Abstract
Purpose
Type 2 diabetes (T2D) is linked with depression due to insulin resistance, oxidative stress and disruption of neurotrophic factors. We evaluated potential benefits of phloridzin in ameliorating depressive symptoms in T2D.
Methods
Adult male Swiss-albino mice (25–30 g) on high-fat-diet (HFD) for 2 weeks were administered with streptozotocin (STZ; 35 mg/kg, intraperitoneal) to induce T2D. Seven days after STZ administration, diabetic mice on HFD were distributed into different groups. Animals were subjected daily to oral treatment of saline (0.25 ml), fluoxetine (10-20 mg/kg) or phloridzin (10-20 mg/kg) for a period of 4 weeks. One hour after last dose, the immobility time of animals was evaluated in forced swim test (FST) and tail suspension test (TST). To further confirm the mechanisms involved in antidepressant effect of phloridzin, biochemical parameters like brain derived neurotropic factor (BDNF), glutathione (GSH), extracellular signal-regulated kinase (ERK), tyrosine receptor kinase B (TrkB) and cAMP-response element binding protein (CREB) were estimated in the brain.
Results
Animals with T2D showed a significant increase in immobility as compared to control in FST and TST. However, 4 weeks administration of fluoxetine or phloridzin attenuated this effect. A significant decline in GSH, BDNF, TrkB, CREB and ERK levels were noticed in the brain of mice with T2D. These changes were also attenuated by administration of phloridzin.
Conclusions
Phloridzin may ameliorates T2D-induced depression by mitigating the oxidative stress, and up-regulation of neurotrophins in the brain. Therefore, phloridzin can be used as a therapeutic intervention for the management of depression co-morbid with T2D.
Keywords: Depression, Diabetes mellitus, Fluoxetine, Phloridzin, Mice
Introduction
The worldwide prevalence of depression and type 2 diabetes (T2D) is estimated to be about 300–400 million [1–3]. Interestingly, a co-occurrence of both conditions is well-established [4, 5]. Mechanisms behind the development of depression in T2D include decreased insulin sensitivity, abnormality in signalling of insulin-like growth factor, glucose toxicity, buildup of advanced glycation end products, cerebrovascular injury and neuro-inflammation. Abnormal blood glucose levels increased formation of reactive oxygen species and oxidative stress, loss of antioxidant defence, functional impairment of neurons and neurotropic factors [6].
Brain derived neurotropic factor (BDNF) is widely distributed in the brain, particularly in the cerebral cortex and the hippocampus [7]. A correlation has already been established between BDNF and depression [4]. For instance, animal studies suggest a reduction in BDNF in depressive disorders, and clinical investigations reported the ability of BDNF to reverse depression-like behaviour [8]. BDNF produced its pro-survival effects by activating BDNF-Tropomyosin receptor kinase B also known as tyrosin receptor kinase B (TrkB) signalling via its high affinity towards TrkB receptor [9]. Behavioural responses to antidepressant drugs were abolished when there is an inhibition of BDNF-TrkB signalling [10]. Therefore, BDNF-TrkB signalling plays a pivotal role in the mechanism of drugs acting as antidepressant. The BDNF-TrkB downstream signalling including PI3K/GSK-3β/AKT and ERK/CREB pathways can modulate neurotransmitters release, postsynaptic responses, viability of cell and apoptosis, which are closely associated with depression [11, 12]. Both animal and human studies have evidenced that BDNF plays a critical role in T2D [13] and associated neurological problems [14]. While decreased BDNF levels in plasma were observed in T2D compared to non-diabetic subjects [15], antidiabetic agents prevented the reduction of BDNF contents centrally [16]. Glutathione (GSH) is an intracellularly present antioxidant plays a major role in removing free radicals and preventing oxidative stress. However, reduced synthesis of GSH has been documented in T2D which depends on the extent of insulin resistance and hyperglycemia [1, 17]. Evidences have shown that decreased GSH is considered to be a predominant marker of depressive behaviour and have proven the presence of oxidative stress in depressive disorders [18]. Thus, BDNF and oxidative stress are the critical elements for the various pathological manifestations of depression associated with T2D.
It is worth noting that around 30% patients of major depressive disorders may not adequately respond to the conventional antidepressants [19, 20]. To overcome this limitation, natural food components have been investigated as a therapeutic intervention for the management of treatment-resistant depression. Natural compounds like polyphenols have been reported to affect antioxidant signalling pathways and may have a putative role in the management of depression [21]. Apple peel extract is a richest source of bioflavonoid phloridzin possessing antioxidant, anti-inflammatory, neuroprotective, anti-cancer, and vasorelaxant properties [22, 23]. Moreover, phloridzin has been known for its proven antidiabetic activity and preventive actions in diabetic complications by virtue of its insulin sensitising action [24]. However, the antidepressant activity of phloridzin in depression co-morbid with T2D, as well as the underlying mechanism remains to be explored.
