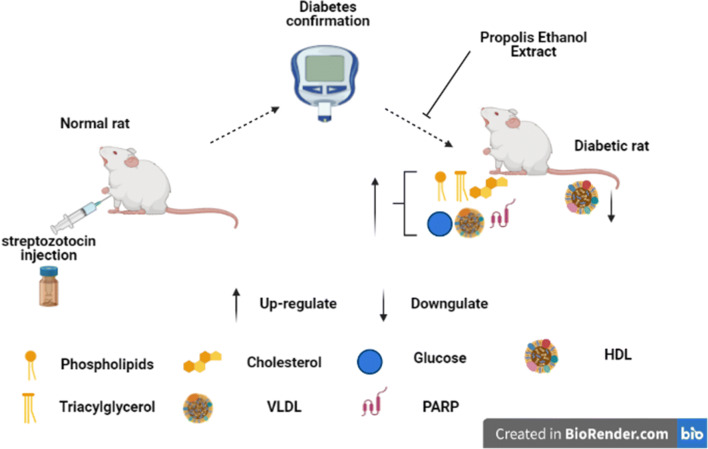
Abstract
Background and aim
Diabetes is a major cause of death worldwide and currently available allopathic drugs presents adverse side effects, thus, necessitating a continuous screening for natural products. This study therefore investigated the effects of Propolis Ethanol Extract (PEE) on blood sugar, lipid metabolism, and poly-(ADP)-ribose polymerase (PARPs) protein level of diabetic male Wistar rats.
Methodology
Seventy rats weighing between (150–180) g used in this study were randomized into seven (7) groups as follows: group 1 (Normal control given Olive oil), group 2 (Diabetic control given Olive oil), group 3 [Diabetic + PEE (200 mg/kg)], group 4 [Diabetic + (PEE 600 mg/kg)], group 5 [Diabetic + Glibenclamide (10 mg/kg)], group 6 [Normal + PEE (200 mg/kg)], and group 7 [Normal + PEE (600 mg/kg)]. Diabetes was induced by a single intraperitoneal injection of streptozotocin (65 mg/kg in 0.1 M citrate buffer pH 4.5), while the vehicle and PEE were orally administered once daily. Treatment with PEE commenced after the confirmation of diabetes. Five rats from each group were sacrificed after the third and sixth weeks of PEE treatment.
Results
Administration of PEE significantly (P < 0.05) lowered the elevated fasting blood sugar, improves body weight, and abated lipotoxicity in the brain, heart, liver and kidney of the treated groups in a dose- and duration-dependent manners. The increased protein level of PARPs and lowered hydroxyl methyl-glutaryl CoA reductase activity were significantly reversed after PEE treatment.
Conclusions
This study concludes that PEE might be a suitable and viable regimen against diabetic complications in rats.
Graphical abstract
Keywords: Propolis, PARPs, Hyperlipidemia, Hyperglycemia, Antioxidant, Diabetes
Introduction
Diabetes mellitus, a complex metabolic disorder is hallmarked by defective insulin action and or secretion culminating to aberrant metabolism of lipids, proteins, and carbohydrates [1]. Several millions of people in the developed world are currently diabetic while about 1 % of the world population are reported be affected by diabetes [2, 3]. Diabetes, as a major risk factor for cardiovascular diseases has been linked to premature death in many countries [4]. Organ failures associated with hyperglycemia and dyslipidemia in the diabetics might be responsible for the ever-increasing mortality [3]. Sustained hyperglycemia as seen in diabetic condition causes glycosylation of cellular proteins such as collagen, causing formation of extracellular matrix and plaques on the arterial walls thereby, causing endothelial dysfunction which contributes to atherosclerosis. Atherosclerosis and dyslipidemia are major risk factors for cardio-cerebrovascular diseases which correlates positively, and have 97 % prevalence in diabetics [3, 5]. Hyperglycemia also aggravates oxidative and nitrosative stress which might activate poly (ADP) ribose polymerase, a nuclear enzyme known to exacerbate endothelial dysfunction and worsening diabetic complications such as cardiomyopathy, neuropathy, nephropathy, and retinopathy [6]. Other roles of PARPs includes cell functioning and viability as well as being involved with cellular reactivity to cytoplasmic stress responses [7]. Due to high prevalence of diabetes, there have been tremendous efforts by scientists concerning identifying blood sugar lowering agents from natural products which are usually considered non-toxic compared with currently available allopathic sources [1].
Propolis, (honey bee glue or hive dross) is a complex resinous material collected by honey bees from buds and exudates of certain plant sources neighbouring its hives with characteristic physico-chemical features which depends largely on geographical location, vegetation, and time of the year during harvest [8, 9]. The chemical compositions of Propolis are mainly flavonoids, aromatic acids and esters, aldehydes and ketones, fatty acids and esters, terpenes, steroids, amino acids, polysaccharides, alcohols, and hydroxyl-benzenes. These metabolites have been reported to possess various biological activities including anticancer, antioxidant, anti-inflammatory, antibiotic, antifungal, anti-hepatotoxic effect, as well as ability to prevent heart diseases and cancer [9, 10]. Ethanol extract of Propolis has been shown to mitigate the elevation of triacylglycerol, low density lipoproteins, and very low density lipoproteins in diabetic rats. This is was suggested to be via regulation of lipogenic enzyme such as hydroxyl-methyl-glutaryl CoA reductase (HMG- CoA reductase) activity and stimulation of reverse cholesterol transport [11]. Considering the disparaging information in the literature about Propolis action on lipid metabolism, and the underplaying molecular mechanism, the present study reports its chemical composition, and a pre-clinical evaluation of the modulatory effects of a South-western Nigerian Propolis on lipid metabolism, and hyperglycaemia in diabetic rats.
Materials and methods
Chemicals and reagents
All chemicals used in this study were of pure and analytical grades available (including streptozotocin), purchased from Sigma Aldrich Co. Ltd (St Louis, United States) while rats Poly (ADP- ribose) polymerase ELISA (cat No. MBS261970) kit was manufactured by MyBioSource, Inc (San Diego, USA). Triacylglycerol and cholesterol kits were products of Randox Laboratories Company (Crumlin, United Kingdom). Glucose oxidase test strips was a product of Lifespan Incorporation (Milpitas, California, USA).
Propolis sample collection
Local Propolis was obtained from honey bee hive at Olorunda, Akobo, Oyo State, South-West Nigeria, stored in an airtight amber bottle and kept at frozen temperature and authenticated at the Department of Forestry and Wildlife Management (FWM), Federal University of Agriculture, Abeokuta. Ogun State, Nigeria.
Preparation of Propolis ethanol extracts (PEE)
Propolis ethanol extract (PEE) was prepared as described by Paviani et al.. [12]. Briefly, 3 g of raw Propolis was dissolved in 10 mL of 95 % ethanol and stirred occasionally for 24 h at room temperature. The insoluble residue was removed by filtration using a Whatman filter paper and the filtrate kept at 4º C. The solvent was evaporated using a rotary evaporator at 60º C to obtain the dried PEE which was reconstituted in Olive oil and used for the study.
Gas chromatography mass spectroscopy (GC-MS) analysis of PEE
GC-MS analysis of PEE was performed using a Fisons GC 8000 gas chromatograph coupled to a Fisons MD 800 mass detector under electron impact ionization (70 eV). Spectra of compounds were collected as they exit a chromatographic column by the mass spectrometer, were then stored on the computer and analyzed. Identification of chemical constituents was accomplished based on mass spectral matching with National Institute of Standards and Technology (NIST 02) and Wiley 275 libraries (≥ 80 % matching) [13], while the retention time was used to compute the Kovat retention index (KI).
