Abstract
Purpose
The aim of the present study was to investigate the effect of spirulina on lipid profiles and glycemic related markers in type 2 diabetes patients.
Methods
PubMed, Scopus, Cochrane Library, ISI Web of Science, and Google Scholar were searched from inception to August 2020. All clinical trials which investigated the effect of spirulina supplementation on glycemic related markers and lipid profile among type 2 diabetes patients were included. Random effects modeling was utilized for pooling analysis to compensate for the between-study heterogeneity.
Results
Eight studies (9 arms) were included in the meta-analysis. We found a significant reduction in fasting blood glucose (−17.88 mg/dl; 95% CI: −26.99, −8.78; I2: 25%), triglyceride (−30.99 mg/dl; 95% CI: −45.20, −16.77; I2: 50%), total-cholesterol (−18.47 mg/dl; 95% CI: −33.54, −3.39; I2: 73%), LDL-C (−20.04 mg/dl; 95% CI: −34.06, −6.02; I2: 75%), VLDL (−6.96 mg/dl; 95% CI: −9.71, −4.22; I2: 33%), in addition to a significant increase in HDL-C (−6.96 mg/dl; 95% CI: −9.71, −4.22; I2: 33%), after spirulina administration. No significant effect was observed on HbA1C or post prandial blood sugar following spirulina consumption.
Conclusion
The present study suggests that spirulina supplementation can elicit beneficial effects on fasting blood glucose and blood lipid profiles.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-021-00760-z.
Keywords: Spirulina, Arthrospira platensis, Diabetes mellitus, Meta-analysis
Background
Type 2 diabetes is a non-communicable disease, manifest via impairment in glucose metabolism, affecting both developed and developing countries [1]. Although many strategies have been suggested for ameliorating or treating diabetes, the incidence of this disease is growing rapidly [2]. Such high incidence imposes a critical burden on health care system utilization, which consequently confers a large economic cost annually [3]. Thus, any viable alternative, complementary, or adjunct therapy that may alleviate some economical and/or health care burden represents an issue of high importance.
In contemporary practice, lifestyle modification, including change in diet and physical activity, is the first step to the treatment of type 2 diabetes [3, 4]. However, many patients find it difficult to adhere to dietary restrictions [5]. On the other hand, many pharmacological agents cause adverse side-effects which limit their palatability and success [6, 7]. In this case, the efficacy of functional food and natural medicines as adjuvant therapies, concomitant with pharmacological agents, has become an interesting area for many researchers [8, 9].
Spirulina (Arthrospira maxima) is a microalga, belonging to the family of cyanobacteria with the most curative and prophylactic components of nutrition [10]; possessing cardioprotective and antioxidant activity due to high amount of phycocyanins, polyphenols, carotenoids, vitamins, essential fatty acids and protein [11]. The beneficial effect of spirulina in many non-communicable diseases has been shown previously [12–14]. Furthermore, animal studies indicated that spirulina can improve metabolic parameters related to glycemic status and lipid profile in diabetic mice [14, 15]. However, there is a lack of consensual evidence from clinical trials. Therefore, the present systematic review and meta-analysis was performed to summarize the current evidence and investigate the effect of spirulina supplementation on glycemic related markers and blood lipid profiles in type 2 diabetes patients.
Methods
The present systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) as a standard guideline [16].
Search strategy
A systematic literature search was carried out through Medline, Scopus, and ISI Web of Science, Cochrane Library, and Google Scholar from inception to August 2020. The comprehensive electronic search was performed by using the following keywords in combination with wildcard ‘*’ and medical subject headings (MeSH): (“Spirulina” OR “Arthrospira”) AND (“diabetes” OR “diabetic” OR “diabetes mellitus” “blood glucose” OR “glucose metabolism disorders” OR “hyperglycemia” OR “Hemoglobin A, Glycosylated”). The references of related clinical trials and pertinent review articles were also hand-searched to identify any additional studies of interest, which might have been missed during the electronic search.
Study selection
To identify eligible studies, two authors independently screened the studies which were included by the primary search. After excluding duplicates, studies were reviewed first by title/abstract and was articles obviously irrelevant were excluded. Subsequently, the full-texts of remaining studies were scanned. All clinical trials which investigated the effect of spirulina supplementation on glycemic related markers and lipid profile among type 2 diabetes patients were included. Studies were excluded if the duration of studies was <1 weeks, spirulina was administrated as part of a complex substance, spirulina was compared with an active agent/component, and the outcome of interest was not reported in the studies. Any discrepancy was settled by the third author. Supplemental Table 1 shows the PICOS (participants, intervention/exposure, comparisons, outcomes, and study design) criteria which is used to define the research question.
