Abstract
Background
Water pre-load affects insulin secretion by altering the level of copeptin (C-terminal component of the arginine vasopressin hormone (AVP)) and preventing obesity by reducing food intake.
Aims
The present randomized controlled trial (RCT) aimed to investigate the effects of pre-meal water intake on type 2 diabetes Mellitus (T2DM).
Materials and methods
In this study, 40 patients with T2DM were randomly assigned to two intervention groups for 8 weeks; a) drinking 1 liter of water per day before each main meal (PW group)., and b) no water consumption before any meal (NPW group). At the beginning and at the end of the study, blood samples were taken to assess glycemic indices, lipid profile, copeptin and anthropometric indices.
Results
Pre-meal water intake was associated with lower energy intake, BMI, waist circumference (WC) and greater weight loss, in compared with the controls (P < 0.0001) after 8 weeks. At the end of the trial, the concentrations of fasting blood sugar (FBS) (P < 0.0001), triglyceride (TG) (P < 0.05), low-density lipoprotein cholesterol (LDL-C) (P < 0.05) and copeptin (P < 0.05) were significantly reduced following water drinking before meals.
Conclusion
To sum up, the present study revealed that pre-meal water intake is associated with lower BMI, body weight, WC, FBS, TG, LDL-C and copeptin levels in patients with T2DM.
Keywords: Diabetes mellitus, Water intake, Copeptin, Glycemic control
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive metabolic disease with a wide range of pathologic conditions, including insulin resistance, hyperglycemia, systemic inflammation, and lipid profile disorders [1, 2]. The role of arginine vasopressin (AVP), a water retention hormone), has been reported in the pathogenesis of diabetes in previous studies [3, 4]. Copeptin (C-terminal component of AVP), has been distinguished as a reliable marker of serum AVP concentration. Hence, elevated plasma copeptin levels are associated with insulin resistance and metabolic disorders such as high waist circumference, hypertension, hypertriglyceridemia, obesity and greater risk of diabetes [3]. Obesity is the most common risk factor for T2DM that may affect insulin resistance and its progression. Additionally, previous studies have reported that weight loss could improve glycemic indices and reduce the risk of cardiovascular diseases in diabetic patients [4–6].
There is a growing trend towards finding ways to treat diabetes. In line with this, some studies have shown that increasing both water intake and weight loss might improve hyperglycemia and insulin resistance [4], and lower body weight [7]. Epidemiological data have shown that the energy intake is estimated to be 9% lower in additional water consumers rather than normal daily consumers [8]. According to the previous reports from The National Health and Nutrition Examination Survey (NHANES), 30% of subjects who managed to lose weight in the United States were given greater amount of water [9]. Some studies have suggested that drinking 500 cc of water before meals led to increased energy expenditure along with decreased food intake in each meal, which could positively affect the weight loss process [10, 11]. In addition, it has been documented that water intake before meals reduces hunger and increases satiety, that resulting in decreased energy intake in overweight and obese adults [12]. In addition, limited studies with inconsistent results examined the effect of higher water intake on serum copeptin levels in patients with T2DM [13, 14]. Therefore, the present RCT aimed to investigate the effects of pre-meal water intake on the levels of serum copeptin, glycemic control, and lipid profile and anthropometric indices in type 2 diabetics.
Materials and methods
Study participants
Enrolled participants
Subjects with diagnosed T2DM in the last 5 years (with serum glucose levels and medication doses that had been stable for at least 6 months), age range of 30–65 years and body mass index (BMI) < 30 Kg/m2 were recruited from outpatients attending diabetes clinic of Boali hospital in Zahedan, Iran. Sample size was calculated according to the previous study regarding to the type Ι error of 5% (α = 0.05) and type II error of 20% [15].
Excluded participants
The patients treated with insulin; women who are pregnant or lactating; consumption of NSAIDs drugs; smokers; patients who taking medication affecting energy intake or appetite, being on a special diet in the last 3 months, anti-hyperlipidemic, and anti-hypertensive medications, having history of underlying illnesses including renal, liver, cardiovascular diseases and other endocrine disorders were excluded from the study.
Informed consent
All participants were informed about the study objectives, procedures using a leaflet and a signed written informed consent was obtained. The procedure of this study was conducted according to the Declaration of Helsinki. The study protocol was approved by the Ethics Committee on Human Experimentation of Zahedan University of Medical Sciences (approval date: 15.07.2018; No. IR.Zamus.Rec.1397.202).This trial was also registered at the Iranian Registry of Clinical Trials (ID number: IRCT 20180910040986 N1, trial ID: 33757).
