Abstract
Purpose
Diabetic Foot Ulcer (DFU) is one of the common and serious complications in patients with Diabetes Mellitus (DM) worldwide. Given the considerable tendency of patients suffering from DFU to use the complementary therapies, the objectives of this study were to: (i) summarize the effects of dietary and herbal supplements on DFU characteristics and metabolic parameters in both animal models and clinical trials, and (ii) evaluate any links between the serum levels of micronutrients and DFU in observational studies.
Methods
A systematic search in five electronic databases including PubMed/Medline, Scopus, Web of Science, Embase, and Cochrane Library was conducted to find relevant English language published from 1990 until 31 December 2018.
Results
Of a total of 8603 studies, 30 eligible papers including animal studies (n = 15), clinical trials (n = 7), and observational works (n = 8) were included in the systematic review. We found that some dietary/herbal supplements and micronutrients had positive effects on the wound healing. However, limited evidence is existed. Also, lower serum levels of vitamin D, C, vitamin E, and selenium in patients with DFU were likely to increase the risk of DFU, leading to impaired wound healing.
Conclusion
Findings suggested that some dietary and herbal supplements such as Vitamin D, Magnesium, Vitamin E, Probiotic, Zinc, and Pycnogenol would be effective on wound healing of DFUs. However, further high-quality randomized controlled clinical trials and prospective cohort studies are needed to clarify the roles of micronutrients and other dietary and herbal supplements on the progress and treatment of DFU.
Keywords: Medicinal herb, Micronutrient, Diabetic foot, Supplement, Systematic review
Introduction
Diabetic foot is a common and serious complication in patients with diabetes mellitus (DM) worldwide [1]. According to the World Health Organization, Diabetic foot ulcer (DFU) is featured as the foot with ulceration, infection and/or destruction of deep tissues, accompanied by neurological dysfunction and various degrees of peripheral vascular problems in the lower limb of patients with DM [2]. Every year, more than 1 million patients with diabetes lose at least a part of their leg due to the DM complications. In other words, every 20 seconds a lower limb is lost because of DM somewhere in the world [3].
DFU imposes a considerable economic and psychological burden on patients, their families, and society [4–6]. Some previous studies have examined various methods to define the risk of the development and the progression of DFU [7–10].
A wide spectrum of adjuvant therapies including oxygen therapies, negative pressure wound therapy, energy-based therapies and oral and topical medications are applied along with standard treatment to accelerate wound healing in patients with DFU [11–14]. However, it is essential to find and develop more effective methods such as complementary medicine for healing wound. As oxidative stress, inflammation, and insulin resistance involve in the pathogenesis of DFU [15–17], it is expected that methods to improve such parameters can achieve the therapeutic goals. On the other hand, wound healing is complicated cellular and biochemical processes depending on nutritional substrates such as energy, protein and micronutrients and can affect enzyme activities contributing to the healing process [18]. Therefore, malnutrition, mal-absorption and insufficient micronutrients can have negative effects on this process. Some studies investigating the association between serum levels of micronutrients [19, 20] and DFU showed that there is an inverse link between low micronutrient concentrations and high risk of DFU [21–24]. Furthermore, the effects of a number of dietary supplements have been evaluated in both animal models [25–29] and clinical trials [30–35] which some of them reported positive effects [25, 26, 30, 31].
Several works have also examined the effects of the medicinal herbs as complementary therapies on diabetic models [25, 36–40]. Some studies reported positive effects [25, 36, 38], whereas the findings were inconsistent. According to the above-mentioned evidence, taking some dietary and herbal supplements along with standard therapy can be helpful. In a meta-analysis performed by Ye et al., on all types of chronic wounds, they found that dietary supplements cannot significantly improve DFU [41]. However, they did not cover publications on neither medical herbs nor the serum levels of micronutrients. In a systematic review by Haiyan et al., positive effects of some dietary supplements on DFU were shown. However, it covered publications up to 2011 and the quality of studies was not examined [42]. Shuo et al., also reported that traditional Chinese medicinal herbs can be effective for healing of DFU. However, they only focus on herbs and dietary supplements were not considered [43]. To the best of our knowledge, no systematic reviews have examined the effects of both dietary supplements and medicinal herbs on DFU. Therefore, the objectives of this study were to (i) summarize the effects of dietary and herbal supplements on DFU characteristics and metabolic parameters in both animal models and clinical trials, and (ii) evaluate any links between the serum levels of micronutrients and DFU in observational studies.
Materials and methods
Search strategy and selection studies
The present study was designed and reported based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [44].
A systematic search in the five electronic databases such as PubMed/Medline, Scopus, Web of Science, Embase, and Cochrane Library was conducted to find relevant English papers published from 1990 until 31 December 2018. Both MeSH and non-MeSH key terms were considered in syntax. Search Strategies were designed by a librarian (R.A) and adopted designed strategy was used for each database. The study protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO): CRD42018116728.
Eligibility criteria
Papers were selected if they met the following criteria: (i) either clinical trials (parallel or crossover design), observational or animal studies, (ii) examining dietary or herbal supplements alone or in combination with other components, or (iii) examining the serum levels of nutrients, and (iv) reporting DFU as a primary outcome. Case report, case series, and grey literatures including interviews, conference papers and theses, unpublished data and topical treatments such as ointments were excluded from the study. To avoid missing any eligible articles, reference lists of all included papers and review studies were hand-searched.
After collecting publications and removing duplications, screening based on title and abstract were performed by two independent investigators (MR.A, M.A). Then, the full-texts of remaining publications from the previous step were evaluated based on the eligibility criteria. The full- texts of possible relevant papers were obtained from some cases via contacting their corresponding authors. Any disagreements between two investigators were resolved by the third investigator (N.N).
Data extraction
From each eligible paper, data were extracted using a pre-defined data extraction form. The primary exposure was dietary/ herbal supplements and the main outcome was DFU. The following data were extracted by two independent reviewers (M.S, N.N) for both human and animal studies (M.R.A, M.A) based on study design: the first author’s last name, publication year, study design, the participants’ characteristics (mean age, gender), sample size at baseline and at the end of study, name of intervention, dosage, duration of intervention, mode of intervention, ulcer characteristics and their changes, biochemical parameters, comparison groups, and controlled confounders. If a study reported the effects of both topical and oral treatments, only findings related to non-topical therapies were extracted. In the case of any discrepancy between the two investigators, the third investigator (E.N) was consulted.
Quality assessments
The quality of the included clinical trials was assessed using the Jadad scale (3 main items) [45]. The maximum score was five and each study obtained minimum three scores was considered as high quality. For observational studies, Newcastle-Ottawa Scale (NOS) was used (three main items) [46]. Based on this scale, a maximum of nine/ten scores can be dedicated to each study and a minimum score of five were considered as high quality. Two investigators (N.N, M.S) independently examined the quality of human studies and then compared their assessments with each other.
For animal studies, SYRCLE checklist (10 items) was used [47]. This tool is based on the Cochrane risk of bias checklist. It has been adjusted for various aspects of bias that play pivotal roles in animal interventions. Each item should be identified by YES/NO/ UNCLEAR. The quality of each animal study was checked by two independent reviewers (MR.A, M.A) and any disagreement was resolved by the third investigator (E.N).
Quantitative data synthesis
Given limited studies conducted on each dietary/herbal supplement and serum levels of micronutrients, quantitative synthesis was not possible and we only summarized the results qualitatively for all nutrients.