Data abstraction and assessment of the quality
Eligible clinical trials were separately reviewed by two authors (A.H and M.P) and the following information was recorded from each study: the first author’s last name, years of publication, country of origin, the total number of participants in each arm as well as their characteristics (mean age, gender), study design, duration of intervention, details of intervention and control groups, the dose of spirulina supplementation and outcomes of interest which reported.
Cochrane Risk of Bias Tool for Randomized Controlled Trials was used to detect the potential risk of bias in included studies (20). This scale included several criteria to evaluate the adequacy of random sequence generation, allocation concealment, blinding as well as detection of incomplete outcome data, reporting selective outcome, and other potential sources of bias. Based on recommendations of the Cochrane Handbook, the judgment of each item appears by “Low”, “High” and “Unclear” risk of bias. Any disagreement in data extraction and quality assessment judgment was resolved by discussion with a third investigator.
Statistical analysis
The whole process of statistical analyses was conducted using the Cochrane Program Review Manager Version 5.3 and STATA software (version 11.0; Stata Corporation). To estimate pooled effect size, data from all variables, including fasting blood glucose (FBS), postprandial blood sugar (PPBS), glycated hemoglobin A1C (HbA1C), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and very-low-density lipoprotein cholesterol (VLDL), which were reported in three or more studies, were extracted as mean difference and standard deviation (SD). In any instance where mean change and SD of change were not reported directly for intervention and control groups, it was calculated following a suggested formula [SD = square root [(SD pre-treatment)2 + (SD post-treatment)2 - (2 r × SD pre-treatment × SD post-treatment)] [17]. To take probable between-study heterogeneity into account, we applied the random-effects model in our analyses to estimate the overall effect size [18, 19]. Sensitivity analysis was conducted by eliminating each study, one at a time, to determine the influence of each study on the overall result. Egger’s regression asymmetry test and Begg’s rank-correlation methods were also performed to explore potential publication bias [20, 21]. To evaluate the potential influence of putative moderators such as baseline measures and duration of administration on changing variables in response to spirulina supplementation, the meta-regression was applied. Results were assumed as statistically significant when P < 0.05.
Results
The study selection process and the reason for study exclusion at each step is illustrated in Fig. 1. The electronic selection process yielded 907 unduplicated trials, in which 896 of them were excluded by title/abstract screening, and 11 studies remained for full-text assessment. Three studies were omitted due to not being conducted on diabetes patients (n = 2) or not reporting outcomes of interest (n = 1). One study administrated 2 different doses of spirulina and was considered as 2 separate arms. Therefore, 8 studies comprising 9 arms met eligibility criteria and were included in the present meta-analysis.
Fig. 1.
Flow chart of the process of the study selection
Studies’ characteristics
The characteristics of included studies are detailed in Table 1. In brief, 8 clinical trials (9 arms) [22–29] comprising 334 patients with diabetes, with a mean age of 51 years old, were included in the meta-analysis. The studies were conducted in various countries including India [22, 23, 25, 27, 28], Iran [24], Romania [29] and South Korea [26], and published between 2001 and 2017. The baseline BMI of participants was only reported in 4 trials [26–29]. Duration of intervention ranged from 45 to 90 days, and the dose of spirulina administration varied between 0.8 and 8 g/day. Only one trial [29] reported that participants received a placebo as a control group. Two trials recruited only male participants [23, 25], whilst of the remaining studies enrolled patients of both genders.
Table 1.
The main characteristics of included studies.