Experimental animals
Seventy (70) male albino rats (weighing between 150 and 180 g) used were procured from the Animal breeding Unit of College of Veterinary Medicine, Federal University of Agriculture, Abeokuta (FUNAAB). They were acclimatized for two weeks prior the commencement of the experiment and maintained on standard chow with free access to clean water ad libitum. The study was approved by the Department of Biochemistry Ethical Committee (FUNBCHPG1718-01). The animals were kept in clean metabolic cage and exposed to 12 h day-light cycle at ambient temperature and were handled humanely according to the guidelines on the use of laboratory animals as described by Sherwin et al.. [14].
Induction of diabetes mellitus in rats
Diabetes was induced in rats as described by Srinivasan and Ramarao [15]. A single dose of streptozotocin-citrate buffer solution (65 mg/kg streptozotocin in 0.1 M citrate buffer pH 4.0) was administered intraperitoneally. Blood samples were collected from the rats’ tail vein 72 h- post induction to confirm diabetes using Glucose oxidase reagent test strips (Lifespan Incorporation, Milpitas, California). Rats with fasting blood sugar ≥ 240 mg/dl were considered diabetic and used for the study. The blood glucose and body weight was determined after the 3rd and 6th weeks post-treatment.
Experimental design
After the confirmation of diabetes, diabetic and non- diabetic animals were randomly divided into seven groups of ten animals each using a simple randomisation method. Olive oil (vehicle) and PEE (reconstituted with Olive oil) were orally administered once daily at 0.5 mL/kg body weight.
Group 1- Normal control (vehicle at 0.5 mL/kg body weight)
Group 2- Diabetic control (also received the vehicle)
Group 3- Diabetic + 200 mg /kg body weight PEE
Group 4- Diabetic + 600 mg/kg body weight PEE
Group 5- Diabetic + 10 mg glibenclamide (Standard drug)
Group 6- 200 mg/ kg body weight PEE alone
Group 7- 600 mg /kg body weight PEE alone
Animal sacrifice and sample collection
At the end of 3rd and 6th weeks of treatment with PEE, five (5) animals each were selected from the groups and sacrificed under light diethyl ether after an overnight fasting. Blood samples were collected via cardiac puncture into lithium- heparinised tube and centrifuged at 4000 rpm for 5 min to obtain the plasma. The tissues (brain, heart, liver, and kidney) were excised and the samples were kept at frozen temperature for biochemical assays.
Biochemical analyses
Determination of lipid profiles
Plasma lipoproteins (high- and low – density lipoproteins) were isolated as described by Ugbaja et al.. [16]. Briefly, 200 µL of the plasma was pippeted into a labelled Eppendorf tube. 10 µL Heparin/manganese chloride mixture was added and centrifuged at 3000 rpm for 10 min. The supernatant (HDL) was separated while the pellet (VLDL + LDL) was reconstituted with 200 µL of distilled water [17]. Homogenate (10 %) prepared by homogenising 0.2 g of the organs in 1.8 mL of chloroform/methanol mixture (2:1) was used for the lipid profile assays [18]. Commercially available test kits used to determine cholesterol and triacylglycerol concentrations were product of Randox laboratory Ltd. (United Kingdom). Phospholipid was determined using Stewart [19] method as described by Ugbaja et al.. [16]. Briefly, an aliquot of the plasma/ lipid extract was evaporated and later mixed with 2 mL of ammonium ferrothiocyanate and mixed for 1 min. Absorbance of the sample was read at 488 nm.
Determination of 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase activity
The activity of 3-hydroxy-3-methyl-glutaryl-CoA (HMG CoA) reductase in the liver was determined using the method described by Rao and Ramakrishnan [20] by determining the ratio of HMG CoA: mevalonic acid. Briefly, liver homogenate (10 % w/v) was prepared in saline arsenate solution (1 g/L). Equal volumes of fresh 10 % (w/v) liver homogenate and dilute Perchloric acid (50 ml/L) were mixed, allowed to stand for 5 min and centrifuged at 2000 rpm for 10 min. Then 1 ml of the filtrate was treated with 0.5 ml of freshly prepared hydroxylamine reagent (alkaline hydroxylamine in the case of HMG CoA and neutral hydroxylamine in the case of mevalonate), mixed and after 5 min, 1.5 ml of ferric chloride reagent was added and shaken. The absorbance was read after 10 min at 540 nm versus a similarly treated saline/arsenate blank.
Determination of plasma poly – (ADP ribose) polymerase level
A fivefold serial dilution of the standard reagent was carried out to yield 24 mIU/L, 12 mIU/L, 6 mIU/L, 3 mIU/L, 1.5 mIU/L. 50 µL of standard solutions was added to the well. 50 µL of streptomycin HRP was added to the standard test. 40 ul of the sample was added to separate wells. After this, 10 µL of PARP antibodies and 50 µL of streptavidin were added to the sample. The plate was covered and mixed gently. The plate was incubated for 60 min at 37℃. After incubation, the plate was washed five times with 30x solution. 50 µL of chromogen solution A and 50 µL of chromogen solution B were added to the well. The plate was shake gently and incubated for 10 min at 37℃. 50ul of stop solution was added. There was a colour change from blue to yellow. A blank well is taken as zero; the optical density was measured at 450 nm. Using the standard concentration and the corresponding optical density (OD) values, a linear curve regression equation was obtained. The OD value of the samples was applied to the regression equation to calculate the corresponding sample’s concentration.
Statistical analysis
Data were analysed using the Statistical Package for Social Sciences (SPSS) version 20.0. Results obtained are expressed as mean ± standard error of mean (SEM). Where homogeneity occurred, One-way analysis of variance (ANOVA) followed by Turkey’s test to determine the level of homogeneity. Values with p < 0.05 were considered significant.
Results
Gas chromatography -mass spectroscopy (GCMS) analysis of PEE
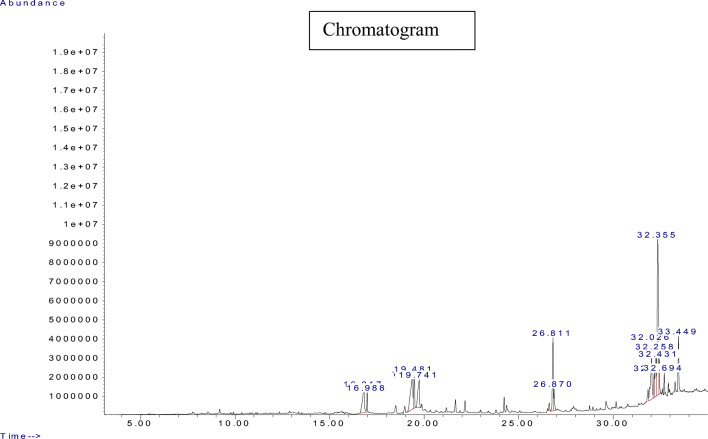
In order to ascertain the different components, present in South -Western Nigeria propolis, the PEE was subjected to GCMS analysis. thirteen distinct peaks (representing 13 components) were observed on the chromatogram revealing a panoply of compounds; ranging from alpha- amyri, 19-Cyclolanost-24-en-3-ol to beta-amyrin; Olean-12-en-3-ol, acetate, 9, 19-Cycloergost-24(28)-en-3-ol, 9, 19-Cyclolanost-24-en-3-ol, taraxasterol, lupeol, Others including hexadecenoic, tridecanoic acid as well as their respective esters were also recorded (Table 1). The chromatogram is shown in Fig. 1.
Table 1.