Study design
This randomized controlled trial was conducted from April to September 2018. A total of 40 patients (17 males and 23 females, mean age: 51.9 ± 8.8 years; age range: 31–65 years) with T2DM were randomly divided 1:1 into two groups (20 participants in each group). Randomization was performed by a qualified statistician based on a computer-generated code in blocks of four. The intervention group was recommended to intake 1 liter of drinking water daily (250 cc; 30 min before breakfast, 500 cc; 30 min before lunch, and 250 cc; 30 min before dinner [10] for 8 weeks (pre-meal water group: PW). The control group did not receive any recommendation regarding water intake (non-pre-meal water group: NPW). Participants in the intervention group were contacted weekly to assess compliance with the recommendations through telephone interviews. We also asked them not to change their level of physical activity during the study.
Assessment of study variables
Anthropometric indices were measured at the beginning of arrival and after 8 weeks of intervention. Body weight was measured using a digital scale (Seca, Hamburg, Germany) with an accuracy of 0.1 kg with minimal coverage and without shoes. Height was measured without shoes using a non-stretched tape to the nearest 0.5 cm. The trained researchers obtained further information about age, medical history, medication and smoking through face-to-face interviews. At the beginning and end of the study, after 12 h of fasting, venous blood samples (10 cc) were taken from each participant. Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), fasting blood sugar (FBS), and glycated hemoglobin (HbA1C) were assessed using standard kits (Pars Azmoon Inc., Tehran, Iran) via auto-analyzer machine (BT-1500, Italy).
Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as (fasting insulin [(μU/ml] - fasting glucose [mmol/l] (/ 22.5 [16].
Serum copeptin (Bioassay Technology lab, No E1129HU / 96 Test, China (and insulin (Monobind Inc., No 5825–300/ 96 test. USA) levels were also measured using enzyme-linked immunoassay kits.
Dietary intake was assessed by three 24-h Diet Recall at the entry and at end of study. Modified Nutritionist IV software (First Data bank) for Iranian foods was used to compute the findings from this questionnaire to calorie and nutrient content [17].
Statistical analysis
The Statistical Package for Social Sciences (SPSS) software version 21 (SPSS, Inc. Chicago, IL, USA) was used for randomization and all statistical analyses. Data were presented as mean ± SD, mean ± SEM with range and percent,as appropriate. Normal distribution of the data was assessed using Kolmogorov–Smirnov test. A repeated measures ANOVA was used to estimate the statistical difference between two groups, including pre- and post-intervention and within each group between pre- and post-intervention. The baseline and end values of the trial were used to measure and percentage of changes in each of the study variables. In order to identify differences between two groups, t-test or Mann–Whitney U test were planned. P values <0.05 were assumed to be statistically significant.
Results
Table 1 outlines the main demographic characteristics of the enrolled patients. All of the selected patients were enrolled to the trial (17 males and 23 females), and they were aged 52 ± 9.2 and 51 ± 8.2 in the intervention and control groups, respectively.
Table 1.