| First author (publication year) | Country | Number and gender (M/F) | Mean age | BMI | Duration of disease (years)/treatment | Clinical Trial design/randomized/Blinding | Duration of study (Days) | Comparison group | Amount Of Spirulina intake | Notes about participants | Outcomes of interest |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serban et al. (1982) [29] | Romania |
Intervention: 15 Control: 15 (NR) |
Range years: 30-70 Intervention: 61.7 ±6.85 Control: 61.6±8.90 |
Intervention: 36.6±6.05 Control: 36.2±6.93 |
> 2 / Metformin | Parallel/Yes/Yes | 60 day |
Metformin + Placebo |
Metformin + Spirulina tablet 0.8 g/day |
Type 2 Diabetes | FBS, HbA1C, TC, TG, HDL-C, LDL-C |
| Mani et al. (2000) [27] | India |
Intervention: 15 Control: 7 (Both gender) |
Intervention: 47.80±9.10 Control: 53.40±6.13 |
Intervention: 29.24±0.30 Control: 25.75±0.13 |
-/- | Parallel/NR/NR | 60 day | - |
Spirulina tablet 2 g/day |
Non-insulin dependent diabetes mellitus | FBS, TC, TG, HDL-C, LDL-C, VLDL |
| Parikh et al. (2001) [28] | India |
Intervention: 15 Control: 10 (Both gender) |
Intervention: 53.8 ± 7.2 Control: 54.6 ± 5.4 |
Intervention: 25.22±5.4 Control: 25.1±2.7 |
12.7/oral hypoglycemic agent | Parallel/Yes/NR | 60 day | - |
Spirulina tablet 2 g/day |
Type 2 Diabetes | FBS, HbA1C, BSPP, TC, TG, HDL-C, LDL-C, VLDL |
| Lee et al. (2008) [26] | Korea |
Intervention: 19 Control: 18 (Both gender) |
Range: years Intervention: 52.1 ± 10.02 Control: 54.5 ± 6.36 |
Intervention: 23.8±2.17 Control: 23.4±2.12 |
2.45/without drug use | Parallel/Yes/NR | 84 day | - |
Spirulina tablet 8.0 g/day |
Type 2 Diabetes | FBS, HbA1C, TC, TG, HDL-C, LDL-C |
| Kaur et al. (2008) [25] | India |
Intervention: (1) 20 (2) 20 Control: 20 (Male) |
Range: 40-60 years Intervention: (1) 46.3±7.60 (2) 45.95±7.15 Control: 47.6±6.70 |
NR | 7.52/ oral hypoglycemic agent | Parallel/NR/NR | 60 day | - |
Spirulina tablet (1) 1 g/day (2) 2 g/day |
Non-insulin dependent diabetes mellitus | FBS, HbA1C, BSPP, TC, TG, HDL-C, LDL-C, VLDL |
| Anitha et al. (2010) [23] | India |
Intervention: 40 Control: 40 (Male) |
Range: 45 – 60 years Intervention: NR Control: NR |
NR | -/- | Parallel/NR/NR | 84 day | - |
Spirulina tablet 1g/day |
Non-insulin dependent diabetes mellitus | FBS, HbA1C, TC, TG, HDL-C, LDL-C, VLDL |
| Alam et al. (2016) [22] | India |
Intervention: 30 Control: 10 (Both gender) |
Range: 41-60 years Intervention: 45.07 ± 7.67 Control: 44.00 ± 9.39 |
NR | -/- | Parallel/Yes/NR | 45 days | Metformin 500 mg/day |
Spirulina powder 7g/day |
Type 2 Diabetes | FBS, HbA1C, BSPP |
| Beihaghi et al. (2017) [24] | Iran |
Intervention: 20 Control: 20 (Both gender) |
Range years: 30-60 Intervention: NR Control: NR |
NR | 5-10 /- | Parallel/Yes/NR | 90 day | - |
Spirulina tablet 8 g/day |
Type 2 Diabetes | FBS, HbA1C |
Lipoprotein; HDL-C: High-Density Lipoprotein; NR: Not Reported.
Abbreviations: FBS: Fasting Blood Sugar; HbA1C: glycated hemoglobin; BSPP: Blood Sugar post prandial; TG: Triglyceride; TC: Total-Cholesterol; LDL-C: Low-Density
Risk of bias assessment
Five trials were randomized [22, 24, 26, 28, 29], however, only one study [22] sufficiently addressed information around allocation concealment. Only 2 studies [22, 29] were blinded. The data regarding attrition and reporting biases were well-addressed in all trials. Table 2 presents the risk in each item of bias among included studies in detail.
Table 2.
The summary of review authors’ judgments about each risk of bias item for included studies.
| Study | Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|
| Serban et l. (1982) [29] | L | U | L | L | L | U |
| Mani et al. (2000) [27] | U | U | H | L | L | U |
| Parikh et al. (2001) [28] | L | U | H | L | L | L |
| Lee et al. (2008) [26] | L | U | H | L | L | L |
| Kaur et al. (2008) [25] | U | U | H | L | L | L |
| Anitha et al. (2010) [23] | U | U | H | L | L | U |
| Alam et al. (2016) [22] | L | L | L | L | L | L |
| Beihaghi et al. (2017) [24] | L | U | H | L | L | U |
H: high risk of bias; L: low risk of bias; U: unclear or unrevealed risk of bias. Criteria defined for risk of bias assessment are according to the Cochrane guidelines.
According to Cochrane criteria, study consider as a poor quality if it had high risk of bias in ≥2 items or unclear risk of bias in ≥3 criteria.