Taking all above into consideration, we planned to explore the antidepressant effect of phloridzin in mice having T2D using the behavioral paradigms like forced swim test (FST) and tail suspension test (TST). Moreover, BDNF, GSH, TrkB, ERK and CREB levels in the brain were estimated to illustrate the molecular mechanisms of phloridzin.
Materials and methods
Animals
Adult male Swiss-albino mice (25-30 g) were used. Animals were kept in acrylic cages under a constant room temperature (25 ± 20 C), relative humidity (50 ± 5 %), 12-hour light/dark cycle and were kept on normal pellet diet (NPD) supplied by Trimurti Feeds, Udaipur, Rajasthan, India and water ad libitum prior to the diet alternation. The experiments conducted in the present study were approved by Institutional Animal Ethics committee (IAEC), Faculty of Pharmacy, Pacific Academy of Higher Education and Research (PAHER) University, Udaipur, Rajasthan, India. All the behavioral studies were conducted between 9:00 and 17:00 hours to minimise the impact of circadian rhythm.
Drugs
All the drug chemicals employed in the treatment protocols were obtained from different sources. Phloridzin (P274313) and streptozotocin (STZ; S0130) were procured from Sigma-Aldrich (USA) and fluoxetine was obtained from Alkem Laboratories, Mumbai, Maharashtra, India as a gift sample. Phloridzin was prepared as a suspension in distilled water with addition of tween 20 (quantum satis). Drug solutions were freshly prepared each day during the experiments.
Treatments
The first set of animals was fed with NPD until the end of the study. They were randomly distributed into 7 different groups (n = 8 per group) and received different oral treatments such as saline, (0.25 ml/mouse), fluoxetine (5, 10 and 20 mg/kg) and phloridzin (5, 10 and 20 mg/kg) for the period of 7 days. Approximately 1 hour after the last dosing, mice were subjected to the FST to determine the effective and sub-effective doses.
Another set of animals was subjected to ad libitum high-fat diet (HFD) with the composition of 58% fat, 17% carbohydrates and 25% proteins (as a % of total kcal) till the end of the experiment. After the 2 weeks of HFD feeding, mice were administered with a single dose of freshly prepared STZ (35 mg/kg, intraperitoneal). After 7 days of STZ injection, the animals with ≥ 300 mg/dl non-fasting blood glucose levels were considered as diabetic. For the confirmation of diabetes induction, the oral glucose tolerance test (OGTT) was conducted in which glucose solution (2 g/kg, oral) was administered in 6-h fasted animals. Blood glucose concentrations were monitored at different time points (0, 30, 60, 90 and 120 minutes) post-glucose administration to measure the area under curve (AUC). This experimental procedure to induce T2D was adopted from previous study [25]. Animals with T2D were divided into 4 groups (n = 8 per group) and administered daily with oral treatments such as saline, fluoxetine (10 mg/kg) and phloridzin (10 and 20 mg/kg) for a period of 4 weeks. In parallel to this, a group of normal control (non-diabetic) mice were also administered with saline. After 60 minutes of last dosing, animals were subjected for the behavioral experiments using TST and FST. The drug treatment was continued till the mice were euthanized at the end of the behavioural experiments for the biochemical estimations of the various biomarkers in the brain. A schematic representation of the treatment protocols is as shown in Fig. 1.
Fig. 1.
Schematic representation of time-line for the treatments and behavioral experiments in normal pellet diet (NPD) fed animals, and high-fat diet (HFD) fed + streptozotocin (STZ) induced type 2 diabetic animals
Behavioural tests
Forced swim test (FST)
After 4 weeks of different treatments, the mice were acclimatized to the experimental area for at least 1 hour prior to behavioral experiments. During FST, animal was dropped into a vertical plexiglas cylinder (diameter: 22.5 cm and height: 30 cm) filled with water (23–35 0 C) to 40 cm depth. The behavioral testing was performed for 5 minutes and the immobility time was recorded once animal acquired an immobility after initial vigorous movements [26, 27].
Tail suspension test (TST)
A horizontal wooden bar having dimension 40 × 46 × 40 cm and about 35 cm above the ground was used for TST. Mouse was suspended by its tail from a wooden bar. Animal was secured to a bar by adhesive tape, which was placed 1-1.5 cm from the tail tip. This kept mouse head approximately 20 cm above the platform. Mouse exhibited escape-like behaviour immediately after suspension and subsequently showed immobility. A trial was performed for 5 min and the total immobility time of each animal was recorded.
Metabolic measurements
Tissue collection
A day after completion of the behavioural tests, the animals were sacrificed by thiopentone (75 mg/kg, intraperitoneal). The brains were isolated and stored at − 80 0 C until further analysis.
Estimation of reduced glutathione (GSH) level
To determine GSH level, brain tissue homogenates were prepared in the freshly prepared phosphate buffer (pH 8) with Triton X (1 %), 10 % tricholoroacectic acid (1ml) and distilled water (1ml). The resultant mixture was then cooled for 10 min and centrifuged (2000 rpm) for 15 min at 4 0 C. To the supernatant obtained, 4 ml of 5,5’-dithiobis-[2-nitrobenzoic acid] (DTNB) and phosphate buffer (1.5 ml) was added. Absorbance was measured at 412 nm wavelength. Standard solution of GSH with different concentrations was prepared for the standard plot. GSH was measured as µg GSH/mg protein.
