Abstract
Background
Evaluating the process of changes in the Metabolic Syndrome (MetS) components over time is one of the ways to study of the MetS natural history. This study aimed to determine the trend of changes in the progression of MetS from its isolated components.
Methods
This longitudinal study was performed on four follow-up periods of the Tehran Lipid and Glucose Study (TLGS) between 1999 and 2015. The research population consisted of 3905 adults over the age of 18 years. MetS was diagnosed based on the Joint Interim Statement (JIS). The considered components were abdominal obesity, hypertension, hyperglycemia, and dyslipidemia.
Results
The highest incidence of MetS from its components was related to hypertension in the short term (3.6-year intervals). In the long run, however, the highest increase in the MetS incidence occurred due to abdominal obesity. Overall, the incidence of MetS increased due to obesity and dyslipidemia, but decreased due to the other factors. Nonetheless, the trend of MetS incidence from all components increased in total. The most common components were dyslipidemia with a decreasing trend and obesity with an increasing trend during the study.
Conclusion
The results indicated that obesity and hypertension components played a more important role in the further development of MetS compared to other components in the Iranian adult population. This necessitates careful and serious attention in preventive and control planning.
Keywords: Metabolic syndrome, Isolated component, Longitudinal development, TLGS
Introduction
Metabolic Syndrome (MetS), as one of the world’s public health challenges, refers to a cluster of metabolic disorders, including central adiposity, hypertension, insulin resistance, and dyslipidemias [1]. These disorders are important predictors of diabetes mellitus, cardiovascular disease, and their mortality as well as all-cause mortality [2, 3]. Evidence has indicated that the risk of diabetes mellitus was five times higher and that of myocardial infarction was three times higher amongst the individuals with MetS compared to healthy people [4]. However, studies on MetS have been mostly limited to the epidemiology, etiology, and prognosis of the disorder [5–7], and there are few studies on the longitudinal changes of its components over time including key information on the pathway of establishment, pathophysiology, and treatment of MetS. In one of these studies, longitudinal changes demonstrated the effect of birth cohort on the prevalence of MetS [8]. In another study using this approach, the relationship between the changes in MetS components over time and the long-term occurrence of MetS was investigated, and the mechanisms of incidence and pathways leading to MetS were reported. Based on the results, long-term changes in the Body Mass Index (BMI) mostly affected the incidence of MetS through hypertension [9]. Investigation of the association between the component changes and the occurrence of complications, such as type 2 diabetes [10, 11], as well as the longitudinal relationship between some biomarkers and the occurrence of MetS components [12] have shown further evidence on the assessment of longitudinal changes in MetS components and their dynamics. In the development process of MetS, components can appear sequentially or simultaneously depending on the individual’s lifestyle. Researchers believe that in this process, the component with highest incidence plays an important role in the progression of MetS [13]. However, discussions in this area are still controversial [14–16]. Exploring the dynamics of MetS components over time can play a significant role in clarifying the pathways of this disorder. In other words, since the components of this disorder occur together as a set, it can be argued that there are common mechanisms for the occurrence of MetS that may be better understood and judged by examining them in a large set. To date, however, there has been a major belief among researchers about the central role of insulin resistance in this process [13, 17]. Considering the progression of MetS from the angle of longitudinal changes of its components over time is a process and pathological view that can play an important role in the evolution of knowledge on how this disorder and similar disorders are established. On the other hand, the dynamics of the incidence and prevalence of MetS components during the time of MetS establishment may contain important information on the interaction of components in forming this disorder, disease prognosis, interventional strategies, and mitigating policies. Therefore, the present study aims to evaluate the longitudinal changes of the MetS components, including hypertension, dyslipidemia (low High Density Lipoprotein (HDL) and high triglycerides), hyperglycemia, and abdominal obesity, and the incidence rate of MetS due to each of the components in the context of a large-population cohort study in order to achieve a clear picture of the role of each component in the process of MetS development.
Methods
Study participants and design
This longitudinal population-based study was done based on the data from four follow-up periods of the Tehran Lipid and Glucose Study (TLGS) whose details have been previously described [18–20]. This study was performed in order to determine the dynamism of the process of changes in the occurrence of MetS from its components among the individuals over the age of 18 years (inclusion criteria). The TLGS study was the first large-population cohort study in Iran designed to determine the prevalence, incidence, changes, and outcomes of the risk factors of chronic non-communicable diseases in 15,005 urban populations over three years of age in Tehran using multi-sampling method since 1999. The sampling phase of this study was conducted between 1997 and 2001 and periodic evaluations in separate phases were followed approximately once in every 3.6 years. The second phase of the study was carried out between 2002 and 2005, the third phase between 2006 and 2008, the fourth phase between 2009 and 2011, and the fifth phase between 2012 and 2015.
Exclusion criteria process
In the first stage of the sampling process, out of a total of 15,005 people, 1532 were eliminated due to the history or the definite diagnosis of diabetes and cardiovascular disease. In the second stage, 4486 people aged 18 years and lower were eliminated and 8987 ones were entered into the next stage. In order to evaluate the trends in a virginal population accurately and purely, 5082 people who had undergone various lifestyle-based interventions were eliminated, as well. Finally, 3905 adults were entered into the process of analysis.
Missing data strategy
After applying the exclusion criteria, depending on the design of the study, missing data values in some variables ranged from 15% to 25% in the follow-up periods, which was expected. Since the missing pattern was Missing At Random (MAR), multiple imputation strategy [21–27] was used to analyze and estimate the missing data using the Markov Chain Monte Carl (MCMC) approach with a maximum of 10 iterations. In the MAR pattern, the probability of a missing value is related only to the observable data and it is possible to estimate the missing values from the observed data. Missing pattern in this case is not completely ‘random’, but it is the most general case where we can ignore the missing mechanism. The final pool estimation was the basis of the analysis.
Measurements and definitions
Joint Interim Statement (JIS) [28] guideline and the cut-off proposed for Waist Circumference (WC) in the Iranian population were used to define MetS in adults [29, 30]. In this context, three out of the five following criteria were needed for diagnosis: (1) abdominal obesity (WC ≥90 cm for both genders), (2) triglycerides ≥150 mg/dL, (3) HDL cholesterol ≤40 mg/dL for males or ≤ 50 mg/dL for females or receiving drugs, (4) systolic/diastolic blood pressure ≥ 130/85 mmHg or receiving medications, and (5) fasting plasma glucose ≥100 mg/dL or receiving drugs.