Meta-analysis
The effect of spirulina supplementation on glycemic related markers
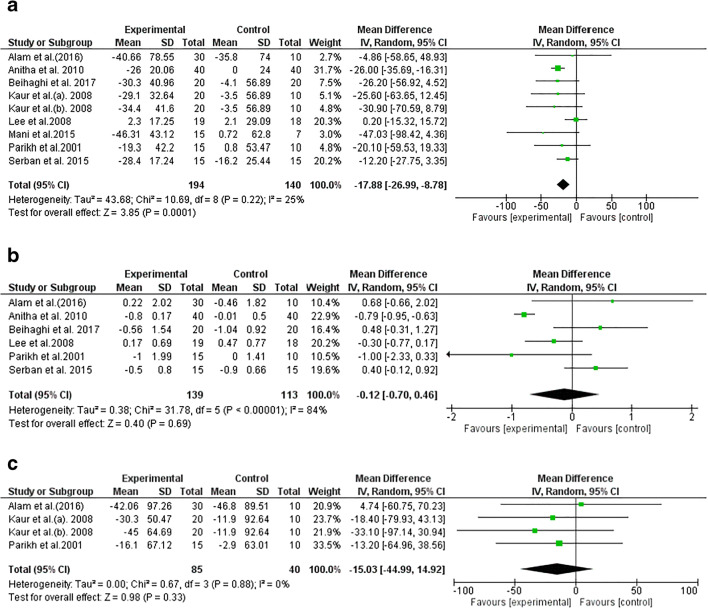
The result of our meta-analysis suggested a significant effect of spirulina supplementation on FBS levels (−17.88 mg/dl; 95% CI: −26.99, −8.78; I2: 25%). However, no notable influence was detected for HbA1C (−0.12%, 95% CI: −0.70, 0.46; I2 = 84%) or PPBS (−15.03%, 95% CI: −44.99, 14.92; I2 = 0%) after spirulina intervention (Fig. 2).
Fig. 2.
Forest plot detailing mean difference and 95% confidence intervals (CI) for the effect of spirulina consumption on fasting blood sugar, hemoglobin A1C, blood sugar post-prandial
The effect of spirulina on blood lipid profiles
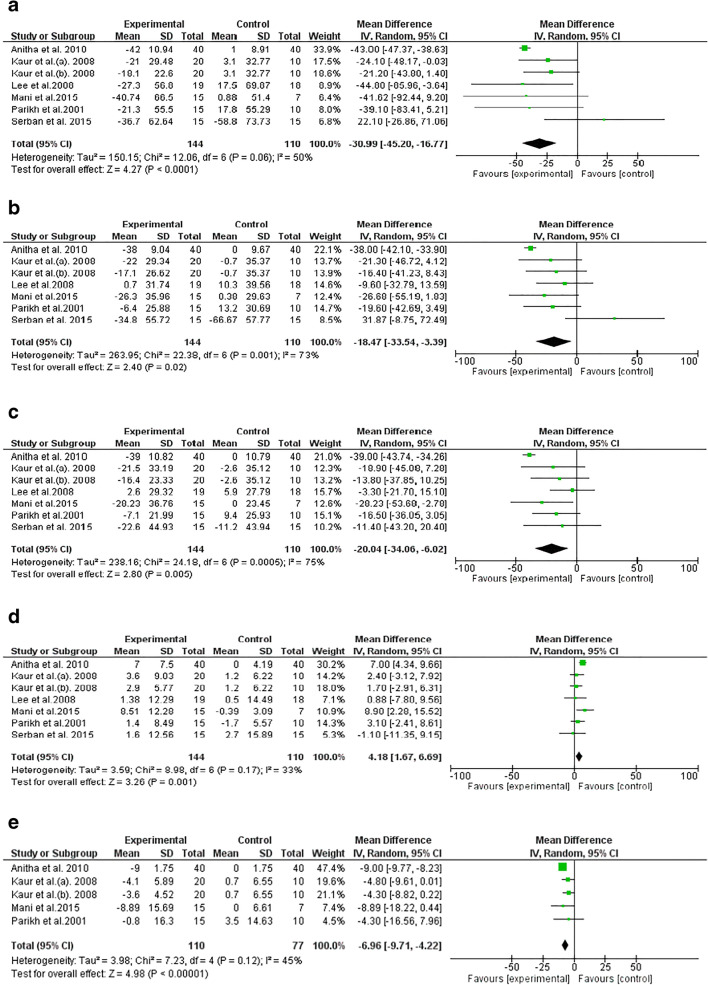
Pooled effect sizes revealed a significant reduction in TG (−30.99 mg/dl, 95% CI: −45.20, −16.77; I2 = 50%), TC (−18.47 mg/dl, 95% CI: −33.54, −3.39; I2 = 73%), LDL-C (−20.04 mg/dl, 95% CI: −34.06, −6.02; I2 = 75%) and VLDL (−6.96 mg/dl, 95% CI: −9.71, −4.22; I2 = 45%) following spirulina supplementation. In addition, the result indicated that spirulina supplementation yielded a significantl increase in HDL-C serum concentration (4.18 mg/dl, 95% CI: 1.67, 6.69; I2 = 33%) (Fig. 3).
Fig. 3.
