Abstract
Background:
HPV-positive oropharynx squamous cell cancer (HPV-OPC) patients were initially described as younger, however incidence has increased among older age-groups. It is unknown why some patients present at a younger age and others at a later age.
Methods:
Multi-institutional prospective study of HPV-OPC cases (n=163) and matched controls (n=345) with detailed behavioral survey, and serum tested for HPV antibodies by fluorescent bead-based technology. Age at diagnosis was used to stratify patients into younger (≤50 years), middle-age (51–65), and older (>65).
Results:
By age, demographic characteristics were largely similar, but HPV biomarkers and sexual acts differed. Younger cases were more likely to be HPV16-positive than older cases (100% vs 77%, p=0.009). Similarly, younger cases were more likely to be HPV16 E6 or E7 seropositive (100% vs 82%, p=0.03). Younger cases had a higher number of oral sex partners per year, a marker of sexual intensity (sex-years, p=0.003), but a similar number of lifetime oral sex partners (measure of cumulative sexual exposure), compared to older cases. While sex-years were higher for younger cases and controls, cases had significantly higher sex-years than matched controls in each age-group (p<0.001).Younger patients were also more likely to perform oral sex at sexual debut, and were younger at sexual debut (each p<0.03).
Conclusions:
Younger, middle-age and older HPV-OPC have distinct biomarker and behavioral profiles. Younger HPV-OPC cases have higher intensity of sexual exposure than older cases and controls, which may in part explain earlier disease onset. The distribution of HPV16-positive tumors among HPV-OPC differs by age group.
Introduction
Human papillomavirus-related oropharynx squamous cell cancer (HPV-OPC) is a well-established entity with unique risk factors, clinical and demographic profiles. Until recently, HPV-OPC patients were characterized as primarily younger in age, white and male1,2. However, it is now appreciated that the incidence of HPV-OPC in the United States (US) is increasing among older, not just younger, age-cohorts3–5 which has resulted in rising median age of presentation. Additionally, the prevalence of HPV-positive OPC tumors has been shown to increase over time among older age groups in analogous fashion with younger age groups6. Similar increases in prevalence trends apply to non-Whites and females in the US7.
Many studies have reported the association between sexual behavior and HPV-OPC8,9. Increased lifetime exposure for each behavior (e.g. oral, vaginal) is associated with a dose-response increase in odds of HPV-positive malignancy. Sexual exposure to HPV is presumed necessary for individuals of any age to develop HPV-OPC, and oral HPV has been shown to precede the diagnosis of HPV-OPC10, although it is not known why some patients present at a younger age and others at a later age, especially if the risk factors are the same. Therefore, this analysis was designed to evaluate whether there are clinical or biological differences between HPV-positive OPC that present at different ages.
Methods
Study Participants
Participants were enrolled in a multicenter case-control study of squamous cell carcinomas called the Papillomavirus Role in Oral cancer Viral Etiology study (PROVE). Cases with newly diagnosed OPC were enrolled between 2013 and 2018 at three NCCN-designated Comprehensive Cancer Centers: the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital (JHH, Baltimore, MD), University of California San Francisco Helen Diller Family Comprehensive Cancer Center (UCSF, San Francisco, CA) and Tisch Cancer Institute at Icahn School of Medicine at Mount Sinai (New York, NY). Case eligibility criteria included known or suspected incident OPC, and no prior systemic chemotherapy or malignancy (except skin cancer). Controls were patients seen for non-cancer conditions in the same otolaryngology clinics, frequency matched to cases by sex, age (by decade), and race/ethnicity (white non-Hispanic, black non-Hispanic, Asian, Hispanic).
Data Collection
At enrollment, each participant completed a survey, blood sample, and tumor sample was obtained, and medical record abstraction was performed. The behavioral risk survey was a computer assisted self-interview (CASI) taken on either a tablet or computer. The survey was translated and available in Mandarin, Spanish and English and included questions on demographics, behavioral risk factors, comorbidities and health status. Serum was obtained before initiation of treatment. Medical record abstraction was performed at the time of diagnosis for tumor site, tumor and nodal stage using American Joint Committee on Cancer (AJCC) 7th edition11, with additional abstraction later to record primary treatment modality. Medical record follow-up was done approximately annually for recurrence and death. Data were stored using RedCap (Vanderbilt University, Nashville TN).
Tumor testing
Centralized tumor HPV testing was performed prospectively at Johns Hopkins and interpreted by two head and neck pathologists (W.H.W. and L.M.R.). P16 immunohistochemistry (MTM Laboratories, Heidelberg, Germany) was performed on each tumor, with p16 expression considered positive if ≥70% strong and diffuse nuclear and cytoplasmic staining pattern was observed12,13. In addition, in situ hybridization (ISH) was performed for HPV16 E6/E7 RNA ISH (RNAscope®, Advanced Cell Diagnostics, Hayward, CA). In cases that were p16-positive but HPV16 RNA ISH-negative, additional testing was done using a cocktail E6/E7 RNA ISH probe (RNAscope, Advanced Cell Diagnostics) that includes 18 high-risk HPV genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82).14
Serum testing
Sera were tested for antibodies to E1, E2, E6 and E7 for oncogenic HPV types 16, 18, 31, 33, 35, 45, 52, and 58. Testing was performed at the German Cancer Research Center (DKFZ, Heidelberg, Germany) using multiplex serology, in combination with fluorescent bead-based technology, as previously described15. Each antibody of interest was considered positive or negative based upon standardized cutoff values for median fluorescence intensity (MFI).