Results
Literature search
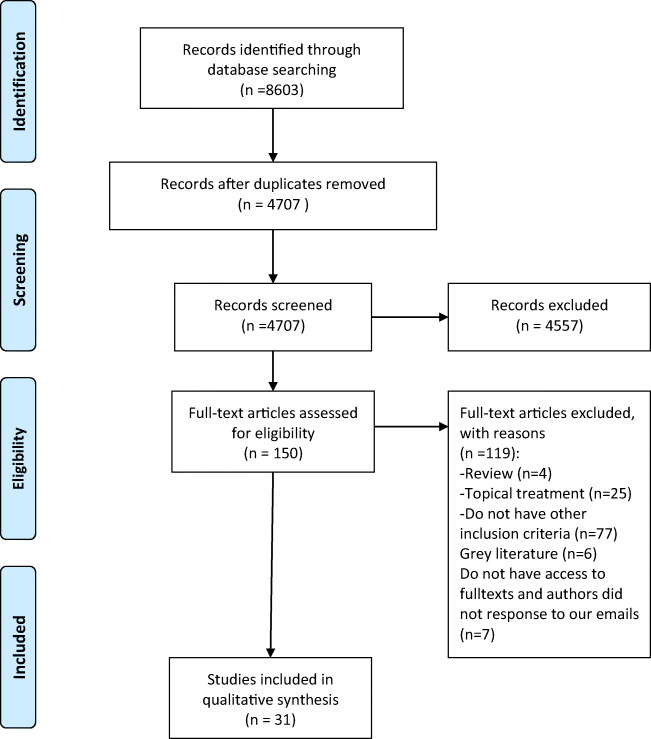
In total, 8603 publications including PubMed (n = 1049), Scopus (n = 3006), Web of Science (n = 1498), and Cochrane library (n = 379), Embase (n = 2671) were found. Of a total of 8603 studies, 3896 were duplicates. Screening was performed on 4707 publications. As depicted in Fig. 1, after the removal of duplicates and screening based on titles and abstracts, 150 publications were considered potentially relevant. With the precise assessment based on the full-texts of papers, publications were excluded due to either not being relevant or the lack of eligibility criteria. Finally, 30 eligible papers including animal studies (n = 15) [25–29, 37–40, 48–53], clinical trials (n = 7) [30, 34, 35, 54–57], observational works (n = 8) [23, 24, 58–62] were included in the systematic review.
Fig. 1.
Paper selection for the present systematic review
Characteristics of the included publications
Animal studies
A total of 15 animal studies conducted on dietary supplements (n = 6) [25, 26, 29, 48, 50, 52] and medicinal herbs (n = 9) were included in the study [25, 27, 28, 37–40, 50, 51]. Of these, 11 papers [26–28, 38–40, 49–53] assessed oral intake and the remaining studies evaluated the impact of intravenous injection of supplements. The effects of each supplement were examined in only one study; just supplementation with hesperidin was explored in two papers [27, 28].
The impacts of vitamin C, Zinc (Zn), vitamin D, vitamin E, amino acids (Argintinand proline), and mixture of nutrients were examined and six types of medicinal herbs (alone or mixture) including Angelica Dahurica, San-huang-sheng-fu, Aloe vera, Radix Astragali & Rehmanniae, and Chinese 2-herb formulas were examined. In addition, three effective components of natural products including hesperidin, naringin, and genistein were also assessed in animal models. All the aforementioned supplements except Zn [48] showed positive effects on wound healing and other metabolic parameters as shown in Table 1. Based on SYRCLE checklist, all animal studies had high risk of bias (Appendix 1).
Table 1.
Characteristics of the included animal studies in the systematic review
| Author (year) | Type of animal/sample size | Comparative groups | Intervention | Dosage | Duration | Type of intervention (oral, injection, etc) | Findings | Quality score |
|---|---|---|---|---|---|---|---|---|
| SusamaChokesuwattanaskul (2018) | nude mice / 48 |
1-Control 2-Diabetes Mellitus [DM] 3-Diabetes Mellitustreated with mesenchymal stem cells [MSCs] 4-DM treated with VitC 5-DM treated with MSCs and VitC |
Vitamin C | - Vit. C (1.5 g/l) | 7 days | Oral |
In the 4th Group: - ↑Angiogenesis -Accelerate diabetic wound healing |
High RoB |
| Yusom Shin (2017) | Rat / 20 |
1-Non-diabetic group 2-A nontreated 3-A nerve-block 4-A nerve-block and IV zinc group |
Zinc | 0.1 ml zinc sulphate hydrate | 10 days | IV |
Among the three DFU groups: The wound-surface-area differences were not statistically significant. |
High RoB |
| Xiao-na Zhang, (2017) | Rat / 45 |
1-Diabetic treated with ADEE 2-Diabetic without drugtreatment 3-Non-diabetic rats served as normal control |
Angelica Dahurica Ethanolic Extract | 1.1 ml/0.2 kg body weight once daily | 14 days | Oral |
-Correcting impaired Wound Healing -Accelerating wound closure -↑ newly formed blood vessels -Improving diabetes-impaired wound healing |
High RoB |
| Wenbin Li 2018 | Rat / 30 |
1-Non-diabetic and non-wounded animals 2-Non-diabetic and wounded animals 3-Diabetic and wounded animals |
Hesperidin |
25, 50 and 100 mg/kg, |
21 days | Oral |
-↑inpercent Wound closure and serum insulin level. -↑Lipid peroxidation (MDA) and Nitric Oxide levels. -↑mRNA expressions |
High RoB |
| YiFeng Yuan et al. 2018 | Mice /45 |
1-Normal group 2- Diabetic group 3-Vitamin D treatment group |
Vitamin D | 100 ng/ kg per day | 14 days | IV |
-↓Level tumor necrosis factor-α, interleukin 6,1β) -Suppressed NF-κB pathway activation -Improves impaired wound |
High RoB |
| Li Wang, 2018 | Rat / 90 |
1-Non-diabetic rats and nonwounded got sterile water for injection (WFI) 2-Non-diabetic rats and wounded received sterile WFI 3-Diabetic rats & wounded (DW) administered with sterile WFI 4-DW rats Administered with aqueous suspension of HSP (10 mg/kg). 5- DW rats Administered with aqueous suspension of HSP (20 mg/kg). 6- DW rats Administered with aqueous suspension of HSP (40 mg/kg). 7- DW rats Administered with aqueous suspension of HSP (60 mg/kg) 8- DW rats Administered with aqueous suspension of HSP (80 mg/kg) 9- DW rats Administered with insulin (10 IU/kg) |
Hesperidin (HSP) Suspension And Insulin |
HSP: 10, 20, 40, 60, and 80 mg/kg And Insulin 10 IU/kg |
20 days |
Hesperidin: Oral And Insulin: subcutaneous |
↑ Expression of VEGF and stimulation of angiogenesis ↓ expression of inflammatory mediators ↑ Expression of growth factors statistically significant (P < 0.05) improvement in wound dimension |
High RoB |
| Amit D. 2014 | Rat /? |
Group I: Normal non-diabetic (ND): without wound received double distilled water (10 mg/kg, p.o.) Group II: non-diabetic Normal wound control: single injection of citrate buffer (vehicle), and then double distilled water (10 mg/kg, p.o.) Group III: diabetic wound control: double distilled water (10 mg/kg, p.o.) Group IV: diabetic animals with wound received naringin (20 mg/kg, p.o.) in double distilled water. Group V: diabetic animals with wound received naringin (40 mg/kg, p.o.) in double distilled water Group VI: diabetic animals with wound received naringin (80 mg/kg, p.o.) in double distilled water Group VII: diabetic animals with wound received insulin (10 IU/kg, s.c.). Group VIII: TCDO: diabetic animals with wound received TCDO(1 drop, twice a day, topically). |
Naringin And Insulin And tetra chlorodecaoxide |