GCMS analysis of PEE
| PEAK NUMBER | KI VALUE | RETENTION TIME | COMPONENTS | %PEAK AREAS |
|---|---|---|---|---|
| 1. | 1687.80 | 16.814 | n-Hexadecanoic acid | 7.0352 % |
| 2. | 1735.94 | 16.986 | Hexadecanoic acid ethyl ester | 1.917 % |
| 3. | 1991.62 | 19.360 | 9-Octadecanoic acid | 11.142 % |
| 4. | 2081.37 | 19.481 | (E) -9-Octadecanoic acid Ethyl ester | 4.577 % |
| 5. | 2467.53 | 19.741 | Octadecanoic acid | 6.553 % |
| 6. | 2700.26 | 26.811 | (Z)-3-(Heptadec-10-en-1-yl) phenol | 8.924 % |
| 7. | 2992.73 | 32.026 | β-Amyrin | 10.351 % |
| 8. | 3194.45 | 32.161 | β-Amyrone | 3.068 % |
| 9. | 3194.48 | 32.258 | 9,19-Cyclolanost-24-en-3-ol | 7.268 % |
| 10. | 3195.69 | 32.355 | 9,19- Cycloergost- 24(28)-en-3-ol | 25.594 % |
| 11. | 3196.19 | 32.431 | 9,19-Cyclolanost-24-en-3-ol | 3.310 % |
| 12. | 3257.21 | 32.694 | 9,19-Cyclonostan-3- ol | 1.792 % |
| 13. | 3275.70 | 33.449 | Taraxasterol | 5.974 % |
Fig. 1.
Chromatogram of Propolis ethanol extracts (PEE). Peaks are shown as retention time (RT); the identified phyto-constituents are found in Table 1
Effect of PEE on fasting blood glucose concentration and body weight changes
As expected, induction of diabetes with streptozotocin caused a marked (p < 0.05) increase in the fasting blood glucose levels at 3rd and 6th weeks respectively when compared to the normal control (Table 2). Treatment with PEE resulted in significant (p < 0.05) reductions in the elevated blood glucose by the 3rd weeks with 200 and 600 mg/ kg body weight PEE respectively. There was no significant difference (p > 0.05) between the standard drug (Glibenclamide) - treated group and the PEE treated- groups. There were significant (p < 0.05) weight loss in diabetic control (untreated) compared to normal control at the 3rd and 6th week. Interestingly, the extract did not have significant effect (p > 0.05) on the body weight in the diabetic group treated with 200 mg/kg and 600 mg/kg body weight dose compared to the normal control. At both 3 and 6 weeks, a significant increase (p < 0.05) was observed in the body weight of the rats treated with 10 mg/kg glibenclamide compared to the diabetic group (Table 3).
Table 2.
Blood glucose concentration (mg/dl) of animals pre- and post-treatment with PEE
| Groups | Day 0 | Week 3 | Week 6 |
|---|---|---|---|
| Normal control | 85.50 ± 4.52a | 72.75 ± 4.22a | 65.50 ± 4.67a |
| Diabetics control | 342.67 ± 43.10c | 332.67 ± 32.31b | 323.67 ± 30.24d |
| Diabetics + 200 mg/ kg PEE | 254.34 ± 5.61b | 311.00 ± 19.16b | 243.34 ± 4.81c |
| Diabetics + 600 mg/ kg PEE | 241.25 ± 11.52b | 233.50 ± 45.8b | 173.50 ± 24.73c |
| Diabetics + 10 mg/ kg glibenclamide | 255.67 ± 11.18b | 234.67 ± 2.85b | 154.00 ± 5.57b |
| Non-diabetics + 200 mg/ kg PEE | 62.20 ± 5.00a | 65.20 ± 4.45a | 54.20 ± 2.66a |
| Non-diabetic + 600 mg/ kg PEE | 76.60 ± 4.77a | 62.80 ± 1.78a | 46.20 ± 2.04a |
Values are expressed as mean ± SEM (n = 5). Values with different letters along the same column are statistically different (p < 0.05). PEE – Propolis ethanol extracts
Table 3.
Changes in body weight (g) of animals after treatment
| Groups | Week 3 | Week 6 |
|---|---|---|
| Normal control | 33.00 ± 2.04c | 31.00 ± 5.78b |
| Diabetic control | -2.60 ± 4.48b | -28.20 ± 3.55a |
| Diabetic + 200 mg/kg PEE | 3.20 ± 1.06b | -26.40 ± 3.65a |
| Diabetic + 600 mg/kg PEE | -22.85 ± 5.61a | -23.80 ± 3.99a |
| Diabetic + 10 mg/kg glibenclamide | 3.00 ± 2.56b | 4.60 ± 5.08c |
| Non-diabetic + 200 mg/kg PEE | 47.60 ± 2.31d | 22.00 ± 5.14b |
| Non-diabetic + 600 mg/kg PEE | 53.00 ± 3.19e | 26.00 ± 6.96b |
Values are expressed as mean ± SEM (n = 5). Values with different letters along the same column are statistically different (p < 0.05). PEE – Propolis ethanol extracts
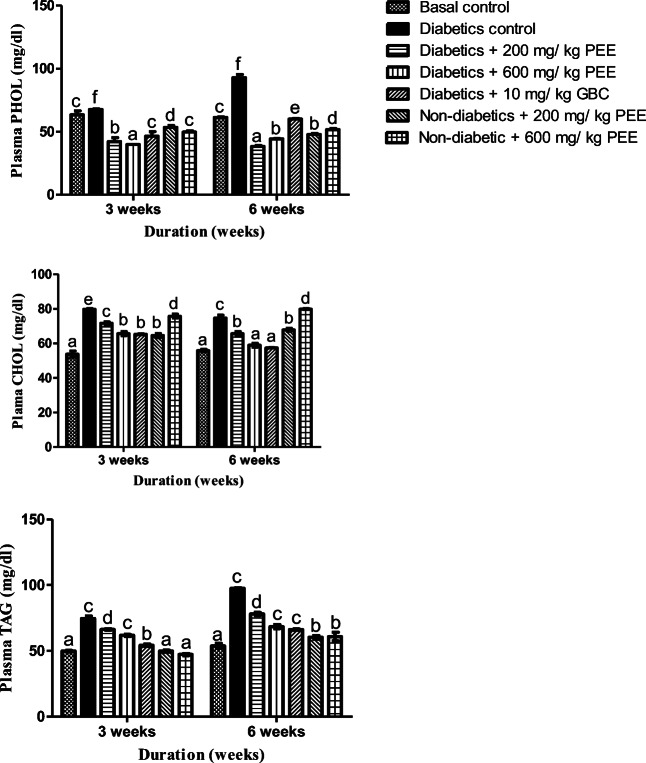
PEE lowered plasma triacylglycerol (TAG), cholesterol (CHOL) and phospholipids (PHOL) in a dose- and duration-dependent manners
Plasma cholesterol increased significantly (p < 0.05) in the untreated diabetic group when compared to the normal control at the 3rd and 6th week respectively. After the treatments however, 200- and 600 mg/kg PEE caused significant (p < 0.05) decrease at 3rd and 6th week when compared to the diabetic untreated group. In the same manner, plasma triacylglycerol (TAG) concentration was increased significantly (p < 0.05) when compared to the normal control group by while plasma phospholipid also increased at the end of week 3 and week 6 respectively. Notwithstanding, the hypertriglyceridemia and hyperphospholipidosis were significantly abated following treatment with 200 and 600 mg /kg PEE at the 3rd week respectively. The reductions were more pronounced by the 6th week when compared to the diabetic untreated group as shown in Fig. 2
Fig. 2.
