Abstract
Aim
The present study aimed to investigate the effect of Zingiber officinale (ZO) extract on weight gain, food intake, locomotor activity, and lipid and glucose metabolism in olanzapine-treated rats.
Methods
The hydroalcoholic extract of ZO was prepared by macerating the coarse dry powder in 70% v/v ethanol for 7 days, filtered, and concentrated under reduced pressure. Animals were divided into six groups containing six animals in each. Three doses of extract (100, 200, and 400 mg/kg, p.o.) were co-administered with olanzapine 2 mg/kg i.p for 21 days. Bodyweight and food intake were recorded at the interval of three days and locomotor activity once a week. At the end of the study oral glucose tolerance test was performed followed by the estimation of lipid profile.
Results
Co-administration of hydroalcoholic extract of ZO with olanzapine ameliorated olanzapine-induced weight gain and hyperphagia. Similarly, ZO extract also improved pancreatic β-cell function and glucose and lipid metabolism.
Conclusions
ZO extract ameliorated olanzapine-induced weight gain and hyperphagia by improving pancreatic β-cell functions and lipid metabolism.
Keywords: Lipid metabolism, Locomotor activity, Olanzapine, Weight gain, Zingiber officinale
Introduction
Previously, typical anti-psychotic molecules were in a great trend for the management of psychiatric illness. However, they are restricted in their clinical utilization except for urgency due to their extrapyramidal syndrome side effects including symptoms of tardive dyskinesia and neurological toxicity which lead to the utilization of atypical anti-psychotic molecules that are also called second-generation antipsychotic drugs [1, 2]. However, atypical antipsychotics like olanzapine linked prescriptions are reported to cause weight gain and metabolic changes in psychiatric illness; identified via clinical and preclinical studies [3–5]. Further, weight gain and dyslipidemia are well-known risk factors for metabolic disorders via insulin resistance and diabetes [6, 7]. Presently, for the management of olanzapine-induced weight gain, few approaches have been made like co-administration of metformin and weight-reducing agents like orlistat with olanzapine or switching the olanzapine with other antipsychotics [8–10]. However, weight gain is the outcome of the polygenic condition in which multiple-proteins are involved in its progression leading to obesity. Hence, it is important to identify a new therapeutic agent that can be supplemented along with olanzapine which should overcome the olanzapine side effects without affecting its clinical benefits.
Ayurveda and other complementary medicines record the multiple herbal medicines which are composed of multiple secondary metabolites and are effective in the management of etiological-, drug-induced weight gain. Among them, Zingiber officinale (ZO) belonging to the family Zingiberaceae, commonly known as Ginger is an enduring herbaceous plant which is commonly available in every kitchen garden of Asian countries including India, Nepal, Pakistan, China and also grown throughout the world; rhizome of the ginger is crushed, turned to powder and stored for its utilization with vegetables to enhance the taste in each dinner/lunch throughout the year. Ayurveda records ZO as “Sunthi” to be utilized for digestive impairment, flatulence with a gurgling sound, rheumatism, abdominal lump, anemia, chronic rhinitis, filariasis, and dyspnoea [11]. Additionally, extracts of ginger rhizome have been investigated to evaluate their efficacy to reduce weight gain and energy expenditure in high-fat diet-fed rodent models [12–16]. Additionally, the ZO has been reported for its anti-depressant activity [17]. Human trials have reported reduced appetite in overweight subjects after supplementation with a ginger powder [18]. However, no studies have been reported to understand its effect on olanzapine-induced weight gain along with glucose and lipid metabolism.
Hence, the present study aimed to investigate the effects of hydroalcoholic extract of ZO on body weight, food intake, and locomotor activity in an established female rat model of olanzapine-induced weight gain. Besides these, the effects of the extract on lipid and glucose metabolism were also examined in olanzapine-treated rats.
Materials and methods
Collection and extract preparation of ZO rhizomes
Rhizomes of ZO were collected from local areas of Belagavi India and rhizomes were washed to remove foreign matter. The rhizomes were then shade dried and turned into a coarse powder. The coarse powder was then macerated with 70% v/v ethanol for seven days, filtered, and concentrated using a rotator evaporator (IKA RV 10) under reduced pressure.
