Abstract
Background:
Tele-rehabilitation (TR) may be an effective alternative or complement to centre-based cardiac rehabilitation (CBCR) with heart failure (HF) patients, helping overcome accessibility problems to CBCR. The aim of this study is to systematically review the literature in order to assess the clinical effectiveness of TR programs in the management of chronic HF patients, compared to standard of care and standard rehabilitation (CBCR).
Methods and Results:
We conducted a systematic review and meta-analysis of randomized controlled trials on the effect and safety of TR programs in HF patients, regarding cardiovascular death, heart failure-related hospitalizations, functional capacity and quality of life. We searched 4 electronic databases up until May 2020, reviewed references of relevant articles and contacted experts. A quantitative synthesis of evidence was performed by means of random-effects meta-analyses. We included 17 primary studies, comprising 2206 patients. Four studies reported the number of hospitalizations (TR: 301; Control: 347). TR showed to be effective in the improvement of HF patients’ functional capacity in the 6 Minute Walk-Test (Mean Difference (MD) 15.86; CI 95% [7.23; 24.49]; I2 = 74%) and in peak oxygen uptake (pVO2) results (MD 1.85; CI 95% [0.16; 3.53]; I2 = 93%). It also improved patients’ quality of life (Minnesota Living with Heart Failure Questionnaire: MD −6.62; CI 95% [−11.40; −1.84]; I2 = 99%). No major adverse events were reported during TR exercise.
Conclusion:
TR showed to be superior than UC without CR on functional capacity improvement in HF patients. There is still scarce evidence of TR impact on hospitalization and cv death reduction. Further research and more standardized protocols are needed to improve evidence on TR effectiveness, safety and cost-effectiveness.
Keywords: Tele-rehabilitation, heart failure, functional capacity, quality of life, hospitalizations
Introduction
Heart Failure (HF) affects overall 26 000 people worldwide, with a higher prevalence among population above 70 years old (>10%). 1 It has a great economic impact, representing 1% to 2% of healthcare budget in developed countries.1-3 HF is also associated with high mortality and hospitalizations, as well as high impact on functional status and quality of life reduction.3,4
Cardiac Rehabilitation (CR) is an important component of HF treatment. 5 The European Society of Cardiology Guidelines 2016 (ESC) recommend regular aerobic exercise in HF patients, to improve functional capacity and HF symptoms, as well as to reduce the risk of HF-hospitalizations. This is a level I and class A recommendation. 1
Cardiac rehabilitation includes patient assessment, management and control of cardiovascular risk factors, physical activity counselling, exercise training prescription, nutritional advice, as well as psychosocial and vocational support. 5 CR programs are usually centre-based (CBCR) and medically supervised comprising 30 to 45 minute length sessions, with a frequency of 2 to 3 times per week for up to 6 months. 2
Accessibility to CBCR is a limiting factor. This is mainly due to personal economical constrains, lack of availability of CBCR programs, 3 and unawareness of the impact of such programs. 4 Accessibility inequities have significant impact on HF prognosis. 4 Thus, this is a true unmet need in the management of HF patients. Covid-19 pandemic crisis increased this need, due to increased limitations to hospital accessibility.6,7
Telerehabilitation (TR) may be an effective and safe alternative or complement to CBCR.5,8,9 It may help improve HF patients’ accessibility to supervised regular physical exercise at their home or community. 10 In TR programs, patient’s information – physical activity, blood pressure, ECG-recordings, heart rate variability, oxygen saturation – are monitored and transmitted to the medical team. The latter can then provide weekly feedback, in order to adequate exercise program to the patient’s status. 11 TR helps improve adherence to exercise and life style modifications. 12 Preliminary evidence shows potential of TR for cost savings and reduction in health-care facilities utilization, 13 and have not reported any major adverse events, such as arrhythmias or death in HF patients. 14 Nevertheless TR still needs to overcome some challenges for a widespread implementation, such as patient-related barriers (low technological and health literacy, physician lack of awareness, legal and ethical issues, interoperability problems, technical issues and reimbursement difficulties). 5
While the concept is appealing, TR is in its early days and there is a lack of information on its impact. Additionally, major heterogeneity is identified among study populations, duration of interventions, type of home-care devices, and communication with the patient (including intensity and frequency). Previous systematic reviews have analysed the effects of TR on HF alongside other cardiovascular conditions8,15,16 and some focused on the comparison of the effectiveness of different types of CR (CBCR, home-based CR and hybrid CR) on functional capacity and QoL of HF patients. 17
Our systematic review focuses on TR effect on HF patients’ management. The aim of the present study is to systematically review the literature, so as to assess the clinical effectiveness of TR in HF outpatient care, when compared with the standard of care in terms of cardiovascular death and heart failure-related hospitalizations. An additional objective comprises the analysis of TR impact on functional capacity, quality of life, cardiovascular safety and cost-effectiveness.
Methods
This systematic review and meta-analysis was implemented according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. 18
Eligibility criteria
Study designs
This systematic review included randomized controlled trials (RCT) comparing TR programs to usual care for the management of chronic heart failure. Narrative reviews, preclinical studies, in vitro studies, editorial or opinion articles and conference papers were excluded. Previous reviews and meta-analysis were assessed as guide, and reference lists were searched to identify additional RCTs.
Participants
We included studies examining adult outpatients (⩾18 years old and with no restrictions regarding sex, ethnicity and socioeconomic background), with a definitive diagnosis of HF according to the 2016 ESC Guidelines, 1 either with reduced or preserved ejection fraction. Participants were enrolled under stable conditions in ambulatory follow-up or at hospital discharge.
Interventions
We defined TR as an intervention including physical exercise prescription by a CR specialist, performed outside the hospital or the CR centre (implemented at home or community). Additionally, TR should include some form of interaction between patients and medical team, in order to adjust patient’s exercise program and therapy.