Effects of PEE on plasma phospholipids (PHOL), cholesterol (CHOL), and triacylglycerol (TAG) levels of diabetic rats. Values are expressed as mean ± SEM (n = 5). Bars with different letters are statistically distinct (p < 0.05). PEE - Propolis ethanol extracts; GBC - Glibenclamide
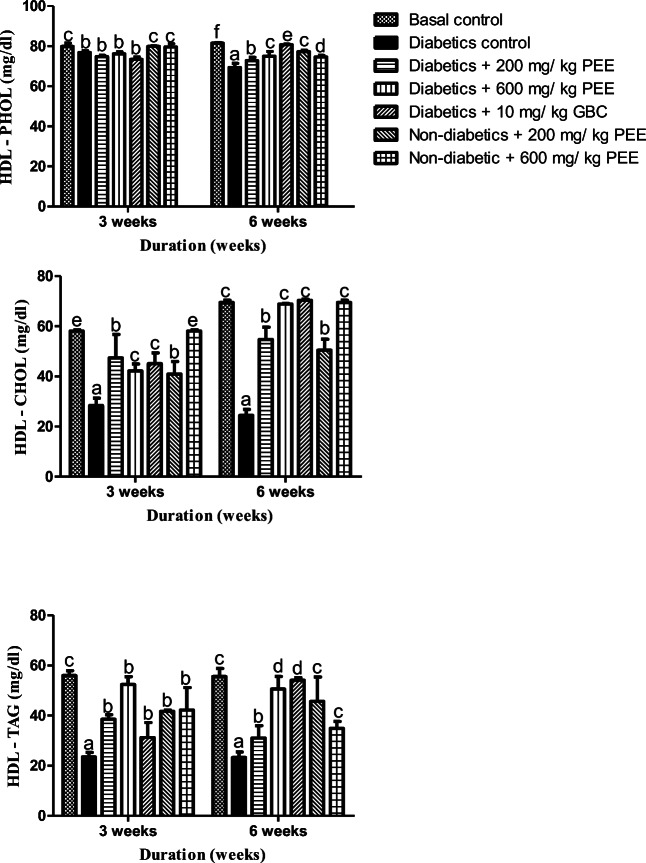
Effect of Propolis ethanol (PEE) extract on high-density lipoprotein (HDL) triacylglycerol (TAG), cholesterol (CHOL) and phospholipids (PHOL) concentration in diabetic rats
To evaluate the effect of Propolis on lipoproteins particles in diabetic rats, cholesterol, triacylglycerol and phospholipids concentrations were estimated in the high-density lipoproteins (Fig. 3). HDL- cholesterol, triacylglycerol and phospholipids were significantly (p < 0.05) reduced significantly (p < 0.05) in the diabetic group at the end of 3rd and 6th week respectively when compared with the normal control group. Nevertheless, administration of PEE significantly (p < 0.05) caused drastic increment in the lipid-carrying HDL particles in varying manners; HDL-CHOL and HDL-TAG increased significantly in a dose-related manner and duration –wise. There was no significant difference in HDL-PHOL at 3rd week however, increments were recorded by the 6th week in 200 and 600 mg /kg PEE groups respectively.
Fig. 3.
Effects of PEE on the high-density lipoprotein (HDL) phospholipids (PHOL) cholesterol (CHOL), and triacylglycerol (TAG) concentrations of diabetic rats. Values are expressed as mean ± SEM (n = 5). Bars with different alphabets are statistically different (p < 0.05). PEE- Propolis ethanol extracts; GBC - Glibenclamide
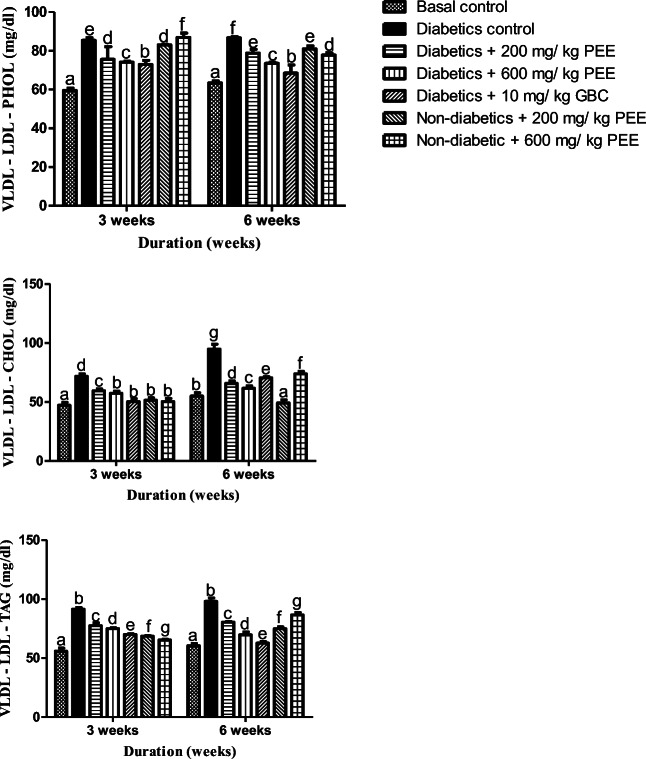
PEE reduces the concentrations of cholesterol, triacylglycerol and phospholipids in very low- density lipoproteins (VLDL-LDL)
The effect of PEE on the concentrations of CHOL, TAG and PHOL in diabetic rats is shown in Fig. 4. VLDL-cholesterol, triacylglycerol and phospholipids concentrations significantly (p < 0.05) in the untreated diabetic groups when compared to the normal control group by at the 3rd and 6th week respectively. However, post-treatment with PEE resulted in significant (p < 0.05) reductions in the elevated parameters in groups treated with 200 and 600 mg/kg PEE at 3rd and 6th week of treatment respectively.
Fig. 4.
Effects of PEE on the very low-density lipoprotein-low density lipoprotein (VLDL-LDL) phospholipids (PHOL), cholesterol (CHOL), and triacylglycerol (TAG) of diabetic rats. Values are expressed as mean ± SEM (n = 5). Bars with different letters are statistically different (p < 0.05). PEE- Propolis ethanol extracts; GBC - Glibenclamide
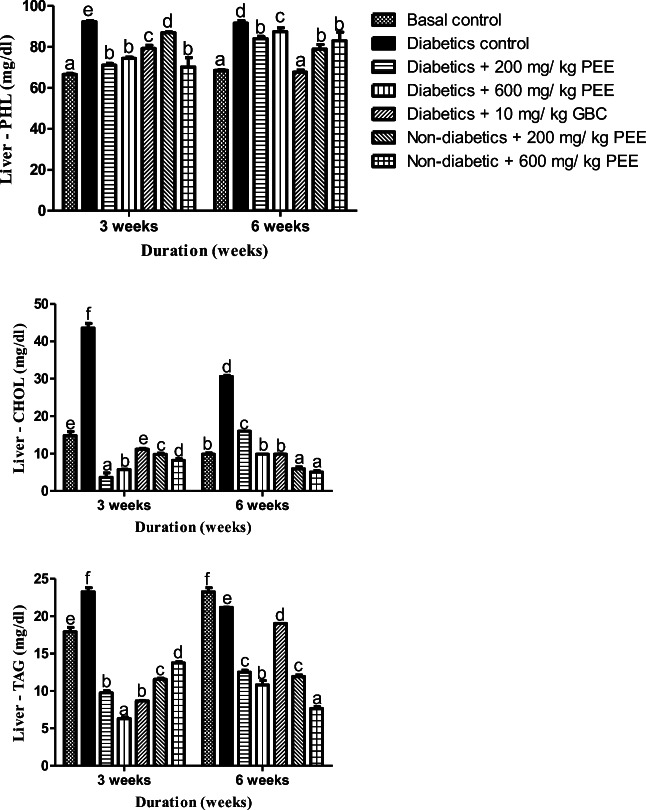
PEE mitigates lipotoxicity in liver of diabetic rats
Lipotoxicity is the accumulation of dangerous lipids in the tissue as a result of aberrant metabolism. Hepatic cholesterol, triacylglycerol, and phospholipids were significantly (p < 0.05) elevated in the diabetic rats by the third the sixth week, an estimated 211. 49 %, 11.18 and 33.73 % increment were recorded when compared with normal group (Fig. 5). Treatment with PEE however lowered these parameters significantly (p < 0.05) by dose- and duration dependently.