Naringin (20, 40 and 80 mg/kg, p.o.), Insulin (10 IU/ kg, s.c.) tetra chlorodecaoxide (TCDO) (1 drop, twice a day, topically) |
16 days |
Naringin: injection Insulin: injection TCDO topical |
↓ wound area ↑ rate of wound contraction ↑Angiogenesis ↓ Inflammatory mediator expression ↑growth factor |
High RoB |
| HyeyoonEo, 2016 | Mice |
1) CON: Nondiabetic mice were fed AIN-93G rodent diet without genistein supplementation. [2] DMC: Diabetic control mice were fed AIN-93 Grodent diet without genistein supplementation. [3] LG: Diabetic mice were fed AIN-93G rodent diet with 0.025% genistein dietary supplementation. [4] HG: Diabetic mice were fed AIN- 93G rodent diet with 0.1% genistein dietary supplementation. |
Genistein |
0.025% and 0.1% genistein dietarysupplementation. |
2 weeks | Oral |
Genistein supplementation: ↓Inflammation and oxidative stress The wound closure rates of LG and HG were accelerated significantly. |
High RoB |
| Jacqueline ChorWing Tam, 2014 | Rat/? |
1:diabetic rats without ischemia and wound induction (Sham); 2: diabetic rats with ischemia surgery and without wound induction(I only); 3: diabetic rats with both ischemia and wound induction followed by water treatment (I + W); 4: diabetic rats with both ischemia and wound induction followed by NF3 treatment (I + W + NF3) |
(NF3)* *[Astragali Radix (AR) and Rehmanniae Radix (RR) in the ratio of 2 to 1] |
NF3 (at clinical relevant dose = 0.98 g/kg) |
7 DAYs | IV | ↓ Wound area of the diabetic foot ulcer | High RoB |
| Wan Y, et al. 2016 | Rat / 25 |
G1: non-diabetes (N), gavaged with saline and applied with sesame oil on pelma of both hind limbs G2: diabetes and sham treatment (DS),gavaged with saline and applied with sesame oil on pelma of both hind limbs G3: metformin (M), gavaged with diluted M and applied with sesame oil on pelma of both hind limbs G4: S, gavaged with saline and applied with S onpelma of both hind limbs G5: combined treatment (CT) gavaged with diluted M and applied with S onpelma of both hind limbs |
San-huang-sheng-fu oil | - | 35 days | Oral |
-↓ Plantar temperature and pain dysesthesia. -↓ Cyclooxygenase-2 (COX-2) - ↑ Vascular endothelial growth factor (VEGF). The wound areas of rats with diabetes in the 4 groups were close to those in group N (with t values from 0.111 to 1.476, P values above 0.05). On PTD (post treatment day) 21. The wound area of rats in group DS was significantly larger than that in group N (t = 5.502, P < 0.01), The wound areas of rats with diabetes in the other 3 groups were close to the area in group N (with t values from 0.544 to 1.676, P values above 0.05). |
High RoB |
| Musalmah M, et al. 2002 | Rats/ 24 |
1-Normal rats Untreated group -α-tocopherols group 2- Diabetic rats -Untreated group -α-tocopherols group |
α-tocopherols (Vit E) |
200 mg/kg | 10 days | Oral |
-↓ plasma malondialdehyde levels, -↑ lutathione peroxidase activity -Accelerated the rate of wound closure in treated rats |
High RoB |
| T.W. Lau, et al. 2008 | Rats / 164 |
1- Control group (water treatment) 2-Intervention group (Herbal treatment) |
Radix Astragali and Radix Rehmanniae |
-RA or RR 3.7 g/kg |
7 days | Oral |
Radix Astragali: There was no significant difference in the rate of change of wound area between the two groups [control & RA] (p = 0.94) Radix Rehmanniae: There was a significant difference in the rate of change of wound area between the two groups[control & RR] (p = 0.04). |
High RoB |
| A. Raynaud-Simon et al. 2012 | Rats / 17 |
1-Standard Diet 2- Arginine + proline Diet 3-Iso nitrogenousisoenergetic control Diet |
Arginine + proline | 120 ml / daily | 5 DAYs | Oral | -Significantly improved wound repair (by angiogenesis) | High RoB |
| Na-Young Park 2011 | Mice |
1-CON (non-diabetic control mice), 2-DM (diabetic mice), 3-VCE (diabetic mice fed 0.5% vitamin C and 0.5% vitamin E supplemented diet), 4-Comb (diabetic mice fed 0.5% vitamin C, 0.5% vitamin E, and 2.5% N-acetylcysteine supplemented diet). |
Vit. C + Vit. E + N-acetylcysteine | 0.5% vitamin C, 0.5% vitamin E, 2.5% N-acetylcysteine | 10 days | Oral |
-Improved blood glucose levels and wound closure rate. -Wound closure rate of the VCE and the Comb groups were accelerated significantly as compared to those of the DM group. |
High RoB |
|
Mohan Daburkar et al. 2014 |
Rats |
1-Untreated control 2-Untreated DFUs 3-DFUs treated with Aloevera gel ethanolic extract 4-DFUs treated with topical A. vera gel 5-DFUs treated with A. vera gel ethanolic extract and topical A. vera gel |
Aloe vera gel ethanolic extract | Aloevera gel ethanolic extract [300 mg/kg, per os (p.o.), twice daily] | 9 days | Oral | ↓ wound size (P < 0.01) | High RoB |
DM: Diabetes Mellitus; Vit C: Vitamin C; MSCs: Mesenchymal stem cells; RoB: Risk of Bias; DFU: Diabetic Foot Ulcer;ADEE:Angelica DahuricaEthanolic Extract; WFI: Water for injection; DW: Diabetic rats& wounded; HSP: Hesperidin; VEGF: Vascular endothelial growth factor; MDA: Malondialdehyde; ND:Non-diabetic;TCDO: Tetra chlorodecaoxide; VEGF: Vascular endothelial growth factor; CT: combined treatment
Observational studies
Eight observational studies [23, 24, 58–63] were finally included in the systematic review (Table 2). All articles except three papers [23, 61, 62] were conducted in Asian countries. Design of the included observational studies was prospective/retrospective cohort (n = 3) [23, 24, 59] and case-control and cross-section (n = 5) [58, 60–63]. In total, four paper evaluated the serum levels of vitamin D [23, 24, 59, 61] and the remaining studies were conducted on vitamin C, vitamin E, and selenium (Se), magnesium (Mg), copper (Cu) and Zn. All the included papers obtained more than half of maximum score of Newcastle Ottawa Quality (NOS) checklist and considered as high quality (Table 2).
Table 2.