Demographic and clinical characteristics in the water and non-water groups before and after the 8 weeks intervention
| Groups Variables |
NPW group (n = 20) |
Pv | PW group (n = 20) |
Pv | ||
|---|---|---|---|---|---|---|
| Baseline | After 8 weeks | Baseline | After 8 weeks | |||
| Age (years) | 51 ± 8.2 | 52 ± 9.2 | ||||
| Weight (Kg) | 77.30 ± 12.7 | 78.4 ± 13.3 | 0.15 | 77.9 ± 13.2 | 76.6 ± 12.3€ | 0.0001 |
| BMI (Kg/m2) | 27 ± 4.4 | 27.4 ± 4.7 | 0. 14 | 27.2 ± 3.9 | 26 ± 3.8 € | 0.0001 |
| WC (Cm) | 104 ± 8.9 | 104.2 ± 10 | 0.15 | 105 ± 8.7 | 102.0 ± 8.3 € | 0.001 |
| WHR | 0.95 ± 0.8 | 0.95 ± 0.8 | 0.48 | 0.98 ± 0.7 | 0.96 ± 0.63 | 0.069 |
| FBS (mg/dL) | 221.7 ± 77.4 | 227 ± 57 | 0.51 | 225.7 ± 56.4 | 193.2 ± 32 € | 0.0001 |
| HbA1C (%) | 8.2 ± 1.9 | 8.3 ± 1.9 | 0.35 | 8 ± 1.4 | 7.8 ± 1.0 | 0.52 |
| Insulin (ml/μIU) | 8.7 ± 1.3 | 9.1 ± 1.8 | 0.14 | 8.5 ± 0.93 | 8.7 ± 1.9 | 0.35 |
| HOMA-IR | 4.0 ± 0.68 | 4.7 ± 0.91 | 0.11 | 3.8 ± 0.49 | 4.2 ± 0.52 | 0.15 |
| TC (mg/dL) | 172.4 ± 62.5 | 167.8 ± 31.9 | 0.13 | 176.6 ± 77 | 160.8 ± 38.1 | 0.19 |
| TG (mg/dL) | 155.6 ± 65.5 | 174.6 ± 72.0 | 0.12 | 150 ± 63.2 | 127.7 ± 50 € | 0.049 |
| HDL-C (mg/dL) | 47.9 ± 12.7 | 45.9 ± 11.5 | 0.37 | 44.4 ± 7.6 | 44.7 ± 7.9 | 0.48 |
| LDL-C (mg/dl) | 92.7 ± 39.1 | 88.5 ± 31.5 | 0.15 | 87.7 ± 35.5 | 74.3 ± 34.1 ϕ | 0.03 |
| aCopeptin (pmol/L) |
10.6 ± 3.4 (0.96–58.1) |
11.4 ± 4.5 (1.64–61.6) |
0.27 |
10.3 ± 3.2 (2.86–61.6) |
7.6 ± 2.2 € (1.38–52.6) |
0.046 |
Data are presented as mean ± SD
aData are presented as mean ± SEM and range, because the data were not normally distributed
Abbreviations: NPW Non-Pre-meal Water, PW Pre-meal Water, BMI Body Mass Index, WC Waist Circumference, WHR Waist to Hip Ratio, FBS Fasting Blood Sugar, HbA1C Hemoglobin A1c, HOMA-IR Homeostatic Model Assessment of Insulin Resistance, TC Total Cholesterol, TG Triglycerides, HDL-C High-Density Lipoprotein-Cholesterol, LDL-C Low-Density Lipoprotein- Cholesterol
€ Difference between two groups after 8 weeks, P < 0.001; ϕ Difference between two groups after 8 weeks, P < 0.05
Pre-meal water drinking was associated with the lower levels of copeptin (P < 0.05 and P < 0.001), serum FBS (P < 0.0001 and P < 0.001), TG (P < 0.05 and P < 0.001) and LDL-C (both P < 0.05) compared to baseline and NPW group, respectively (Table 2). Additionally, body weight (P < 0.001), BMI (P < 0.0001) and WC (P < 0.001) were significantly decreased in group receiving pre-meal water in compare with the NPW (Table 2). In contrast, total cholesterol, HbA1c, serum insulin, HOMA-IR and HDL-C were not statistically significant affected by pre-meal water intake (P > 0.05) (Table 2).
Table 2.