Fig. 5.
Effects of PEE on the hepatic phospholipids (PHOL), cholesterol (CHOL), and triacylglycerol (TAG) of diabetic rats. Values are expressed as mean ± SEM (n = 5). Bars with different letters are statistically different (p < 0.05). PEE- Propolis ethanol extracts; GBC - Glibenclamide
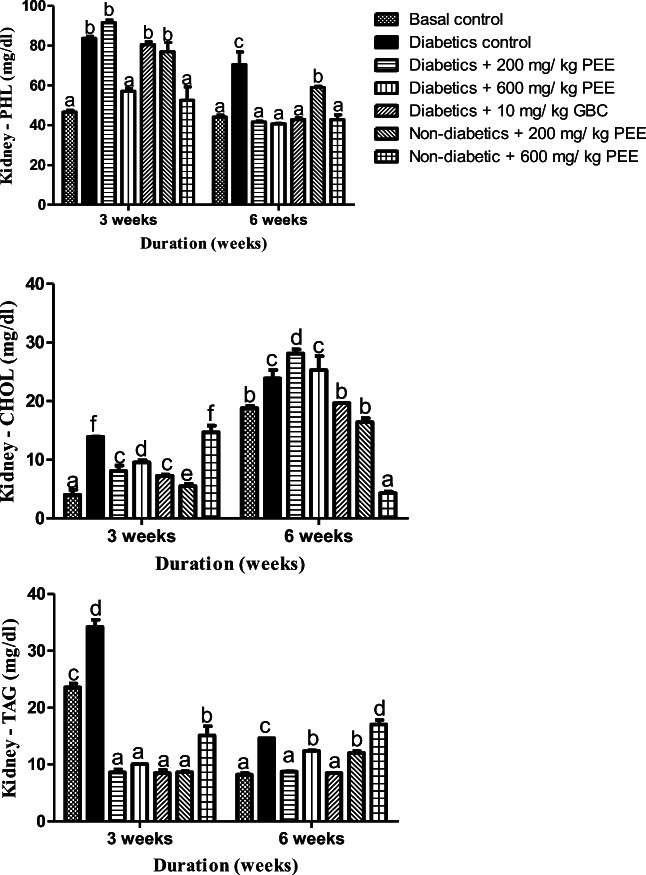
PEE downs lipotoxicity in kidney of diabetic rats
There were significant increases (p < 0.05) in renal cholesterol concentration in the diabetic control group compared to the normal control at week 3 and 6 respectively. After treatment for 3 weeks however, significant decrease were observed in the kidney cholesterol concentration in the treated groups with 200 and 600 mg/kg PEE respectively compared to the diabetic control. But at 6 weeks, significant increase (p < 0.05) was observed in kidney cholesterol concentration (Fig. 6). For TAG levels however, significant increase (p < 0.05) was observed in the diabetic group compared to the normal control. At 3 weeks, significant decrease was observed in the kidney triacylglycerol concentration in the diabetic group treated with 200 mg/kg and 600 mg/kg body weight respectively compared to the diabetic control. But at 6 weeks, significant increase (p < 0.05) was observed in kidney cholesterol concentration in the diabetic group treated with 200 mg/kg and 600 mg/kg body weight respectively compared to the diabetic control. There was a significant increase (p < 0.05) in the kidney phospholipid concentration in the diabetic control group compared to the normal control. At 3 weeks, there was no significant difference (p > 0.05) compared with the diabetic treated groups. At 6 weeks though, there were significant decreases (p < 0.05) in phospholipid concentration in diabetic group treated with 200 mg/kg and 600 mg/kg body weight respectively compared to the diabetic control group.
Fig. 6.
Effects of PEE on the renal phospholipids (PHOL), cholesterol (CHOL), and triacylglycerol (TAG) of diabetic rats. Values are expressed as mean ± SEM (n = 5). Bars with different letters are statistically different (p < 0.05). PEE- Propolis ethanol extracts; GBC - Glibenclamide
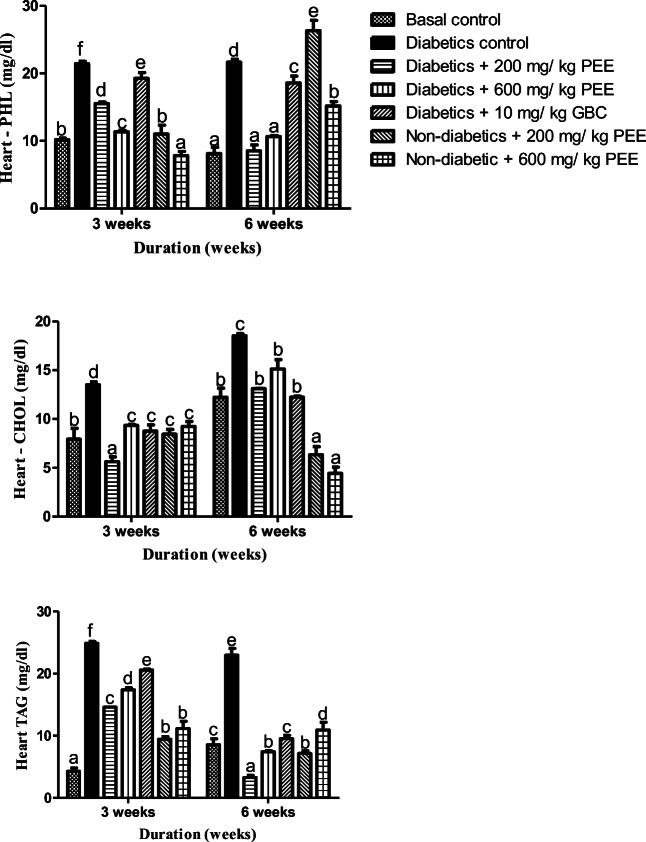
PEEs modulate lipids in the heart and brain of diabetic rats
Figure 7 shows the effect of ethanol extract of Propolis on heart cholesterol, triacylglycerol and phospholipids concentrations. After 3 and 6 weeks respectively, there were significant (p < 0.05) increases in the heart cholesterol, triacylglycerol concentration and phospholipid in the diabetic control when compare to normal group. On treatment, there were significant (p < 0.05) decrease in cholesterol levels while triacylglycerol and phospholipids decreased in groups treated with 200 mg/kg and 600 mg/kg body weight PEE compared to the diabetic control by weeks 3. Significant decrease (p < 0.05) were however observed in the cholesterol concentration levels, triacylglycerol as well as phospholipids in diabetic group treated with 200 mg/kg and 600 mg/kg body weight PEE respectively when compared to the diabetic control at week 6.
Fig. 7.