Analysis
This analysis for cases was restricted to HPV-positive participants who answered the “ever” oral sex survey question (97% included). Cases were considered HPV-positive if they were p16 and ISH-positive (RNA and/or DNA). Analyses were stratified by age groups of interest (younger: ≤50, middle-age 51–65 and older ≥65 years). Prevalence of HPV biomarkers and sexual behaviors were compared by age category, and differences were evaluated by X2 test. For continuous variables, medians and interquartile ranges (IQR) were compared using test of medians.
Sexual exposure was evaluated using traditional metrics (lifetime and recent number of sexual partners) as well as a novel metric of intensity coined “sex-years”. Sex-years was calculated as the cumulative lifetime number of sexual partners divided by the number of years of sexual activity (years from sexual initiation to when survey taken) and multiplied by 10 to represent the number of partners per 10 years. To evaluate whether the observed changes in sexual behavior among cases were explained by generational changes in behavior, we also compared sexual behavior of cases in each age group to matched controls.
Results
The characteristics of younger, middle-aged and older HPV-OPC cases were similar (Table 1; N=163), except that younger age cases had a higher household income level (p<0.001) and were less likely to have diabetes (p=0.003). In terms of risk behaviors, there were no differences in alcohol or tobacco exposure, although ever smokeless tobacco (20.0% vs. 5.3% vs. 3.1%, p=0.01) and ever marijuana use (91.4% vs. 75.8%, vs. 62.5%, p=0.02) were significantly more common among the younger age group.
Table 1.
Characteristics of HPV-positive OPC study population by age
| Younger ≤ 50 | Middle-Aged 51–65 | Older >65 | P-value | |
|---|---|---|---|---|
| N= 35 | N= 96 | N= 32 | ||
| Sex | 0.67 | |||
| Male | 88.6 | 86.3 | 81.3 | |
| Race | 0.27 | |||
| White Non-Hispanic | 91.40% | 87.40% | 84.40% | |
| Black Non-Hispanic | 8.60% | 6.30% | 3.10% | |
| Hispanic/Asian/Multiracial/Other | 0.00% | 6.30% | 12.50% | |
| Relationship status | ||||
| Currently married or living with a partner | 71.40% | 81.00% | 71.90% | 0.37 |
| Income | 0.0001 | |||
| < $50,000 | 5.90% | 0.50% | 43.30% | |
| $50,000–99,999 | 23.50% | 32.50% | 13.40% | |
| $>100,000 | 70.60% | 57.00% | 43.30% | |
| Highest degree | ||||
| No college | 22.90% | 21.10% | 21.90% | 0.67 |
| Some college/ college grad | 54.20% | 56.80% | 43.70% | |
| Advanced/Professional degree | 22.90% | 22.10% | 34.40% | |
| Tumor subsite | ||||
| Tonsil | 74.30% | 52.60% | 40.60% | 0.10 |
| Oropharynx | 0.00% | 3.20% | 6.30% | |
| Base of tongue | 22.80% | 36.80% | 50.00% | |
| Other | 2.90% | 7.40% | 3.10% | |
| RISK BEHAVIORS | ||||
| Cigarette smoking | ||||