Study characteristics of the included observational studies
| Author (year) |
Location | Study design | Measured nutrient | Comparative groups | Sample size/ sex (M/F) |
Mean age (year) |
Type of diabetes | Baseline BMI (kg/m2) | Baseline FBS (mg/dL)/HbA1c (%) |
Mean Duration of diabetes (year) | Findings | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Greenhagen 2019 |
USA |
Retrospective Cohort |
Serum vitamin D levels Albumin creatinine |
Diabetic patients with and without CN*, PAD*, DFI*, DFU, DPN* |
50 Non-CN 28/22 |
55.7 | T1DM | 30.9 | 176/7.7 | – |
-Vitamin D with and without CN Lower levels of vitamin D: ↑PAD ↑DFI ↑DFU Lower levels of albumin and higher serum Creatinine: ↑PAD, DFI, DPN, and DFU. |
8/10 |
|
61 Non- DFI 29/32 |
55.9 | 33.8 | 163/7.7 | |||||||||
|
39 DFI 27/ 12 |
56.2 | 31.8 | 184/7.7 | |||||||||
|
79 Non- PAD 39/40 |
55.6 | 33.3 | 169/7.8 | |||||||||
|
22 PAD 17/5 |
58.5 | 33.0 | 179/7.5 | |||||||||
|
11 Non- DPN 7/4 |
52.7 | 30.2 | 141/6.9 | |||||||||
|
89 DPN 37/52 |
56.2 | 32 | 175/7.8 | |||||||||
|
46 No DFU 17/29 |
55.5 | 32.6 | 159/7.5 | |||||||||
|
54 DFU 39/15 |
56.1 | 32.2 | 182/7.9 | |||||||||
| CN | 55.9 | 33.8 | 175/7.7 | |||||||||
| Sever Cağlar 2018 | Turkey | Prospective study |
Vitamin D OPG* CRP Creatinine63.6 51.4 |
Patients with DFU (study group) |
Total= 105 58 DFU (42/16) |
T2DM | – | 7.8 |
14.3 4.2 |
lower OPG levels: ↑DFU lower vitamin D D levels: ↑DFU OPG level: ↑CRP (r = 0.379, p < 0.05). OPG level: ↑Creatinine (r = 0.562, p < 0.05) |
7/9 | |
|
Newly diagnosed T2DM (control group) |
47 (27/20) |
8 | ||||||||||
| Joachim Feldkamp 2018 | Germany | Cross-sectional |
Vitamin D Calcium, Creatinine |
Controls | 99 | 69.6 | T2DM | Not reported | – | – |
↑Armstrong classification Vitamin D level↓ (r = −0.241; p < 0.01) Vitamin D level: Controls: 27.3 ± 12.2 Diabetes type 2: 19.0 ± 14.4 Diabetic foot syndrome: 11.8 ± 11.3 Inpatients: 10.8 ± 9.5 Outpatients: 13.4 ± 13.1 |
8/9 |
| T2DM | 103 | 69.4 | 60.65 | 16.8 | ||||||||
| Diabetic foot syndrome | 104 | 70.2 | 59.56 | 17.5 | ||||||||
| Inpatients | 63 | 70.2 | 65.36 | 18.1 | ||||||||
| Outpatients | 41 | 69.6 | 51.91 | 17.4 | ||||||||
|
Bolajokol 2016 |
Nigeria | Cross sectional | vitamin C, vitamin E, copper, selenium, zinc |
DM + FU/ non-diabetic |
70 | – | T2DM | – | 12.98 mmol/L/ 8.63% | – |
↑DM + FU: Lower Vitamin C, Vitamin E, Selenium, -DM + FU: Copper and Zinc Lower vitamin C: ↑FPG: (r = 0.250, p = 0.037) Lower Cu: ↑HbAIc: (r = 0.131, p = 0.365) |
7/9 |
| 50 | 5.09 mmol/L/4.08% | |||||||||||
|
ŞakirzgürKeşkek 2013 |
Turkey | cross-sectional | Magnesium | Patients with T2DM and DFU |
Total= 147 F = 24 |
56.6 | T2DM | – | 221.5/10.0 | 12.4 |
Lower magnesium levels DFU ↑ (p < 0.001) |
7/9 |
| Patients with T2DM without DFU | F = 24 | 53.4 | 184.9/8.5 | 12.1 | ||||||||
| Healthy subjects | F = 26 | 53.1 | 90.1/5.5 | – | ||||||||
| Zubair 2013 | India | Prospective cohort hospital based study | Vitamin D |
Patients with Ulcer (DFU) |
103/59 | 46.29 | T2DM | 24.84 | 9.6 |
<10 years DFU:111(68.5) non-DFU: 123(75.9) >10 years DFU: 51(31.4) non-DFU: 39(24.0) |
↓Vitamin D DFU: 8.4(7.1–9.2) non-DFU: 29.8 (15.6–44.2) <0.005 |
7/9 |
|
Patients without Ulcer (non-DFU) |
102/58 | 47.10 | 24.03 | 7.9 | ||||||||
|
Singh 2008 |
India | Cross-sectional |
Vitamin E Malondialdehyde SOD* |
DFU |
Total= 32 (26/6) |
53.81 | T2DM | – | – | – |
Vitamin E: Group A vs B= 5.35 < 0.001 B vs C= 2.34 < 0.05 |
7/9 |
|
Non-DPN and Non-PAD |
15 | 53.4 | ||||||||||
| Healthy control | 15 | 50.66 | ||||||||||
| Martha Rodríguez-Morán2001 | México | Cross-sectional | Magnesium |
Diabetic patients with and without ulcer |
With DFU: 33 (17/16) |
62.3 | T2DM | 26.1 | 291.8 | 13.9 |
Lower levels ofMagnesium: ↑DFU OR: 2.9, 95%CI: 1.7–6.8 Hypomagnesemia: With DFU: 49 (74.2%) Without DFU: 31 (93.9%) |
7/9 |
| Without DFU: (31/66) | 61.2 | 26.7 | 273.2 | 14.2 |
WG: Wagner grade; CN: Charcot neuroarthropathy; PAD: peripheral arterial disease; DFI: Diabetic Foot infection; DPN: Peripheral neuropath; OPG: Osteoprotegerin; SOD: Superoxide dismutase enzyme
Greenhang et al., compared the serum levels of vitamin D in patients suffering from DM and without Charcot neuroarthropathy (CN), infection, DFU, peripheral arterial disease and peripheral neuropathy in a retrospective cohort study. They found that 78% of patients had insufficient/deficient vitamin D and no significant difference were found between those with and without CN [23]. However, serum vitamin D concentrations in patients with DFU was significantly lower than those without this complication (p = 0.04) [23]. Bolajokol et al., also indicated that serum levels of vitamin C, vitamin E, and Se in diabetic patients with DFU were considerably lower than those without DM. However, no differences were found between two the groups with respect to the levels of Cu and Zn [58]. Feldkamp et al., concluded that the serum levels of vitamin D in 55.8% of patients with DFU was below 10 ng/mL. In addition, they reported a positive link between DFU and vitamin D deficiency [61].Singh et al., also reported that patients with DFU had lower serum levels of vitamin E compared to those with DM and healthy subjects (control group) [60].
Clinical trials
As presented in Table 3, seven clinical trials [30, 34, 35, 54–57] were considered for the current systematic review. All the included trials were double blind placebo controlled clinical trials. All publications except two papers were conducted in Iran. In their study, Afzali et al., showed that Magnesium oxide (250 mg/day) combined with Vitamin E (400 IU/day) could decrease length, depth and width of ulcer compared to placebo after 12 weeks in patients with grade III DFU [56]. The study of Mohseni et al., demonstrated that daily supplementation with probiotic for 12 weeks had positive effects on wound healing and it reduced length, depth and width of ulcer as well as improvement in FBS and insulin sensitivity in diabetic patients [55]. In another study, 50,000 IU vitamin D (every two weeks)also suggested an improvement in DFU after 12 weeks [34]. The double blind clinical trial performed by Momen-heravi et al., showed that 220 mg/day zinc sulfate decreased the dimension of ulcer as well as improvement in metabolic status [54]. Razzaghi et al., indicated positive effects of 250 mg/day magnesium on diabetic ulcer and glydemic status after 12 weeks [35]. A mixture of Arginine (7 g), glutamine (7 g), and calcium b-hydroxy-b-methylbutyrate (1.5 g) daily showed beneficial effects on wound healing after the 16-week intervention [30]. Furthermore, other clinical trials showed the positive impact of Arginine supplement on DFU [64, 65].
Table 3.