Effects of PEE on the brain phospholipids (PHOL), cholesterol (CHOL), and triacylglycerol (TAG) of diabetic rats. Values are expressed as mean ± SEM (n = 5). Bars with different letters are statistically different (p < 0.05). PEE- Propolis ethanol extracts; GBC - Glibenclamide
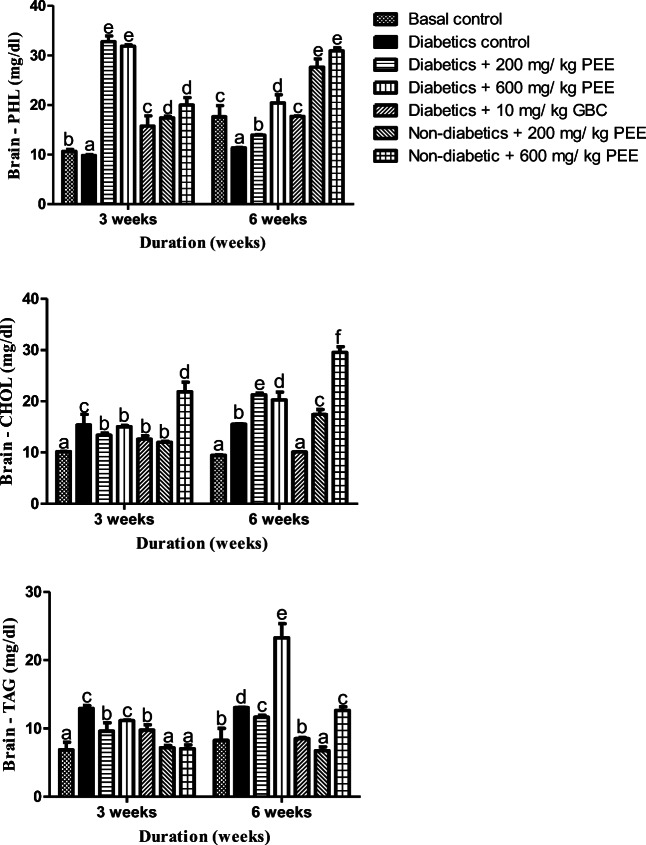
In the rats’ brain, an increase of cholesterol and TAG was observed parallel with decrease in phospholipids in the untreated diabetic groups at the 3rd week as shown in Fig. 8. Treatment with 200 and 600 mg/ kg body weight Propolis ethanol caused a significant (p < 0.05) decrease in cholesterol level, and triacylglycerol level while phospholipids also reduced by the third week of treatment. At 6 weeks however, only 200 mg/kg body weight. Propolis ethanol extract significantly decrease (p < 0.05) the brain triacylglycerol concentration while increasing the brain phospholipid concentrations in diabetic rats treated with 200 mg/kg and 600 mg/kg body weight ethanol Propolis extract compared to normal control, while there was significant decrease in cholesterol level as well.
Fig. 8.
Effects of PEE on the heart phospholipids (PHOL), cholesterol (CHOL), and triacylglycerol (TAG) of diabetic rats. Values are expressed as mean ± SEM (n = 5). Bars with different letters are statistically different (p < 0.05). PEE- Propolis ethanol extracts; GBC - Glibenclamide
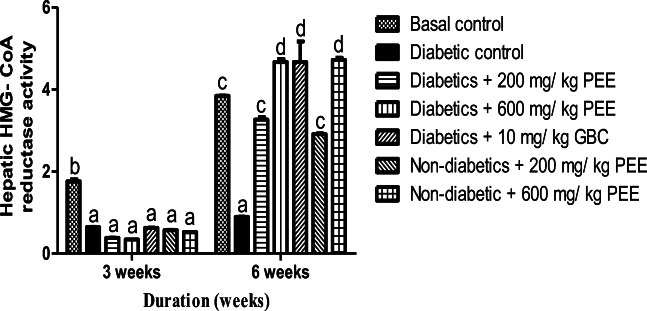
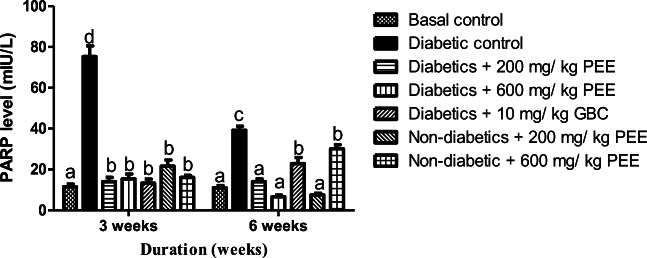
Effect of PEE on hepatic HMG- CoA reductase activity and PARPs level in diabetic rats
Figure 9 shows the effect of Propolis ethanol extract on hepatic HMG-CoA to mevalonate ratio. The ratio of HMG-CoA to Mevalonate in the liver is inversely proportional to the activity of the enzyme, HMG-CoA reductase. A significant decrease (p < 0.05) was observed in HMG-CoA to mevalonate ratio in diabetic control group compared to normal control at 3 and 6 weeks respectively. At 6 weeks, 200 mg/kg and 600 mg/kg doses significantly increase HMG-CoA to mevalonate ratio in the diabetic treated group compared to the diabetic control. At 6 weeks, diabetic group treated with 600 mg/kg dose shows the highest cholesterogenesis inhibition. The effect of PEE on PARPs level is shown in Fig. 10. The concentration of poly-(ADP)-ribose polymerase in the plasma was significantly (p < 0.05) increased in diabetic control rats compared to normal control. At 3 and 6 weeks 200 mg/kg and 600 mg/kg doses significantly (p < 0.05) decreased high concentration of PARPs observed in diabetic treated groups.
Fig. 9.
Effect of PEE on HMG- CoA/ mevalonate ratio in the liver of diabetic rats. Bars represents mean ± SEM (n = 5). Bars with different alphabet are statistically distinct. PEE – Propolis ethanol extract; GBC - Glibenclamide
Fig. 10.
Effects of PEE on plasma Poly (ADP- ribose) polymerase (PARP) level of diabetic rats. Bars with different letters are significantly different (p < 0.05). PEE- Propolis ethanol extract; GBC- glibenclamide
Discussion
Propolis embodies a plethora of biologic and pharmacologic attributes which has been extensively studied in different experimental models, with hundreds of bioactive principles identified in Propolis samples collected from various locations around the world, making it a viable source of therapeutic regimen [21]. The main bioactive substances found in South-western Nigeria Propolis in the present study include α- and β- amyrin, mono- and polyunsaturated fatty acid, phenolic compounds, taraxasterol, lupeol, among others as shown by the GC-MS analysis (Fig. 1). Due to variability in the chemical structures of Propolis from different geographical locations, season of the year, collection methods and other factors, Propolis can exert diverse biological and pharmacological effects such as antioxidant, anti-inflammatory, cytoprotective, anti-cholytic, anti-hyperglycemic and hypolipidemic effects [22]. Glucotoxicity, hyperlipidemia, and lipotoxicity are hallmarks of diabetes and its associated complications such as insulin resistance and cardiovascular diseases [22]. Lipotoxicity refers to an array of aberrant conditions of lipid metabolism including elevated non-esterified fatty acid, increased tissue fat content, altered fat topography and adiposopathy [23]. Hyperglycemia, a major risk factor for microvascular complications might be reduced up to about 25–35 % by a mere 1 % reduction in glycated haemoglobin (HbA1c). Microvascular complications are major causes of mortality due to incidence of stroke and heart attack. Stroke and heart attack are thus, linked to dysmetabolism of lipids, glucose, and proteins, and are important predisposing factors of diabetes [21, 22].
Studies have shown blood lipids and sugar lowering effect of Propolis in rabbits [23] human subjects [11], Mice [24], and rats [25]. Although many of the studies investigated hyperlipidemia and hyperglycemia associated with diabetes, most of these studies failed to investigate lipotoxicity in vital organs leaving a gap in the link between diabetes and accumulation of lipids in these tissues thus failing punctuate the incipient dangers in tissue accumulation of lipids. This present study therefore explored the modulatory effects of PEE on lipotoxicity and dyslipidemia as well as poly (ADP- ribose) polymerase protein level in diabetic male rats.