Variation of the studied variables after 8 week intervention versus baseline between two groups
| Variables | Groups | Mean | Pv |
|---|---|---|---|
| Weight (Kg) | NPW | 0.9 | 0.0001 |
| PW | −1.35 | ||
| BMI (Kg/m2) | NPW | 0.40 | 0.0001 |
| PW | −1.28 | ||
| WC (Cm) | NPW | 0.20 | 0.0001 |
| PW | −3.0 | ||
| WHR | NPW | 0.00 | 0.46 |
| PW | −0.01 | ||
| FBS (mg/dL) | NPW | 5.3 | 0.003 |
| PW | −32.6 | ||
| HbA1C (%) | NPW | 0.1 | 0.96 |
| PW | −0.2 | ||
| Insulin (ml/μIU) | NPW | 0.45 | 0.13 |
| PW | 0.22 | ||
| HOMA-IR | NPW | 0.74 | 0.17 |
| PW | 0.42 | ||
| TC (mg/dL) | NPW | −13.57 | 0.61 |
| PW | −15.75 | ||
| TG (mg/dL) | NPW | −19.00 | 0.01 |
| PW | 22.05 | ||
| LDL (mg/dL) | NPW | −4.2 | 0.04 |
| PW | −13.37 | ||
| HDL (mg/dL) | NPW | −2 | 0.79 |
| PW | −0.30 | ||
| *Copeptin (pmol/L) | NPW | 0.8 | 0.01 |
| PW | −2.7 |
Abbreviations: NPW Non-Pre-meal Water, PW Pre-meal Water, BMI Body Mass Index, WC Waist Circumference, WHR Waist to Hip Ratio, FBS Fasting Blood Sugar, HbA1C Hemoglobin A1c, HOMA-IR Homeostatic Model Assessment of Insulin Resistance, TC Total Cholesterol, TG Triglycerides, HDL-C High-Density Lipoprotein-Cholesterol, LDL-C Low-Density Lipoprotein- Cholesterol
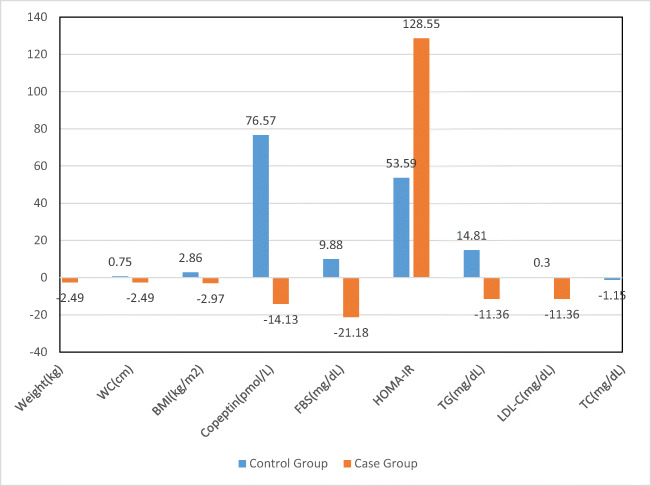
As shown in Fig. 1, compared to controls, the percentage of changes was higher for anthropometric indices, FBS, and LDL-C, and lower for total cholesterol, triglyceride and copeptin levels in treatment group.
Fig. 1.
Variation percent of studied variables
Table 3 presents the distribution of energy and dietary intakes in two studied groups at the baseline and end of intervention. Pre-meals water intake significantly decreased energy (P < 0.0001) and fat (P < 0.001) intakes rather than baseline in the PW group. Moreover, the consumption of protein and energy in PW group was significantly higher than NPW group after 8 weeks of intervention (P < 0.01). In multiple linear regression analysis adjusted for age and BMI, none of the parameters was significantly corrected with copeptin.
Table 3.
Dietary intake and physical activity in water and non-water groups before and after the 8 week intervention
| Groups Variables | NPW (n = 20) |
Pv | PW (n = 20) |
Pv | ||
|---|---|---|---|---|---|---|
| Baseline After 8 weeks | Baseline After 8 weeks | |||||
| Energy (Kcal) | 2190 ± 331 | 2169 ± 413 | 0.1 | 2265 ± 254 | 2091 ± 307 € | 0.0001 |
| Carbohydrate (gr) | 225 ± 38 | 231 ± 51 | 0. 053 | 234 ± 38 | 231 ± 37 | 0.25 |
| Protein(gr) | 80 ± 14 | 77 ± 17 | 0.07 | 82 ± 18 | 87 ± 17 € | 0.20 |
| Fat(gr) | 108 ± 34 | 104 ± 39 | 0.45 | 112 ± 27 | 91 ± 27 | 0.001 |
Data are presented as mean ± SD
Abbreviations: NPW Non- Pre-meal water, PW Pre-meal Water
€ Difference between two groups after 8 weeks, P < 0.01
Discussion
In the present study, we found a significant improvement of serum levels of copeptin, FBS, TG, LDL-C, body weight, BMI and WC, after increasing pre-meals water intake for 8 weeks compared to the habitual water intake in patients with T2DM. In addition, no parameters were significantly corrected with copeptin in multiple linear regression analysis adjusted for age and BMI, which could follow the small sample size and short duration of intervention.