ELISA kit assay
BDNF, CREB, ERK and TrkB concentrations were determined in the brain tissues by ELISA method (Elabscience®, India). Optical density was obtained at 450 nm using a microplate reader (Varioskanfash, Thermoscientific, USA) within 15 min of the addition of stop solution.
Statistical analysis
GraphPad Prism software was used for statistical analyses. The data are presented as mean ± SEM. The data obtained from behavioral experiments and biochemical estimations were analysed using one-way analysis of variance (ANOVA) and the post-hoc comparison of individual means was performed by Bonferroni’s multiple comparison test. P < 0.05 was considered as significant.
Results
Oral glucose tolerance test (OGTT)
In OGTT, significantly higher blood glucose levels were observed in normal NPD-fed and T2D (HFD-fed) animals at 30 minutes time-point. However, in HFD-STZ animals, very high glucose levels were noticed at all the time-points (30, 60, 90 and 120 mins; p < 0.001) (Fig. 2a). The AUC plot of glucose in HFD-STZ animals was significantly higher (p < 0.001) than that in the NPD-fed and HFD-fed animals (Fig. 2b). The delayed in disappearance of elevated blood glucose levels confirms the development of diabetes in HFD-STZ animals.
Fig. 2.
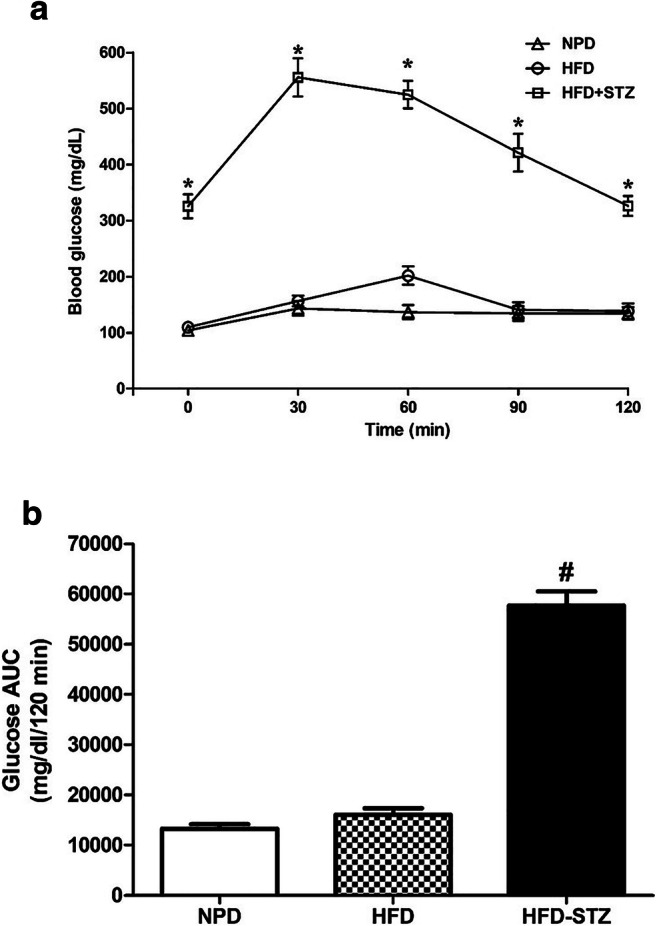
Blood glucose level during oral glucose tolerance test (a) and area under the curve (AUC) of the blood glucose (b). Different groups of mice were fed for 2 weeks with normal pellet diet (NPD) and high-fat diet (HFD). After 2 weeks, animals on HFD received single intraperitoneal streptozotocin (STZ) injection (35 mg/kg). One week thereafter, the blood glucose tests were performed. The data were analysed with one-way ANOVA followed by post-hoc Bonferroni's multiple comparison test. Each line (a) represents the mean of blood glucose level ± SEM, whereas each bar (b) represents the mean of AUC of blood glucose levels ± SEM of each group (n=8 per group). *p<0.001 vs NPD at respective time points in (a); #p<0.001 vs NPD in (b)
Evaluation of effect of phloridzin on depression
Effect of phloridzin on the immobility time in FST
Normal NPD-fed animals showed reduction in immobility time following treatment with phloridzin [F(3,31) = 5.87, p = 0.003] and fluoxetine [F(3,31) = 8.82, p = 0.0003]. After post-hoc analysis it was observed that, the phloridzin treatment showed dose dependent decrease in immobility time at 10 mg/kg (p < 0.05) and 20 mg/kg (p < 0.01) as compared to the saline treated animals (Fig. 3a). Similarly, after the treatment with fluoxetine, animals showed dose dependent decrease in immobility time at 10 mg/kg (p < 0.05) and 20 mg/kg (p < 0.01) as compared to the saline-treated mice (Fig. 3b). Lower dose of phloridzin and fluoxetine (5 mg/kg) was found to be sub-effective as it did not affect the immobility time significantly (p > 0.05).
