scholar]
- 34.Ueda M, Hayashibara K, Ashida H. Propolis extract promotes translocation of glucose transporter 4 and glucose uptake through both PI3K-and AMPK-dependent pathways in skeletal muscle. Biofactors. 2013;39(4):457–466. doi: 10.1002/biof.1085. [DOI] [PubMed] [Google Scholar]
- 35.Kang LJ, Lee HB, Bae HJ, Lee SG. Antidiabetic effect of propolis: reduction of expression of glucose-6-phosphatase through inhibition of Y279 and Y216 autophosphorylation of GSK-3α/β in HepG2 cells. Phytother Res. 2010;24(10):1554–1561. doi: 10.1002/ptr.3147. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Si Y, Song G, Luo T, Wang J, Qin S. Ethanolic extract of propolis promotes reverse cholesterol transport and the expression of ATP-binding cassette transporter A1 and G1 in mice. Lipids. 2011;46(9):805–811. doi: 10.1007/s11745-011-3568-7. [DOI] [PubMed] [Google Scholar]
- 37.Iio A, Ohguchi K, Maruyama H, Tazawa S, Araki Y, Ichihara K, Nozawa Y, Ito M. Ethanolic extracts of Brazilian red propolis increase ABCA1 expression and promote cholesterol efflux from THP-1 macrophages. Phytomedicine. 2012;19(5):383–388. doi: 10.1016/j.phymed.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Chen M, Xuan H, Hu F (2012) Effects of encapsulated propolis on blood glycemic control, lipid metabolism, and insulin resistance in type 2 diabetes mellitus rats. Evid Based Complement Alternat Med [DOI] [PMC free article] [PubMed]
- 39.Bahmani F, Tajadadi-Ebrahimi M, Kolahdooz F, Mazouchi M, Hadaegh H, Jamal AS, Mazroii N, Asemi S, Asemi Z. The consumption of Synbiotic bread containing Lactobacillus sporogenes and inulin affects nitric oxide and malondialdehyde in patients with type 2 diabetes mellitus: randomized, double-blind, placebo-controlled trial. J Am Coll Nutr. 2016;35(6):506–513. doi: 10.1080/07315724.2015.1032443. [DOI] [PubMed] [Google Scholar]
- 40.Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, Hadaegh H, Hijijafari M, Abedi F, Asemi Z. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab. 2014;65(1):34–41. doi: 10.1159/000365153. [DOI] [PubMed] [Google Scholar]
- 41.Shang H, Bhagavathula AS, Aldhaleei WA, Rahmani J, Karam G, Rinaldi G, Clark C, Salehisahlabadi A, Yuan Q. Effect of propolis supplementation on C-reactive protein levels and other inflammatory factors: a systematic review and meta-analysis of randomized controlled trials. Journal of King Saud University-Science. 2020;32(2):1694–1701. doi: 10.1016/j.jksus.2020.01.003. [DOI] [Google Scholar]
- 42.Zhu A, Wu Z, Zhong X, Ni J, Li Y, Meng J, Du C, Zhao X, Nakanishi H, Wu S. Brazilian green Propolis prevents cognitive decline into mild cognitive impairment in elderly people living at high altitude. Journal of Alzheimer's disease : JAD. 2018;63(2):551–560. doi: 10.3233/jad-170630. [DOI] [PubMed] [Google Scholar]
- 43.Silveira MAD, Teles F, Berretta AA, Sanches TR, Rodrigues CE, Seguro AC, Andrade L. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: a randomized, double-blind, placebo-controlled trial. BMC Nephrol. 2019;20(1):140. doi: 10.1186/s12882-019-1337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mujica V, Orrego R, Perez J, Romero P, Ovalle P, Zuniga-Hernandez J, Arredondo M, Leiva E. The role of Propolis in oxidative stress and lipid metabolism: a randomized controlled trial. Evidence-based complementary and alternative medicine : eCAM. 2017;2017:4272940–4272911. doi: 10.1155/2017/4272940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afsharpour F, Javadi M, Hashemipour S, Koushan Y, Haghighian HK. Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Complementary therapies in medicine. 2019;43:283–288. doi: 10.1016/j.ctim.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Tolba MF, Azab SS, Khalifa AE, Abdel-Rahman SZ, Abdel-Naim AB. Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: a review on its anti-inflammatory, neuroprotective, hepatoprotective, and cardioprotective effects. IUBMB Life. 2013;65(8):699–709. doi: 10.1002/iub.1189. [DOI] [PubMed] [Google Scholar]
- 47.Kurek-Górecka A, Rzepecka-Stojko A, Górecki M, Stojko J, Sosada M, Swierczek-Zieba G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules (Basel, Switzerland) 2013;19(1):78–101. doi: 10.3390/molecules19010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khayyal MT, el-Ghazaly MA, el-Khatib AS, Hatem AM, de Vries PJ, el-Shafei S, Khattab MM. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam Clin Pharmacol. 2003;17(1):93–102. doi: 10.1046/j.1472-8206.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 49.Fukuda T, Fukui M, Tanaka M, Senmaru T, Iwase H, Yamazaki M, Aoi W, Inui T, Nakamura N, Marunaka Y. Effect of Brazilian green propolis in patients with type 2 diabetes: a double-blind randomized placebo-controlled study. Biomedical reports. 2015;3(3):355–360. doi: 10.3892/br.2015.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Sharkawy HM, Anees MM, Van Dyke TE. Propolis improves periodontal status and glycemic control in patients with type 2 diabetes mellitus and chronic periodontitis: a randomized clinical trial. J Periodontol. 2016;87(12):1418–1426. doi: 10.1902/jop.2016.150694. [DOI] [PubMed] [Google Scholar]
- 51.Afsharpour F, Hashemipour S, Khadem-Haghighian H, Koushan Y (2017) Effects of Iranian propolis on glucose metabolic changes, inflammatory factors, liver enzymes levels in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. Journal of Nutritional Sciences and Dietetics.
- 52.Zakerkish M, Jenabi M, Zaeemzadeh N, Hemmati AA, Neisi N. The effect of Iranian Propolis on glucose metabolism, lipid profile, insulin resistance, renal function and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Sci Rep. 2019;9(1):7289. doi: 10.1038/s41598-019-43838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koo HJ, Lee KR, Kim HS, Lee BM. Detoxification effects of aloe polysaccharide and propolis on the urinary excretion of metabolites in smokers. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2019;130:99–108. doi: 10.1016/j.fct.2019.05.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)
Data Availability Statement
Not applicable.