Animals and ethical clearance
Healthy Sprague-Dawley female rats weighing 180 ± 10 grams were purchased from the committee for the purpose of control and supervision of experiments on animals registered vendor and housed in pathogen-free conditions after ethical approval from Institutional animal ethical clearance at KLE College of Pharmacy Belagavi; resolution no. KLECOP/CPCSEA-Reg.No.221/Po/Re/S/2000/CPCSEA,Res.28 − 12/10/2019. Animals were acclimatized under a 12 light/dark cycle for 7 days before the study.
Study design and grouping of animals
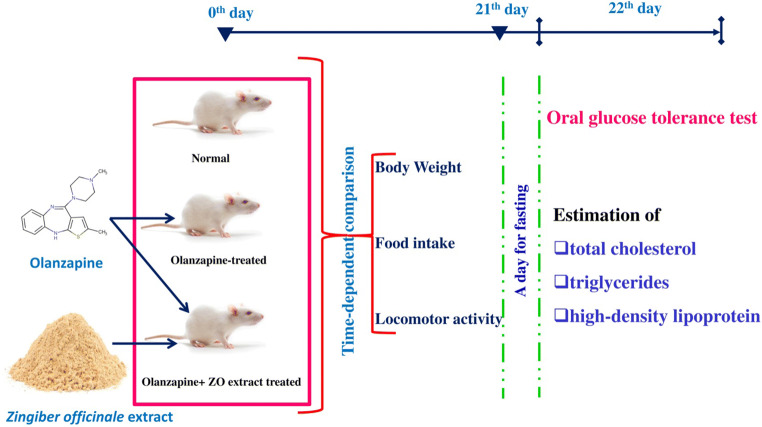
Animals were randomized into 5 different groups containing six animals in each using computer-generated random numbers namely (a) control (CON): receives vehicle; (b) OLZ: receives olanzapine 2 mg/kg, i.p., b.i.d. [19]; (c) OLZ + ZO100: receives olanzapine 2 mg/kg, i.p., b.i.d.+ZO extract 100 mg/kg, p.o., OD; (d) OLZ + ZO200: olanzapine 2 mg/kg, i.p., b.i.d.+ ZO extract 200 mg/kg, p.o., OD; (e) OLZ + ZO400: olanzapine 2 mg/kg, i.p., b.i.d.+ZO extract 400 mg/kg. Each group contained three cages with two animals in each and animals of all 5 groups were fed with normal pellet diet. The detailed study design of the present study is presented in Fig. 1.
Fig. 1.
Representation of the experimentation to evaluate the hydroalcoholic extract of ZO in olanzapine-induced weight gain and metabolic changes
Measurement of body weight and food intake
The body weight and food intake were recorded from 1st to 21st day. The percentage gain in body weight for the corresponding day was calculated using the following formula
Similarly, mean food intake in each group in a week was calculated using the following formula
Locomotor activity
The animal locomotor behavior was examined using Actophotometer as explained by Dews [20]. Briefly, individual animals were placed in an actophotometer for 5 min to record the basal locomotor of animals followed by administration of the drug; after 30 min locomotor activity was recorded as digital counts. The increased counts reflected the increased locomotor activity.
Oral Glucose Tolerance Test (OGTT)
OGTT was performed after 21 days of treatment as explained by Kumar et al. [21] The XY plot of glucose level vs. time (min) was used to calculate the total area under the curve (AUC).
Plasma biochemical estimations
Fasting blood glucose level was measured using a glucometer (Janaushadi, India). Similarly, triglyceride (TG), total cholesterol (TC), and high-density lipid (HDL) were measured using commercially available kits (ERBA diagnostics). The LDL and VLDL levels were measured using the following formula
Statistical analysis
All the data are expressed in mean ± SD. Bodyweight, food intake, locomotor activity, blood glucose during OGTT were analyzed using two-way ANOVA followed by Bonferroni test whereas total area under the curve of glucose during OGTT, TG, TC, HDL, LDL, and VLDL were analyzed using one-way followed by Tukey’s Test for Post-Hoc Analysis using GraphPad Prism 5. Differences among the means of groups were considered statistically significant at p < 0.05.