Interviews using validated questionnaires [31–33] were used to collect demographic data, medical records, and status of MetS components during the follow-up period. Moreover, WC and blood pressure were measured during regular medical examinations in accordance with the existing standards. Blood pressure was measured twice after a five-minute rest in sitting position and the average value was considered. WC was also measured over the umbilicus [34]. All blood tests were performed in fasting state. Glucose oxidase assay was used to measure fasting blood glucose and enzymatic methods were used to measure lipid factors (triglycerides, HDL, and cholesterol). The five components of MetS were central obesity (abdominal obesity), hypertension, hyperglycemia, increased level of triglycerides, and decreased level of HDL. Because the combination of triglycerides elevation and HDL decrease has been introduced as dyslipidemia in the majority of sources, these two components were merged in the current study.
Statistical analysis
Mean and standard deviation were used to describe the quantitative variables, while frequency and percentage were utilized for qualitative variables. For quantitative variables, Kolmogorov-Smirnov test was used to confirm the normality assumption. In addition, chi-square and Cochrane test were used to compare the differences between the two genders and significance of the components trend. First, the prevalence of each component and MetS in each period was calculated. Then, the component-specific incidence rate of MetS was used to investigate the dynamic trend of changes in MetS incidence rates, wherein the net population of each component was considered the denominator and the number of people with MetS from the same component was regarded as the numerator in any period. To calculate the incidence rate, the baseline (F0) was considered the beginning of the period and the first, second, third, and fourth incidences were calculated in F1 (follow-up period 1), F2, F3, and F4, respectively. The incidence rate of MetS was calculated similarly in healthy individuals without metabolic disorders (no components). In order to assess the stability of MetS in patients with MetS over time, the persistent MetS rate index was computed. It should be noted that stability implied staying in the MetS state or disease persistence in different periods. IBM SPSS Statistics for Windows, version 24 (IBM Corp, Armonk, NY) and Microsoft Excel 2016 were used for all these steps, and the level of significance was set at <0.05.
Ethical considerations
This study was performed using the TLGS data and, consequently, it was ethically subject to the ethical considerations taken into account in that project (TLGS). It was independently the result of a research project approved by the National Ethics Committee on Biomedical Research of Iran (code: IR.SUMS.REC.1398.835).
Results
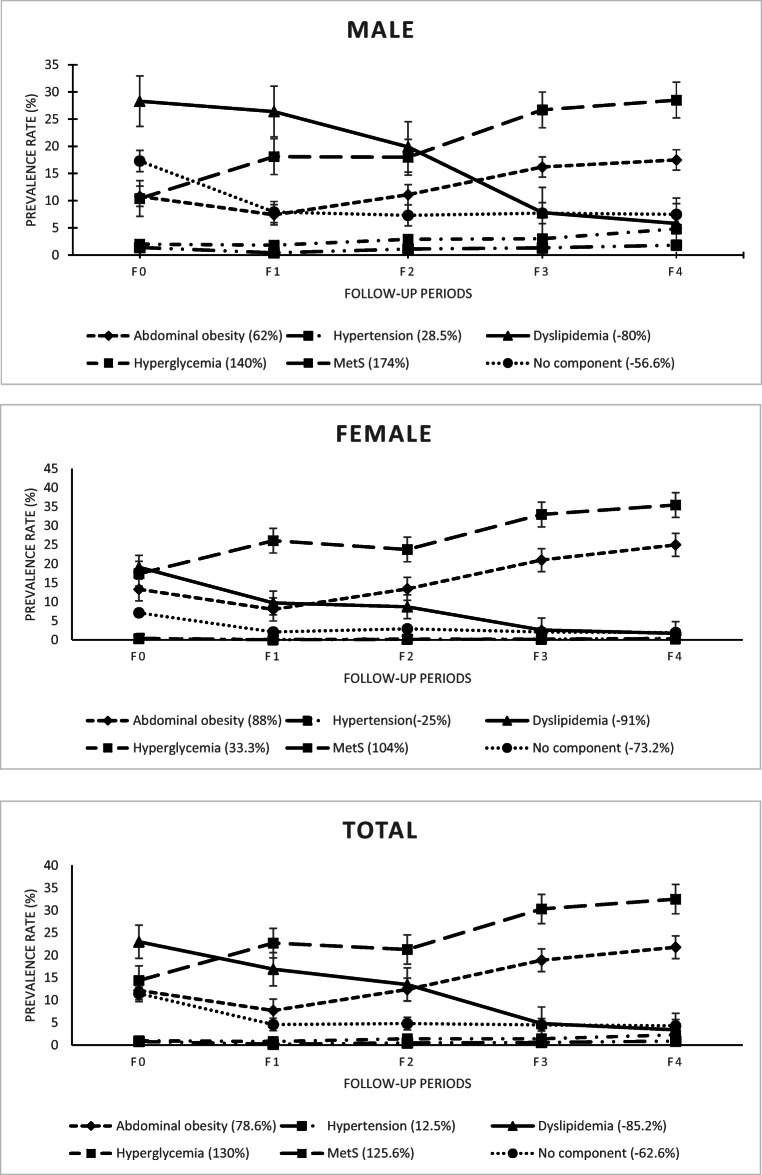
Out of a total of 3905 people, 1681 (43%) were male and 2224 (57%) were female. The median age of the participants was 37 years at the beginning of the study and 50 years at the end. The median age was one year higher for males compared to females. The most common component at the beginning of the study was dyslipidemia in males, females, and in total. However, the most common component was obesity in all three groups (males, females, and in total) in the final period of the study. On the other hand, hypertension was the rarest component in all three groups. Based on the prevalence rates, obesity followed an ascending trend in all three groups, the trend of hypertension was constant and irregular, dyslipidemia followed a descending trend, and the trend of hyperglycemia was initially stable and increasing at the end. Furthermore, the trend of MetS was increasing in all three groups and the trend of the number of healthy people (no components) during the study was relatively constant. The trend of changes in MetS as well as in its components was statistically significant over time. Moreover, there were significant differences between males and females regarding the trend of hypertension in the last three periods, dyslipidemia in all periods, hyperglycemia in the second, fourth, and final periods, obesity in all periods, and MetS in all periods (p < 0.001). Furthermore, the incidence of obesity was higher among females in all periods, while that of hypertension, dyslipidemia, hyperglycemia, and healthy status (no components) was higher amongst males in all periods. However, the prevalence of MetS was higher in females in all periods. Separate information of the samples based on their gender groups in the follow-up periods has been presented in Table 1. In addition, the trend of changes in the middle ranges has been shown in Fig. 1.