Fig. 3.
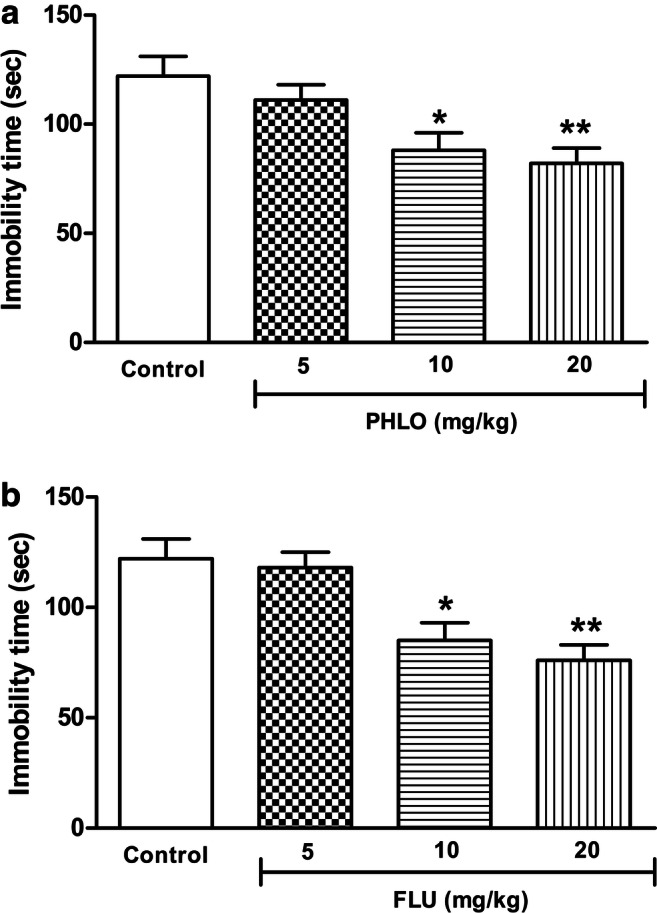
Dose dependent effect of phloridzin (PHLO) (a) and fluoxetine (FLU) (b) on immobility time of normal animals in forced swim test (FST). Different groups were treated orally with saline (control), FLU (5-20 mg/kg), and PHLO (5-20 mg/kg) for a period of 7 days, and one hour after last dosing they were subjected to the FST. The data were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparison test. Each bar represents the mean±SEM of each group (n=8 per group). *p<0.05, **p<0.01 vs respective control group
In TST, HFD-STZ-induced T2D animals showed significant increase in immobility time (Fig. 4a). However, treatment with phloridzin significantly reduced immobility time [F(4,39) = 18.40, p < 0.0001]. After post-hoc analysis it was revealed that, fluoxetine (10 mg/kg) significantly reduced immobility time as compared to the diabetic control (p < 0.001). Similar to this, phloridzin treatment (10 mg/kg; p < 0.01 and 20 mg/kg; p < 0.001) showed significant decrease in immobility time as compared to the diabetic control group.
Fig. 4.
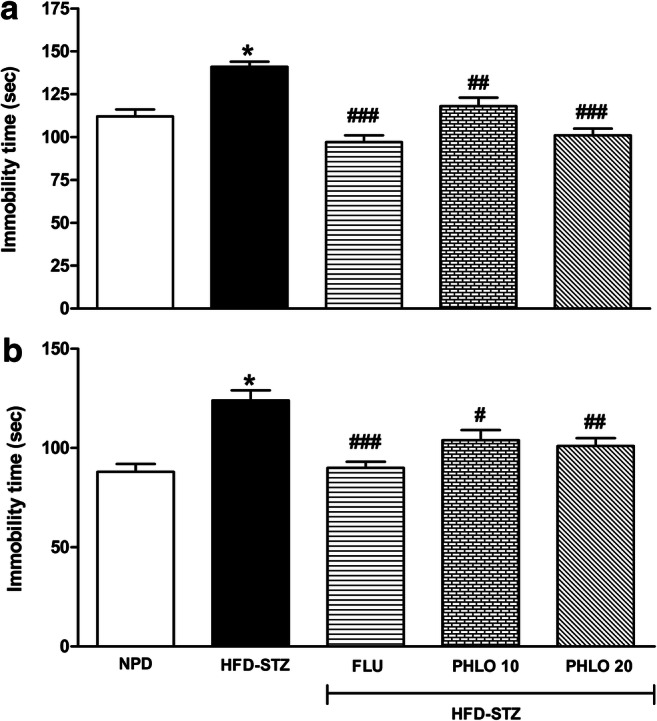
Effect of phloridzin (PHLO) on immobility time of diabetic mice in forced swim test (a) and tail suspension test (b). While normal pellet diet (NPD) fed control animals received oral saline, different groups of high-fat diet (HFD) + streptozotocin (STZ)-induced type 2 diabetic mice were treated daily with saline, phloridzin (PHLO; 10-20 mg/kg) and fluoxetine (FLU; 10 mg/kg) via oral route for a period of 4 weeks. The data were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparison test. Each bar represents the mean±SEM of each group (n=8 per group). *p<0.001 vs NPD control group; #p<0.05, ##p<0.01, ###p<0.001 vs HFD-STZ-diabetic group
Effects of phloridzin on the immobility time in TST
In consistent with the results of FST, diabetic animals showed significant increase in immobility time in TST (p < 0.001) (Fig. 4b). However, treatment with phloridzin significantly reduced immobility time [F(4,39) = 11.36, p < 0.0001]. After pots-hoc analysis it was revealed that, fluoxetine (10 mg/kg, p < 0.001) and phloridzin (10 mg/kg; p < 0.05 and 20 mg/kg; p < 0.01) treatment significantly decreased immobility time as compared to the diabetic control mice.