Regarding copeptin, our result complements the finding of previous studies indicating relationship between water intake and the levels of copeptin. Several studies have reported that extra water intake was associated with the lower level of copeptin in adults [16, 18]. Lemetais et al. demonstrated that plasma copeptin concentrations could be modulated through increasing water intake in healthy adults [19]. They stated that the high intake of water might be a potential approach to reduce the risk of metabolic disorders. In addition, in several cohort studies, increased circulating copeptin has been related to the occurrence of metabolic diseases [14, 19]. Several mechanisms have been reported for this relationship [20–22]. The presence of vasopressin receptors V1a and V1b in the pancreas and liver is a reasonable explanation for the association among water intake, copeptin concentrations, and metabolic diseases [19, 23]. In addition, previous studies have obtained a favorable effect of increased water intake on decreasing the risk of some chronic diseases such as cardiovascular disease, chronic kidney disease, and type 2 diabetes [24, 25]. In a clinical study, water intake for 6 weeks in patients with chronic kidney disease showed an inverse effect between a high water intake of up to 1.5 l / day and the serum copeptin levels [26].
In line with our findings, Dennis et al. [27] have reported that water drinking decreases serum LDL-C and TG levels. In the study of Enhörning et al. [14], 1 week of high water intake did not change serum FBS and insulin levels.
Diabetes is one of the complications of obesity [5, 7]. Hence, it has been hypothesized that improvement of body weight in diabetic patients would be associated with appropriate management of diabetes [4]. Drinking a lot of water is often recommended for weight loss [9]. The exact mechanism responsible for the greater weight loss with increased water consumption is presently unknown and further investigations are needed to explain the underlying mechanisms. However, an animal study reported a helpful role of high intake of water and subsequently reduced circulating vasopressin for health conditions [23]. Another studies reported that drinking water might cause weight loss through reducing energy intake [13, 24]. Several studies also reported that consuming water before or with a meal declines the sensations of hunger, and intensifies satiety [11, 12]. Additionally, substituting energy-containing sweet drinks and juices in the diet with water may lead to a decrease in energy intake [28]. Consequently, reducing energy intake in meals is thought to be a beneficial weight loss strategy. In our study, pre-meals water intake was associated with a significant reduction of body weight, BMI and WC in intervention group when compared to the baseline and NPW group. Stookey et al. [29] found that overweight women who reported drinking ≥ 1 liter/day of water over a 12 months period experienced more weight loss compared to those who did not drink such amount water (≈ 2 kg). In the study of Dennis et al. [26] water intake reduced body weight and WC in adults.
The present study also demonstrated that water intake before eating each main meal significantly reduced energy and fat intakes in type 2 diabetic patients. In agreement with our study, Dennis et al. [26] found an inverse association between drinking water and energy intake in adults. They also reported that combining a hypocaloric diet with consuming 500 ml water before each main meal could lead to lower energy intake and greater weight loss. Moreover, Davy et al. [12] reported that pre-meal water intake decreased meal energy intake in overweight and obese adults.
The relationship between serum copeptin and anthropometric indices has been reported in few studies. In a studies, copeptin levels were markedly higher in obese children than in thin children, but there was no significant correlation between metabolic syndrome and copeptin [30]. However, another study found that obese people had lower levels of copeptin than people with normal weight [31]. In another study, the copeptin levels were indirectly related to BMI and WC as indicators of metabolic syndrome [32].
Our findings should be interpreted while considering the study limitations; i) we could not collect other valuable anthropometric and biochemical parameters which linked to the obesity status in these patients such as evaluation of body composition and adipose tissue hormones ii) This study was performed in a short term with low sample size. Therefore, future studies with greater sample size and longer period are needed to evaluate other specific markers that may be related to possible mechanisms of high water intake in energy expenditure and body weight loss.
Conclusion
In conclusion, the present study showed that increasing daily water intake before each main meal leads to an improvement of body composition, lipid profile, serum glucose and copeptin levels. Pre-meals water intake could also resulted in the lower energy and fats intake, which are play an important role in obesity, diabetes and cardio-metabolic disorders.
Acknowledgements
This article was extracted from a master’s degree thesis in nutrition. The authors are grateful to Research Vice Chancellor of Zahedan University of Medical Sciences for financial support and to all patients for their sincere cooperation to this study.
Author contributions
FM and GS contributed to study design and concept. MAK visited patients. GS and SS contributed to data collection. FM and AD contributed to data analyses. FM, GS, MK and MAK contributed to drafting and reviewing the final manuscript. All authors read and approved the final manuscript for publication.
Funding
Research Deputy of Zahedan University of Medical Sciences, Zahedan, Iran financially supported the present study (grant No. 8984).