Table 1.
The prevalence (%) of components and MetS stratified by gender and follow-up periods
| Components | Follow-up periods | P value | |||||
|---|---|---|---|---|---|---|---|
| F0# (%) | F*1 (%) | F2 (%) | F3 (%) | F4 (%) | |||
| Males | Age (median) | 38 | 41 | 44 | 47 | 51 | – |
| Abdominal obesity | 181 (10.8) | 124 (7.4) | 187 (11.1) | 272 (16.2) | 294 (17.5) | < 0.001& | |
| Hypertension | 23 (1.4) | 7 (0.4) | 18 (1.1) | 22 (1.3) | 30 (1.8) | < 0.001& | |
| Dyslipidemia | 475 (28.3) | 444 (26.4) | 334 (19.9) | 131 (7.8) | 97 (5.8) | < 0.001& | |
| Hyperglycemia | 33 (2) | 30 (1.8) | 48 (2.9) | 51 (3) | 80 (4.8) | < 0.001& | |
| MetS | 175 (10.4) | 305 (18.1) | 303 (18) | 448 (26.7) | 479 (28.5) | < 0.001& | |
| No components | 291 (17.3) | 132 (7.9) | 122 (7.3) | 130 (7.7) | 126 (7.5) | < 0.001& | |
| Females | Age (median) | 37 | 40 | 43 | 46 | 50 | – |
| Abdominal obesity | 296 (13.3) | 177 (8) | 297 (13.4) | 466 (21) | 556 (25) | < 0.001& | |
| Hypertension | 8 (0.4) | 0 | 3 (0.1) | 2 (0.1) | 7 (0.3) | < 0.001& | |
| Dyslipidemia | 424 (19.1) | 216 (9.7) | 194 (8.7) | 57 (2.6) | 37 (1.7) | < 0.001& | |
| Hyperglycemia | 7 (0.3) | 3 (0.1) | 5 (0.2) | 5 (0.2) | 10 (0.4) | < 0.001& | |
| MetS | 386 (17.4) | 581 (26.1) | 529 (23.8) | 735 (33) | 789 (35.5) | < 0.001& | |
| No components | 157 (7.1) | 46 (2.1) | 64 (2.9) | 46 (2.1) | 42 (1.9) | < 0.001& | |
| Total | Age (median) | 37 | 40 | 43 | 47 | 50 | – |
| Abdominal obesity | 477 (12.2) | 301 (7.7) | 484 (12.4) | 738 (18.9) | 850 (21.8) | < 0.001& | |
| Hypertension | 31 (0.8) | 7 (0.2) | 21 (0.5) | 24 (0.6) | 37 (0.9) | < 0.001& | |
| Dyslipidemia | 899 (23) | 660 (16.9) | 528 (13.5) | 188 (4.8) | 134 (3.4) | < 0.001& | |
| Hyperglycemia | 40 (1) | 33 (0.8) | 53 (1.4) | 56 (1.4) | 90 (2.3) | < 0.001& | |
| MetS | 561 (14.4) | 886 (22.7) | 832 (21.3) | 1183 (30.3) | 1268 (32.5) | < 0.001& | |
| No components | 448 (11.5) | 178 (4.6) | 186 (4.8) | 176 (4.5) | 168 (4.3) | < 0.001& | |
#Baseline, *follow-up period, & significant; two-sided p value at 0.05 significance level in Cochrane test.
Fig. 1.
The trend changes in the prevalence rate of MetS and its isolated components by gender
As shown in Fig. 1, the decreasing trend of dyslipidemia in the middle of the study period was slower in males than in females. Overall, however, the rate of decrease in dyslipidemia was higher in females than in males. The progress trend of other components in both male and female groups in the middle of the study period was consistent and relatively the same.
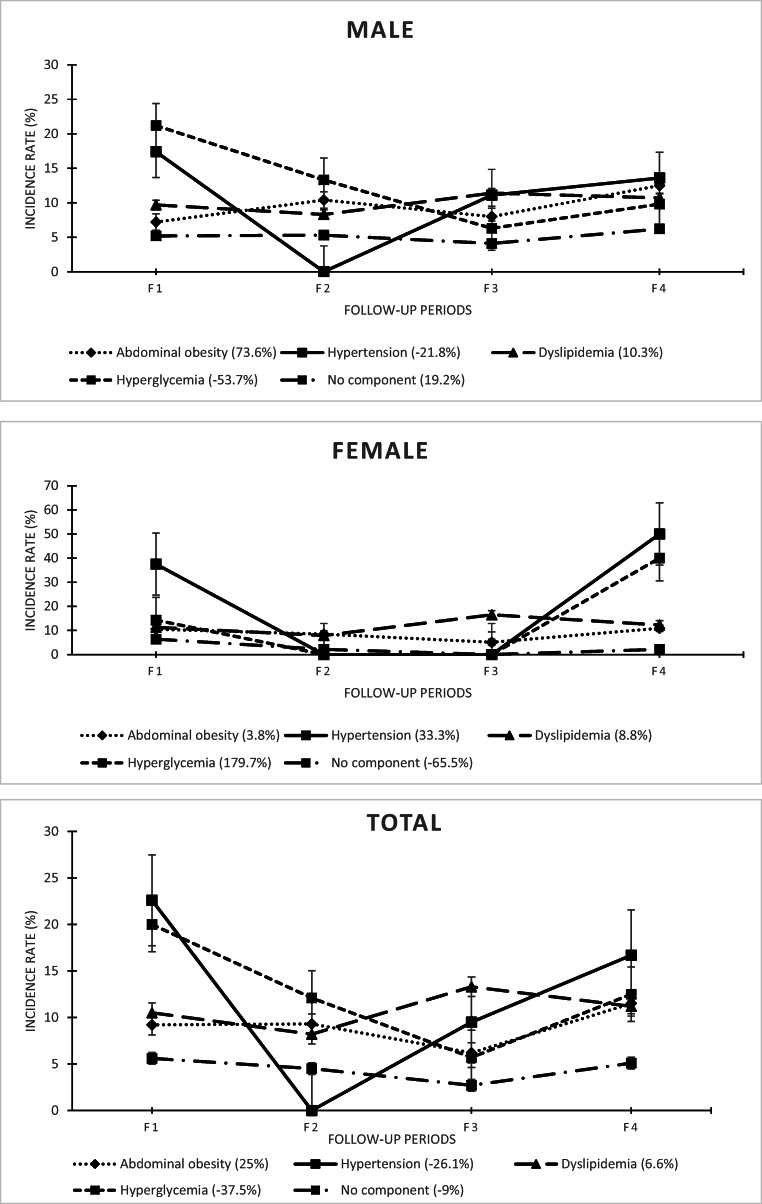
In the first follow-up period, in proportion to the population at risk in each component and period (dynamic at risk population), the highest incidence rate of MetS from the components was related to hyperglycemia, hypertension, and hypertension in males, females, and in total, respectively. In addition, the overall incidence of MetS from the isolated components was calculated to be 8.5% among males in the first period. In the second, third, and fourth follow-up periods, the highest overall incidence rates of MetS in males were related to hyperglycemia, dyslipidemia, and hypertension, respectively. As for females, it was associated with obesity, dyslipidemia, and hypertension. In total also, it was related to hyperglycemia, dyslipidemia, and hypertension.