| Never | 42.90% | 50.50% | 34.40% | 0.10 |
| Former | 45.70% | 41.10% | 65.60% | |
| Current | 11.40% | 8.40% | 0.00% | |
| Other tobacco products | ||||
| E-cigarettes-ever | 8.60% | 5.30% | 0.00% | 0.26 |
| Pipe Smoking-ever | 0.00% | 5.30% | 12.50% | 0.08 |
| Cigar smoking-ever | 34.30% | 15.80% | 18.80% | 0.07 |
| Smokeless tobacco-ever (includes loose leaf, plug, dipping, snus or snuff used orally) | 20.00% | 5.30% | 3.10% | 0.01 |
| Current drinker | 51.70% | 50.60% | 59.30% | 0.86 |
| Drugs^ | ||||
| Ever used marijuana Yes | 91.40% | 75.80% | 62.50% | 0.02 |
| Ever used methamphetamine Yes | 5.70% | 6.30% | 9.40% | 0.80 |
| Ever used cocaine Yes | 34.30% | 36.80% | 18.80% | 0.17 |
| Ever used heroin Yes | 5.70% | 3.20% | 6.30% | 0.68 |
| COMORBIDITY & PHYSICAL HEALTH | ||||
| Comorbidities | ||||
| Asthma Yes | 11.80% | 7.50% | 12.50% | 0.61 |
| Diabetes Yes | 5.70% | 2.10% | 18.70% | 0.003 |
| Coronary heart disease Yes | 2.90% | 3.20% | 12.90% | 0.08 |
| Stroke Yes | 0.00% | 2.10% | 6.30% | 0.24 |
| Anemia Yes | 2.90% | 7.40% | 3.10% | 0.47 |
| HIV Yes | 2.90% | 2.20% | 0.00% | 0.65 |
| STI Yes | 29.40% | 23.40% | 34.40% | 0.44 |
| Autoimmune disorder Yes | 5.70% | 3.20% | 6.40% | 0.68 |
| Ever prior cancer Yes | 14.30% | 22.10% | 25.00% | 0.51 |
| Ever prior HPV related cancer* Yes | 5.70% | 9.50% | 6.30% | 0.71 |
| Ever organ transplant Yes | 5.70% | 1.10% | 0.00% | 0.15 |
| History of tonsillectomy | ||||
| Yes | 38.90% | 41.20% | 50.00% | 0.74 |
| Body mass index | ||||
| Underweight <18.5 | 2.90% | 0.00% | 3.10% | 0.29 |
| Normal 18.5 to <25 | 31.40% | 28.70% | 21.90% | |
| Overweight 25 to <30 | 31.40% | 45.80% | 56.30% | |
| Obese 30+ | 34.30% | 25.50% | 18.70% | |
| Oral hygiene | ||||
| Number of times brush teeth/day median (IQR) | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.07 |
| Number of times floss/week median (IQR) | 2 (0–5) | 3 (1–7) | 5 (0.5–7) | 0.18 |
| Regular mouth wash Yes | 45.70% | 44.20% | 53.1% | 0.68 |
HPV-related cancers include anal, cervical, penile, and oropharyngeal cancer
People who did not answer the question on drug use were assumed to not have used that substance
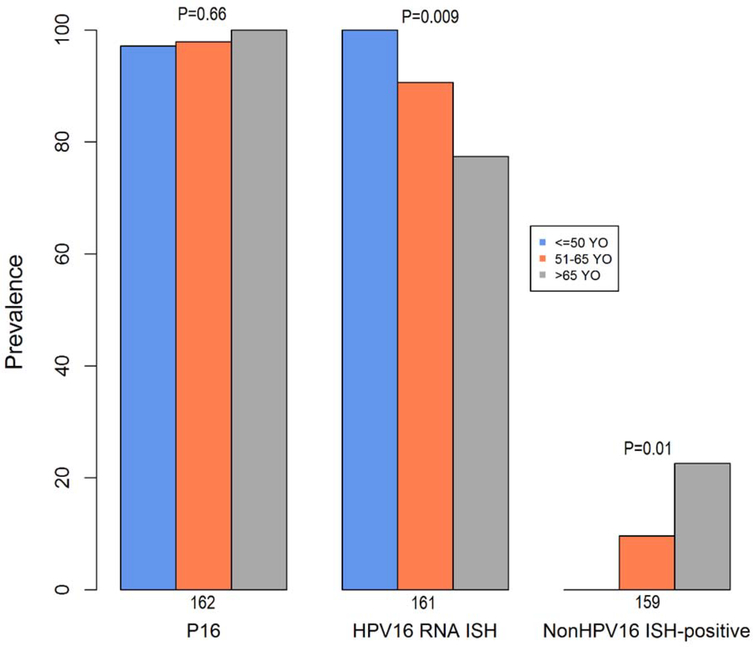
Differences in biomarkers for HPV-OPCs were explored across age groups (Table 2). The prevalence of p16 was similar for each age group (range 97–100%). HPV16 was detected by RNA ISH in all of the young cases but was less common in middle-aged (90%) and older (77%) participants (p=0.009; Figure 1). Conversely, the proportion of non-HPV16 ISH-positive tumors was significantly lower in the younger age group (0.0%) compared to the middle aged (9.6%) and older (22.6%) patients, p=0.01.
Table 2.
HPV biomarker prevalence among younger (≤50 years old), middle aged (51–65 years old) and older (>65 years old) HPV-related^ OPC cases.