Characteristics of the included clinical trials in the systematic review
| Author (year) | Location | Study design (randomization/ blindness) |
Sample size/ Characteristics of participants | Intervention | Dosage | Duration (week) |
Baseline BMI (kg/m2) | Baseline FBS/HbA1c In intervention group |
Mean Duration of diabetes (year) | Findings | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Afzali et al. (2019) |
Iran | Double blind |
57/ patients with grade 3 DFU (45:m, 12:f) |
Magnesium oxide + Vitamin E |
250 mg + 400 IU |
12 | 30 |
FBS: 173 HbA1c:7.4 |
– | ↓Ulcer length | 4 |
| ↓Ulcer width | |||||||||||
| ↓Ulcer depth | |||||||||||
| ↓FBS | |||||||||||
| ↓Insulin | |||||||||||
| IR | |||||||||||
| HbA1c | |||||||||||
| ↑Insulin sensitivity | |||||||||||
| ↓TG | |||||||||||
| ↓LDL-C | |||||||||||
| ↑VLDL | |||||||||||
| ↓ESR | |||||||||||
| ↓hs-CRP | |||||||||||
| ↓MDA | |||||||||||
| ↑HDL-C | |||||||||||
| ↑TAC | |||||||||||
| Mohseni et al. (2018) | Iran | Double blind | 60 (20:m, 10:f) |
Probiotic (Lactobacillus acidophilus, Lactobacillus casei,Lactobacillus FermentumandBifidobacteriumbifidum |
2 × 109 CFU/g each | 12 | 25 |
FBS:225 HbA1C:8 |
– | ↓Ulcer length | 4 |
| ↓Ulcer width | |||||||||||
| ↓Ulcer depth | |||||||||||
| ↓FBS | |||||||||||
| ↓Insulin | |||||||||||
| IR | |||||||||||
| HbA1c | |||||||||||
| ↑Insulin sensitivity | |||||||||||
| ↓TG | |||||||||||
| ↓LDL-C | |||||||||||
| ↑VLDL | |||||||||||
| ↓TC | |||||||||||
| ↓hs-CRP | |||||||||||
| ↓MDA | |||||||||||
| ↑HDL-C | |||||||||||
| ↑TAC | |||||||||||
| ↓MDA | |||||||||||
| -GSH | |||||||||||
| Razzaghi et al. (2018) | Iran | Double blind | 70 patients with grade 3 | Magnesium | 250 mg | 12 | 27 |
FBS: 226 HbA1c: 8.3 |
↓Ulcer length | 4 | |
| ↓Ulcer width | |||||||||||
| ↓Ulcer depth | |||||||||||
| ↓FBS | |||||||||||
| ↓Insulin | |||||||||||
| ↓IR | |||||||||||
| ↓HbA1c | |||||||||||
| ↑Insulin sensitivity | |||||||||||
| -HOMA-IR | |||||||||||
| -TG, TC, LDL-C, HDL-C | |||||||||||
| Razzaghi et al. (2017) | Iran | Double blind | 60 patients with grade 3 | Vitamin D | 50,000 | 12 | ↓Ulcer length | 4 | |||
| ↓Ulcer width | |||||||||||
| ↓Ulcer depth | |||||||||||
| ↓FBS | |||||||||||
| ↓Insulin | |||||||||||
| ↓IR | |||||||||||
| ↓HbA1c | |||||||||||
| ↑Insulin sensitivity | |||||||||||
| -TG | |||||||||||
| ↓LDL-C | |||||||||||
| -VLDL | |||||||||||
| ↓ESR | |||||||||||
| ↓hs-CRP | |||||||||||
| ↓MDA | |||||||||||
| -HDL-C | |||||||||||
| ↓Erythema rate | |||||||||||
| -GSH | |||||||||||
|
Momen-Heravi et al. (2017) |
Iran | Double blind | 60 patients with grade 3 (42:m,18 = f) | Zinc sulphate | 220 mg | 12 | 25.8 |
FBS:190 HbA1c:7.9 |
– | ↓Ulcer length | 5 |
| ↓Ulcer width | |||||||||||
| ↓Ulcer depth | |||||||||||
| ↓FBS | |||||||||||
| ↓Insulin | |||||||||||
| ↓IR | |||||||||||
| ↓HbA1c | |||||||||||
| ↑Insulin sensitivity | |||||||||||
| -TG | |||||||||||
| ↓LDL-C | |||||||||||
| -VLDL | |||||||||||
| ↓ESR | |||||||||||
| ↓hs-CRP | |||||||||||
| ↓MDA | |||||||||||
| ↑HDL-C | |||||||||||
| ↑TAC | |||||||||||
| -GSH | |||||||||||
| Armstrong et al. (2014) | USA | Double blind | 270 | Mixture of Arginine (7 g), glutamine (7 g), calcium b-hydroxy-b-methylbutyrate (1.5 g) contained 79 kcal drink | Twice/day | 16 | 32 | NR | 15 |
In patients with Alb≤40 g/L and/or ankle-brachial index <1: significant wound healing -No differences in healing in non-ischaemic or those with normal Alb |
5 |
|
Gianni Belcaro et al. (2006) |
Germany | Double blind | 16 diabetic patients with severe microangiopathy causingfoot ulcerations |
Oral treatment: 150 mg/d Pycnogenol (50 mg) |
3 times daily | 6 weeks | NR | NR | NR | Healing rate: 85% | 2 |
NR: Not Reported; FBS: fasting blood glucose; IR: Insulin Resistance; TG: triglyceride; LDL-C low-density lipoprotein; VLDL-C very low-density lipoprotein; ESR: Erythrocyte sedimentation rate; hs-CRP: high-sensitivity C-reactive protein; MDA: malondialdehyde; HDL-C: high-density lipoprotein; TAC: total antioxidant capacity; GSH: glutathione; HOMA: Homeostatic Model Assessment for Insulin Resistance; MDA: Malondialdehyde
Discussion
The present systematic review showed the positive effects of dietary and herbal supplements with anti-oxidant properties on the process of wound healing in both animal models and clinical trials. Additionally, it was revealed that serum levels of vitamin D, C, vitamin E, and Se in subjects with DFU were lower than those in either healthy or patients without DFU. However, there are limited investigations in this area.
To the best of our knowledge, no systematic reviews have examined the link between serum levels of nutrients and DFU in observational studies. In their study, Dai et al., examined the association between serum levels of vitamin D and DFU in a systematic review and meta-analysis and found that severe vitamin D deficiency increased the risk for DFU by 3.2 [66]. In addition, a literature review performed by Macido et al., pointed out an inverse link between 25(OH) D3 levels and the presence of ulcer in patients with diabetes mellitus [67].
Based on previous works, malnutrition can play a key role in the progress of DFU and infection [68]. It has been shown that in patients with DFU, apart from body mass index (BMI), serum albumin, other metabolic parameters, and infection, nutritional status affect wound healing and metabolic profile [68, 69]. Apart from macronutrients, several micrunitrients including vitamins and minerals may involve in the treatment or the progress of DFU [70].
Limited observational studies which mostly had case-control design were included in the study. As such types of studies cannot reveal any cause and effect link between serum levels of nutrients and DFU, we cannot make a definitive decision regarding possible supplementations which are effective in DFU healing.
Although there were few studies on each specific nutrient, based on quality assessment tool all the included studies had high quality. Therefore, risk of bias seems not to be able to affect the results. We found that the levels of several nutrients with anti-oxidative properties in patients with DFU were lower than those in controls. Some studies compared the levels of nutrients in patients suffering from DFU with non-diabetic subjects with any ulcers. As there is a mutual link between DM and some nutrients, it seems reasonable to compare people with DM with non-diabetic subjects.
Some review studies summarized the effects of supplements on the DFU. A recent systematic review included only three clinical trials, two on protein-based supplementation and one on medicinal herb. In a study conducted by Ye et al., no nutrient supplements were included. They reported that nutritional supplementations had no positive effect on the DFU [41].