Comparators
‘Usual care’ was defined as the standard multidisciplinary management programs proposed by the 2016 ESC Guidelines, 1 including regular follow-up planned appointments (usual care with or without exercise prescription) targeting safety and optimal dosing of medicines, as well as early detection of decompensation or disease progression requiring change in the management scheme. In some cases, patients in the comparator group were advised to do exercise but were not included in a structured and supervised cardiac rehabilitation program.
Outcomes and effect measures
The assessed outcomes included: (i) heart-failure hospitalizations (defined as an admission to a healthcare facility for ⩾24 hours); (ii) cardiovascular mortality (CV mortality); (iii) functional capacity and exercise tolerance, measured with 6-minute walk test (6MWT), peak oxygen uptake (pV02), cardiopulmonary exercise test (CEPT), incremental shuttle walk test (ISWT); (iv) general and disease specific quality of life, measured with Minnesota Living with Heart Failure Questionnaire (MLHFQ), Short Form Health Survey (SF-36), Health related Quality of Life (HRQoL), EuroQol-5D. Other outcomes comprised cardiovascular safety, self-care and therapeutic adherence, mental health, cognitive function, frailty, as well as healthcare costs and cost effectiveness.
Search strategy and information sources
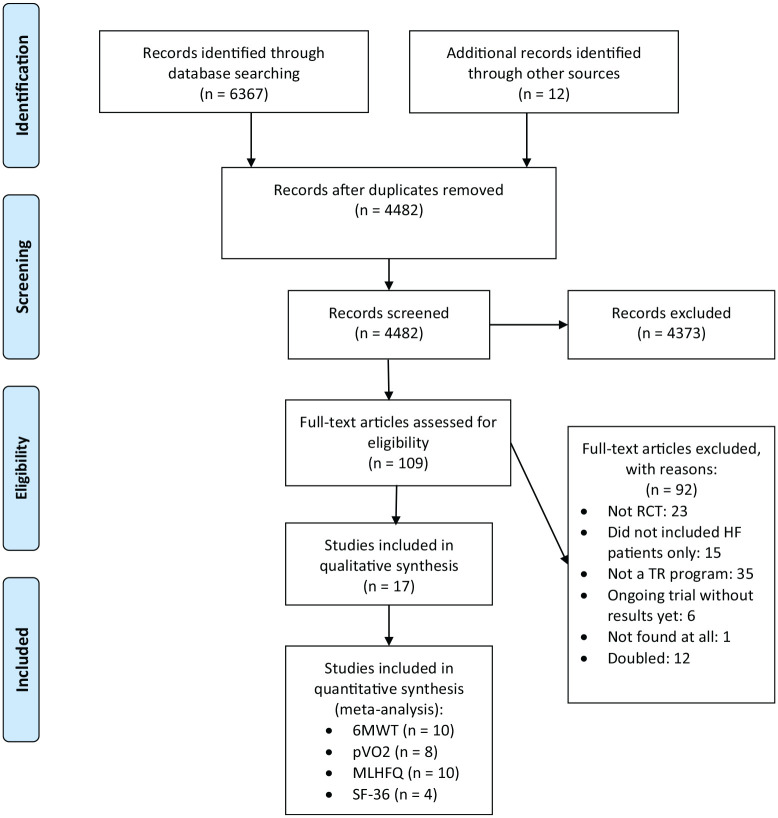
We searched for studies meeting our eligibility criteria in 4 bibliographic databases (MEDLINE, Scopus, Science Citation Index Expanded – Web of Science and Cochrane Central Register of Controlled Trials) up to May 2020. Our full queries are displayed in Figure 1. Search was performed on 3rd June 2020.
Figure 1.
Flow chart of included studies.
Search strategy used different combinations of Medical Subject Headings (MeSH) terms, such as tele-rehabilitation, telecardiology, telecare, remote rehabilitation, virtual rehabilitation (the query used is available at Supplemental Files). We also performed manual searching through grey literature across clinicaltrials.gov in order to retain efficacy in the identification of additional published, unpublished or ongoing trials. In addition, we browsed trial registers, contacted study authors, and searched the references of all relevant primary studies, as well as of other relevant systematic reviews. We included all studies published until May of 2020. No limitation concerning language of publication was applied.
Selection process
Two authors independently screened the titles and abstracts of all records. Subsequently, relevant full-text articles were obtained and read by 2 independent authors. Inter-reviewer discrepancies were solved by discussion and consensus or by a third reviewer when agreement was not reached. When needed, authors were contacted in order to ask for additional information for study eligibility assessment, or to request the full text when unavailable. In the end, only 1 reference could not be found. 19
Data collection process
Data was independently collected by 2 authors, using a standardized form. We retrieved data on patients’ demographic information, study methodology, intervention details, number of total participants and number of participants developing each outcome. Data regarding primary and secondary outcomes were also collected. Inter-reviewer disagreements were solved either by consensus or by a third reviewer when agreement could not be reached. Study authors were contacted to provide missing information.
Study risk of bias and certainty assessment
Statistical analysis was performed using Review Manager 5.3., to assess the possible risk of bias (RoB) for each study, we collected information using the Cochrane Collaboration RoB tool. Each study was classified as ‘high risk’, ‘low risk’ or ‘unclear risk’ of bias. We computed graphic representations of potential bias within and across studies using the same software. Assessments of quality of evidence were performed using the GRADE approach for every outcome. 20
Synthesis methods
A meta-analysis regarding functional capacity (measured with 6MWT, PVO2) and quality of life (measured with MLHFQ and SF-36) was performed using a random effects model with the DerSimonian and Laird method, taking into account the high heterogeneity observed. Data for each outcome was combined and calculated using the RevMan 5.3 software. To overcome the limitations associated with some missing values for important data, we strictly followed Cochrane recommendations. To calculate the standard deviation (SD) of the change between baseline and post-test assessments we used the mean correlation coefficient and SD values of baseline and post-trial measures. Three studies did not report the SD from baseline and post-trial measures. For that reason, we assumed the mean value of SD across the analysed studies.