Results
Effect on body weight
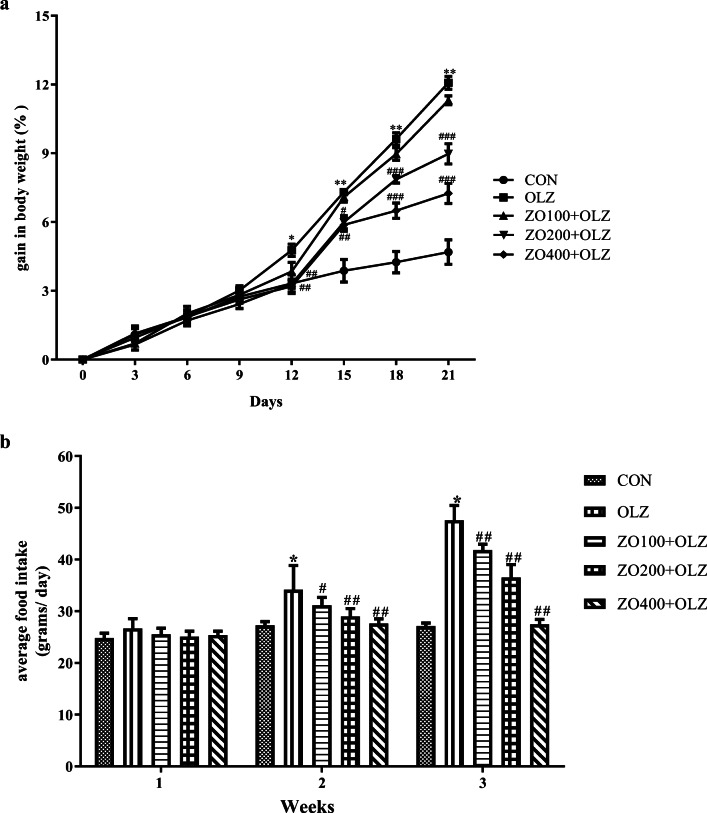
At the end of the study, the gain in body weight (%) was significantly higher (p < 0.001, F = 57.25, R2 0.9016) in olanzapine treated rats compared to the control. This increase in body weight gain was significantly higher (p < 0.001) from the 12th day until the 21st day. Co-administration of ZO at the dose of 200 and 400 mg/kg with olanzapine significantly decreased (p < 0.001) olanzapine-induced body weight gain from the 12th day to 21st day (Fig. 2a).
Fig. 2.
Effect of ZO extract on body weight and food intake of rats treated with olanzapine. a *p <0.01, **p <0.001 compared to CON, #p <0.05, ##p <0.01, ###p <0.001 compared to OLZ; b *p <0.001 compared to normal, #p <0.05, ##p <0.001 compared to OLZ
Effect on food intake
There was a significant difference (p < 0.0001 in food intake week wise, F = 242.3 vs. 86.47 as weeks vs. food intake) in food intake which was duration dependent and was affected by the co-administration of ZO extract with olanzapine. The average food intake during 2nd week (8th -15th day) and 3rd week (16th -21st day) were significantly higher (p < 0.001) in olanzapine treated rats compared to the control. Co-administration of ZO (100, 200, and 400 mg/kg) of olanzapine significantly decreased hyperphagia induced by olanzapine at both 2nd and 3rd week period (p < 0.05, 0.001) (Fig. 2b).
Effect on locomotor activity
We observed a significant difference (p < 0.0001 time-dependent locomotor counts, F = 31.92 vs. 11.72 as count vs. days) in locomotor activity after the co-administration of ZO extract with olanzapine. There was a significant decrease (p < 0.001) in locomotor activity in the olanzapine group compared to normal on the 14th and 21st days. Co-administration of ZO extract (400 mg/kg) with olanzapine showed a significantly increased (p < 0.05) in the decrease of locomotor activity induced by olanzapine (Fig. 3).
Fig. 3.
Effect of ZO extract on locomotor activity of rats treated with olanzapine. *p < 0.001 compared to normal, #p < 0.05 compared to olanzapine
Effect on ZO on oral glucose tolerance test
At the end of 3rd week of the study, there was a significant difference in blood glucose level during OGTT which was time-dependent and group dependent (p < 0.0001 for both time-dependent glucose level, F = 26.46 vs. 45.80 as glucose level vs. time). Likewise, there was a significant difference (P = 0.0022, F = 5.737, R2 0.4888) in the total area under the curve of glucose of the groups. Similarly, fasting blood glucose level and area under the curve for OGTT of olanzapine treated rats were significantly higher (p < 0.05) than control. Co-administration of ZO extract had a non-significant decrease in fasting blood glucose levels. However, co-administration of ZO (200 and 400 mg/kg) with olanzapine significantly decreased (p < 0.05, 0.01) the total area under the curve and glucose levels at all three intervals (30, 60, and 120 min) of measurement to olanzapine treated rats (Fig. 4).