| Younger | Middle-Aged | Older | P-values | ||
|---|---|---|---|---|---|
| TUMOR | N | N=35 | N=96 | N=32 | |
| P16 | 162 | 97.1% | 97.9% | 100.0% | 0.66 |
| HPV16 RNA ISH | 161 | 100.0% | 90.6% | 77.4% | 0.009 |
| P16-positive, ISH16-RNA negative | 160 | 0.0% | 9.4% | 23.3% | 0.007 |
| P16-positive, non-HPV16 ISH-positive | 159 | 0.0% | 9.6% | 22.6% | 0.01 |
| Additional testing | |||||
| High-risk HPV RNA | 16 | --- | 100.0% | 100.0% | --- |
| SERUM ANTIBODIES | N | N=31 | N=87 | N=28 | |
| HPV16 E6 or E7 | 146 | 100.0% | 93.1% | 82.1% | 0.03 |
| HPV16 E-proteins: positive for any 3 out of 4 (E1, E2, E6, E7) for HPV16 | 146 | 87.1% | 81.6% | 53.6% | 0.003 |
| HPV18 E-proteins: positive for any 3 out of 4 (E1, E2, E6, E7) for HPV18 | 146 | 0.0% | 3.5% | 0.0% | 0.35 |
|
Type-specific antibodies | |||||
| HPV16 L1 | 146 | 77.4% | 69.0% | 60.7% | 0.38 |
| HPV16 E6 | 146 | 96.8% | 92.0% | 75.0% | 0.01 |
| HPV16 E7 | 146 | 87.1% | 73.6% | 39.3% | <0.001 |
| HPV18 E6 | 146 | 0.0% | 3.5% | 3.6% | 0.57 |
| HPV18 E7 | 146 | 3.2% | 6.9% | 0.0% | 0.30 |
| HPV31 E6 | 146 | 0.0% | 2.3% | 10.7% | 0.051 |
| HPV31 E7 | 146 | 54.8% | 56.3% | 39.3% | 0.28 |
| HPV33 E6 | 146 | 51.6% | 52.9% | 32.1% | 0.15 |
| HPV33 E7 | 146 | 61.3% | 60.9% | 42.9% | 0.21 |
| HPV35 E6 | 146 | 0.0% | 8.1% | 10.7% | 0.21 |
| HPV35 E7 | 146 | 61.3% | 57.5% | 35.7% | 0.09 |
| HPV45 E6 | 146 | 0.0% | 2.3% | 3.6% | 0.61 |
| HPV45 E7 | 146 | 0.0% | 5.8% | 3.6% | 0.38 |
| HPV52 E6 | 146 | 3.2% | 5.8% | 10.7% | 0.47 |
| HPV52 E7 | 146 | 35.5% | 22.8% | 25.0% | 0.32 |
| HPV58 E6 | 146 | 16.1% | 19.5% | 28.6% | 0.46 |
| HPV58 E7 | 146 | 35.5% | 31.0% | 32.1% | 0.90 |
HPV-related defined as positive for tumor HPV16 DNA or RNA, or p-16 positive with another HPV-related biomarker. P16 positive cases who were negative for HPV DNA and RNA were not considered HPV-related
non-HPV16 ISH positive was defined as anyone who was HPV16 RNA ISH negative and HPV HR ISH RNA was positive (or HPV16 RNA ISH negative).
Figure 1.
For each HPV tumor biomarker, proportion positive is considered by age group at diagnosis. P-value represents difference across age groups.
Prevalence of HPV type-specific serum antibodies were also evaluated. Serum was available for 89.5% of the study population. HPV16 L1 seropositivity, a marker of lifetime HPV16 exposure, was similar across HPV-OPC cases of each age group (range 60.7–77.4%; p=0.38). However, a significantly higher proportion of younger than older patients were seropositive to HPV16 E6 or E7 oncogenes (100% vs. 93.1% vs. 82.1%, p=0.03, Table 2). Seroprevalence of oncogenes for other HPV types was similar by age.
We next explored differences in sexual exposures for HPV-OPCs across age groups (Table 3). Ever oral sex and vaginal sex were ubiquitously reported by all age groups (range 97–100%). Median age of sexual debut (16 vs. 16 vs. 18 years, p<0.001) and first performing oral sex on women (17 vs. 18. vs. 21 years, p<0.001) was significantly lower among the younger than middle-aged or older age cases. Despite these statistical differences, it is of note that across all age groups most cases (75th percentile) initiated sex before age 20 and first performed oral sex by age 25.
Table 3.
Prevalence of sexual behaviors among HPV-related OPC cases, by age
| N | Younger N= 35 | Middle-Aged N= 96 | Older N= 32 | P-value | |
|---|---|---|---|---|---|
| Ever perform oral sex | 163 | 100% | 99% | 97% | 0.49 |
| Age of sexual debut: median (IQR) | 162 | 16 (15, 17) | 16 (15, 18) | 18 (17, 20) | <0.001 |
| Age of first performing oral sex on a woman (among heterosexual men): median (IQR) | 133 | 17 (16, 18) | 18(17, 21) | 21(19, 25) | <0.001 |
| First sexual act | |||||
| Vaginal sex only | 158 | 47% | 67% | 82% | 0.028 |
| Vaginal and performed oral sex | 44% | 25% | 9% | ||
| Performed oral sex only | 9% | 8% | 9% | ||
| Lifetime number of sexual partners | |||||
| Open mouth kissing partners: median (IQR) | 161 | 30 (20–75) | 28 (10–50) | 27 (9–51) | 0.93 |
| Vaginal sex: median (IQR) | 157 | 18 (10–49) | 19 (7–30) | 12 (6–30) | 0.93 |
| Performed oral sex on: median (IQR) | 163 | 10 (8–25) | 8 (3–25) | 10 (3–20) | 0.77 |
| Intensity of sexual exposure (sex-years)^ | Median (IQR) | ||||
| Vaginal sex: median (IQR) | 157 | 5.6 (3.3, 16.6) | 4.2 (1.71, 7.5) | 2.2 (1.17, 5.9) | 0.08 |
| Oral sex: median (IQR) | 162 | 3.6 (2.6, 8.3) | 2.0 (0.82, 6.6) | 1.9 (0.48, 4.2) | 0.003 |
| Anal sex: median (IQR) | 161 | 0.6 (0.30, 1.3) | 0.25 (0.00, 0.8) | 0.17 (0.00, 0.4) | <0.001 |
Number of lifetime partners per 10 years of age after sexual debut)
Notable differences were observed in ordering of sexual acts among cases of different age groups. More than half of the younger cases (53%) performed oral sex at sexual debut compared to a third or less of middle-age (33%) and older participants (18%; p=0.03). Performing both oral and vaginal sex at debut was also more common among younger, than either middle-age or older participants (44% vs. 25% vs. 9%, p=0.03). Several other sexual dynamics of interest did not differ by age, including ever paying for sex (p=0.97), sex at a young age with an older partner (p=0.47) and having sex outside of their relationship or suspecting that a partner did (p=0.22). Although lifetime number of partners for any sex type and open mouth kissing were generally similar across age groups, none of the young patients reported one or less lifetime oral sex partners while 19% of older patients did (p=0.01).