A qualitative description was performed for other outcomes that could not be included in the meta-analysis. Heterogeneity was analysed with the Chi-square2 test and I-square2 statistic (I2). Moderate or severe heterogeneity was considered if I2 > 40% and I2 > 90%, respectively.
To assess potential moderators of heterogeneity, the following subgroup analysis were performed:
- 1. HF Classification
- a. HFrEF (<40%)
- b. HFrEF + HFpEF
- 2. Presence of telemonitoring
- a. With telemonitoring
- b. Without telemonitoring
- 3. Bias assessment
- a. Low or Unclear risk of bias
- b. High risk of bias
- 4. Follow-up intensity
- a. Regular Follow-up
- b. Intense Follow-up
Sensitivity analysis was performed with a classic take-one-out approach.
Results
Study selection
Out of the 6367 studies initially identified, 17 studies were included in the meta-analyses. Figure 1 shows the selection process and the reasons for article exclusion during the full-text assessment.
Studies’characteristics
This review included a total of 2226 HF patients, counting 1145 patients undergoing TR and 1081 patients undergoing standard of care (258 with CR and 803 without CR). Most patients were male, diagnosed with HF with reduced ejection fraction and were in NYHA class II. All studies were set in developed countries. The included studies were based on several different interventions, although the most frequent was aerobic exercise (walking) with or without strength exercises. In 11 studies (n = 823 patients) UC didn’t include any type of exercise prescription (Table 1).
Table 1.
General characteristics of studies and patients.
| First author Year, country |
HF population | Recruitment setting | Intervention during session | Monitoring during exercise | Feedback | Comparison | Primary outcome | Enrolled patients (retention, %) | Duration of trial/follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Babu 2011, India |
CHF NYHA II-IV |
University teaching hospital | Walking and strength exercise | No | Weekly calls | Usual care | 6MWT | 30 (90) | 8w/8w |
| Bernocchi 2018, Italy |
NYHA II-IV With COPD |
3 rehabilitation centres | Cycling and strength exercise | ECG, pulse oximeter | Weekly calls | Usual care | 6MWT | 112 (71.4) | 4M/6M |
| Chen 2018, Taiwan |
HFrEF NYHA <IV |
Outpatient, general ward and UCI | Aerobic exercise | No | Calls every 2w | Usual care | VO2p, QoL 6MWT |
75 (49.3) | 3M/3M |
| Cowie 2014, Scotland |
HFrEF NYHA II-III |
National Health Service Scotland | Interval aerobic training | No | Calls every 2w | Hospital CR OR Usual care | ISWT, QoL | 60 (76.7) | 8w/8w |
| Hwang 2017, Australia |
NYHA<IV Recent hospitalized |
Cardiology and general medical ward | Aerobic and strength exercises | ECG, pulse oximeter | During the session | Outpatient CR | 6MWT | 53 (92.4) | 12w/24w |
| Lang 2018, Scotland |
HFpEF NYHA <IV |
Single center (Tayside, Scotland) | Walking or chair-based exercises | No | As needed | Usual care | ISWT, QoL, hospitalization | 50 (90) | 12w/6M |
| Servantes 2012, Brazil |
HFrEF 30-70yo NYHA II-III |
Medical center from São Paulo Federal University | Walking and strength exercises | No | Weekly calls | Usual care | VO2p, QoL Strength endurance |
50 (90) | 3M/3M |
| Karapolat 2009, Turkey |
HFrEF NYHA II-III |
Ege University Hospital | Walking, strength, flexibility exercises | No | Weekly calls | Hospital based CR | VO2 peak 6MWT, QoL |
74 (91.9) | 8w/8w |
| Keast 2013, Canada |
EF 20%-35% NYHA II-III | Tertiary cardiac care center, Ottawa | Nordic walk | Online supervision | During the session | Outpatient CR | 6MWT | 54 (79.6) | 12w/12w |
| Piotrowicz 2010, Poland |
HFrEF NYHA II-III |
Institute of Cardiology, Warsaw | Walking | ECG, vitals | Daily calls | Outpatient CR | VO2p, QoL 6MWT |
152 (86.2) | 8w/8w |
| Piotrowicz 2015, Poland |
HFrEF NYHA II-III |
Institute of Cardiology, Warsaw | Nordic walk | ECG, vitals | Daily calls | Usual care | VO2p | 111 (96.4) | 8w/8w |
| SafiyariHafiz 2016, Canada |
HFrEF NYHA <IV |
Not reported | HIIT (walking) and resistance training | HR and pedometer | Calls 2-3x/w | Usual care | 6MWT VO2p, QoL |
40 (72.5) | 12w/12w |
| Frederix 2017, Belgium |
HF NYHA <IV |
Multi-center trial | Walking | Accelerometer | Calls or SMS 1x/w | Outpatient CR | VO2p | 140 (85) | 12w /2y |
| Peng 2018, China |
HFrEF NYHA I-III |
Teaching hospital in Chengdu | Aerobic and strength | Online supervision | At session, weekly calls | Usual care | QoL, 6MWT, HADS | 98 (84.7) | 2M/6M |
| Zielinska 2006, Poland |
HFrEF NYHA II-III |
3 clinics and 1 hospital in Poland | 3w outpatient CR 9w aerobic exercise | ECG, HR | No regular feedback | Usual care | QoL, duration of stress test | 61 (100) | 12w/12w |
| Piotrowicz 2019, Poland |
HFrEF NYHA I-III |
Institute of Cardiology, Warsaw | Nordic walk and strength exercises | Tele-ECG, vitals | Daily calls | Usual care | Mortality and hospitalizations | 850 (91.8) | 9w/26M |
| Dalal 2019, UK |
HFrEF NYHA I-IV |
Four 1ry and 2ry care centres in UK | Walking training or chair-based exercises | No | Not specified | Usual care (some had rehabilitation) | HRQoL at 12 months using MLHFQ | 216 (87) | 12w/12M |
Abbreviations: CHF, congestive heart failure; CPET, cardiopulmonary exercise test; HADS, hospital anxiety and depression score; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; HRQoL, health related quality of life; ISWT, incremental shuttle walk test; LVEF, left ventricule ejection fraction; M, months; MLHFQ, Minnesota Living with Heart Failure Questionnaire; 6MWT, 6-minute walk test; Outpatient, standard cardiac rehabilitation; Pvo2, peak aerobic capacity; QoL, quality of life; Usual care, no exercise prescription; w, weeks.