In the first follow-up period, the lowest rate of MetS in all three groups (males, females, and total) had occurred in the obesity component. In the second, third, and fourth periods, the lowest incidence had respectively occurred in hypertension, hyperglycemia, and hyperglycemia in males and in hypertension and hyperglycemia (concurrently), hypertension, and hyperglycemia and dyslipidemia (concurrently) in females. In total, it had occurred in hypertension, hyperglycemia, and dyslipidemia.
The MetS persistence rate in patients with MetS was generally stable with low fluctuations in the first to fourth follow-up periods. However, the progression declined in females as well as in the total mode. On the other hand, the incidence rate of MetS in healthy people (no components) was higher in males than in females at the end of the study, and only increased among males. The overall incidence of MetS from the isolated components also increased in all three groups, with a superiority among males.
In terms of trend, the trend of the MetS incidence rate from no components, obesity, and dyslipidemia increased in males at the end of the study. The highest increase in the MetS incidence rate was related to obesity. The incidence trend of all components also increased in males. Considering females, the incidence trend in all components, except for no components, increased over time. In this group, the highest increase in the MetS incidence rate was related to hyperglycemia. The incidence trend of all components increased, as well. In the total mode, the results revealed an increase in the incidence from obesity and dyslipidemia, but a decrease in the incidence from other factors. Moreover, the trend of MetS incidence related to all components was increasing. The trend of MetS incidence changes related to all components in all three groups was also significant over time. In terms of gender comparisons, the rate of change in the MetS incidence rate from hyperglycemia and hypertension was inversely proportional; both were higher in females than in males. In other words, the incidence rate of MetS from these two components increased in females, but decreased in males. Nonetheless, progression in other components was consistent in males and females. Among the components with consistent trends, the highest difference between males and females was related to obesity; the rate of increase in the incidence trend was 73.6% in males and 3.8% in females. Among the components with heterogeneous trends, the highest difference between the two genders was related to hyperglycemia; the change rate in the trend was −53.7% in males and 179.7% in females. In the total mode, the highest increase in the MetS incidence was related to obesity. Other details have been presented in Table 2.
Table 2.
Dynamics of the incidence rate (%) of MetS by components, gender, and follow-up periods
| Components | At-risk population | 3.6-yr incidence rate, % (F*1) | At-risk population | 3.6-yr incidence rate, % (F2) | At-risk population | 3.6-yr incidence rate, % (F3) | At-risk population | 3.6-yr incidence rate, % (F4) | Change %& | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count | Rate % | Count | Rate % | Count | Rate % | Count | Rate % | |||||||
| Male | Abdominal obesity to MetS | 181 | 13 | 7.2 | 124 | 13 | 10.4 | 187 | 15 | 8 | 272 | 34 | 12.5 | 73.6 |
| Hypertension to MetS | 23 | 4 | 17.4 | 7 | 0 | 0 | 18 | 2 | 11.1 | 22 | 3 | 13.6 | −21.8 | |
| Dyslipidemia to MetS | 475 | 46 | 9.7 | 444 | 37 | 8.3 | 334 | 38 | 11.4 | 131 | 14 | 10.7 | 10.3 | |
| Hyperglycemia to MetS | 33 | 7 | 21.2 | 30 | 4 | 13.3 | 48 | 3 | 6.3 | 51 | 5 | 9.8 | −53.7 | |
| MetS to MetS | 175 | 92 | 52.6 | 305 | 135 | 44.3 | 303 | 187 | 61.7 | 448 | 254 | 56.7 | 7.7 | |
| No components to MetS | 291 | 15 | 5.2 | 132 | 7 | 5.3 | 122 | 5 | 4.1 | 130 | 8 | 6.2 | 19.2 | |
| Overall | 1003 | 85 | 8.5 | 737.0 | 61.0 | 8.3 | 709.0 | 63.0 | 8.9 | 606.0 | 64.0 | 10.6 | 24.6 | |
| Female | Abdominal obesity to MetS | 296 | 31 | 10.5 | 177 | 15 | 8.5 | 297 | 15 | 5.1 | 466 | 51 | 10.9 | 3.8 |
| Hypertension to MetS | 8 | 3 | 37.5 | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 1 | 50 | 33.3 | |
| Dyslipidemia to MetS | 424 | 48 | 11.3 | 216 | 17 | 7.9 | 194 | 32 | 16.5 | 57 | 7 | 12.3 | 8.8 | |
| Hyperglycemia to MetS | 7 | 1 | 14.3 | 3 | 0 | 0 | 5 | 0 | 0 | 5 | 2 | 40 | 179.7 | |
| MetS to MetS | 386 | 274 | 71 | 581 | 310 | 53.4 | 529 | 385 | 72.8 | 735 | 477 | 64.9 | −8.5 | |
| No components to MetS | 157 | 10 | 6.4 | 46 | 1 | 2.2 | 64 | 0 | 0 | 46 | 1 | 2.2 | −65.6 | |
| Overall | 892.0 | 93.0 | 10.4 | 442.0 | 33.0 | 7.5 | 563.0 | 47.0 | 8.3 | 576.0 | 62.0 | 10.8 | 3.2 | |
| Total | Abdominal obesity to MetS | 477 | 44 | 9.2 | 301 | 28 | 9.3 | 484 | 30 | 6.2 | 738 | 85 | 11.5 | 25 |
| Hypertension to MetS | 31 | 7 | 22.6 | 7 | 0 | 0.0 | 21 | 2 | 9.5 | 24 | 4 | 16.7 | −26.1 | |
| Dyslipidemia to MetS | 899 | 94 | 10.5 | 660 | 54 | 8.2 | 528 | 70 | 13.3 | 188 | 21 | 11.2 | 6.6 | |
| Hyperglycemia to MetS | 40 | 8 | 20.0 | 33 | 4 | 12.1 | 53 | 3 | 5.7 | 56 | 7 | 12.5 | −37.5 | |
| MetS to Mets | 561 | 366 | 65.2 | 886 | 445 | 50.2 | 832 | 572 | 68.8 | 1183 | 731 | 61.8 | −5.2 | |
| No components to MetS | 448 | 25 | 5.6 | 178 | 8 | 4.5 | 186 | 5 | 2.7 | 176 | 9 | 5.1 | −8.9 | |
| Overall | 1895.0 | 178.0 | 9.4 | 1179.0 | 94.0 | 8.0 | 1272.0 | 110.0 | 8.6 | 1182.0 | 126.0 | 10.7 | 13.5 | |
*follow up period, &p value for trend <0.001
The trend of changes in the incidence rates in the middle time intervals (between the first follow-up period and the end) has been depicted in Fig. 2. In all three groups, the incidence rates of MetS from hypertension and hyperglycemia were declining and consistent in the middle of the study. Additionally, the trends of the incidence rates of MetS from obesity and dyslipidemia were against each other in the middle of the study. However, the distance between the incidence values of these two components (obesity and dyslipidemia) increased by moving towards the end of the study. Furthermore, the incidence of MetS from hypertension reached zero in the middle of the study and increased afterwards in all three groups (males, females, and in total).