Effect of phloridzin on biochemical parameters in the brain
In HFD-STZ diabetic animals, a significant decrease in the GSH levels was observed as compared to the control non-diabetic animals (p < 0.001). However, phloridzin treatment showed significant increase in GSH levels in diabetic animals [F(3,31) = 25.31, p < 0.0001] (Fig. 5). Post-hoc analysis revealed a significant increase in GSH levels after phloridzin treatment at 10 and 20 mg/kg doses (p < 0.001).
Fig. 5.
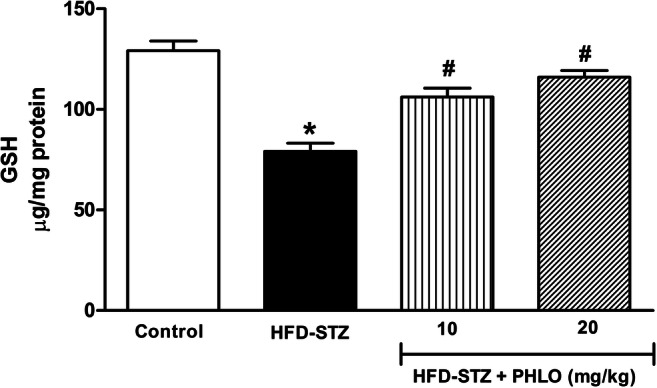
Effect of phloridzin (PHLO) on glutathione (GSH) levels in the brain. While normal control animals received oral saline, different groups of high-fat diet (HFD) + streptozotocin (STZ)-induced type 2 diabetic mice were treated daily with saline and phloridzin (PHLO; 10 and 20 mg/kg) via oral route for a period of 4 weeks. Thereafter, the brain tissue homogenate supernatants of all groups were subjected to the biochemical analysis. Each bar represents the mean±SEM of each group (n=8 per group). *p<0.001 vs control; #p<0.001 vs HFD-STZ-diabetic group
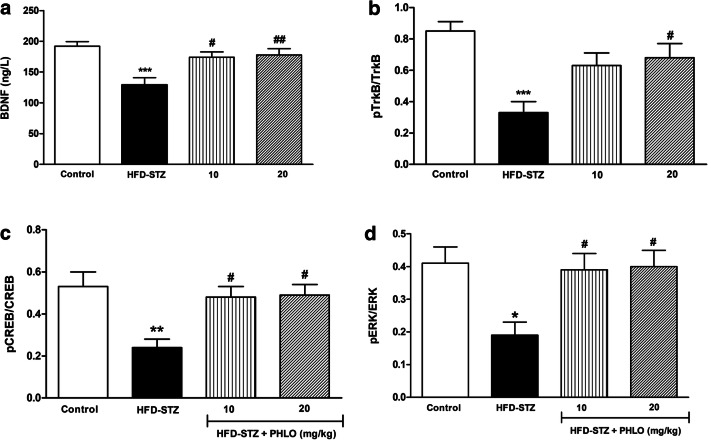
As compared to the control (NPD-fed) group, the HFD-STZ-induced T2D animals showed a significant decrease in the levels of BDNF (p < 0.001), CREB (p < 0.01), ERK (p < 0.05) and TrkB (p < 0.001). Interestingly, phloridzin treatment showed a significant reversal in declined levels of BDNF [F(3,31) = 7.83, p = 0.0006], CREB [F(3,31) = 6.04, p = 0.002], ERK [F(3,31) = 4.87, p = 0.007] and TrkB [F(3,31) = 8.15, p = 0.0005] in the brain of diabetic group (Fig. 6a-d). After post-hoc analysis, it was revealed that, a significant increase (p < 0.05) in all the biochemical parameters was observed in the brain of diabetic mice following administration of phloridzin at 10 and 20 mg/kg doses.
Fig. 6.