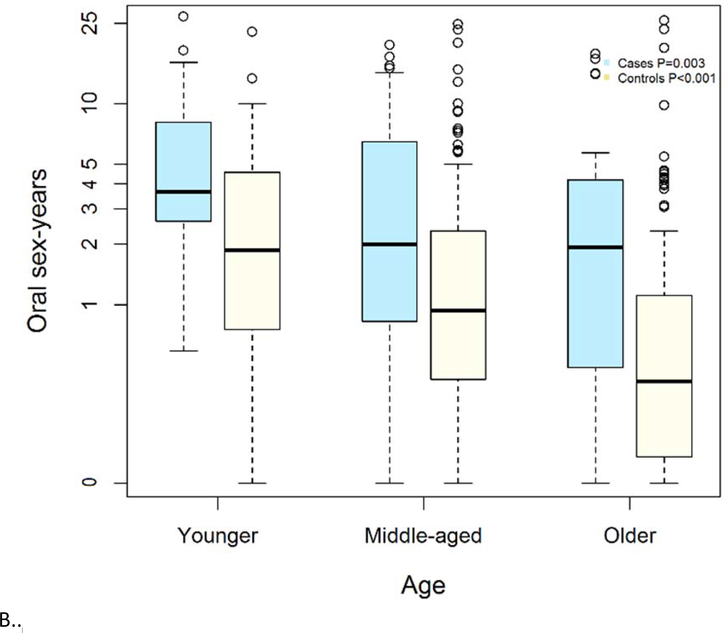
Despite similar number of lifetime sexual partners across age groups, younger age groups had a higher intensity of sexual exposure. When considering the rate of sexual exposure, or number of partners performed oral sex on per ten years (referred to as oral sex-years), younger participants had a median of 3.6 sex-years compared to almost half that rate among older participants (1.9 sex-years, p=0.003; Figure 1). For example, this difference in rates would represent a median of 10 lifetime oral sex partners for both a 42 and a 65 year-old HPV-OPC who had sexual debut at 15, but the 42 year old would have had twice as many partners per year to accumulate that same cumulative number of partners. Oral sex-years was significantly higher among young HPV-OPC cases than middle-age or older cases (p=0.003).
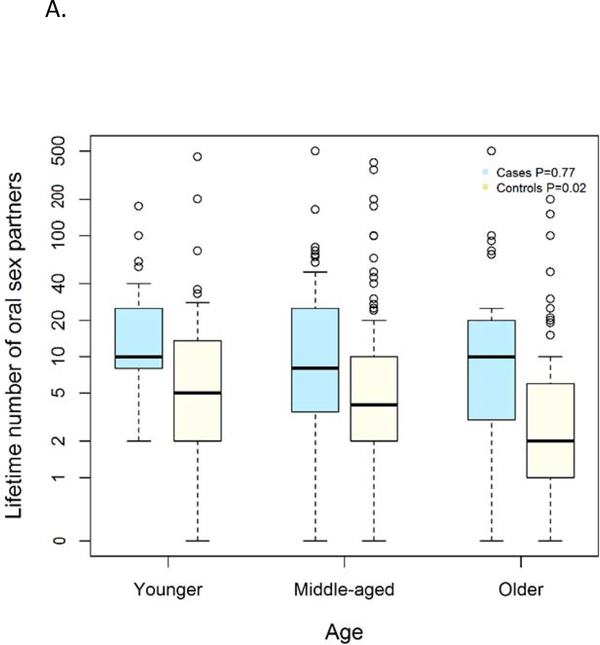
To understand whether differences in sexual behavior among HPV-OPC were explained by generational changes in behavior, we next compared sexual behavior of cases to matched controls (Figure 2). As expected, among every age group, the median number of oral sex partners was significantly higher among cases than controls: younger (10 vs 5 partners, p=0.003), middle-aged (8 vs 4 partners p=<0.001) and older (10 vs 2 partners p=0.003). However, while the numbers of lifetime oral sexual partners was similar among HPV-OPC cases of different ages (p=0.77), the number of partners changed significantly among controls by age group, with higher number of partners among young than old controls (5 vs 2, p=0.02). When examining sexual intensity, similar differences were observed with significantly higher sex-years among cases than controls, and increasing sex-years among younger individuals than older individuals. A higher median number of oral sex-years was observed among cases than similarly aged controls in each age group including: younger (3.63 vs 1.87 partners/10 years, p=0.01), middle-aged (2.00 vs 0.94 partners/10 years p=<0.001) and older (1.93 vs 0.42 partners/10 years p=0.004).