Risk of bias in studies
Overall bias classification was high risk. Out of the total of 17 studies, 3 were classified as unclear risk,21-23 5 as low risk24-28 and 9 as high risk.29-37
Selection bias related to random sequence used was low risk in almost all trials. A total of 3 studies reported significant differences in baseline characteristics of control and intervention groups.31,36,37
Allocation concealment was low risk in 9 trials.24,25,27,28,30,31,33,35,36 Another 7 studies didn’t present a detailed description of this procedure neither their authors could clarify this topic, so they were classified as unclear.21-23,29,30,32,34 Another trial was classified as high risk because patients’ assignment was based on their acceptance of the intervention. 37
All studies were classified as high risk for performance bias because all were non-blinded due to the nature of interventions.
Considering detection bias, the most frequent classification was unclear: 7 studies didn’t clarify that point,21-24,29,30,37 2 studies did not blind outcome assessors31,32 and 8 studies performed a blind assessment.25-28,33-36
Both attrition and reporting bias domains were most commonly rated as having low risk. Only 1 study did not report all pre-specified outcomes in the protocol. 29
We analysed the risk of intensive monitoring and feedback influence adherence to the intervention. For that topic, 8 studies were considered to have low risk of bias,22-24,27,31,32,36,37 3 were unclear risk25,26,28 and 6 had high risk.21,29,30,33,34,35 These last group reported intensive contacts (daily or weekly) to assess compliance with the program and showed high adherence rates.
Figure 2.
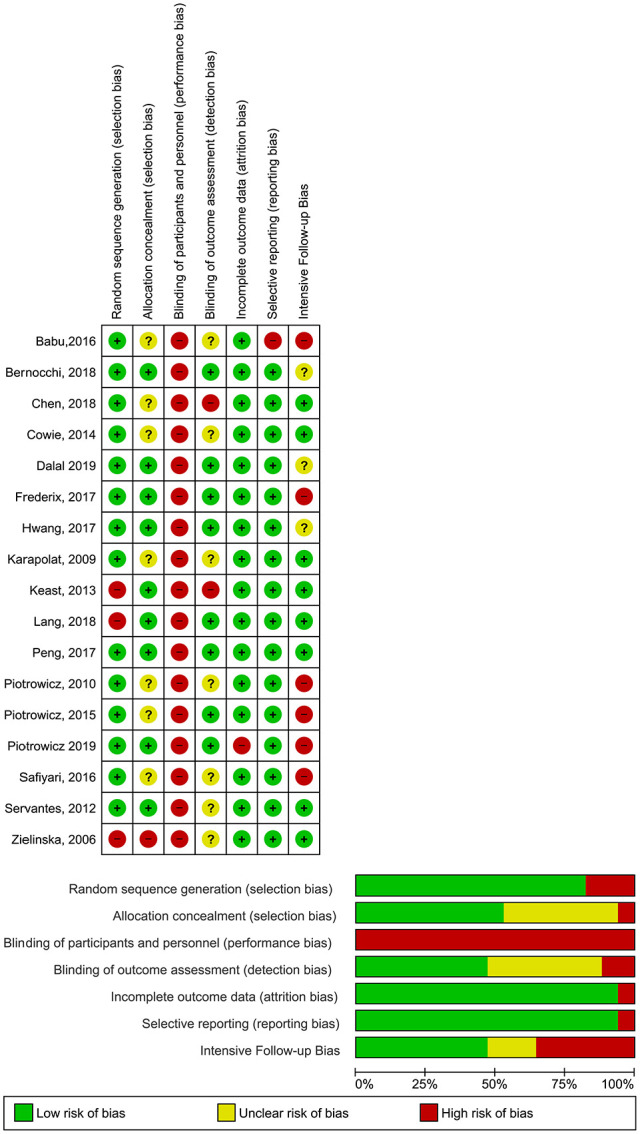
Risk of bias across studies.
Results of synthesis
TR Programs: Description and adherence
Most of TR programs combined aerobic exercise (walking, cycling) with another type of exercises (strength, balance or flexibility), performed 2 to 5 times/week.
In most cases, aerobic exercise started at moderate intensity. In 11 studies the initial intensity level was adjusted to the patients’ status.21,22,24-26,28,29,33,34,35,36 The most common aerobic exercise was walking. In 1 study, exercise was performed as high intensity training. 21 In 7 studies, aerobic exercises were combined with strength exercises.22,24,26-29,33
Regarding feedback from the health team, the majority of programs included a scheme of regular contacts of 1 to 2 phone calls/week from the professional team.