Forest plot detailing mean difference and 95% confidence intervals (CI) for the effect of spirulina consumption on lipid profiles
Meta-repression
The meta-regression revealed that the effect of spirulina supplementation on TC and LDL-C was inversely associated with baseline values (TC: coefficient = −0.89, P = 0.01; LDL-C: coefficient = −1.13, P = 0.005). In addition, the change in TG blood concentrations in response to spirulina intervention was related to the duration of the intervention (TG: coefficient = −0.87, P = 0.03). However, the change in of the remaining variables were independent of the dose of spirulina supplementation, duration of intervention, and baseline measures, respectively.
Sensitivity analysis and publication bias
Sensitivity analysis, by removing each RCT one by one, indicated that the pooled effect size of TG was non-significant after excluding Mani et al. (−16.77 mg/dl; 95% CI: −33.97, 0.43; I2: 77%). In addition, by removing Anitha et al. from TG overall effect size, the heterogeneity was altered from 50% to 7%, while the results remained significant (−24.51 mg/dl; 95% CI: −38.62, −10.39). The overall results of the remaining variables were not influenced by individual studies.
No evidence of publication bias was detected according to Egger’s regression asymmetry test and Begg’s rank-correlation methods in FBS (Begg’s test P = 0.67; Egger’s test P = 0.86), HbA1C (Begg’s test P = 0.85; Egger’s test P = 0.77), BSPP (Begg’s test P = 1.0; Egger’s test P = 0.99), TG (Begg’s test P = 0.65; Egger’s test P = 0.10), HDL-C (Begg’s test P = 0.54; Egger’s test P = 0.12), VDLD (Begg’s test P = 0.99; Egger’s test P = 0.12). Although Egger’s regression asymmetry test indicated significant evidence of publication bias in TC (Egger’s test: P = 0.005) and LDL-C (Egger’s test: P = 0.01); however, these results were not confirmed by Begg’s rank-correlation methods (TC: P = 0.45; LDL-C: P = 0.65).
Discussion
The present systematic review and meta-analysis suggests that spirulina supplementation can elicit a beneficial impact on metabolic parameters including FBS, TG, TC, LDL-C, HDL-C and VLDL. However, no favorable effect was observed in HbA1C and PPBS; which might be due to the low number of included studies that reported on these parameters. In addition, as HbA1C levels change over longer periods of time, it might that the duration of the included studies was not sufficient to truly reflect the efficacy of spirulina on decreasing in HbA1C. The meta-regression indicated that the change in blood TC and LDL-C concentrations was associated with baseline values; so that, higher baseline measures of TC or LDL-C led to greater reductions in blood concentration of these parameters. In addition, a greater reduction in TG was observed when the duration of spirulina supplementation was longer.
Patients with type 2 diabetes suffer from innumerable complications and are at risk of several additional diseases, such as non-alcoholic fatty liver [30] and cardiovascular disease [31]. The current study revealed a significant reduction in FBS and lipid profile following spirulina consumption. Although the mechanisms underlying the beneficial activity of spirulina are not well-understood, although several putative pathways are attributed to spirulina’s hypoglycemic and hypolipidemic activity. One of the bioactive components of spirulina is C-phycocyanin, a protein which can inhibit lipid peroxidation, scavenge free radicals, as well as enhance GSH peroxidase and superoxide dismutase activity [32, 33]. Spirulina can inhibit pancreatic lipase activity via glycolipid H-b2 [34, 35], and regulate cholesterol and prostaglandin synthesis via its gamma-linolenic acid components [36, 37]. Spirulina also possesses hypoglycemic properties via stimulation of insulin secretion from β-cell, or elevation of blood glucose transport to peripheral tissues by its protein and amino acid constituents [38].
The beneficial effect of spirulina on type 2 diabetes is not only related to the aforementioned parameters, but also associated with body weight and inflammatory factors, where both are involved with this disease [39–41]. Increases in body weight, especially abdominal obesity and inflammation, are associated with insulin resistance [42, 43], such that spirulina can also improve diabetes by weight loss activity and alleviation of inflammation through suppressing the NF-KB activity and reducing pro-inflammatory cytokines production [44, 45].
Spirulina is regarded to be generally safe in commonly-used doses and only rare cases of unwanted effect have been reported [46, 47]. Except for one study [24], none of the included studies reported evidence of adverse effects attributed to spirulina consumption. Beihaghi et al. [24] reported that participants experienced side-effects such as abdominal discomfort and diarrhea after 8 g/d spirulina consumption, which was alleviated in many of them after a few days. In addition, it has been shown that feeding mice for 7 days with 10 g/kg and 30 g/kg of body weight of dried and fresh spirulina, respectively, did not cause any form of toxicity [48]. However, there is a concern about contamination with low levels of mercury and other heavy metals from open water sources [47, 49], which should be avoided by controlling the growth and processing of spirulina.