Figure 2.
Panel A depicts number of people performed oral sex on in lifetime (y-axis) by age group (x-axis). Panel B represents oral sex-years on y-axis (number of people performed oral sex on per 10 years of age) and age group on x-axis. Age groups are younger (≤50 years), middle-aged (51–65 years) and older (65 and older).
Discussion
Recent findings have shown that HPV-OPC is not uniformly a disease of individuals who are younger, white, and non-smokers, as initially described1,9,16, but is also increasing among older individuals and non-whites3,6,7. To date, HPV-OPC has been regarded as a homogeneous entity; HPV-positive patients are often described to have higher number of oral and vaginal sex partners, lower tobacco exposure and increased marijuana use relative to HPV-negative OPC patients8. With the exception of prognostic groups16, few analyses have investigated sources of epidemiologic heterogeneity among HPV-OPC. In this multi-institutional prospective cohort analysis, we show that HPV-positive OPC is a heterogeneous entity. Indeed, people who develop this disease at different ages appear to have distinct HPV genotype distribution in tumor and serum antibodies and different behavioral exposures. We also present a novel metric (sex-years) which may have utility as a risk-communication measure.
For HPV-OPCs, striking differences in HPV genotype distribution were observed between younger, middle-aged, and older HPV-OPC patients: A significantly higher proportion of younger patients had HPV16 (vs. other nonHPV16 oncogenic HPV types) (Table 2, Figure 1). This difference was observed both by tumor RNA ISH and by HPV serum antibody testing. Of note, none of the younger patients at diagnosis had a non-HPV16-positive tumor, as compared to 23% of older patients (p=0.01). This is consistent with several prior studies which compared HPV16-positive and non-HPV16-positive OPC tumors20–23, although age differences were not elaborated on. Further supporting the plausibility of these findings, these same differences by age have been shown in the cervical cancer literature where HPV16 is the predominant type in younger women and decreases in prevalence with increasing age24. Of note, among women without cervical dysplasia, HPV16 infection has a higher risk of progression to CIN3+ than other high risk HPV types25, which suggests that HPV16 is a more potent carcinogen (more likely to persist and quicker to progress) than other high-risk HPV types26,27. The higher prevalence of non16 oncogenic HPV types among older OPC cases is consistent with the hypothesis that these types take longer to progress and cause OPC. However, there were a modest number of older HPV-OPC in this study, and these findings will need to be reproduced in other studies. Reasons for differences in natural history for oral HPV16 as compared to non-HPV16 high-risk types are unknown. These findings underscore the importance of long-term natural history studies for oral HPV infection to determine the factors associated with persistence and malignant transformation for each oral HPV type, as well time between infection, carcinogenesis and clinical presentation. It will be important to understand whether there are immunologic underpinnings to a later susceptibility to non-HPV16 infection, especially in the context of presumed prior exposure and clearance of HPV16.
There remains a question in the literature regarding whether prognosis for non-HPV16-related OPC differs from that of HPV16. Some studies have suggested higher overall survival for HPV16 than non-HPV16 OPC, while others have not.21,23,28 It is possible that age was a confounder in these studies explaining the apparent effect of HPV type modulating tumor sensitivity to treatment. Presently, HPV tumor testing is recommended in clinical standard of care, but only to inform prognosis, not to influence treatment decision-making. If non-HPV16 is indeed associated with worse survival after accounting for age, then clarification of HPV type, not just tumor status may be needed.
Differences in HPV serology type were observed by age consistent with the observed differences in tumor HPV type distribution. All of the younger HPV-OPC patients were HPV16 E6 or E7 seropositive, compared to only 82% of older patients. HPV16 E6 but not E7 seropositivity was similar to tumor HPV16 status, supporting the potential for E6 serology as a biomarker for HPV-OPC. When looking at individual non-HPV16 oncogenic HPV types seroprevalence was not significantly different by age, although some types appeared to be less common in younger than older patients (e.g. HPV31 E6 was 0% vs 11%, p=0.051). It has been hypothesized that immunosenescence may contribute to re-activation or later age of presentation with HPV-OPC or cervical cancer29. However, the proportion of older patients who was seropositive to HPV16 E6 or E7 oncogenes mirrored that with HPV16-positive tumors, which does not support this theory of immunosenescence in older cases.