Fig. 2.
The trend of changes in the incidence rate of MetS from its isolated components by gender
Discussion
In this study, the dynamic behavior of the four components in direct transition to the MetS state was investigated in the form of incidence rates. The results showed that the highest incidence rate of MetS from the components resulted from hypertension in the total mode in the short term (3.6 years). In long-term trends, however, the highest increase in the MetS incidence rate occurred due to obesity. Additionally, the trend of MetS incidence was ascending due to obesity and dyslipidemia and descending from the other factors in the total mode at the end of the study. There was also an increase in the incidence trend of MetS from the set of components. In terms of the prevalence of the components, the most common component was dyslipidemia with a decreasing trend and obesity with an increasing trend during the study.
The present study aimed to determine the MetS incidence rate using a different approach compared to other studies in this field. Moreover, the purpose of determining the incidence rate was to evaluate the incidence trend of MetS from each component rather than calculating the incidence rate as generally done in other studies. Hence, similar studies are very rare and the incidence rate computed in the current study is not comparable to the values reported in other studies. In other words, the incidence rate computed in other studies is not component-specific and is the result of considering all components together without separating the share of each. Considering the occurrence of MetS from the perspective of the component-based natural history, it can be said that the occurrence of this disorder can be calculated both directly from the isolated components and as a whole (all components together). In most studies, there is no evidence of isolated components and the proportion of the isolated components contributing to the MetS incidence is not clear. This is exactly the gap that was tried to be filled in this study and can play an important role in a more accurate understanding of the pathophysiology of this disorder and its treatment.
In the process of MetS natural history, except for the no components state, there were four isolated states [13]: isolated abdominal obesity, isolated hypertension, isolated dyslipidemia, and isolated hyperglycemia. Among the existing studies in this area, some have generally determined the incidence rate (the first approach), while some rare ones have determined the share of each of the abovementioned components in the occurrence of MetS by utilizing complex and new statistical approaches (second approach). What follows includes the examination and comparison of some aspects of the two approaches.
One comparable aspect between the present study and other studies was the timing of the incidence rate calculation. Although the results of the studies using the first approach are not comparable to the current research, the findings of the Baltimore cohort study demonstrated that the MetS incidence rate was 25.5% in males and 14.8% in females over a six-year period [35]. In a large cohort study conducted on 2000 people aged 20–74 years in central Iran between 2005 and 2015 [36], the 10-year incidence of MetS was reported to be 56.1% (5.61% per year) in healthy individuals. In another study [37] performed on patients with type 2 diabetes (without MetS) followed up for 11.7 years in Iran, the incidence rate of MetS was reported to be 33.4%. In another study conducted in China [38] over a nine-year period, the incidence rate of MetS was found to be 48.28% among females and 42.12% among males. In one other study in Iran, based on the data obtained from the four follow-up periods of the TLGS study [39], the cumulative incidence of MetS was estimated to be about 39%. In another study on the same population in Iran [40] also, the incidence rate of MetS was reported to be 20.4% in a three-year period. There are also several reports on the incidence of this disorder in other populations (Portugal [41], France [42], and Japan [43]), in which the incidence rate was reported to be 81.4%; 10.5% in males, 8% in females, and 15% in total. In the final period of the present study, the overall incidence of MetS was reported to be 10.7%, which was consistent with some studies and inconsistent with some others. The variations might be attributed to several reasons. All studies made use of two criteria, namely NCEP (National Cholesterol Education Program) and JIS, and there was no difference in this regard. It seems that the most important difference was related to the present study’s approach to calculating the incidence rate as well to shorter time intervals. In this study, a component-based approach was taken to compute the incidence rate by calculation of the incidence rate in each of the components as well as in healthy people (no components) at the beginning and in the follow-up periods. However, in most studies, component separation has not been performed to calculate the incidence rates. On the other hand, there was a dynamic and two-way connection between each component or a combination of components and MetS over time. This implied that in each time interval, a person might improve or the number of components in a person might increase or decrease. Therefore, considering the incidence rate based on the components might provide a more accurate estimate of the net incidence rate of MetS. Furthermore, the participants were assessed in five periods (four follow-up periods) in the current investigation, while they were evaluated over a period of time in other studies. Finally, and yet importantly, because the number of at-risk people decreased and varied over time in the present research, both the number of cases (numerator) and the denominator decreased over time.