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E. Chronic complications of diabetes mellitus: a mini review. Curr Diabetes Rev. 2017;13(1):3–10. doi: 10.2174/1573399812666151016101622. [DOI] [PubMed] [Google Scholar]
- 2.Tabrizi R, Nowrouzi-Sohrabi P, Hessami K, Rezaei S, Jalali M, Savardashtaki A, et al. Effects of Ginkgo biloba intake on cardiometabolic parameters in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of clinical trials. 2020. [DOI] [PubMed] [Google Scholar]
- 3.Wannamethee SG, Welsh P, Papacosta O, Lennon L, Whincup PH, Sattar N. Copeptin, insulin resistance, and risk of incident diabetes in older men. J Clin Endocrinol Metabol. 2015;100(9):3332–3339. doi: 10.1210/JC.2015-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark AG, Dennis Parker EA, Savla JS, Davy KP. Is increased water consumption among older adults associated with improvements in glucose homeostasis? 2013. [Google Scholar]
- 5.Agrawal S, Gensure R. Commentary on the impact of obesity on pediatric diabetes. Clin Ther. 2018;40(10):1631–1637. doi: 10.1016/j.clinthera.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Nowrouzi-Sohrabi P, Tabrizi R, Rezaei S, Jafari F, Hesami K, Abedi M, et al. The effect of voglibose on metabolic profiles in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of clinical trials. 2020. p. 104988. [DOI] [PubMed] [Google Scholar]
- 7.Daniels MC, Popkin BM. Impact of water intake on energy intake and weight status: a systematic review. Nutr Rev. 2010;68(9):505–521. doi: 10.1111/j.1753-4887.2010.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popkin BM, Barclay DV, Nielsen SJ. Water and food consumption patterns of US adults from 1999 to 2001. Obes Res. 2005;13(12):2146–2152. doi: 10.1038/oby.2005.266. [DOI] [PubMed] [Google Scholar]
- 9.Muckelbauer R, Sarganas G, Grüneis A, Müller-Nordhorn J. Association between water consumption and body weight outcomes: a systematic review. Am J Clin Nutr. 2013;98(2):282–299. doi: 10.3945/ajcn.112.055061. [DOI] [PubMed] [Google Scholar]
- 10.Parretti HM, Aveyard P, Blannin A, Clifford SJ, Coleman SJ, Roalfe A, Daley AJ. Efficacy of water preloading before main meals as a strategy for weight loss in primary care patients with obesity: RCT. Obesity. 2015;23(9):1785–1791. doi: 10.1002/oby.21167. [DOI] [PubMed] [Google Scholar]
- 11.Van Walleghen EL, Orr JS, Gentile CL, Davy BM. Pre-meal water consumption reduces meal energy intake in older but not younger subjects. Obesity. 2007;15(1):93–99. doi: 10.1038/oby.2007.506. [DOI] [PubMed] [Google Scholar]
- 12.Davy BM, Dennis EA, Dengo AL, Wilson KL, Davy KP. Water consumption reduces energy intake at a breakfast meal in obese older adults. J Am Diet Assoc. 2008;108(7):1236–1239. doi: 10.1016/j.jada.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbasi A, Corpeleijn E, Meijer E, Postmus D, Gansevoort RT, Gans RO, et al. Sex differences in the association between plasma copeptin and incident type 2 diabetes: the prevention of renal and vascular Endstage disease (PREVEND) study. Diabetologia. 2012;55(7):1963–1970. doi: 10.1007/s00125-012-2545-x. [DOI] [PubMed] [Google Scholar]
- 14.Enhörning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, et al. Plasma copeptin and the risk of diabetes mellitus. Circulation. 2010;121(19):2102–2108. doi: 10.1161/CIRCULATIONAHA.109.909663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enhörning S, Tasevska I, Roussel R, Bouby N, Persson M, Burri P, Bankir L, Melander O. Effects of hydration on plasma copeptin, glycemia and gluco-regulatory hormones: a water intervention in humans. Eur J Nutr. 2019;58(1):315–324. doi: 10.1007/s00394-017-1595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ioannou GN, Bryson CL, Boyko EJ. Prevalence and trends of insulin resistance, impaired fasting glucose, and diabetes. J Diabetes Complications. 2007;21(6):363–370. doi: 10.1016/j.jdiacomp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Emami S, Saraf-Bank S, Rouhani MH, Azadbakht L. Diet quality and total daily price of foods consumed among Iranian diabetic patients. Int J Prev Med. 2019;10:50. 10.4103/ijpvm.IJPVM_334_16. [DOI] [PMC free article] [PubMed]