In addition to the differences in HPV tumor and antibody type detected between younger, middle-aged and older patients, striking differences in behavioral characteristics, albeit nuanced, were observed. While both behavioral and serologic markers of lifetime sexual exposure (e.g. number of oral sexual partners and HPV16 L1 seroprevalence) are similar for HPV-OPC patients of different ages, the timing and type of initial sexual act differs for patients who present at a younger rather than older age. Patients who presented at a younger age were significantly younger at their sexual debut, and age of first performing oral sex (p<0.001 for each) and less commonly had vaginal sex exclusively during first sexual act (p=0.03). It is therefore not surprising, given the suspected lead time of at least ten years from initial infection to diagnosis of cancer17,18, that younger patients had oral exposure to HPV at a younger age, although the differences in median age of coitarche by group are substantially less than median age of diagnosis with OPC. Given that infection with HPV alone is likely to be insufficient for carcinogenesis17, other co-factors which are yet to be identified are likely involved in oncogenesis or alternate sexual exposures prior to later diagnosis with similar lead time not detected in the survey is possible. Interestingly, younger patients more commonly reported using smokeless tobacco and marijuana, which supports their evaluation in future prospective natural history studies.
This analysis shows for the first time that while sexual exposure is a risk factor for all HPV-OPC, individuals diagnosed with cancer at a younger age have had a higher intensity of exposure (more partners per year) while individuals diagnosed later in life had a comparatively lower intensity of sexual exposures but over a longer time (Table 3, Figure 2). While many previous studies have shown the strong association between number of lifetime sexual partners and oral HPV prevalence18 or odds of HPV-OPC8, to our knowledge, the rate of sexual exposure has not been previously explored for OPC or other sexually transmitted malignancies. Sex-years is a measure of intensity analogous to tobacco pack-years, a well-established epidemiologic and clinical assessment of exposure, and provides a different metric in which to consider sexual exposure. Indeed, epidemiologic studies have demonstrated that increasing tobacco intensity, as measured by pack-years, is associated with increased risk for malignancies. This measure does not address whether exposure during certain periods of life (e.g. immediately after sexual debut, or after a mid-life divorce) may have difference in risk. It does suggest that among those who go on to develop HPV-OPC a higher rate of early exposure to multiple partners may lead to cancer development more quickly than that same number of partners over a longer period of time.
Previous hypotheses for why some HPV-positive OPC patients have a younger age of presentation included earlier sexual debut, cohort effect from sexual revolution and sexual dynamics19. While this study shows that younger patients do indeed have earlier sexual debut, the actual differences in median age between young, middle and older patients are disproportionate (relatively small when compared to the differing ages of presentation). Median age of sexual debut for young and older cases were 16 and 18, respectively, while median age of diagnosis was 47 and 70. A cohort (or generational) effect in number of lifetime oral sexual partners was observed among controls, but not cases. However, it is noteworthy that while lifetime number of sexual partners was similar across age groups for HPV-OPC, intensity (measured by sex-years) was significantly higher in the young cases and declined with aging.
Lastly, differences in sexual dynamics were not observed between age groups; when considering sex outside of long-term relationships, and sex with an older individual at a young age, no statistical differences across age groups were observed suggesting that differences in sexual dynamics do not explain differences in age at presentation of HPV-OPC.
Other notable behavioral differences across age groups that were observed included differences in exposure to marijuana and smokeless tobacco. Younger patients were significantly more likely to have used both marijuana and smokeless tobacco and cocaine, although this was not statistically different. These behaviors further emphasize that patients who present younger have distinct behavioral exposures as compared to older patients; it is not known whether these represent generational differences (cohort effect) or have a role as co-factors for HPV persistence.
Strengths of this study include being multi-institutional and prospective with centralized tumor and serologic testing, and that the study population is from a contemporary time period. Despite the large study population overall, the age stratification did result in relatively small numbers in each group. We acknowledge the limitation of sample size and power which may reduce the precision of estimates and thus the certainty of the observed age-related differences. A sensitivity analysis that was performed dividing the cases into two instead of three age groups had similar findings. While the differences observed by age in sex-years suggest there may be intensity differences in disease etiology, we cannot exclude the possibility that relevant exposure periods are similar in young and older cases and the longer onset among older cases is explained by another unknown cofactor which creates the appearance of a difference in intensity that is not causal.
In summary, patients who present with HPV-OPC at a younger age are more likely to have HPV16-positive tumors and HPV16-seropositive status, and higher intensity sexual exposure when measured by a novel epidemiologic tool. These data suggest, for the first time, that HPV-OPC is a heterogeneous epidemiologic entity. These findings emphasize the importance of understanding the natural history of type-specific oral HPV are important for future epidemiologic studies, and clinical trials.