In this study, the highest increase in the incidence rate was associated with obesity in the total mode. In other words, the highest increase in the incidence of MetS occurred due to obesity in the long term. In the short term (3.6 years), however, the highest incidence was observed in hypertension, hyperglycemia, dyslipidemia, and hypertension in the first to fourth periods, respectively. Similarly, Baltimore cohort study [35], Tang [44], Palaniappan [45], and Hwang [14] have identified obesity as the most important component leading to MetS in adults. In contrast, Jia et al. [13] maintained that hypertension was the most important component leading to MetS in adults. On the other hand, a large study conducted in central Iran [36] and a study in China [46] introduced hyperglycemia as the most important component related to the development of MetS. Among the aforementioned studies, four were performed using the second approach [13, 14, 44, 46]. Since the present study and these four studies were conducted with a longitudinal approach, their results might be more comparable. Among these four studies, the results of two [14, 44] were consistent with the long-term findings of the present investigation in reporting the higher significance of obesity in the occurrence of MetS. In addition, the results of one study [13] were in agreement with the short-term findings on the more significant role of hypertension. However, the results of the last study [46] were inconsistent with the overall findings of the current research. It is worth mentioning that the present study was the first to examine the component-based occurrence process of MetS in Iran. Regardless of the differences among the results of the studies in this field, it can be said that depending on the lifestyles of different communities, the role of each component in the development and occurrence of MetS can be different. Regarding the roles of hypertension and obesity in the development of MetS amongst adults, the difference might be attributed to the differences in the lifestyle and culture of the study populations. Additionally, the increase in the incidence rate of the MetS in people with hypertension could be mediated by obesity and the changes that occurs in the body to facilitate the conditions of hypertension. Since the components of MetS have common roots in terms of the pathophysiology of the disease, it may not be possible to distinguish between the roles of obesity and hypertension in the development of MetS. Nonetheless, the results presented in Fig. 2 revealed that in the process of MetS development from its components, hypertension and hyperglycemia were relatively similar and obesity and dyslipidemia were also similar. This makes a slight distinction between the common roots of the components. In terms of mechanism also, it puts obesity and dyslipidemia in a different level from hypertension and hyperglycemia in the development of MetS. Hypertension and hyperglycemia have been known as vascular disorders and are likely to share their effects in this way, especially among males. As in the present study, the prevalence of obesity and dyslipidemia was higher in females, while that of hypertension and hyperglycemia was higher among males. On the other hand, obesity leads to dyslipidemia through metabolic changes as well as metabolites and lipid abnormalities. Moreover, the common link among obesity, dyslipidemia, and MetS has been largely attributed to the phenomenon of insulin resistance in peripheral tissues [47].
In the current study, the stability rate of MetS followed a descending trend in total and in females, but an ascending trend in males from the first to the fourth follow-up periods. Stability rates can indicate a change in the direction of some patients towards recovery, a return to recovery, or even a progression of the disease. This index can be called persistent MetS, as well [11]. The decreasing trend of this index among females might be a sign of the instability of the disease in this group, which could be affected by various drug or behavioral interventions over time. On the other hand, the increasing trend of this index among males could be a sign of the higher stability of the disease in this group, disease resistance to treatment, or ineffectiveness of the treatments and interventions over time.
The present study findings revealed the increasing trend of the MetS incidence among healthy males (no components) and its decreasing trend among females as well as in the total mode during the research. Besides, the overall incidence of MetS from all the isolated components increased more in males than in females. Overall, it could be concluded that the overall progression of MetS was likely to be more severe amongst males. On the contrary, evidence has suggested a higher prevalence of the disease among females. In the studies carried out by Jia [13], Chen [46], Tang [44], and Hwang [14], the probability of transmission from the healthy status (no components) directly to MetS as well as the possibility of maintaining and stabilizing the disease status over time was higher in males than in females. These findings were in line with those of the present investigation, showing that MetS persistence was higher in males compared to females over time.
One of the important limitations of this study was the incomparability of the calculated incidence rates with other studies, which limited the ability to accurately compare the findings and determine the possible causes. Other study limitations included not exploring the interplay between the components and the effective covariates in this process as well as the impossibility to generalize the results to the total population of Tehran or Iran due to differences in culture, lifestyle, and access to health services. Nevertheless, paying attention to the component-specific calculation of MetS incidence as an innovation in this study is strongly recommended in other longitudinal studies to accurately identify the natural history of MetS and to compute its net incidence rate.
Conclusion
The results indicated that isolated abdominal obesity, as the most important component of MetS, played a fundamental role in the development of MetS in the Iranian population through the creation and development of other components. This needs to be taken into consideration in preventive and control policies as well as in further studies.
Acknowledgments
This article is the result of Pezhman Bagheri’s PhD thesis with registration code: SUMS.98/19936. We have to express our sincere thanks to all the personnel of the Shahid Beheshti University of Medical Sciences (SBMU) research institute for endocrine sciences for respectable cooperation in data collection phase that lead to the outcome of this project.
Authors’ contributions
P.B. developed the theory and performed the analysis, the literature search, assessed the literature, extracted data, wrote the manuscript with support from D.K. and A.R... A.R. with D.K. also encouraged and supervised the findings of this work, design and implementation of the research. F.A. in data establishment and the planning process of the main study has played a major role. M.S., EKM, and EB. verified the analytical methods and procedures.
Funding
This study was financially supported by the Vice-Chancellor for Research and Technology of Shiraz University of Medical Sciences (SUMS), which is worthy of thanks and appreciation.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflicts of interest/competing interests
The authors declare that they have no competing interests.
Ethics approval
This study was based on the TLGS cohort study data, therefore, it was ethically subject to the ethical considerations considered in this project and was independently the result of a research project approved by the National Ethics Committee on Biomedical Research of Iran under code IR.SUMS.REC.1398.835.