- 18.Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30(3):747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 19.Lemetais G, Melander O, Vecchio M, Bottin JH, Enhörning S, Perrier ET. Effect of increased water intake on plasma copeptin in healthy adults. Eur J Nutr. 2018;57(5):1883–1890. doi: 10.1007/s00394-017-1471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villela-Torres MDLL, Higareda-Mendoza AE, Gómez-García A, Alvarez-Paredes AR, García-López E, Stenvikel P, Gu HF, Rashid-Qureshi A, Lindholm B, Alvarez-Aguilar C. Copeptin plasma levels are associated with decline of renal function in patients with type 2 diabetes mellitus. Arch Med Res. 2018;49(1):36–43. doi: 10.1016/j.arcmed.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflügers Archiv-European J Physiol. 2008;456(6):1005–1024. doi: 10.1007/s00424-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoiser B, Mörtl D, Hülsmann M, Berger R, Struck J, Morgenthaler N, et al. Copeptin, a fragment of the vasopressin precursor, as a novel predictor of outcome in heart failure. Eur J Clin Invest. 2006;36(11):771–778. doi: 10.1111/j.1365-2362.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 23.Taveau C, Chollet C, Waeckel L, Desposito D, Bichet DG, Arthus M-F, Magnan C, Philippe E, Paradis V, Foufelle F, Hainault I, Enhorning S, Velho G, Roussel R, Bankir L, Melander O, Bouby N. Vasopressin and hydration play a major role in the development of glucose intolerance and hepatic steatosis in obese rats. Diabetologia. 2015;58(5):1081–1090. doi: 10.1007/s00125-015-3496-9. [DOI] [PubMed] [Google Scholar]
- 24.Clark WF, Sontrop JM, Macnab JJ, Suri RS, Moist L, Salvadori M, Garg AX. Urine volume and change in estimated GFR in a community-based cohort study. Clin J Am Soc Nephrol. 2011;6(11):2634–2641. doi: 10.2215/CJN.01990211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sontrop JM, Dixon SN, Garg AX, Buendia-Jimenez I, Dohein O, Huang S-HS, Clark WF. Association between water intake, chronic kidney disease, and cardiovascular disease: a cross-sectional analysis of NHANES data. Am J Nephrol. 2013;37(5):434–442. doi: 10.1159/000350377. [DOI] [PubMed] [Google Scholar]
- 26.Dennis EA, Dengo AL, Comber DL, Flack KD, Savla J, Davy KP, Davy BM. Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity. 2010;18(2):300–307. doi: 10.1038/oby.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffey KJ, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity. 2007;15(11):2739–2747. doi: 10.1038/oby.2007.326. [DOI] [PubMed] [Google Scholar]
- 28.Stookey JD, Constant F, Popkin BM, Gardner CD. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity. 2008;16(11):2481–2488. doi: 10.1038/oby.2008.409. [DOI] [PubMed] [Google Scholar]
- 29.Rothermel J, Kulle A, Holterhus PM, Toschke C, Lass N, Reinehr T. Copeptin in obese children and adolescents: relationships to body mass index, cortisol and gender. Clin Endocrinol (Oxf) 2016;85(6):868–873. doi: 10.1111/cen.13235. [DOI] [PubMed] [Google Scholar]
- 30.Lewandowski KC, Lewiński A, Skowrońska-Jóźwiak E, Stasiak M, Horzelski W, Brabant G. Copeptin under glucagon stimulation. Endocrine. 2016;52(2):344–351. doi: 10.1007/s12020-015-0783-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saleem U, Khaleghi M, Morgenthaler NG, Bergmann A, Struck J, Mosley TH, Jr, Kullo IJ. Plasma carboxy-terminal provasopressin (copeptin): a novel marker of insulin resistance and metabolic syndrome. J Clin Endocrinol Metabol. 2009;94(7):2558–2564. doi: 10.1210/jc.2008-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenderenda-Banasiuk E, Wasilewska A, Filonowicz R, Jakubowska U, Waszkiewicz-Stojda M. Serum copeptin levels in adolescents with primary hypertension. Pediatr Nephrol. 2014;29(3):423–429. doi: 10.1007/s00467-013-2683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]