In the present study, PEE administration to diabetic rats significantly (p < 0.05) lowered the elevated blood glucose levels when compared with the untreated groups in a dose- and duration dependent manner at the end of third and sixth weeks of treatment with 200 and 600 mg/ kg PEE respectively. Interestingly, there was no significant difference between the 600 mg / kg PEE treated group and the glibenclamide- treated group validating the blood sugar lowering effect of Propolis. This lowering effect of propolis on blood sugar has been reported before by Al-Hariri et al. [26] who suggested improved pancreatic function and increased insulin immune-reactive area due to propolis administration. In the same vein, Oršolić et al.. [27] posited possible mechanisms of PEE- mediated improvement in blood sugar level. Firstly, increased in glucose utilization by peripheral tissues; secondly, inhibition of alpha-glucosidase action; and thirdly, increased glucokinase activity and reduction in gluconeogenic enzymes. However, the exact mechanism remains enigmatic. The results obtained in the animal body weight shows significant reductions in weight of diabetic animals when compared to the normal control group. This reduction is interestingly more pronounced in the PEE treated group especially at the third week of administration for both doses of the extract. Oršolić et al.. [27] observed similar trend in body weights of the animals administered with PEE. Reductions in blood sugar level and reduced weight gain suggests a positive physiologic effect of Propolis, especially as this may be an added advantage in mitigating against over-weightiness - a very important risk factor for obesity and type − 2 diabetes (T2D).
Diabetes has been shown to cause damage to several organs of the body including the brain, eyes, heart, liver and kidney due to varying complications arising from glucotoxicity and lipotoxicity [28]. The findings in this study indicate a modulatory role of PEE on diabetic lipotoxic and non-lipotoxic perturbation in tissues’ and plasma lipids homeostasis in the investigated organs (brain, heart, liver, and kidney), as well as lipoproteins particles characterized by dysregulated cholesterol (CHOL), triacylglycerol (TAG) and phospholipids (PHOL) concentrations. Plasma and tissues’ cholesterol, triacylglycerol, and phospholipids were markedly (p < 0.05) elevated as expected in the diabetic untreated group however, PEE showed tremendous abatement effect on these lipidosis in a dose- duration dependent manner for both 200 and 600 mg/ kg PEE treated groups. Reduction of circulating blood lipids is a plausible step in the prevention and management of cardiovascular and associated complications [25]. This reductions in plasma and tissues’ CHOL and TAG levels is consistent with study of Elissa et al. [10], and we report here of the reduction in PHOL as well following PEE administration. The decrease in the level of cholesterol of the blood and organs may be attributed to a reduction in the activities of the enzyme, hepatic 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase (Fig. 9). This is also in accordance with [28]. PEE (600 mg/kg) body weight have the highest HMG-CoA to mevalonate ratio implying an inhibition of endogenous cholesterogenesis.
HDL are lipids-carrying particles whose presence in the cell is important in the protection against cardiovascular disease by avoiding oxidation of low-density lipoproteins (LDL) or neutralizing the atherogenic effects of oxidized LDL in the arterial walls [11]. We demonstrated in this study of the ability of PEE to reduce the concentration of LDL particles with a concomitant increment in the HDL fractions. There were significant reductions in VLDL + LDL- cholesterol, triacylglycerol and phospholipids concentrations in a dose- and duration dependent manners after week 3 and 6 respectively with and opposite effect on the HDL- CHOL, TAG and PHOL. Our observation agrees with the studies of Mustafa et al. [29] and Usman et al. [30] that reported the same trends after treatment with PEE. ATP-binding cassette transporter A1 and G1 (ABCA1 and ABCG1) expressions are associated with cholesterol efflux from peripheral tissues and Propolis has been shown to enhance their expression suggesting that PEE modulate the formation of HDL particles leading to an increase in HDL fractions [11, 31].
PARP, a nuclear protein coordinates several physiological functions including, but not limited to DNA replication, cell division, and cell death [32]. PARPs activation is critical in the pathogenesis of diabetic complications mediated by apoptosis [32, 33]. Furthermore, increased level of PARPs has been linked to oxidative stress and inflammation [32]. Many additional functions of PARPs isoforms in biochemical and molecular signalling have now been demonstrated. According to Obrosova et al. [34], PARPs upregulation plays important role in the pathogenesis of cardiovascular and neurodegenerative diseases. This study further accentuates the role of PARPs in the pathophysiology of diabetes. Increased level of PARPs protein in the plasma of the diabetic rats is consistent with other studies [21, 34], suggesting diabetes-induced organ damage. The mechanism of PARPs activation was proposed to be due to oxidative stress induced by the streptozotocin [21]. However, the main reason for PARP-induced cell death is via depletion of NAD+ and ATP pools [35], which lead to organ failure. PEE attenuated the expression of PARPs significantly (p < 0.05) in the treated group when compared with the untreated animals. We could propose two possible mechanisms for this observation; firstly, Streptozotocin enters into the β-cells via a glucose transporter (GLUT2), where it causes alkylation of DNA molecule and its eventual damage. Pancreatic damage reflects massive activation of PARPs in the islets [36]. But, α and β amyrin, cycloartenol (9,19-Cyclolanost-24-en-3- ol) present in Propolis have been reported to exhibit anti-oxidant, cardiotonic, antihyperlipidemic, and anti-inflammatory effects [37]. We pose that PEE could penetrate the pancreatic B- cell (being lipophilic) and exert its action via its enormous antioxidant ability to mitigate against the streptozotocin- induced oxidative damage to pancreatic DNA leading to improved insulin secretion and actions. Secondly, Propolis or any of its metabolites could act as an inhibitor of PARPs thus, mitigating against the DNA damage and reduced adenine nucleotides pool depletion. On the basis of our findings, attenuation of PARPs level in the PEE-treated group might be indicative its anti-inflammatory effect, as well as suppression of oxidative stress. Furthers studies are needed to evaluate the effect of PEE on expression of key lipogenic enzymes especially those linked with diabetes to ascertain the main receptor for the Propolis.
Conclusion
Taken together, amelioration of hyperglycemia, hyperlipidemia, lipotoxicity, reduction of weight gain and attenuation of hepatic PARPs level are just but few beneficial effects exhibited by Propolis as shown in this study, thus, maybe a source of readily available, cheap and effective regimen against diabetes and its associated complications.
Declarations
Conflict of interest
We declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malini P, Kanchana G, Rajadurai MU. Antibiabetic efficacy of ellagic acid in streptozotocin-induced diabetes mellitus in albino Wistar rats. Asian J Pharm Clin Res. 2011;4(3):124–8. [Google Scholar]
- 2.Stryer L. Biochemistry, editors. New York: W. H. Freeman and company; 2000; pp. 779–780.