Fig. 4.
Effect of ZO on OGTT of rats treated with olanzapine. a*p < 0.05, **p < 0.001 compared to CON, #p < 0.05, ##p < 0.001 compared to OLZ b *p < 0.05 compared to CON, #p < 0.05, ##p < 0.01 compared to OLZ
Effect of ZO on lipid profile
There was significant difference in TG (p < 0.0001, F = 18.02, R2 0.7662), TC (p = 0.0096, F = 4.219, R2 0.4030), HDL (p < 0.0001, F = 20.24, R2 0.7714), LDL (p < 0.0001, F = 38.75, R2 0.8611), VLDL (p < 0.0001, F = 18.05, R2 0.7665) among the groups. There was a significant increase in triglycerides (p < 0.001), total cholesterol (p < 0.01), LDL (p < 0.001), and VLDL (p < 0.001) level and a decrease in HDL (p < 0.001) in the olanzapine group compared to normal. Co-administration of ZO at the dose of 400 mg/kg showed a significant increase (p < 0.001)in HDL level and decreased TG (p < 0.01) and VLDL (p < 0.01)compared to olanzapine. Similarly, co-administration of ZO extract with olanzapine significantly decreased (p < 0.001) the LDL level at 100, 200, and 400 mg/kg compared to olanzapine. However, co-administration of ZO extract with olanzapine had no influence on total cholesterol at the dose of 100, 200, and 400 mg/kg (Table 1).
Table 1.
Effect of ZO on lipid profile in rats treated with olanzapine
| Groups | TG (mg/dl) | TC (mg/dl) | HDL (mg/dl) | LDL (mg/dl) | VLDL (mg/dl) |
|---|---|---|---|---|---|
| Normal | 92.59 ± 1.16 | 76.70 ± 1.04 | 42.33 ± 1.99 | 14.82 ± 3.56 | 18.51 ± 0.23 |
| Olanzapine | 133.30 ± 11.37** | 98.59 ± 8.68* | 21.74 ± 5.24** | 51.07 ± 7.03** | 26.66 ± 2.28** |
| ZO 100 mg/kg | 125.70 ± 6.76 | 89.33 ± 13.80 | 25.40 ± 3.65 | 37.40 ± 4.23## | 25.14 ± 1.35 |
| ZO 200 mg/kg | 120.20 ± 8.04 | 88.48 ± 10.85 | 28.57 ± 3.74 | 35.73 ± 6.24## | 24.04 ± 1.61 |
| ZO 400 mg/kg | 111.00 ± 12.47# | 82.19 ± 9.86 | 34.34 ± 5.94## | 29.63 ± 3.88## | 22.20 ± 2.50# |
All data are represented in Mean ± SD (n = 6), *p < 0.01, **p < 0.001 compared to normal, #p < 0.01, ##p < 0.001 compared to olanzapine
Discussion
Consistent with the previous report, rats after treatment with olanzapine (2 mg/kg) for 21 days developed increased weight gain associated with hyperphagia [19]. Treatment of a high-fat diet with ZO extract or its active contents reported reducing body weight gain, obesity, and serum leptin content [22]. Similarly, a randomized clinical trial study reported that the supplementation of 2 grams/day dry ZO powder caused a reduction of BMI and appetite score in obese women [18]. Our data suggest that ZO has a significant effect on preventing olanzapine-induced weight gain in this rat model. Rats receiving combined treatment showed a trend for less weight gain during all intervals of body weight measurement till the end of the study. Further, the food intake of these rats was less than olanzapine-treated rats. These data suggest that the ability of ZO to decrease olanzapine-induced body weight gain; may be due to reducing effect on olanzapine-induced hyperphagia.
Previous studies have reported decreased locomotors activity in rat’s model of olanzapine-induced weight gain and suggested that reduced energy expenditure may be partially responsible for olanzapine-induced weight gain [23, 24]. Consistent with these reports, in the present study, olanzapine treated rats showed reduced locomotor activity during the second and third weeks of study. Under the conditions of our study, ZO treatment did not improve olanzapine-induced hyper locomotor activity except partial movement in the third week with the highest dose (400 mg/kg) of ZO. These observations suggest that ZO may not ameliorate olanzapine-induced weight gain by modulating energy expenditure through increased physical activity. Probably this could be one of the possible explanations for partial prevention of weight gain in olanzapine and ZO co-treated rats.