In the present study, all animal and clinical trials assessing the effects of non-topical dietary and herbal supplements were included. It seems that only three studies had examined the effect of herbs orally or intravenously on animal models. All studies conducted on herbal supplements showed positive effects on DFU and some metabolic parameters. Antioxidant effects of mentioned plants seem to be the main reason for such positive effects [71].
However, given few animal studies conducted on each nutrient and herb as well as low quality of methodology, we have to interpret results with great caution. In the present systematic review, we used SYRCLE checklist that had been developed in 2014 [47]. Therefore, it seems reasonable that papers published before this date, did not obtain acceptable score. This systematic review revealed that high-quality animal studies are needed to make a decision regarding the efficacy of supplements. In addition, to examine the novel supplements, more attention should be paid for the methodology and study design to minimize bias.
Wound healing is a complicated process containing four steps as follows: hemostasis, inflammation, proliferation and tissue remodeling [72]. One of possible mechanisms through which nutrients affect DFU is reducing free radicals and oxidative parameters followed by inducing a balance between oxidant and antioxidant components [73, 74]. Production of reactive oxygen species (ROS) play a crucial role in the etiology of complications related to diabetes including DFU. Following hyperglycemia, the levels of free radicals increase and can damage tissue, leading to non-healing DFU and infection [75]. Accordingly, anti-oxidant ingredients can be helpful to equilibrate oxidant and anti-oxidant components.
Another pathway contributing to wound healing is reducing systemic inflammatory parameters [76]. Anti-inflammatory effects of several nutrients including vitamin D have been proposed [77]. Hyperglycemia can disturb normal procedures for the production of cytokines. Therefore, uncontrolled glucose level can provide conditions for increasing inflammation, ulcer, and infection [78]. Duration of diabetes, age and BMI impact upon inflammatory cytokine production [78]. Therefore, apart from dosage, duration of intervention by a specific supplement with anti-inflammatory effects, the aforementioned factors can lead to different findings following the supplementation.
Vitamin D as an immune modulator plays a pivotal role in the activation of T and B cells by macrophages. Besides, it can decrease inflammatory parameters including C-reactive protein (CRP), and tumor necrosis- alpha (TNF- alpha) [23]. It has been reported that insufficient vitamin D increase the release of inflammatory cytokine (IL-1beta, IL-6, TNF-alpha) in subjects with DFU and infection, particularly when serum levels of 25 (OH) D3 is lower than 10 ng/mL [78]. Other proposed mechanisms for vitamin D are as follows: (i) improvement of glycemic status following supplementation with vitamin D, (ii) effects on insulin resistance, and (iii) beta-cell function [66]. In the present systematic review, studies examining the effects of vitamin D on DFU are greater than other nutrients and this issue highlighted the possible significant role of vitamin D on DFU. However, more high-quality studies are needed to clarify its efficacy.
Not only did some nutrients including Mg, vitamin D, vitamin E, and vitamin C have been shown positive effects on DFU, but also improved glycemic status, lipid profile, inflammatory and oxidative stress. The mentioned nutrients would be involved in several biological functions and therefore the low levels of such necessary nutrients cause to increase insulin resistance, beta-cell dysfunction, and deterioration of metabolic status.
Limitations of the study
Main limitations of this systematic review would include the following: First, given few studies conducted on each nutrient and supplement we were not able to do a quantitative synthesis. Second, studies published in languages other than English were not included. Third, due to few cohort studies, cause and effect relationship between nutrients and DFU remained unclear.
Strengths of this systematic review are as follows: (i) to the best of our knowledge this is the first systematic review to assess the effects of serum levels of nutrients on DFU healing (ii), it examined methodological quality for both animal and human studies and, (iii) address all types of supplements including medicinal herbs and dietary supplements.
Conclusion
We found that some dietary and herbal supplements can shorten the process of wound healing in both animal models and clinical trials, but the relevant studies included in the review are limited. Also, we revealed that lower serum levels of vitamin D, C, vitamin E, and Se in patients with DFU were likely to increase the risk for DFU, whereas any cause and effect relationship remained unclear due to limited cohort studies. Therefore, it would be recommended that further high-quality randomized controlled clinical trials and prospective cohort studies are needed to shed light the impacts of micronutrients and other dietary and herbal supplements in the progress and treatment of DFU.
Appendix
Table 4.
SYRCLE’s tool for assessing risk of bias
Funding
Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
Declarations
Ethics approval
Not Applicable.
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Registration number CRD42018116728.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahnaz Sanjari, Email: mahnaz.sanjari@gmail.com.
Nazli Namazi, Email: nazli.namazi@yahoo.com.
References
- 1.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 2.Sotodehasl N, Malek F, Tamadon MR. Vitamin D deficiency and depression: a short review article. Middle East J Rehabil Health. 2015;2(3):e26961.
- 3.Bakker K, Apelqvist J, Lipsky B, Van Netten J, Schaper N, Foot IWGotD The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016;32:2–6. doi: 10.1002/dmrr.2694. [DOI] [PubMed] [Google Scholar]
- 4.Vileikyte L, Rubin RR, Leventhal H. Psychological aspects of diabetic neuropathic foot complications: an overview. Diabetes Metab Res Rev. 2004;20(S1):S13–SS8. doi: 10.1002/dmrr.437. [DOI] [PubMed] [Google Scholar]
- 5.Ali S, Fareed A, Humail S, Basit A, Ahmedani M, Fawwad A, et al. The personal cost of diabetic foot disease in the developing world—a study from Pakistan. Diabet Med. 2008;25(10):1231–1233. doi: 10.1111/j.1464-5491.2008.02529.x. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri A, Gatt A, Formosa C. Inter-rater reliability of four validated diabetic foot ulcer classification systems. J Tissue Viability. 2020;29:284–90. [DOI] [PubMed]
- 7.Lim JZM, Ng NSL, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017;110(3):104–109. doi: 10.1177/0141076816688346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Netten J, Price PE, Lavery L, Monteiro-Soares M, Rasmussen A, Jubiz Y, et al. Prevention of foot ulcers in the at-risk patient with diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32:84–98. doi: 10.1002/dmrr.2701. [DOI] [PubMed] [Google Scholar]
- 9.Verrone Quilici MT, Del Fiol FdS, Franzin Vieira AE, Toledo MI. Risk factors for foot amputation in patients hospitalized for diabetic foot infection. J Diab Res. 2016:8931508. [DOI] [PMC free article] [PubMed]
- 10.Paisey R, Abbott A, Paisey C, Walker D, Birch R, Bowen B, et al. Diabetic foot ulcer incidence and survival with improved diabetic foot services: an 18-year study. Diabet Med. 2019;36(11):1424–1430. doi: 10.1111/dme.14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153–165. doi: 10.1111/nyas.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toygar I, Simsir IY, Cetinkalp S. Evaluation of three different techniques for measuring wound area in diabetic foot ulcers: a reproducibility study. J Wound Care. 2020;29(9):518–524. doi: 10.12968/jowc.2020.29.9.518. [DOI] [PubMed] [Google Scholar]