Adherence evaluation to TR programs used different criteria across studies. This way, we could not perform a meta-analysis on this outcome. In 6 studies, adherence to the program, was defined as ‘attending all sessions’.22,24,26,29,34,37 In these cases, adherence rates ranged from 70% to 100% in the intervention groups. In 4 studies, adherence was defined as ‘attendance to more than 80% of sessions’, ranging from 71% to 95% in experimental group.23,32,33,36
CV mortality
Only 1 study presented data on mortality during the follow-up. 33 This study reported a CV mortality rate of 8.3% in the TR group and of 8.8% in the control group (P = .95). All-cause mortality was similar at 2 years of follow-up between the 2 groups (12.5% in TR vs 12.4% in control group; P = .86).
Heart failure-related hospitalizations
Heart failure-related hospitalizations were reported in 4 studies. Dalal et al reported a total of 4 admissions related to HF in the intervention group, and 10 in the control group, after 1 year of follow-up. 25 Lang et al followed patients for 3 months after the end of the trial and registered 4 admissions in the intervention group and 7 admissions in the control group. 36 Frederix et al revaluated patients 2 years after intervention and reported 32 cardiovascular admissions in the intervention group and 60 in the control group. 35 Piotrowicz et al reported 104 hospitalizations in TR group and 103 in usual care at the end of follow-up. 33 Due to lack of analysis of hospital admissions in all trials, we could not perform a statistical analysis of these data. Some studies presented the total number of admissions without further group discrimination.
Functional capacity
6MWT
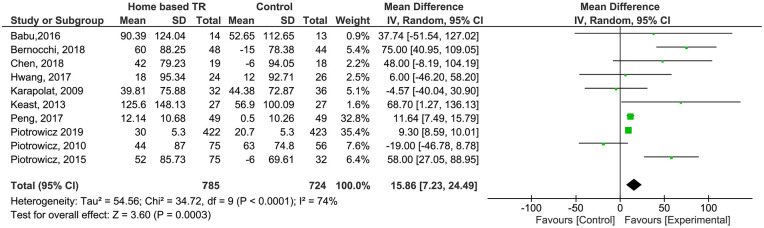
Functional capacity (FC) change measured with 6MWT, was reported in 10 studies comparing TR programs and usual care (n = 1509 patients). In 5 studies, patients in the control group were advised by the medical team to do exercise but were not participating in a structured and centre-based program (n = 168 patients).22,26,30,31,35 The remaining controls didn’t perform any type of exercise (n = 619 patients). Patients in TR group showed a higher improvement in functional capacity than controls (Mean Difference [MD] 15.86; CI 95% [7.23, 24.49]). Moderate heterogeneity was found (I2 = 74%). GRADE assessment considered evidence as moderate quality (Supplemental Files).
Peak VO2
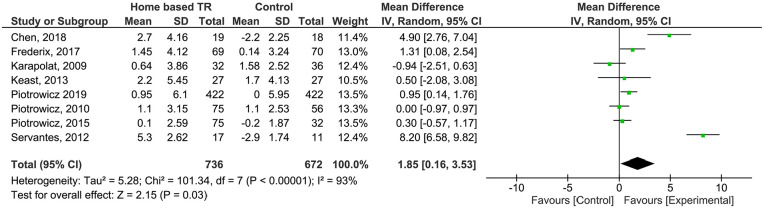
Peak VO2 (pVO2) change was reported in 8 studies,22,24,30-35 comprising 1408 patients. In 4 studies, controls were submitted to standard CR (n = 209 patients)22,30,31,35 and in the remaining 4, patients were under usual care without exercise prescription. Patients in the TR group showed a higher improvement in pVO2 (MD 1.85; CI 95% [−0.16; 3.53]), but the pooled estimate was not statistically significant. Severe heterogeneity was found (I2 = 93%). GRADE assessment considered evidence as very low (Supplemental Files).
Figure 3.
Analysis of 6MWT outcome.
Figure 4.
Analysis of pVO2 outcome.
Quality of Life
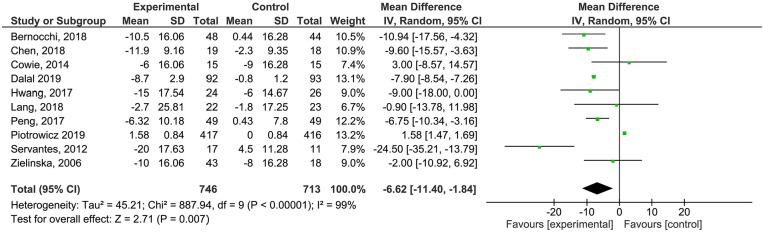
Quality of life (QoL) was evaluated with the Minnesota Living Heart Failure Questionnaire (MLHFQ) in 10 studies,23-28,32,33,36,37 at pre- and post-test, including 1459 patients. Only in 1 study analysing QoL compared TR with standard CR (n = 29 patients). 26 We found a statistical significant higher score in intervention group (Mean Difference −6.62; CI 95% [−11.40; −1.84]) compared to controls. Severe heterogeneity was found (I2 = 99%). GRADE assessment considered evidence as high quality (Supplemental Files).
In 2 studies using EQ5D no differences between groups regarding QoL were found.26,35
In 5 studies, QoL was assessed using Short Form Health Survey (SF36), but only results from 4 studies were included due to lack of information.22,23,29,30 No significant differences were found in the physical score (Mean Difference 0.24; CI 95% [−5.79;6.26]; participants = 256). Considerable heterogeneity was found (I2 = 80%). GRADE assessment considered evidence as very low quality (Supplemental Files). No significant differences were found in mental score, either (MD 0.38; CI 95% [−4.93; 5.70]; participants = 256). Significant heterogeneity was found (I2 = 81%). GRADE assessment considered evidence as very low quality (Supplemental Files).