There are several limitations which should be acknowledged in the present study. First, the number of included studies was somewhat low, and the duration of studies was relatively short. Second, there are several factors related to type 2 diabetes, such as insulin levels, insulin resistance, and homeostatic model assessment of insulin resistance (HOMA-IR), which are essential in the etiology of this disease. However, none of the included studies reported on these factors, and future studies should be conducted to investigate the effect of spirulina on these parameters. Finally, the quality of methodology of the included studies was low, and they had a significant risk of bias in several items. In this case, although the overall results indicated a promising effect on metabolic parameters in diabetic patients, these findings are not conclusive enough to utilize in clinical practice, and more clinical trials, with high-quality methodology are needed to affirm the efficacy of spirulina in diabetic treatment.
Conclusion
The present meta-analysis highlights that spirulina supplementation can yield improvements in FBS as well as lipid profiles. This study summarizes the currently available information from clinical trials and provides better insight into the effect of spirulina supplementation on type 2 diabetes. Spirulina is a natural functional agent, and generally safe supplement with a low cost, along with a beneficial impact on improving metabolic abnormalities manifest in type 2 diabetes. The favorable effects of spirulina suggest it may be a beneficial adjuvant therapy in conjunction with conventional medicine. However, the results of the present study should be considered as primary findings and further studies are needed to confirm the veracity of the results.
Supplementary Information
(DOCX 12 kb)
Abbreviations
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- FBS
fasting blood glucose
- PPBS
postprandial blood sugar;
- HbA1C
glycated hemoglobin A1C
- TG
triglyceride; total cholesterol: total cholesterol
- LDL-C
low-density lipoprotein cholesterol
- HDL-C
high-density lipoprotein cholesterol
- VLDL
very-low-density lipoprotein cholesterol
- SD
standard deviation
Authors’ contributions
M.P., A.N. and A.H. carried out the concept, design and drafting of this study. A.S., S.GH. and M.P. searched databases, screened articles and extracted data. E.H., M.A., and F.J. performed the acquisition, analysis, and interpretation of data. C.C. and F.M-G. critically revised the manuscript. All authors approved the final version of the manuscript. F.J. and F.M-G. are the guarantors of this study.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Farahnaz Joukar, Email: farajov@gmail.com.
Fariborz Mansour-Ghanaei, Email: ghanaie@yahoo.com.
References
- 1.Kaiser AB, Zhang N, Van Der Pluijm W. Global prevalence of type 2 diabetes over the next ten years (2018–2028). Am Diabetes Assoc: 2018;67(Supplement 1):202-LB.
- 2.Organization WH . WHO global report on trends in prevalence of tobacco smoking 2000–2025. Geneva: World Health Organization; 2018. [Google Scholar]
- 3.Taylor SI. The high cost of diabetes drugs: disparate impact on the Most vulnerable patients. Diabetes Care. 2020;43(10):2330–2332. doi: 10.2337/dci20-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B, Mu X-L, Zhao J, Jiang H-P, Li S-S, Yan G, Hua YY, Ren XY, Xing LX, Liang Y, Zhang SD, Zhao YC. Effects of lifestyle interventions on rural patients with type 2 diabetes mellitus. World J Diabetes. 2020;11(6):261–268. doi: 10.4239/wjd.v11.i6.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadi A, Pourmasoumi M, Najafgholizadeh A, Kafeshani M, Sahebkar A. Effect of purslane on blood lipids and glucose: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2019;33(1):3–12. doi: 10.1002/ptr.6203. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee M, Khursheed R, Yadav AK, Singh SK, Gulati M, Pandey DK, Prabhakar PK, Kumar R, Porwal O, Awasthi A, Kumari Y, Kaur G, Ayinkamiye C, Prashar R, Mankotia D, Pandey NK. A systematic review on synthetic drugs and Phytopharmaceuticals used to manage diabetes. Curr Diabetes Rev. 2020;16(4):340–356. doi: 10.2174/1573399815666190822165141. [DOI] [PubMed] [Google Scholar]
- 7.Strain WD, Paldanius P. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57. doi: 10.1186/s12933-018-0703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pourmasoumi M, Hadi A, Najafgholizadeh A, Joukar F, Mansour-Ghanaei F. The effects of cranberry on cardiovascular metabolic risk factors: a systematic review and meta-analysis. Clin Nutr. 2019;39(3):774–88. [DOI] [PubMed]