Effects of phloridzin (PHLO) on BDNF/TrkB/CREB/ERK levels in the brain. While normal control animals received oral saline, different groups of high-fat diet (HFD) + streptozotocin (STZ)-induced type 2 diabetic mice were treated daily with saline and phloridzin (PHLO; 10 and 20 mg/kg) via oral route for a period of 4 weeks. Thereafter, the brain tissue homogenate supernatants of all groups were subjected to the biochemical analysis. Each bar represents the mean±SEM of each group (n=8 per group). *p<0.05, **p<0.01, ***P<0.001 vs control; #p<0.05, ##p<0.01 vs HFD-STZ-diabetic group
Discussion
T2D and depression are strongly associated with each other [28]. In the present investigation, HFD-STZ model of diabetes was employed to mimic T2D in humans. Consistent with the previous reports, we observed that T2D animals showed depression symptoms [29, 30]. However, chronic phloridzin administration for 4-weeks significantly attenuated T2D-induced depressive behaviour with the reversal of increased immobility duration in FST and TST. Correlation of T2D and depression is complex since the mechanism behind its inter-relationship is still not well explored. Decreased antioxidant mechanisms with remarkable decline in levels of GSH and BDNF in the brain of diabetic animals is associated with various disorders like depression, Parkinsonian and Huntington’s disease [31]. In the present study, we observed that, diabetic animals showed significantly increased immobility time as a sign of depression in FST and TST with declined in the levels of GSH and BDNF levels in the brain. However, long term phloridzin administration, showed attenuation of depressive symptoms and increased GSH and BDNF levels in the brain. The results are in consistent with the previous study, where it is established that, both acute and chronic treatment with antidepressants increases BDNF expression in the cortex and the hippocampus [32]. In addition to this, phloridzin administration increased BDNF and decreased neuro-inflammatory markers in the hippocampus of amnesic animals. Moreover, it also promotes the synthesis of GSH, a non-enzymatic antioxidant defense [33]. The aforementioned data and results obtained in our study illustrated that, phloridzin attenuated T2D-induced depression by increasing antioxidant defence mechanism and improved signalling of BDNF. These finding are consistent with the previous reports stating, flavonoid compounds showed up-regulation of BDNF levels to attenuate depression-like symptoms [34].
A protein tyrosine-kinase receptor, TrkB is highly expressed in the brain, and primarily mediated the actions of BDNF. Since BDNF-TrKB signalling links with the pathophysiology of depression [35, 36], it is considered as the therapeutic target for novel antidepressant drugs. Furthermore, plethora of published evidences supports that, BDNF attenuates depression by binding to the TrkB receptors leading to the autophosphorylation of TrkB-tyrosine residues and activation of downstream signalling molecules, such as ERK1/2 which further phosphorylates CREB [37]. It is also evidenced that, BDNF activates full length TrkB for its autophosphorylation which stimulates ERK by enhancing cAMP levels to further trigger transcription of gene regulated by CREB, and this mechanism also activates BDNF transcription [38]. In the present study, down-regulation of BDNF/CREB/ERK/TrkB signalling in T2D was reversed by phloridzin treatment. This finding indicates the role of phloridzin in up-regulating BDNF expression by regulating BDNF/CREB/ERK/TrkB pathway to exert anti-depressant activity. In addition to this, BDNF-TrkB interaction triggers dimerization, phosphorylation of tyrosine residues and consequent activation of pathways like MAPK/ERK/CREB, PI3K/AKT and phospholipase C-ϒ [39]. Amongst these, PI3K/AKT and EKT are the two predominant signalling pathways in TrkB-mediated survival pathways involved in neuronal survival and protects against neuronal apoptosis. BDNF/TrkB signalling stimulates BDNF production through CREB via stimulation of PI3K/ERK signalling. This interaction is emerging as a positive-feedback mechanism [40–43]. Figure 7 describes the T2D-induced oxidative stress, neuro-inflammatory changes, and perturbation of possible signalling pathways which can influenced BDNF production, and the ameliorative effect of phloridzin on these signalling perturbations.
Fig. 7.
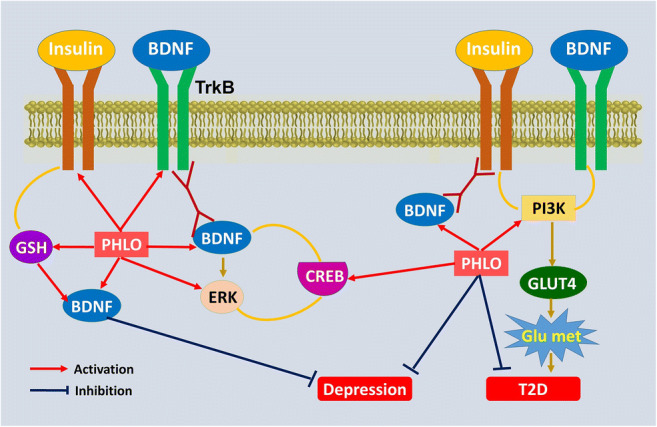
Proposed antidepressant-like mechanisms of phloridzin (PHLO) with the interaction among BDNF, TrkB, CREB, ERK, PI3K, GSH and glucose metabolism (Glu met). The findings of the current study show that the activation of diabetes-induced oxidative stress decreases neurotrophic and antioxidative support leading to neuronal damage. Treatment with PHLO regulates TrkB/CREB/ERK pathway to alleviate depression through increasing GSH and BDNF
Our research findings and aforementioned studies led us to suggest that, phloridzin being having an antidepressant action elevates BDNF concentration in the brain by stimulating TrKB/ERK/CREB pathway. Hence, phloridzin treatment in T2D could prevent or attenuate depression by its insulin sensitising action, antioxidant effects and by modulating neuronal mechanism or various biochemical pathways in the brain. We also suggest that, phloridzin has a potential to be used as a therapeutic intervention for the clinical management of depression associated with diabetes. Nevertheless, further molecular and genetic studies are required to elucidate interactions between diabetes and depression, and the separate, simultaneous evaluation of effects of phloridzin in diabetes induced-depression would be performed in the future studies.