Figure 5.
Analysis of MLHFQ outcome.
Mental health
Overall, 3 studies used the Hospital Anxiety and Depression Symptoms (HADS)25,27,31 to analyse the impact of TR on the presence of anxiety and depressive symptoms. Peng et al found a positive impact of TR on anxiety and depression reduction in both standards (P = .030 for anxiety; P = .032 for depression). 27 Keast et al reported a significant reduction only in the depression score (P = .014). 31 No significant change between baseline and follow-up, neither between intervention nor control groups were found in Dalal et al. 25
TR safety
Safety evaluation criteria varied widely among trials. Majority of authors classified adverse events as major (such as death, life-threatening arrhythmias, hospitalization) or minor (such as angina, diaphoresis, ankle pain, etc). Nevertheless, none of the studies reported major adverse events related to TR program and interventions were considered safe. Minor adverse events were limited and reported as total number of adverse events (see Supplemental Files).
Cost-effectiveness analysis for TR programs and long-term feasibility
Overall 4 studies made a cost-analysis about TR program.25,26,35,36 In 2 studies a total cost per patient in intervention and control group was calculated.26,35 Frederix et al reported a cost of 3252€ and 4140€, respectively, with a total saving of 888€ per patient with TR program. 35 Hwang et al presented a cost of 2325€ in TR group and 3915€ in control, leading to a saving of 1590€ per patient with TR program. 26 A total of 2 other studies only reported the cost per patient in the TR group.25,36 Lang et al reported a 37 059€ cost 36 and Dalal et al a 46 242€ cost per patient in TR program. 25
Considering long-term feasibility, 6 trials extended the follow-up period beyond the duration of the training program – minimum 2 months and maximum of 2 years.25-28,33,36 In 2 of these studies authors found sustained improvements of FC and QoL in TR group27,28 and 1 found a partial decline. 25 The other 3 found a sustained benefit in QoL assessment.25,26,36
Other secondary outcomes mentioned in the initial protocol – self-care and therapeutic adherence, cognitive function, frailty, difficulties with technology – were not analysed because they were not reported in any of the trials.
Subgroup analysis
A set of pre-specified subgroup analysis of the potential moderators of heterogeneity in TR were conducted. We found better improvements for TR groups in all outcomes after subgroup division (Supplemental Files).
Sensitivity analysis
We identified a decrease in heterogeneity in 6MWT, after leaving out of the analysis the study by Bernocchi et al (74%-61%) 28 and Piotrowicz et al (74%-68%), 34 using take-one-out approach. Regarding pVO2, we have observed a decrease in heterogeneity, after removing the study of Servantes et al (93%-78%). 24 In what concerns QoL measured with MLHFQ, we have also found a decrease in heterogeneity after removing Piotrowicz et al study (99%-53%) 33 (Supplemental Files).
Discussion
In this systematic review, we have analysed the effectiveness of TR programs in the management of HF patients. Overall, 17 primary studies were identified, including a total of 2226 patients.
Evidence on TR programs globally revealed a paucity of effective programs and a huge heterogeneity in terms of settings, forms of intervention and monitoring.
We were not able to perform meta-analysis regarding the primary outcome CV mortality and heart failure hospitalizations, since only 4 studies reported on this kind of events. We hypothesize that this may be due to the short period of intervention and follow-up of most studies. Nevertheless, the 2 most recent studies provided a more detailed analysis of this outcome and longer follow-ups, which may represent a new trend in study designs.17,38
Patients submitted to TR showed significant better results on functional capacity compared to usual care without exercise prescription. It is also relevant to note that patients under TR showed a significant improvement in both 6MWT and pVO2, which highlights TR validity.
Patients assigned to TR also showed a consistent improvement in QoL measured with MLHFQ, when compared to usual care (with or without CBCR). This is possibly associated to the fact that TR is performed at home, with family support, less time constrains, easier logistics and it is an opportunity to the patient feel more useful and involved in management of his condition. Nevertheless, when QoL was assessed with SF-36, no statistical differences between both groups were identified. This may be explainable by the higher specificity of MLHFQ in assessing health-related QoL in HF patients.
Studies included in this meta-analysis were extremely heterogenous regarding adherence evaluation, allowing no definite conclusion. This highlights the need to create a globally acceptable definition of patient adherence in this setting, that could be uniformly used in future trials.
TR cost-effectiveness evaluation was not feasible since only 4 trials presented some results regarding costs per patient, but no formal cost-effectiveness analysis. We strongly suggest that this should be considered in future trials, in order to evaluate the feasibility of TR programs.
Our study analysed the effectiveness of TR in HF patients. Previous systematic reviews assessing the effectiveness of TR included cardiac patients8,16,17 or focused on home-based and hybrid cardiac rehabilitation models, which included other modalities than TR. 18 Despite these differences, our results corroborate previous ones, regarding feasibility and effectiveness of TR or home-based CR on functional capacity and quality of life.
This way, the above-mentioned evidence shows that TR can improve patients’ functional capacity, autonomy and psychological well-being, being superior to usual care without CBCR. Nevertheless, the paucity of available studies together with a high heterogeneity, calls for prudence in the interpretation of this data. Bearing in mind the methodological heterogeneity observed between studies, future study protocols on TR effectiveness should include the following features in order to promote comparability and safety:
ECG measures before the training sessions;
data regarding telemonitoring surveillance of exercise sessions;
data regarding individualized TR program adaptations;
data regarding contacts with the rehabilitation team;
standardized measured of therapeutic adherence and satisfaction with TR
In order to better understand the value of TR in HF management, future studies should also include CBCR in the comparator arm and the analysis of long-terms effects.