Increased expression of pck1, a gene controlling hepatic gluconeogenesis, and decreased expression of GLUT4 in adipose tissue and skeletal muscle are suggested to cause insulin resistance leading to hyperglycemia in a rat model of olanzapine-induced weight gain [25]. Consistent with these reports in the present study, olanzapine treated rats showed fasting hyperglycemia and impaired glucose tolerance. Treatment of rats with ginger water reported increasing hepatic glucose uptake and its oxidation through GLUT-2 and PK mRNA expression [26]. Further, gingerol from ZO reported enhancing glycogen synthesis through increasing translocation of GLUT-4 in skeletal muscle [27] of diabetic mice. These effects of ZO may explain improved glucose metabolism in rats co-treated with olanzapine and ZO in the present study.
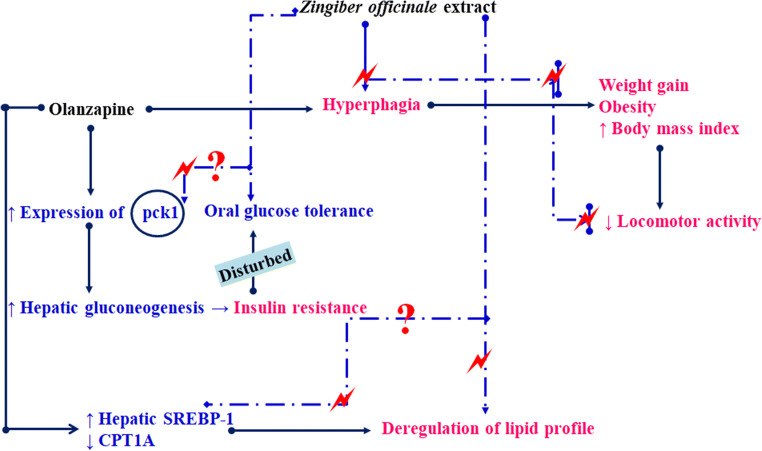
In the present study, rats treated with olanzapine for 3 weeks showed increased plasma level of total cholesterol, triglycerides, and decreased HDL levels which are consistent with previous reports [24]. Olanzapine is reported to upregulate the expression of the hepatic SREBP-1 gene involved in lipogenesis and down-regulate the expression of CPT1A involved in lipolysis through fatty acid β-oxidation in a rat model of weight gain [28, 29]. In our study, ZO co-treatment prevented the olanzapine-induced elevation of total cholesterol, triglycerides, and low-density lipoprotein levels. ZO is reported to prevent high-fat diet-induced dyslipidemia by decreasing the expression of SREP1-C and increasing the expression of CPT-1 in rats and such effects of ZO could be responsible for the results of the present study. The schematic representation for the probable mechanism of action of the present study is presented in Fig. 5.
Fig. 5.
Schematic representation for the probable mechanism of action of ZO in olanzapine-induced weight gain and metabolic changes. Solid and dotted lines represent the effect of olanzapine and ZO respectively. “  ” represents the probable checkpoint affected by ZO extract and “?” represents the effect of ZO extract yet to be investigated on a particular pathway
” represents the probable checkpoint affected by ZO extract and “?” represents the effect of ZO extract yet to be investigated on a particular pathway
Further, it is to be understood that a plant extract composes multiple secondary metabolites and a broad biological spectrum reflecting the probability to interact with multiple proteins/pathways [30–32]. Similarly, for the present study, it is a necessity to identify the probable interaction of multiple secondary metabolites from ZO extract with numerous targets related to an olanzapine-induced weight gain which is the future scope of the present study.
Conclusions
The present study evaluated the effect of hydroalcoholic extract of ZO on olanzapine-induced weight gain and associated metabolic changes. The study revealed the potency of ZO extract to manage weight gain and food intake if co-administered with olanzapine. Additionally, it demonstrated the potency of ZO to ameliorate glucose intolerance and dyslipidemia.
Acknowledgements
The authors are thankful to Principal KLE College of Pharmacy Belagavi for providing the necessary facilities to complete the work.
Data availability
Data will be made available from the corresponding author in case of a request.