- 9.Hadi A, Pourmasoumi M, Mohammadi H, Symonds M, Miraghajani M. The effects of silymarin supplementation on metabolic status and oxidative stress in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of clinical trials. Complement Ther Med. 2018;41:311–319. doi: 10.1016/j.ctim.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 10.van den Driessche JJ, Plat J, Konings MCJM, Mensink RP. Effects of spirulina and wakame consumption on intestinal cholesterol absorption and serum lipid concentrations in non-hypercholesterolemic adult men and women. Eur J Nutr. 2019;59:2229–2236. doi: 10.1007/s00394-019-02073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ovando CA. Carvalho JCd, Vinícius de Melo Pereira G, Jacques P, Soccol VT, Soccol CR. Functional properties and health benefits of bioactive peptides derived from Spirulina: a review. Food Rev Int. 2018;34(1):34–51. doi: 10.1080/87559129.2016.1210632. [DOI] [Google Scholar]
- 12.Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH. Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol. 2017;29(2):949–982. doi: 10.1007/s10811-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Ocampo M, Rodriguez B, Chen J. Resveratrol and Spirulina: Nutraceuticals that potentially improving cardiovascular disease. J Cardiovasc Med Cardiol. 2020;7:138–145. doi: 10.17352/2455-2976.000129. [DOI] [Google Scholar]
- 14.Hu S, Fan X, Qi P, Zhang X. Identification of anti-diabetes peptides from Spirulina platensis. J Funct Foods. 2019;56:333–341. doi: 10.1016/j.jff.2019.03.024. [DOI] [Google Scholar]
- 15.Zhao B, Cui Y, Fan X, Qi P, Liu C, Zhou X, Zhang X. Anti-obesity effects of Spirulina platensis protein hydrolysate by modulating brain-liver axis in high-fat diet fed mice. PLoS One. 2019;14(6):e0218543. doi: 10.1371/journal.pone.0218543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Hoboken: John Wiley & Sons; 2011. [Google Scholar]
- 18.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne J, Bradburn M. Meta-analysis in Stata. In: Egger M, Smith G, Altman D, editors. Systematic Reviews in Health Care 2London. London: BMJ Publishing Group; 2001. pp. 347–372. [Google Scholar]
- 22.Alam A, Siddiqui M, Quamri A, Fatima S, Roqaiya M, Ahmad Z. Efficacy of Spirulina (Tahlab) in Patients of Type 2 Diabetes Mellitus (Ziabetus Shakri)-A Randomized Controlled Trial. J Diabetes Metab. 2016;7(10).
- 23.Anitha L, Chandralekha K. Effect of supplementation of Spirulina on blood glucose, glycosylated hemoglobin and lipid profile of male non-insulin dependent diabetics. Asian J Exp Biol Sci. 2010;1(1):36–46. [Google Scholar]
- 24.Beihaghi M, Taherzadeh Z. The effects of oral administration of spirulina platensis (cultured Iranian) on blood glucose and glycosylated hemoglobin blood in type ii diabetes mellitus patients. Iran J Diabetes Metab. 2017;16(3):183–190. [Google Scholar]
- 25.Kaur K, Sachdeva R, Grover K. Effect of supplementation of Spirulina on blood glucose and lipid profile of the non-insulin dependent diabetic male subjects. Kidney. 2008;1:5. [Google Scholar]
- 26.Lee EH, Park J-E, Choi Y-J, Huh K-B, Kim W-Y. A randomized study to establish the effects of spirulina in type 2 diabetes mellitus patients. Nutr Res Pract. 2008;2(4):295–300. doi: 10.4162/nrp.2008.2.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani U, Desai S, Iyer U. Studies on the long-term effect of spirulina supplementation on serum lipid profile and glycated proteins in NIDDM patients. J Nutraceuticals, Func Med Foods. 2000;2(3):25–32. doi: 10.1300/J133v02n03_03. [DOI] [Google Scholar]
- 28.Parikh P, Mani U, Iyer U. Role of Spirulina in the control of glycemia and lipidemia in type 2 diabetes mellitus. J Med Food. 2001;4(4):193–199. doi: 10.1089/10966200152744463. [DOI] [PubMed] [Google Scholar]
- 29.Serban M-C, Stoichescu-Hogea G, Gurban C, PETCU F, Jeyakumar D, Andrica F, et al. The role of Spirulina platensis in the control of type 2 diabetes mellitus. BOARD. 1982;96(61.7):27. [Google Scholar]