Acknowledgements
We are thankful to the Dean, Faculty of Pharmacy, Pacific Academy of Higher Education and Research (PAHER) University, Udaipur, Rajasthan, India for providing the resources and facilities for the completion of research work.
Author contributions
SPK: design and execution of the research work, analysis of the results and manuscript writing. AR and KTN: provided help in interpretation and analysis of the data and reviewing final content of the manuscript. All authors read and approved the manuscript for the publication.
Declarations
Conflict of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sartorius N. Depression and diabetes. Dialogues Clin Neurosci. 2018;20:47–52. doi: 10.31887/DCNS.2018.20.1/nsartorius. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–30. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res. 2020;126:134–40. [DOI] [PubMed]
- 4.Wang J, He M. Levels of serum brain-derived neurotrophic factor in Type 2 diabetes mellitus patients with and without depressive symptoms. Acta Biochim Biophys Sin (Shanghai) 2015;47:137–8. doi: 10.1093/abbs/gmu117. [DOI] [PubMed] [Google Scholar]
- 5.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–90. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips C. Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plast. 2017;2017:7260130. doi: 10.1155/2017/7260130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 9.Diniz BS, Reynolds CF, Begley A, Dew MA, Anderson SJ, Lotrich F, et al. Brain-derived neurotrophic factor levels in late-life depression and comorbid mild cognitive impairment: a longitudinal study. J Psychiatr Res. 2014;49:96–101. doi: 10.1016/j.jpsychires.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F, Kang Z, Li W, Xiao Z, Zhou X. Roles of brain-derived neurotrophic factor/tropomyosin-related kinase B (BDNF/TrkB) signalling in Alzheimer’s disease. J Clin Neurosci. 2011;19:946–9. doi: 10.1016/j.jocn.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Li W, He QZ, Wu CQ, Pan XY, Wang J, Tan Y, et al. PFOS disturbs BDNF-ERK-CREB signalling in association with increased microRNA-22 in SH-SY5Y cells. Biomed Res Int. 2015;2015:302653. doi: 10.1155/2015/302653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Luo Y, Zhang R, Shi H, Zhu W, Shi J. Neuropeptide trefoil factor 3 reverses depressive-like behaviours by activation of BDNF-ERK-CREB signaling in olfactory bulbectomized Rats. Int J Mol Sci. 2015;16:28386–400. doi: 10.3390/ijms161226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suwa M, Kishimoto H, Nofuji Y, Nakano H, Sasaki H, Radak Z, Kumagai S. Serum brain-derived neurotrophic factor level is increased and associated with obesity in newly diagnosed female patients with type 2 diabetes mellitus. Metabolism. 2006;55:852–7. doi: 10.1016/j.metabol.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Passaro A, Dalla Nora E, Morieri ML, Soavi C, Sanz JM, Zurlo A, et al. Brain-derived neurotrophic factor plasma levels: relationship with dementia and diabetes in the elderly population. J Gerontol A Biol Sci Med Sci. 2015;70:294–302. doi: 10.1093/gerona/glu028. [DOI] [PubMed] [Google Scholar]
- 15.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–8. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 16.Yoo DY, Kim W, Nam SM, Yoo KY, Lee CH, Choi JH, et al. Reduced cell proliferation and neuroblast differentiation in the dentate gyrus of high fat diet-fed mice are ameliorated by metformin and glimepiride treatment. Neurochem Res. 2011;36:2401–8. doi: 10.1007/s11064-011-0566-3. [DOI] [PubMed] [Google Scholar]
- 17.Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J. 2016;24:547–53. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freed RD, Hollenhorst CN, Weiduschat N, Mao X, Kang G, Shungu DC, et al. A pilot study of cortical glutathione in youth with depression. Psychiatry Res Neuroimaging. 2017;270:54–60. doi: 10.1016/j.pscychresns.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–88. doi: 10.2147/PPA.S29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ionescu DF, Rosenbaum JF, Alpert JE. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci. 2015;17:111–26. doi: 10.31887/DCNS.2015.17.2/dionescu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sureda A, Tejada S. Polyphenols and depression: from chemistry to medicine. Curr Pharm Biotechnol. 2015;16:259–64. doi: 10.2174/1389201016666150118133313. [DOI] [PubMed] [Google Scholar]