Compliance with ethical standards
Conflict of interest
There are no conflicts of interest to declare.
Consent for publication
Not Applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boyda HN, Tse L, Procyshyn RM, Honer WG, Barr AM. Preclinical models of antipsychotic drug-induced metabolic side effects. Trends Pharmacol Sci. 2010;31(10):484–97. doi: 10.1016/j.tips.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Kirk SL, Glazebrook J, Grayson B, Neill JC, Reynolds GP. Olanzapine-induced weight gain in the rat: Role of 5-HT2C and histamine H1 receptors. Psychopharmacology. 2009;207(1):119–25. doi: 10.1007/s00213-009-1639-8. [DOI] [PubMed] [Google Scholar]
- 3.Pouzet B, Mow T, Kreilgaard M, Velschow S. Chronic treatment with antipsychotics in rats as a model for antipsychotic-induced weight gain in human. Pharmacol Biochem Behav. 2003;75(1):133–40. doi: 10.1016/s0091-3057(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 4.Choi S, DiSilvio B, Unangst J, Fernstrom JD. Effect of chronic infusion of olanzapine and clozapine on food intake and body weight gain in male and female rats. Life Sci. 2007;81(12):1024–30. doi: 10.1016/j.lfs.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanal P, Patil BM, Unger BS. Zebrafish shares common metabolic pathways with mammalian olanzapine-induced obesity. Futur J Pharm Sci. 2020;6:36. doi: 10.1186/s43094-020-00049-7. [DOI] [Google Scholar]
- 6.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–84. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erion DM, Park HJ, Lee HY. The role of lipids in the pathogenesis and treatment of type 2 diabetes and associated co-morbidities. BMB Rep. 2016;49(3):139–48. doi: 10.5483/bmbrep.2016.49.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joffe G, Takala P, Tchoukhine E, Hakko H, Raidma M, Putkonen H, Eronen M, Räsänen P. Orlistat in clozapine- or olanzapine-treated patients with overweight or obesity: a 16-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):706–11. doi: 10.4088/jcp.v69n0503. [DOI] [PubMed] [Google Scholar]
- 9.Praharaj SK, Jana AK, Goyal N, Sinha VK. Metformin for olanzapine-induced weight gain: a systematic review and meta-analysis. Br J Clin Pharmacol. 2011;71(3):377–82. doi: 10.1111/j.1365-2125.2010.03783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva VA. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231–41. doi: 10.2147/NDT.S113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Government of India. Ministry of Health and Family Welfare. Department of AYUSH. The Ayurvedic Pharmacopoeia of India. Part 1, vol. 1, pp. 138–139.
- 12.Misawa K, Hashizume K, Yamamoto M, Minegishi Y, Hase T, Shimotoyodome A. Ginger extract prevents high-fat diet-induced obesity in mice via activation of the peroxisome proliferator-activated receptor δ pathway. J Nutr Biochem. 2015;26(10):1058–67. doi: 10.1016/j.jnutbio.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Zhou L. Inhibitory effect 6-gingerol on adipogenesis through activation of the Wnt/catenin signaling pathway in 3T3-L1 adipocytes. Toxicol In Vitro. 2015;30:394–401. doi: 10.1016/j.tiv.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Sampath C, Rashid MR, Sang S, Ahmedna M. Specific bioactive compounds in ginger and apple alleviate hyperglycemia in mice with high fat diet-induced obesity via Nrf2 mediated pathway. Food Chem. 2017;226:79–88. doi: 10.1016/j.foodchem.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 15.Suk S, Kwon GT, Lee E, Jang WJ, Yang H, Kim JH, Thimmegowda NR, Chung MY, Kwon JY, Yang S, Kim JK, Park JHY, Lee KW. Gingerenone A, a polyphenol present in ginger, suppresses obesity and adipose tissue inflammation in high-fat diet‐fed mice. Mol Nutr Food Res. 2017;61(10):1700139. doi: 10.1002/mnfr.201700139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nammi S, Sreemantula S, Roufogalis BD. Protective effects of ethanolic extract of Zingiber officinale rhizome on the development of metabolic syndrome in high-fat diet‐fed rats. Basic Clin Pharmacol Toxicol. 2009;104(5):366–73. doi: 10.1111/j.1742-7843.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- 17.Singh RP, Jain R, Mishra R, Tiwari P. Antidepressant activity of hydroalcoholic extract of Zingiber officinale. Int Res J Pharm. 2012;3(2):149–51. http://irjponline.com/admin/php/uploads/860_pdf.pdf.