Acknowledgments
Funding: This work has been funded by R35DE026631 (NIDCR), P50 DE019032 (NIDCR) and Oral Cancer Foundation
Footnotes
Conflicts: The authors have no conflicts
References
- 1.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011 [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2015;33(29):3235–3242. doi: 10.1200/JCO.2015.61.6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumsteg ZS, Cook-Wiens G, Yoshida E, et al. Incidence of Oropharyngeal Cancer Among Elderly Patients in the United States. JAMA Oncol. 2016;2(12):1617–1623. doi: 10.1001/jamaoncol.2016.1804 [DOI] [PubMed] [Google Scholar]
- 4.Rettig EM, Zaidi M, Faraji F, et al. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of Human Papillomavirus is attenuated among older patients: Analysis of the National Cancer Database. Oral Oncol. 2018;83:147–153. doi: 10.1016/j.oraloncology.2018.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rettig EM, Fakhry C, Khararjian A, Westra WH. Age Profile of Patients With Oropharyngeal Squamous Cell Carcinoma. JAMA Otolaryngol-- Head Neck Surg. 2018;144(6):538–539. doi: 10.1001/jamaoto.2018.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Windon MJ, D’Souza G, Rettig EM, et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer. 2018;124(14):2993–2999. doi: 10.1002/cncr.31385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Souza G, Westra WH, Wang SJ, et al. Differences in the Prevalence of Human Papillomavirus (HPV) in Head and Neck Squamous Cell Cancers by Sex, Race, Anatomic Tumor Site, and HPV Detection Method. JAMA Oncol. December 2016. doi: 10.1001/jamaoncol.2016.3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025 [DOI] [PubMed] [Google Scholar]
- 9.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497 [DOI] [PubMed] [Google Scholar]
- 10.Agalliu I, Gapstur S, Chen Z, et al. Associations of Oral α-, β-, and γ-Human Papillomavirus Types With Risk of Incident Head and Neck Cancer. JAMA Oncol. 2016;2(5):599–606. doi: 10.1001/jamaoncol.2015.5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming ID. AJCC Cancer Staging Manual. Fifth Edition. Philadelphia: Lippincott-Raven; 1997. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC5thEdCancerStagingManual.pdf [Google Scholar]
- 12.Fakhry C, Lacchetti C, Rooper LM, et al. Human Papillomavirus Testing in Head and Neck Carcinomas: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists Guideline. J Clin Oncol Off J Am Soc Clin Oncol. September 2018:JCO1800684. doi: 10.1200/JCO.18.00684 [DOI] [PubMed] [Google Scholar]
- 13.Lewis JS, Beadle B, Bishop JA, et al. Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Arch Pathol Lab Med. December 2017. doi: 10.5858/arpa.2017-0286-CP [DOI] [PubMed] [Google Scholar]
- 14.Bishop JA, Ma X-J, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36(12):1874–1882. doi: 10.1097/PAS.0b013e318265fb2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzinger D, Wichmann G, Baboci L, et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int J Cancer. 2017;140(12):2748–2757. doi: 10.1002/ijc.30697 [DOI] [PubMed] [Google Scholar]
- 16.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreimer AR, Campbell CMP, Lin H-Y, et al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. The Lancet. 2013;382(9895):877–887. doi: 10.1016/S0140-6736(13)60809-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillison ML, Broutian T, Pickard RKL, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307(7):693–703. doi: 10.1001/jama.2012.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Souza G, Cullen K, Bowie J, Thorpe R, Fakhry C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PloS One. 2014;9(1):e86023. doi: 10.1371/journal.pone.0086023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman MT, Saraiya M, Thompson TD, et al. Human papillomavirus genotype and oropharynx cancer survival in the United States of America. Eur J Cancer. 2015;51(18):2759–2767. doi: 10.1016/j.ejca.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varier I, Keeley BR, Krupar R, et al. Clinical characteristics and outcomes of oropharyngeal carcinoma related to high-risk non-human papillomavirus16 viral subtypes. Head Neck. 2016;38(9):1330–1337. doi: 10.1002/hed.24442 [DOI] [PubMed] [Google Scholar]
- 22.Mazul AL, Rodriguez-Ormaza N, Taylor JM, et al. Prognostic significance of non-HPV16 genotypes in oropharyngeal squamous cell carcinoma. Oral Oncol. 2016;61:98–103. doi: 10.1016/j.oraloncology.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bratman SV, Bruce JP, O’Sullivan B, et al. Human Papillomavirus Genotype Association With Survival in Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2016;2(6):823–826. doi: 10.1001/jamaoncol.2015.6587 [DOI] [PubMed] [Google Scholar]
- 24.Hammer A, Rositch A, Qeadan F, Gravitt PE, Blaakaer J. Age-specific prevalence of HPV16/18 genotypes in cervical cancer: A systematic review and meta-analysis. Int J Cancer. 2016;138(12):2795–2803. doi: 10.1002/ijc.29959 [DOI] [PubMed] [Google Scholar]
- 25.Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102(19):1478–1488. doi: 10.1093/jnci/djq356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sand FL, Munk C, Frederiksen K, et al. Risk of CIN3 or worse with persistence of 13 individual oncogenic HPV types. Int J Cancer. September 2018. doi: 10.1002/ijc.31883 [DOI] [PubMed] [Google Scholar]
- 27.Gargano JW, Nisenbaum R, Lee DR, et al. Age-group differences in human papillomavirus types and cofactors for cervical intraepithelial neoplasia 3 among women referred to colposcopy. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2012;21(1):111–121. doi: 10.1158/1055-9965.EPI-11-0664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin LX, D’Souza G, Westra WH, et al. Prognostic factors for human papillomavirus–positive and negative oropharyngeal carcinomas. The Laryngoscope. 2018;128(8):E288–E296. doi: 10.1002/lary.27130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gravitt PE, Rositch AF, Silver MI, et al. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J Infect Dis. 2013;207(2):272–280. doi: 10.1093/infdis/jis660 [DOI] [PMC free article] [PubMed] [Google Scholar]