- 22.Francini A, Sebastiani L. Phenolic compounds in apple (Malus x domesticaBorkh.): compounds characterization and stability during postharvest and after processing. Antioxidants (Basel) 2013;2:181–93. doi: 10.3390/antiox2030181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutr J. 2004;3:5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Londzin P, Siudak S, Cegieła U, Pytlik M, Janas A, Waligóra A, et al. Phloridzin, an apple polyphenol, exerted unfavorable effects on bone and muscle in an experimental model of type 2 diabetes in rats. Nutrients. 2018;10:1701. doi: 10.3390/nu10111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–20. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 27.Costa AP, Vieira C, Bohner LO, Silva CF, Santos EC, De Lima TC, et al. A proposal for refining the forced swim test in Swiss mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:150–5. doi: 10.1016/j.pnpbp.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Badescu SV, Tataru C, Kobylinska L, Georgescu EL, Zahiu DM, Zagrean AM, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9:120–5. [PMC free article] [PubMed] [Google Scholar]
- 29.Nakhate KT, Yedke SU, Bharne AP, Subhedar NK, Kokare DM. Evidence for the involvement of neuropeptide Y in the antidepressant effect of imipramine in type 2 diabetes. Brain Res. 2016;1646:1–11. doi: 10.1016/j.brainres.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Yan T, He B, Wan S, Xu M, Yang H, Xiao F, et al. Antidepressant-like effects and cognitive enhancement of Schisandrachinensis in chronic unpredictable mild stress mice and its related mechanism. Sci Rep. 2017;7:6903. doi: 10.1038/s41598-017-07407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markham A, Bains R, Franklin P, Spedding M. Changes in mitochondrial function are pivotal in neurodegenerative and psychiatric disorders: how important is BDNF? Br J Pharmacol. 2014;171:2206–29. doi: 10.1111/bph.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433–49. doi: 10.2147/NDT.S5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Oliveira MR. Phloretin-induced cytoprotective effects on mammalian cells: A mechanistic view and future directions. Biofactors. 2016;42(1):13–40. doi: 10.1002/biof.1256. [DOI] [PubMed] [Google Scholar]
- 34.Hritcu L, Ionita R, Postu PA, Gupta GK, Turkez H, Lima TC, et al. Antidepressant flavonoids and their relationship with oxidative stress. Oxid Med Cell Longev. 2017;2017:5762172. doi: 10.1155/2017/5762172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang XY, Chen DC, Tan YL, Tan SP, Wang ZR, Yang FD, et al. The interplay between BDNF and oxidative stress in chronic schizophrenia. Psychoneuroendocrinology. 2015;51:201–8. doi: 10.1016/j.psyneuen.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JC, Yao W, Hashimoto K. Brain-derived neurotrophic factor (BDNF)-TrkB Signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol. 2016;14:721–31. doi: 10.2174/1570159X14666160119094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin P, Wang C, Xu B, Gao S, Guo J, Zhao X, et al. The VGF-derived peptide TLQP62 produces antidepressant-like effects in mice via the BDNF/TrkB/CREB signaling pathway. Pharmacol Biochem Behav. 2014;120:140–8. doi: 10.1016/j.pbb.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 38.He Q, Wang S, Liu X, Guo H, Yang H, Zhang L, et al. Salvianolate lyophilized injection promotes post-stroke functional recovery via the activation of VEGF and BDNF-TrkB-CREB signaling pathway. Int J Clin Exp Med. 2015;8:108–22. [PMC free article] [PubMed] [Google Scholar]
- 39.Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121:1846–57. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu CH, Hung TH, Chen CC, Ke CH, Lee CY, Wang PY, et al. Post-injury treatment with 7,8-dihydroxyflavone, a TrkB receptor agonist, protects against experimental traumatic brain injury via PI3K/Akt signaling. PLoS One. 2014;9:e113397. doi: 10.1371/journal.pone.0113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakhate K, Borkar C, Bharne A. Chapter 2 - functional neuroanatomy and disorders of cognition. Cognitive Informatics, computer modelling, and cognitive science, volume 2: Application to neural engineering, robotics, and STEM. Academic Press; 2020. p. 21–47.
- 42.Kamdi SP, Raval A, Nakhate KT. Phloridzin attenuates lipopolysaccharide-induced cognitive impairment via antioxidant, anti-inflammatory and neuromodulatory activities. Cytokine 2021;139:155408. [DOI] [PubMed]
- 43.Kamdi SP, Raval A, Nakhate KT. Effect of apple peel extract on diabetes-induced peripheral neuropathy and wound injury. J Diabetes Metab Disord. 2021. 10.1007/s40200-020-00719-6. [DOI] [PMC free article] [PubMed]