Consent to participate
In TLGS project, people in admission time, declare their consent to participate as part of ethical considerations.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Davood Khalili, Email: dkhalili@endocrine.ac.ir.
Pezhman Bagheri, Email: bpegman@yahoo.com.
Mozhgan Seif, Email: m_seif@sums.ac.ir.
Abbas Rezaianzadeh, Email: rezaiana@gmail.com.
Esmaeil Khedmati Morasae, Email: E.E.Khedmati-Morasae@exeter.ac.uk.
Ehsan Bahramali, Email: ebahramali@gmail.com.
Fereidoun Azizi, Email: azizi@endocrine.ac.ir.
References
- 1.Zafar U, Khaliq S, Ahmad HU, Manzoor S, Lone KP. Metabolic syndrome: an update on diagnostic criteria, pathogenesis, and genetic links. Hormones (Athens, Greece) 2018;17(3):299–313. doi: 10.1007/s42000-018-0051-3. [DOI] [PubMed] [Google Scholar]
- 2.Zambon S, Zanoni S, Romanato G, Corti MC, Noale M, Sartori L, et al. Metabolic syndrome and all-cause and cardiovascular mortality in an Italian elderly population: the Progetto Veneto Anziani (pro.V.a.) study. Diabetes Care. 2009;32(1):153–159. doi: 10.2337/dc08-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet (London, England) 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 5.Carnethon M, Loria C, Hill J, Sidney S, Savage P, Liu K. Risk factors for the metabolic syndrome the coronary artery risk development in young adults (CARDIA) study, 1985–2001. Diabetes Care. 2004;27:2707–2715. doi: 10.2337/diacare.27.11.2707. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Li W, Lun Z, Zhang H, Sun Z, Kanu JS, et al. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health. 2016;16:296. doi: 10.1186/s12889-016-2870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu LT, Shen YF, Hu L, Zhang MY, Lai XY. Prevalence and associated factors of metabolic syndrome in adults: a population-based epidemiological survey in Jiangxi province. China BMC Public Health. 2020;20(1):133. doi: 10.1186/s12889-020-8207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuzuya M, Ando F, Iguchi A, Shimokata H. Age-specific change of prevalence of metabolic syndrome: longitudinal observation of large Japanese cohort. Atherosclerosis. 2007;191(2):305–312. doi: 10.1016/j.atherosclerosis.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Van Hemelrijck M, Ulmer H, Nagel G, Peter R, Fritz J, Myte R, et al. Longitudinal study of body mass index, dyslipidemia, hyperglycemia, and hypertension in 60,000 men and women in Sweden and Austria. PLoS One. 2018;13:e0197830. doi: 10.1371/journal.pone.0197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee M-K, Han K, Kim MK, Koh ES, Kim ES, Nam GE, Kwon HS. Changes in metabolic syndrome and its components and the risk of type 2 diabetes: a nationwide cohort study. Sci Rep. 2020;10(1):2313. doi: 10.1038/s41598-020-59203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huh JH, Ahn SG, Kim YI, Go T, Sung KC, Choi JH, Koh KK, Kim JY. Impact of longitudinal changes in metabolic syndrome status over 2 years on 10-year incident diabetes mellitus. Diabetes Metab J. 2019;43(4):530–538. doi: 10.4093/dmj.2018.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, Fox CS, Meigs JB, Levy D, Larson MG, Selhub J, D’Agostino RB, Sr, Wang TJ, Vasan RS. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors. Circulation. 2007;116(9):984–992. doi: 10.1161/CIRCULATIONAHA.107.708537. [DOI] [PubMed] [Google Scholar]
- 13.Jia X, Chen Q, Wu P, Liu M, Chen X, Xiao J, et al. Dynamic development of metabolic syndrome and its risk prediction in Chinese population: a longitudinal study using Markov model. Diabetol Metab Syndr. 2018;10:24. doi: 10.1186/s13098-018-0328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang L-C, Bai C-H, You S-L, Sun C-A, Chen C-J. Description and prediction of the development of metabolic syndrome: a longitudinal analysis using a Markov model approach. PLoS One. 2013;8(6):e67436. doi: 10.1371/journal.pone.0067436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haring R, Rosvall M, Völker U, Völzke H, Kroemer H, Nauck M, Wallaschofski H. A network-based approach to visualize prevalence and progression of metabolic syndrome components. PLoS One. 2012;7(6):e39461. doi: 10.1371/journal.pone.0039461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barcelo MA, Rodriguez-Poncelas A, Saez M, Coll-de-Tuero G. The dynamic behaviour of metabolic syndrome and its components in an eight-year population-based cohort from the Mediterranean. PLoS One. 2017;12(5):e0176665. doi: 10.1371/journal.pone.0176665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 18.Azizi F, Zadeh-Vakili A, Takyar M. Review of Rationale, Design, and Initial Findings: Tehran Lipid and Glucose Study. Int J Endocrinol Metab. 2018;16(4 (Suppl)):e84777. doi: 10.5812/ijem.84777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azizi F. Tehran Lipid and Glucose Study: A National Legacy. Int J Endocrinol Metab. 2018;16(4 Suppl):e84774-e. doi: 10.5812/ijem.84774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azizi F. Tehran lipid and glucose study: a legacy for prospective community-based research. Archives of Iranian medicine. 2014;17(6):392–393. [PubMed] [Google Scholar]
- 21.Huque MH, Moreno-Betancur M, Quartagno M, Simpson JA, Carlin JB, Lee KJ. Multiple imputation methods for handling incomplete longitudinal and clustered data where the target analysis is a linear mixed effects model. Biometric J. 2020;62(2):444–466. doi: 10.1002/bimj.201900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huque MH, Carlin JB, Simpson JA, Lee KJ. A comparison of multiple imputation methods for missing data in longitudinal studies. BMC Med Res Methodol. 2018;18(1):168. doi: 10.1186/s12874-018-0615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Silva AP, Moreno-Betancur M, De Livera AM, Lee KJ, Simpson JA. Multiple imputation methods for handling missing values in a longitudinal categorical variable with restrictions on transitions over time: a simulation study. BMC Med Res Methodol. 2019;19(1):14. doi: 10.1186/s12874-018-0653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Silva AP, Moreno-Betancur M, De Livera AM, Lee KJ, Simpson JA. A comparison of multiple imputation methods for handling missing values in longitudinal data in the presence of a time-varying covariate with a non-linear association with time: a simulation study. BMC Med Res Methodol. 2017;17(1):114. doi: 10.1186/s12874-017-0372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patrician PA. Multiple imputation for missing data. Res nursing health. 2002;25(1):76–84. doi: 10.1002/nur.10015. [DOI] [PubMed] [Google Scholar]