Strengths and limitations
This study has some limitations, mostly derived from the big heterogeneity among primary included studies. Ten studies were classified as presenting a ‘high risk of bias’ and all were non-blinded to intervention.22,24-26,30,32,33-35,37 Another limitation concerns to missing data (even all authors were contacted) and poor reporting. These limitations hampered further comparison analysis, namely sub-group analysis.
This study has also important strengths. It was performed according to recommended guidelines, with rigorous data selection and analysis. Other strengths include search in multiple bibliographic databases with no language restrictions, the exploration of sources of heterogeneity by means of subgroup analysis, and the assessment of the provided evidence by adopting the GRADE approach.
Conclusion
Despite being a class I recommendation of the ESC guidelines for HF patients, CBCR is not accessible to most of the patients, due to manifest logistic limitations. Considering the high prevalence of HF there simply are not enough centres to perform cardiac rehabilitation to all patients. In fact, considering that there are 15 million HF patients in Europe, it’s not conceivable a scenario where all could do hospital-based rehabilitation.
This systematic review and meta-analysis suggests that TR is superior to Usual Care and non-inferior to CBCR in improving functional capacity and QoL in HF patients. If supported by future and better designed trials, TR may become a striking alternative to standard CBCR, allowing to reduce inequities in the accessibility to CR and, thus, contribute to the promotion of prognosis in patients with HF.
Supplemental Material
Supplemental material, sj-pdf-1-his-10.1177_11786329211021668 for Effectiveness of Tele-rehabilitation Programs in Heart Failure: A Systematic Review and Meta-analysis by Ana Helena Cavalheiro, José Silva Cardoso, Afonso Rocha, Emí lia Moreira and Luís Filipe Azevedo in Health Services Insights
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was supported by NORTE-01-0145-FEDER-032069 – “AdHeart – Engage with your heart”, financed by NORTE2020 under PORTUGAL2020; Project - NORTE-01-0145-FEDER-000026 – “Symbiotic technology for societal efficiency gains: Deus ex Machina”, financed by NORTE2020 under PORTUGAL2020; National Funds through FCT - Fundação para a Ciência e a Tecnologia, I.P., within CINTESIS, R&D Unit (reference UIDB/4255/2020).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Ana Helena Cavalheiro contributed in the implementation of the study in all phases (study design, data collection and analysis, manuscript writing)
José Silva Cardoso was responsible for the study design and for the supervision of all study phases, as well as for the manuscript review.
Afonso Rocha contributed in the data collection as one of the study reviewers and in the manuscript review.
Emília Moreira contributed in the study design, supervision of data collection and in the manuscript writing.
Luís Filipe Azevedo contributed in the study design, supervision of data collection and analysis and in the manuscript review.
Registration and Protocol: The protocol was registered on PROSPERO with register name of ‘Effectiveness of tele-rehabilitation programs in the follow-up of adult patients diagnosed with heart failure: protocol for a systematic review’ and register number CRD42019119409. It can be accessed at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42019119409.
ORCID iDs: Ana Helena Cavalheiro  https://orcid.org/0000-0002-3116-955X
https://orcid.org/0000-0002-3116-955X
Afonso Rocha  https://orcid.org/0000-0003-0824-4598
https://orcid.org/0000-0003-0824-4598
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200. [DOI] [PubMed] [Google Scholar]
- 2. Conraads VM, Deaton C, Piotrowicz E, et al. Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2012;14:451-458. [DOI] [PubMed] [Google Scholar]
- 3. Piotrowicz E. How to do: telerehabilitation in heart failure patients. Cardiol J. 2012;19(3):243-248. [DOI] [PubMed] [Google Scholar]
- 4. Wenger NK. Current status of cardiac rehabilitation. J Am Coll Cardiol. 2008;51:1619-1631. [DOI] [PubMed] [Google Scholar]
- 5. Ambrosetti M, Abreu A, Corrà U, Davos CH, Hansen D, Frederix I, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. Published online March 30, 2020. doi: 10.1177/2047487320913379. [DOI] [PubMed] [Google Scholar]
- 6. Keesara S, Jonas A, Schulman K. Covid-19 and health care’s digital revolution. N Engl J Med. 2020;382:e82. [DOI] [PubMed] [Google Scholar]
- 7. Neubeck L, Hansen T, Jaarsma T, Klompstra L, Gallagher R. Delivering healthcare remotely to cardiovascular patients during COVID-19: a rapid review of the evidence. Eur J Cardiovasc Nurs. 2020;19:486-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cristo D, Nascimento NP, Dias A, Sachetti A. Telerehabilitation for cardiac patients: systematic review. Int J Cardiovasc Sci. 2018;31:443-450. [Google Scholar]
- 9. Brouwers RWM, van Exel HJ, van Hal JMC, et al. Cardiac telerehabilitation as an alternative to centre-based cardiac rehabilitation. Neth Heart J. 2020;28:443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Batalik L, Filakova K, Batalikova K, Dosbaba F. Remotely monitored telerehabilitation for cardiac patients: a review of the current situation. World J Clin Cases. 2020;8:1818-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Piotrowicz E, Piotrowicz R. Cardiac telerehabilitation: current situation and future challenges. Eur J Prev Cardiol. 2013;20:12-6. [DOI] [PubMed] [Google Scholar]
- 12. Piotrowicz E, Piotrowicz R, Opolski G, Pencina M, Banach M, Zareba W. Hybrid comprehensive telerehabilitation in heart failure patients (TELEREH-HF): a randomized, multicenter, prospective, open-label, parallel group controlled trial-Study design and description of the intervention. Am Heart J. 2019;217:148-158. [DOI] [PubMed] [Google Scholar]