- 30.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Paquette M, Bernard S, Ruel I, Blank DW, Genest J, Baass A. Diabetes is associated with an increased risk of cardiovascular disease in patients with familial hypercholesterolemia. J Clin Lipidol. 2019;13(1):123–128. doi: 10.1016/j.jacl.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Ho S-H, Li R, Zhang C, Ge Y, Cao G, Ma M, et al. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019;159:77–86. doi: 10.1016/j.watres.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Memije-Lazaro IN, Blas-Valdivia V, Franco-Colín M, Cano-Europa E. Arthrospira maxima (Spirulina) and C-phycocyanin prevent the progression of chronic kidney disease and its cardiovascular complications. J Funct Foods. 2018;43:37–43. doi: 10.1016/j.jff.2018.01.013. [DOI] [Google Scholar]
- 34.Shao W, Ebaid R, El-Sheekh M, Abomohra A, Eladel H. Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): an overview. Grasas Aceites. 2019;70(1):292. doi: 10.3989/gya.0690181. [DOI] [Google Scholar]
- 35.Fan X, Cui Y, Zhang R, Zhang X. Purification and identification of anti-obesity peptides derived from Spirulina platensis. J Funct Foods. 2018;47:350–360. doi: 10.1016/j.jff.2018.05.066. [DOI] [Google Scholar]
- 36.Karkos P, Leong S, Karkos C, Sivaji N, Assimakopoulos D. Spirulina in clinical practice: evidence-based human applications. Evid Based Complement Alternat Med. 2011;2011:1–4. doi: 10.1093/ecam/nen058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serban M-C, Sahebkar A, Dragan S, Stoichescu-Hogea G, Ursoniu S, Andrica F, Banach M. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin Nutr. 2016;35(4):842–851. doi: 10.1016/j.clnu.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Hannan J, Ansari P, Azam S, Flatt PR, Wahab YHA. Effects of Spirulina platensis on insulin secretion, DPP-IV activity and both carbohydrate digestion and absorption indicate potential as an adjunctive therapy for diabetes. Br J Nutr. 2020;124(10):1021–34. [DOI] [PMC free article] [PubMed]
- 39.Sena CM, Carrilho F, Seiça RM. Endothelial dysfunction in type 2 diabetes: targeting inflammation. Endothelial Dysfunction: Old Concepts and New Challenges. 2018;24:23110.
- 40.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physio Pathophysiol Pharmacol. 2019;11(3):45. [PMC free article] [PubMed] [Google Scholar]
- 41.Oh TJ, Moon JH, Choi SH, Lim S, Park KS, Cho NH, Jang HC. Body-weight fluctuation and incident diabetes mellitus, cardiovascular disease, and mortality: a 16-year prospective cohort study. J Clin Endocrinol Metab. 2019;104(3):639–646. doi: 10.1210/jc.2018-01239. [DOI] [PubMed] [Google Scholar]
- 42.Barazzoni R, Cappellari GG, Ragni M, Nisoli E. Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord. 2018;23(2):149–157. doi: 10.1007/s40519-018-0481-6. [DOI] [PubMed] [Google Scholar]
- 43.Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, Clement N, Moes S, Colombi M, Meier JA, Swierczynska MM, Jenö P, Beglinger C, Peterli R, Hall MN. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128(4):1538–1550. doi: 10.1172/JCI96139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gemma C, Mesches MH, Sepesi B, Choo K, Holmes DB, Bickford PC. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar β-adrenergic function and increases in proinflammatory cytokines. Journal of Neuroscience. 2002;22(14):6114–20. [DOI] [PMC free article] [PubMed]
- 45.de Freitas BA, Silva AS, Ferreira PB, Tavares RL, Neto MM. Spirulina platensis prevents oxidative stress and inflammation promoted by strength training in rats: dose-response relation study. Scientific Reports (Nature Publisher Group). 2020;10(1):6382. 10.1038/s41598-020-63272-5. [DOI] [PMC free article] [PubMed]
- 46.Bashir S, Sharif MK, Javed MS, Amjad A, Khan AA, F-u-H S, et al. Safety assessment of Spirulina platensis through Sprague dawley rats modeling. Food Sci Technol. 2020;40(2):376–381. doi: 10.1590/fst.41918. [DOI] [Google Scholar]
- 47.Marles RJ, Barrett ML, Barnes J, Chavez ML, Gardiner P, Ko R, Mahady GB, Dog TL, Sarma ND, Giancaspro GI, Sharaf M, Griffiths J. United States pharmacopeia safety evaluation of Spirulina. Crit Rev Food Sci Nutr. 2011;51(7):593–604. doi: 10.1080/10408391003721719. [DOI] [PubMed] [Google Scholar]
- 48.Hutadilok-Towatana N, Reanmongkol W, Satitit S, Panichayupakaranant P, Ritthisunthorn P. A subchronic toxicity study of Spirulina platensis. Food Sci Technol Res. 2008;14(4):351. doi: 10.3136/fstr.14.351. [DOI] [Google Scholar]
- 49.Scaglioni PT, Pagnussatt FA, Lemos AC, Nicolli CP, Del Ponte EM, Badiale-Furlong E. Nannochloropsis sp. and Spirulina sp. as a source of antifungal compounds to mitigate contamination by Fusarium graminearum species complex. Curr Microbiol. 2019;76(8):930–938. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12 kb)
Data Availability Statement
Not applicable.