- 26.Duffy ME. Handling missing data: a commonly encountered problem in quantitative research. Clinical nurse specialist CNS. 2006;20(6):273–276. doi: 10.1097/00002800-200611000-00005. [DOI] [PubMed] [Google Scholar]
- 27.El-Masri MM, Fox-Wasylyshyn SM (2005) Missing data: an introductory conceptual overview for the novice researcher. The Canadian journal of nursing research = Revue canadienne de recherche en sciences infirmieres;37(4):156–71. [PubMed]
- 28.Alberti KGMM, Eckel Robert H, Grundy Scott M, Zimmet Paul Z, Cleeman James I, Donato Karen A, et al. Harmonizing the metabolic syndrome. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 29.Hosseini-Esfahani F, Bahadoran Z, Moslehi N, Asghari G, Yuzbashian E, Hosseinpour-Niazi S, et al. Metabolic Syndrome: Findings from 20 Years of the Tehran Lipid and Glucose Study. Int J Endocrinol Metab. 2018;16(4 Suppl):e84771-e. doi: 10.5812/ijem.84771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azizi F, Khalili D, Aghajani H, Esteghamati A, Hosseinpanah F, Delavari A, Larijani B, Mirmiran P, Mehrabi Y, Kelishadi R, Hadaegh F. Appropriate waist circumference cut-off points among Iranian adults: the first report of the Iranian National Committee of obesity. Archives of Iranian medicine. 2010;13(3):243–244. [PubMed] [Google Scholar]
- 31.Rose GA, Blackburn H. Cardiovascular survey methods. Monograph series World Health Org. 1968;56:1–188. [PubMed] [Google Scholar]
- 32.Ainsworth BE, Jacobs DR, Jr, Leon AS. Validity and reliability of self-reported physical activity status: the lipid research clinics questionnaire. Med Sci Sports Exerc. 1993;25(1):92–98. doi: 10.1249/00005768-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Center AC (1997) ARIC Manuals of Operation: Cohort component procedures. (2)
- 34.Ma W-Y, Yang C-Y, Shih S-R, Hsieh H-J, Hung CS, Chiu F-C, Lin MS, Liu PH, Hua CH, Hsein YC, Chuang LM, Lin JW, Wei JN, Li HY Measurement of waist circumference: midabdominal or iliac crest? Diabetes Care 2013;36(6):1660–6, 1666. [DOI] [PMC free article] [PubMed]
- 35.Scuteri A, Morrell CH, Najjar SS, Muller D, Andres R, Ferrucci L, et al. Longitudinal paths to the metabolic syndrome: can the incidence of the metabolic syndrome be predicted? The Baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. 2009;64(5):590–598. doi: 10.1093/gerona/glp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarebanhassanabadi M, Jalil Mirhosseini S, Mirzaei M, Namayandeh SM, Soltani MH, Pedarzadeh A, et al. The incidence of metabolic syndrome and the Most powerful components as predictors of metabolic syndrome in Central Iran: a 10-year follow-up in a cohort study. Iran Red Crescent Med J. 2017;19(7):e14934. [Google Scholar]
- 37.Janghorbani M, Amini M. Incidence of metabolic syndrome and its risk factors among type 2 diabetes clinic attenders in Isfahan. Iran Endokrynologia Polska. 2012;63(5):372–380. [PubMed] [Google Scholar]
- 38.Jiang B, Li B, Wang Y, Han B, Wang N, Li Q, et al. The nine-year changes of the incidence and characteristics of metabolic syndrome in China: longitudinal comparisons of the two cross-sectional surveys in a newly formed urban community. Cardiovasc Diabetol. 2016;15:84. doi: 10.1186/s12933-016-0402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadaegh F, Hasheminia M, Lotfaliany M, Mohebi R, Azizi F, Tohidi M. Incidence of metabolic syndrome over 9 years follow-up; the importance of sex differences in the role of insulin resistance and other risk factors. PloS one. 2013;8(9):e76304-e. doi: 10.1371/journal.pone.0076304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zabetian A, Hadaegh F, Sarbakhsh P, Azizi F. Weight change and incident metabolic syndrome in Iranian men and women; a 3 year follow-up study. BMC Public Health. 2009;9:138. doi: 10.1186/1471-2458-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos AC, Severo M, Barros H. Incidence and risk factors for the metabolic syndrome in an urban south European population. Prev Med. 2010;50(3):99–105. doi: 10.1016/j.ypmed.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Balkau B, Vernay M, Mhamdi L, Novak M, Arondel D, Vol S, Tichet J, Eschwège E, D.E.S.I.R. Study Group The incidence and persistence of the NCEP (National Cholesterol Education Program) metabolic syndrome. The French D.E.S.I.R. study. Diabetes Metab. 2003;29(5):526–532. doi: 10.1016/s1262-3636(07)70067-8. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Yatsuya H, Iso H, Tamakoshi K, Toyoshima H. Incidence of metabolic syndrome according to combinations of lifestyle factors among middle-aged Japanese male workers. Prev Med. 2010;51(2):118–122. doi: 10.1016/j.ypmed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Tang X, Liu Q. Prediction of the development of metabolic syndrome by the Markov model based on a longitudinal study in Dalian City. BMC Public Health. 2018;18(1):707. doi: 10.1186/s12889-018-5599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palaniappan L, Carnethon MR, Wang Y, Hanley AJG, Fortmann SP, Haffner SM, et al. Predictors of the incident metabolic syndrome in adults. Diabetes Care. 2004;27(3):788–793. doi: 10.2337/diacare.27.3.788. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Chen Q, Chen L, Zhang P, Xiao J, Wang S. Description and prediction of the development of metabolic syndrome in Dongying City: a longitudinal analysis using the Markov model. BMC Public Health. 2014;14:1033. doi: 10.1186/1471-2458-14-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.