- 13. Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil Rehabil. 2009;31:427-447. [DOI] [PubMed] [Google Scholar]
- 14. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666. [DOI] [PubMed] [Google Scholar]
- 15. Frederix I, Vanhees L, Dendale P, Goetschalckx K. A review of telerehabilitation for cardiac patients. J Telemed Telecare. 2015;21:45-53. [DOI] [PubMed] [Google Scholar]
- 16. Hwang R, Bruning J, Morris N, Mandrusiak A, Russell T. A systematic review of the effects of telerehabilitation in patients with cardiopulmonary diseases. J Cardiopulm Rehabil Prev. 2015;35:380-389. [DOI] [PubMed] [Google Scholar]
- 17. Imran HM, Baig M, Erqou S, Taveira TH, Shah NR, Morrison A, et al. Home-based cardiac rehabilitation alone and hybrid with center-based cardiac rehabilitation in heart failure: a systematic review and meta-analysis. J Am Heart Assoc. 2019;8(16):e012779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xueyu L, Hao Y, Shunlin X, Rongbin L, Yuan G. Effects of low-intensity exercise in older adults with chronic heart failure during the transitional period from hospital to home in china: a randomized controlled trial. Res Gerontol Nurs. 2017;10:121-128. [DOI] [PubMed] [Google Scholar]
- 20. Schünemann H, Brozek J, Guyatt G, Oxman A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. GRADE Working Group; 2013. [Google Scholar]
- 21. Safiyari-Hafizi H, Taunton J, Ignaszewski A, Warburton DE. The health benefits of a 12-week home-based interval training cardiac rehabilitation program in patients with heart failure. Can J Cardiol. 2016;32:561-567. [DOI] [PubMed] [Google Scholar]
- 22. Karapolat H, Demir E, Bozkaya YT, et al. Comparison of hospital-based versus home-based exercise training in patients with heart failure: effects on functional capacity, quality of life, psychological symptoms, and hemodynamic parameters. Clin Res Cardiol. 2009;98:635-642. [DOI] [PubMed] [Google Scholar]
- 23. Cowie A, Moseley O. Home- versus hospital-based exercise training in heart failure: an economic analysis. Br J Cardiol. 2014;21:76. [Google Scholar]
- 24. Servantes DM, Pelcerman A, Salvetti XM, et al. Effects of home-based exercise training for patients with chronic heart failure and sleep apnoea: a randomized comparison of two different programmes. Clin Rehabil. 2012;26:45-57. [DOI] [PubMed] [Google Scholar]
- 25. Dalal HM, Taylor RS, Jolly K, et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: the REACH-HF multicentre randomized controlled trial. Eur J Prev Cardiol. 2019;26:262-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hwang R, Bruning J, Morris NR, Mandrusiak A, Russell T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J Physiother. 2017;63:101-107. [DOI] [PubMed] [Google Scholar]
- 27. Peng X, Su Y, Hu Z, et al. Home-based telehealth exercise training program in Chinese patients with heart failure: a randomized controlled trial. Medicine (Baltimore). 2018;97:e12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernocchi P, Vitacca M, La Rovere MT, et al. Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial. Age Ageing. 2018;47:82-88. [DOI] [PubMed] [Google Scholar]
- 29. Babu AS, Desai CV, Maiya AG, Guddattu V, Padmakumar R. Changes in derived measures from six-minute walk distance following home-based exercise training in congestive heart failure: a preliminary report. Indian Heart J. 2016;68:527-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Piotrowicz E, Baranowski R, Bilinska M, et al. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. Eur J Heart Fail. 2010;12:164-171. [DOI] [PubMed] [Google Scholar]
- 31. Keast ML, D’Angelo MES, Nelson CRM, et al. Randomized trial of Nordic walking in patients with moderate to severe heart failure. Can J Cardiol. 2013;29:1470-1476. [DOI] [PubMed] [Google Scholar]
- 32. Chen YW, Wang CY, Lai YH, et al. Home-based cardiac rehabilitation improves quality of life, aerobic capacity, and readmission rates in patients with chronic heart failure. Medicine (Baltimore). 2018;97:e9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piotrowicz E, Pencina MJ, Opolski G, Zareba W, Banach M, Kowalik I, et al. Effects of a 9-week hybrid comprehensive telerehabilitation program on long-term outcomes in patients with heart failure: the Telerehabilitation in Heart Failure Patients (TELEREH-HF) randomized clinical trial. JAMA Cardiol. 2020;5:300-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piotrowicz E, Zieliłski T, Bodalski R, et al. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: a randomised controlled study. Eur J Prev Cardiol. 2015;22:1368-1377. [DOI] [PubMed] [Google Scholar]
- 35. Frederix I, Hansen D, Coninx K, et al. Telerehab III: a multi-center randomized, controlled trial investigating the long-term effectiveness of a comprehensive cardiac telerehabilitation program – rationale and study design. BMC Cardiovasc Disord. 2015;15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lang CC, Smith K, Wingham J, et al. A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF Pilot Study. BMJ Open. 2018;8:e019649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zielińska D, Rynkiewicz A, Zajt-Kwiatkowska J, Bellwon J, Bakuła S. The impact of cardiac rehabilitation on exercise capacity and quality of life in patients with impaired left ventricle function. Folia Cardiol. 2006;13:208-217. [Google Scholar]
- 38. Zwisler AD, Norton RJ, Dean SG, et al. Home-based cardiac rehabilitation for people with heart failure: a systematic review and meta-analysis. Int J Cardiol. 2016;221:963-969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-his-10.1177_11786329211021668 for Effectiveness of Tele-rehabilitation Programs in Heart Failure: A Systematic Review and Meta-analysis by Ana Helena Cavalheiro, José Silva Cardoso, Afonso Rocha, Emí lia Moreira and Luís Filipe Azevedo in Health Services Insights