- 18.Ebrahimzadeh Attari V, Malek Mahdavi A, Javadivala Z, Mahluji S, Zununi Vahed S, Ostadrahimi A. A systematic review of the anti-obesity and weight lowering effect of ginger (Zingiber officinale Roscoe) and its mechanisms of action. Phytother Res. 2018;32(4):577–85. doi: 10.1002/ptr.5986. [DOI] [PubMed] [Google Scholar]
- 19.Patil BM, Kulkarni NM, Unger BS. Elevation of systolic blood pressure in an animal model of olanzapine induced weight gain. Eur J Pharmacol. 2006;551(1–3):112–5. doi: 10.1016/j.ejphar.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Dews PB. The measurement of the influence of drugs on voluntary activity in mice. Brit J Pharmacy Chemotherap. 1953;8:46–8. doi: 10.1111/j.1476-5381.1953.tb00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R, Patel DK, Prasad SK, Sairam K, Hemalatha S. Antidiabetic activity of alcoholic root extract of Caesalpinia digyna in streptozotocin-nicotinamide induced diabetic rats. Asian Pac J Trop Biomed. 2012;2(2):934-40. doi: 10.1016/S2221-1691(12)60340-2. [DOI] [Google Scholar]
- 22.Nazish I, Ansari SH, Arora P, Ahmad A. Antiobesity activity of Zingiber officinale. Pharmacogn J. 2016;8(5):440–6. doi: 10.5530/pj.2016.5.5. [DOI] [Google Scholar]
- 23.Cooper GD, Pickavance LC, Wilding JP, Harrold JA, Halford JC, Goudie AJ. Effects of olanzapine in male rats: enhanced adiposity in the absence of hyperphagia, weight gain or metabolic abnormalities. J Psychopharmacol. 2007;21(4):405–13. doi: 10.1177/0269881106069637. [DOI] [PubMed] [Google Scholar]
- 24.Weston-Green K, Huang XF, Deng C. Olanzapine treatment and metabolic dysfunction: a dose response study in female Sprague Dawley rats. Behav Brain Res. 2011;217(2):337–46. doi: 10.1016/j.bbr.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Young AJ, Ehli EA, Nowotny D, Davies PS, Droke EA, Soundy TJ, Davies GE. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sayed S, Ahmed M, El-Shehawi A, Alkafafy M, Al-Otaibi S, El-Sawy H, Farouk S, El-Shazly S. Ginger water reduces body weight gain and improves energy expenditure in rats. Foods. 2020;9(1):38. doi: 10.3390/foods9010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samad MB, Mohsin MNAB, Razu BA, Hossain MT, Mahzabeen S, Unnoor N, Muna IA, Akhter F, Kabir AU, Hannan JMA. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Leprdb/db type 2 diabetic mice. BMC Complement Altern Med. 2017;17(1):395. doi: 10.1186/s12906-017-1903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Lian J, Hu CH, Deng C. Betahistine co-treatment ameliorates dyslipidemia induced by chronic olanzapine treatment in rats through modulation of hepatic AMPKα-SREBP-1 and PPARα-dependent pathways. Pharmacol Res. 2015;100:36–46. doi: 10.1016/j.phrs.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Liu XM, Zhao XM, Deng C, Zeng YP, Hu CH. Simvastatin improves olanzapine-induced dyslipidemia in rats through inhibiting hepatic mTOR signaling pathway. Acta Pharmacol Sin. 2019;40(8):1049–57. doi: 10.1038/s41401-019-0212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanal P, Patil BM. In vitro and in silico anti-oxidant, cytotoxicity and biological activities of Ficus benghalensis and Duranta repens. Chin Herb Med. 2020;12(4):406–413. doi: 10.1016/j.chmed.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khanal P, Patil BM. Gene ontology enrichment analysis of α-amylase inhibitors from Duranta repens in diabetes mellitus. J Diabetes Metab Disord. 2020. 10.1007/s40200-020-00554-9. [DOI] [PMC free article] [PubMed]
- 32.Khanal P, Patil BM. Integration of network and experimental pharmacology to decipher the antidiabetic action of Duranta repens L. J. Integr. Med. 2020. 10.1016/j.joim.2020.10.003. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available from the corresponding author in case of a request.