- 13.Aagaard TV, Moeini S, Skou ST, Madsen UR, Brorson S. Benefits and harms of exercise therapy for patients with diabetic foot ulcers: a systematic review. Int J Low Extrem Wounds 2020;15:34734620954066. [DOI] [PubMed]
- 14.Lazo-Porras M, Bernabe-Ortiz A, Taype-Rondan A, Gilman RH, Malaga G, Manrique H, Neyra L, Calderon J, Pinto M, Armstrong DG, Montori VM, Miranda JJ. Foot thermometry with mHeath-based supplementation to prevent diabetic foot ulcers: a randomized controlled trial. Wellcome Open Res. 2020;5(23):23. doi: 10.12688/wellcomeopenres.15531.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):2085. doi: 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gary Sibbald R, Woo KY. The biology of chronic foot ulcers in persons with diabetes. Diabetes Metab Res Rev. 2008;24(S1):S25–S30. doi: 10.1002/dmrr.847. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien TD. Impaired dermal microvascular reactivity and implications for diabetic wound formation and healing: an evidence review. J Wound Care. 2020;29(Sup9):S21–SS8. doi: 10.12968/jowc.2020.29.Sup9.S21. [DOI] [PubMed] [Google Scholar]
- 18.Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T. Basics in nutrition and wound healing. Nutrition. 2010;26(9):862–866. doi: 10.1016/j.nut.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Bishop A, Witts S, Martin T. The role of nutrition in successful wound healing. J Community Nurs. 2018;32(4):44–50. [Google Scholar]
- 20.Bereznicki L. Factors affecting wound healing. Aust Pharm. 2012;31(6):484. [Google Scholar]
- 21.Zubair M, Malik A, Meerza D, Ahmad J. 25-Hydroxyvitamin D [25 (OH) D] levels and diabetic foot ulcer: is there any relationship? Diabetes Metab Syndr Clin Res Rev. 2013;7(3):148–153. doi: 10.1016/j.dsx.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez Morán M, Guerrero-Romero F. Low serum magnesium levels and foot ulcers in subjects with type 2 diabetes. Arch Med Res. 2001;32(4):300–303. doi: 10.1016/s0188-4409(01)00298-3. [DOI] [PubMed] [Google Scholar]
- 23.Greenhagen RM, Frykberg RG, Wukich DK. Serum Vitam D Diabet Foot Complicat. 2019;10(1):1579631. doi: 10.1080/2000625X.2019.1579631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caglar S, Caglar A, Pilten S, Albay G, Beytemur O, Sari H. Osteoprotegerin and 25-hydroxy vitamin D levels in patients with diabetic foot. Eklem Hastaliklari Ve Cerrahisi-Joint Diseases and Related Surgery. 2018;29(3):170–175. doi: 10.5606/ehc.2018.60797. [DOI] [PubMed] [Google Scholar]
- 25.Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chem Biol Interact. 2014;219:101–112. doi: 10.1016/j.cbi.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Chokesuwattanaskul S, Sukpat S, Duangpatra J, Buppajarntham S, Decharatanachart P, Mutirangura A, Patumraj S. High dose oral vitamin C and mesenchymal stem cells aid wound healing in a diabetic mouse model. J Wound Care. 2018;27(5):334–339. doi: 10.12968/jowc.2018.27.5.334. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Kandhare AD, Mukherjee AA, Bodhankar SL. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: role of TGF-ss/Smads and Ang-1/Tie-2 signaling pathways. Nutrients. 2018;17:399–419. doi: 10.17179/excli2018-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, He T, Fu AD, Mao ZJ, Yi L, Tang S, Yang J. Hesperidin enhances angiogenesis via modulating expression of growth and inflammatory factor in diabetic foot ulcer in rats. Eur J Inflamm. 2018;16:205873921877525. [Google Scholar]
- 29.Yuan YF, Das SK, Li MQ. Vitamin D ameliorates impaired wound healing in streptozotocin-induced diabetic mice by suppressing NF-kappa B-mediated inflammatory genes. Biosci Rep 2018;38:BSR20171294. [DOI] [PMC free article] [PubMed] [Retracted]
- 30.Armstrong DG, Hanft J, Driver V, Smith A, Lazaro-Martinez J, Reyzelman A, et al. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabet Med. 2014;31(9):1069–1077. doi: 10.1111/dme.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Momen-Heravi M, Barahimi E, Razzaghi R, Bahmani F, Gilasi HR, Asemi Z. The effects of zinc supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Wound Repair Regen. 2017;25(3):512–520. doi: 10.1111/wrr.12537. [DOI] [PubMed] [Google Scholar]
- 32.Maktabi M, Jamilian M, Amirani E, Chamani M, Asemi Z. The effects of magnesium and vitamin E co-supplementation on parameters of glucose homeostasis and lipid profiles in patients with gestational diabetes. Lipids Health Dis. 2018;17(1):163. doi: 10.1186/s12944-018-0814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohseni S, Bayani M, Bahmani F, Tajabadi-Ebrahimi M, Bayani MA, Jafari P, Asemi Z. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Diabetes Metab Res Rev. 2018;34(3):e2970. doi: 10.1002/dmrr.2970. [DOI] [PubMed] [Google Scholar]
- 34.Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, Asemi Z. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complicat. 2017;31(4):766–772. doi: 10.1016/j.jdiacomp.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Razzaghi R, Pidar F, Momen-Heravi M, Bahmani F, Akbari H, Asemi Z. Magnesium supplementation and the effects on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2018;181(2):207–215. doi: 10.1007/s12011-017-1056-5. [DOI] [PubMed] [Google Scholar]
- 36.Masoum PS, Bagheri M, Borhani HA, Novitsky Y, Sadeghi B, Gharib DF, et al. Effect of ANGIPARS™, a new herbal drug on diabetic foot ulcer: a phase 2 clinical study. 2008;16:33-41.
- 37.Tam JCW, Ko CH, Lau KM, To MH. Kwok HF, Chan YW, et al. A Chinese 2-herb formula (NF3) promotes hindlimb ischemia-induced neovascularization and wound healing of diabetic rats. J Diabetes Complicat. 2014;28(4):436–447. doi: 10.1016/j.jdiacomp.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Eo H, Lee HJ, Lim Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem Biophys Res Commun. 2016;478(3):1021–1027. doi: 10.1016/j.bbrc.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 39.Wan Y, Yang YJ, Li YS, Li XJ, Zhang W, Liu M, Tang HB. Effects of san-huang-sheng-fu oil on peripheral circulatory disorders and foot ulcers in diabetic rats and the mechanisms. Zhonghua shao shang za zhi = Zhonghua shaoshang zazhi = Chin J Burns. 2016;32(3):168–175. doi: 10.3760/cma.j.issn.1009-2587.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Zhang XN, Ma ZJ, Wang Y, Sun B, Guo X, Pan CQ, et al. Angelica Dahurica ethanolic extract improves impaired wound healing by activating angiogenesis in diabetes. Plos One. 2017;12(5):e0177862. [DOI] [PMC free article] [PubMed]
- 41.Ye J, Mani R. A systematic review and meta-analysis of nutritional supplementation in chronic lower extremity wounds. Int J Low Extrem Wounds. 2016;15(4):296–302. doi: 10.1177/1534734616674624. [DOI] [PubMed] [Google Scholar]
- 42.Maier HM, Ilich JZ, Kim J-S, Spicer MT. Nutrition supplementation for diabetic wound healing: a systematic review of current literature. Skinmed. 2013;11(4):217–224. [PubMed] [Google Scholar]
- 43.Shuo C, Jianwei M, Limei X, Tianhui N, Jing D, Wenjun L, Qi H. Safety and effectiveness of traditional Chinese medicinal herbs for diabetic foot: a systematic review and meta-analysis. J Tradit Chin Med. 2017;37(6):735–745. [PubMed] [Google Scholar]
- 44.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, Fergusson D, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999;20(5):448–452. doi: 10.1016/s0197-2456(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 46.Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute: Ottawa; 2011. [Google Scholar]
- 47.Hooijmans CR, Rovers MM, De Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1):43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin Y, Park TW, Kim JW, Jung MJ, Park HB. The effect of nerve block alone or with zinc on wound healing in a rat model of diabetic foot ulcer. 2017;28(7):3222–7.