- 3.Shankarprasad DS, Gundalli S, Mahantesh B, Kashinakunti SV, Sunitha P. Lipid profile in diabetes mellitus. Indian J Pathol Oncol. 2015;2(4):290–4. doi: 10.5958/2394-6792.2015.00030.7. [DOI] [Google Scholar]
- 4.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Mathews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Spectr. 2012;25(3):154–71. doi: 10.2337/diaspect.25.3.154. [DOI] [PubMed] [Google Scholar]
- 5.Chattanda SP, Mgonda YM. Diabetic dyslipidemia among diabetic patients attending specialized clinics in Dar es Salaam. Tanzan Med J. 2008;23(1):8–11. doi: 10.4314/tmj.v23i1.39221. [DOI] [Google Scholar]
- 6.Pacher P, Szabó C. Role of poly (ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid Redox Signal. 2005;7(11–12):1568–80. doi: 10.1089/ars.2005.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vyas S, Chang P. New PARP targets for cancer therapy. Nat Rev Cancer. 2014;14(7):502–9. doi: 10.1038/nrc3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sayed ES, Abo-Salem OM, Aly HA, Mansour AM. Potential antidiabetic and hypolipidemic effects of Propolis extract in streptozotocin-induced diabetic rats. Pak J Pharm Sci. 2009;22(2):168–74. [PubMed] [Google Scholar]
- 9.Kang LJ, Lee HB, Bae HJ, Lee SG. Antidiabetic effect of propolis: reduction of expression of glucose-6‐phosphatase through inhibition of Y279 and Y216 autophosphorylation of GSK‐3α/β in HepG2 cells. Phytother Res. 2010;24(10):1554–61. doi: 10.1002/ptr.3147. [DOI] [PubMed] [Google Scholar]
- 10.Elissa LA, Elsherbiny NM, Magmomah AO. Propolis restored adiponectin level in type 2 diabetes through PPARγ activation. Egypt J Basic Appl Sci. 2015;2(4):318–26. [Google Scholar]
- 11.Mujica V, Orrego R, Pérez J, Romero P, Ovalle P, Zúñiga-Hernández J, Arredondo M, Leiva E. The role of propolis in oxidative stress and lipid metabolism: a randomized controlled trial. Evid Based Complementary Altern Med. 2017;2017:4272940. [DOI] [PMC free article] [PubMed]
- 12.Paviani LC, Saito E, Dariva C, Marcucci MC, Sánchez-Camargo AP, Cabral FA. Supercritical CO2 extraction of raw propolis and its dry ethanolic extract. Braz J Chem Eng. 2012;29(2):243–51. doi: 10.1590/S0104-66322012000200005. [DOI] [Google Scholar]
- 13.Kartal M, Kaya S, Kurucu S. GC-MS analysis of propolis samples from two different regions of Turkey. Z Naturforsch C. 2002;57(9–10):905–9. doi: 10.1515/znc-2002-9-1025. [DOI] [PubMed] [Google Scholar]
- 14.Sherwin CM, Christiansen SB, Duncan IJ, Erhard HW, Lay DC, Jr, Mench JA, O’connor CE, Petherick JC. Guidelines for the ethical use of animals in applied ethology studies. Appl Anim Behav Sci. 2003;81(3):291–305. doi: 10.1016/S0168-1591(02)00288-5. [DOI] [Google Scholar]
- 15.Srinivasan K, Ramarao P. Animal model in type 2 diabetes research: An overview. Indian J Med Res. 2007;125(3):451. [PubMed] [Google Scholar]
- 16.Ugbaja RN, Onunkwor BO, Omoniyi DA. Lead induced dyslipidemia: The comparative effects of ascorbate and chelation therapy. Afr J Biotechnol. 2013;12(15):1845–1852. doi: 10.5897/AJB2012.2982. [DOI] [Google Scholar]
- 17.Gidez LI, Miller GJ, Burstein M, Slagle S, Eder HA. Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res. 1982;23(8):1206–23. doi: 10.1016/S0022-2275(20)38059-7. [DOI] [PubMed] [Google Scholar]
- 18.Folch J, Lees M, Stanley GS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 19.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104(1):10–4. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 20.Rao AV, Ramakrishnan S. Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin Chem. 1975;21(10):1523–5. doi: 10.1093/clinchem/21.10.1523. [DOI] [PubMed] [Google Scholar]
- 21.Agca CA, Tykhomyrov AA, Baydas G, Nedzvetsky VS. Effects of a propolis extract on the viability of and levels of cytoskeletal and regulatory proteins in rat brain astrocytes: an In Vitro study. Neurophysiology. 2017;49(4):261–71. doi: 10.1007/s11062-017-9680-4. [DOI] [Google Scholar]
- 22.Nogueira AO, Oliveira YI, Adjafre BL, de Moraes ME, Aragao GF. Pharmacological effects of the isomeric mixture of alpha and beta amyrin from Protium heptaphyllum: a literature review. Fundam Clin Pharmacol. 2019;33(1):4–12. doi: 10.1111/fcp.12402. [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270–87. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H, WHO Multinational Study Group Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44(2):14. doi: 10.1007/PL00002934. [DOI] [PubMed] [Google Scholar]
- 25.Nader MA, El-Agamy DS, Suddek GM. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res. 2010;33(4):637–43. doi: 10.1007/s12272-010-0420-1. [DOI] [PubMed] [Google Scholar]
- 26.Al-Hariri MT, Eldin TA, Al-Harb MM. Protective effect and potential mechanisms of propolis on streptozotocin-induced diabetic rats. J Taibah Univ Med Sci. 2016;11(1):7–12. [Google Scholar]
- 27.Oršolić N, Landeka Jurčević I, Đikić D, Rogić D, Odeh D, Balta V, Perak Junaković E, Terzić S, Jutrić D. Effect of propolis on diet-induced hyperlipidemia and atherogenic indices in mice. Antioxidants. 2019;8(6):156. doi: 10.3390/antiox8060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Athesh K, Karthiga D, Brindha P. Anti-obesity effect of aqueous fruit extract of Carica papaya L. in rats fed on high fat cafeteria diet. Int J Pharm Pharm Sci. 2012;4(5):327–30. [Google Scholar]
- 29.Mustafa AH, Eltayeb BI, Ali MA, Shaddad AS, Mohammad HA. Antidiabetic and hypolipidaemic effects of Cicer arientinum seedsextracts in hyperglycemic and diabetic rats. J Pharm Biomed Sci. 2013;30(30):1046–52. [Google Scholar]
- 30.Usman UZ, Bakar AB, Mohamed M. Phytochemical composition and activity against hyperglycaemia of Malaysian propolis in diabetic rats. Biomed Res. 2016;27(1):1–8. [Google Scholar]
- 31.Yu Y, Si Y, Song G, Luo T, Wang J, Qin S. Ethanolic extract of propolis promotes reverse cholesterol transport and the expression of ATP-binding cassette transporter A1 and G1 in mice. Lipids. 2011;46(9):805–11. doi: 10.1007/s11745-011-3568-7. [DOI] [PubMed] [Google Scholar]
- 32.Mohammad G, Siddiqui MM, Abu El-Asrar AM. Poly (ADP-ribose) polymerase mediates diabetes-induced retinal neuropathy. Mediators Inflamm. 2013;2013:510451. [DOI] [PMC free article] [PubMed]
- 33.Zheng L, Szabó C, Kern TS. Poly (ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-κB. Diabetes. 2004;53(11):2960–7. doi: 10.2337/diabetes.53.11.2960. [DOI] [PubMed] [Google Scholar]
- 34.Obrosova IG, Li F, Abatan OI, Forsell MA, Komjáti K, Pacher P, Szabo C, Stevens MJ. Role of poly (ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53(3):711–20. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- 35.Guzyk MM, Tykhomyrov AA, Nedzvetsky VS, Prischepa IV, Grinenko TV, Yanitska LV, Kuchmerovska TM. Poly (ADP-ribose) polymerase-1 (PARP-1) inhibitors reduce reactive gliosis and improve angiostatin levels in retina of diabetic rats. Neurochem Res. 2016;41(10):2526–37. doi: 10.1007/s11064-016-1964-3. [DOI] [PubMed] [Google Scholar]
- 36.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537–46. [PubMed] [Google Scholar]
- 37.Silva-Carvalho R, Baltazar F, Almeida-Aguiar C. Propolis: a complex natural product with a plethora of biological activities that can be explored for drug development. Evid Based Complementary Altern Med. 2015;2015:206439. [DOI] [PMC free article] [PubMed]