- 49.Raynaud-Simon A, Belabed L, Le Naour G, Marc J, Capron F, Cynober L, Darquy S. Arginine plus proline supplementation elicits metabolic adaptation that favors wound healing in diabetic rats. Am J Phys Regul Integr Comp Phys. 2012;303(10):R1053–R1061. doi: 10.1152/ajpregu.00003.2012. [DOI] [PubMed] [Google Scholar]
- 50.Park NY, Lim Y. Short term supplementation of dietary antioxidants selectively regulates the inflammatory responses during early cutaneous wound healing in diabetic mice. Nutr Metab (Lond) 2011;8(1):80. doi: 10.1186/1743-7075-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau TW, Sahota DS, Lau CH, Chan CM, Lam FC, Ho YY, Fung KP, Lau CBS, Leung PC. An in vivo investigation on the wound-healing effect of two medicinal herbs using an animal model with foot ulcer. Eur Surg Res. 2008;41(1):15–23. doi: 10.1159/000122834. [DOI] [PubMed] [Google Scholar]
- 52.Daburkar M, Lohar V, Rathore AS, Bhutada P, Tangadpaliwar S. An in vivo and in vitro investigation of the effect of Aloe vera gel ethanolic extract using animal model with diabetic foot ulcer. J Pharm Bioallied Sci. 2014;6(3):205–212. doi: 10.4103/0975-7406.135248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daburkar M, Lohar V, Rathore AS, Bhutada P, Tangadpaliwar S. An in vivo and in vitro investigation of the effect of Aloe vera gel ethanolic extract using animal model with diabetic foot ulcer. J Pharm Bioallied Sci. 2014;2(3):e26961. [DOI] [PMC free article] [PubMed]
- 54.Momen-Heravi M, Barahimi E, Razzaghi R, Bahmani F, Gilasi HR, Asemi Z. The effects of zinc supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Wound Repair Regen. 2017;25(3):512–520. doi: 10.1111/wrr.12537. [DOI] [PubMed] [Google Scholar]
- 55.Mohseni S, Bayani M, Bahmani F, Tajabadi-Ebrahimi M, Bayani MA, Jafari P, et al. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. 2018;34(3):e2970. [DOI] [PubMed]
- 56.Afzali H, Jafari Kashi AH, Momen-Heravi M, Razzaghi R, Amirani E, Bahmani F, et al. The effects of magnesium and vitamin E co-supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2019:27(3):277–84. [DOI] [PubMed]
- 57.Belcaro G, Cesarone M, Errichi B, Ledda A, Di Renzo A, Stuard S, et al. Diabetic ulcers: microcirculatory improvement and faster healing with pycnogenol. Clin Appl Thromb Hemost. 2006;12(3):318–323. doi: 10.1177/1076029606290133. [DOI] [PubMed] [Google Scholar]
- 58.Bolajokol EB, Akinosui OM, Anetor JI, Mossanda KS. Micronutrient status and its effect on glycaemic indices in type 2 diabetics with foot ulcer in Ibadan, Nigeria. Afr J Med Med Sci. 2016;45(1):83–90. [PubMed] [Google Scholar]
- 59.Zubair M, Malik A, Meerza D, Ahmad J. 25-hydroxyvitamiv D [25(OH)D] levels and diabetic foot ulcer: is there any relationship? Diabetes. 2013;62:A171–A1A2. doi: 10.1016/j.dsx.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Singh SK, Sahay RK, Krishna A. Oxidative stress in diabetic foot ulcer. Diabetes Metab Syndr Clin Res Rev. 2008;2(2):109–113. [Google Scholar]
- 61.Feldkamp J, Jungheim K, Schott M, Jacobs B, Roden M. Severe vitamin D3 deficiency in the majority of patients with diabetic foot ulcers. Horm Metab Res. 2018;50(08):615–619. doi: 10.1055/a-0648-8178. [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez-Morán M, Guerrero-Romero F. Low serum magnesium levels and foot ulcers in subjects with type 2 diabetes. Arch Med Res. 2001;32(4):300–3. [DOI] [PubMed]
- 63.Şakirzgurk, Kırım S, Karaca A, Saler T. Low serum magnesium levels and diabetic foot ulcers. PaK J Med Sci. 2013;29(6):1329. [DOI] [PMC free article] [PubMed]
- 64.Neyens J, Cereda E, Meijer E, Lindholm C, Schols J. Arginine-enriched oral nutritional supplementation in the treatment of pressure ulcers: a literature review. Wound Med. 2017;16:46–51. [Google Scholar]
- 65.Gündoğdu RH, Temel H, Bozkırlı BO, Ersoy E, Yazgan A, Yıldırım Z. Mixture of arginine, glutamine, and β-hydroxy-β-methyl butyrate enhances the healing of ischemic wounds in rats. J Parenter Enter Nutr. 2017;41(6):1045–1050. doi: 10.1177/0148607115625221. [DOI] [PubMed] [Google Scholar]
- 66.Dai J, Jiang C, Chen H, Chai Y. Vitamin D and diabetic foot ulcer: a systematic review and meta-analysis. Nutr Diabetes. 2019;9(1):1–6. doi: 10.1038/s41387-019-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macido A. Diabetic foot ulcers and vitamin D status: a literature review. SAGE Open Nurs. 2018;4:2377960818789027. doi: 10.1177/2377960818789027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang S-S, Tang Z-Y, Fang P, Qian H-J, Xu L, Ning G. Nutritional status deteriorates as the severity of diabetic foot ulcers increases and independently associates with prognosis. Exp Ther Med. 2013;5(1):215–222. doi: 10.3892/etm.2012.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shahin ES, Meijers J, Schols J, Tannen A, Halfens R, Dassen T. The relationship between malnutrition parameters and pressure ulcers in hospitals and nursing homes. Nutrition. 2010;26(9):886–889. doi: 10.1016/j.nut.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Pena G, Kuang B, Cowled P, Howell S, Dawson J, Philpot R, Fitridge R. Micronutrient status in diabetic patients with foot ulcers. Adv Wound Care. 2020;9(1):9–15. doi: 10.1089/wound.2019.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Majumder P, Paridhavi M. A novel PolyHerbal formulation hastens diabetic wound healing with potent antioxidant potential. 2019;11:324–31.
- 72.ACdO G, Costa TF, ZdA A, ARAP M. Wound healing-a literature review. An Bras Dermatol. 2016;91(5):614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maier HM. Nutritional status and the relationship of dietary and serum advanced Glycation end-products with inflammation, oxidative stress and healing of diabetic foot ulcers. Thesis. 2013.
- 74.Hussan F, Yahaya MF, Teoh SL, Das S. Herbs for effective treatment of diabetes mellitus wounds: medicinal chemistry and future therapeutic options. Mini-Rev Med Chem. 2018;18(8):697–710. doi: 10.2174/1389557517666170927155707. [DOI] [PubMed] [Google Scholar]
- 75.Shaikh AK, Suryakar AN. Oxidative stress and antioxidant status before and after supplementation of AZ anti-oxidant tablets in coronary artery disease. 2009;20(2):136–40.
- 76.Hawksworth JS, Stojadinovic A, Gage FA, Tadaki DK, Perdue PW, Forsberg J, Davis TA, Dunne JR, Denobile JW, Brown TS, Elster EA. Inflammatory biomarkers in combat wound healing. Ann Surg. 2009;250(6):1002–1007. doi: 10.1097/sla.0b013e3181b248d9. [DOI] [PubMed] [Google Scholar]
- 77.Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PloS One. 2015;10(11):e0141770. [DOI] [PMC free article] [PubMed]
- 78.Tiwari S, Pratyush DD, Gupta SK, Singh SK. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr. 2014;112(12):1938–1943. doi: 10.1017/S0007114514003018. [DOI] [PubMed] [Google Scholar]