Abstract
Screening for cancer is a proven and recommended approach to prevent deaths from cancer; screening can locate precursor lesions and/or cancer at early stages when it is potentially curable. Racial and ethnic minorities and other medically underserved populations exhibit lower uptake of cancer screening than nonminorities in the United States. The COVID-19 pandemic, which disproportionately affected minority communities, has curtailed preventive services including cancer screening to preserve personal protective equipment and prevent spread of infection. While there is evidence for a rebound from the pandemic-driven reduction in cancer screening nationally, the return may not be even across all populations, with minority population screening that was already behind becoming further behind as a result of the community ravages from COVID-19. Fear of contracting COVID-19, limited access to safety-net clinics, and personal factors like, financial, employment, and transportation issues are concerns that are intensified in medically underserved communities. Prolonged delays in cancer screening will increase cancer in the overall population from pre-COVID-19 trajectories, and elevate the cancer disparity in minority populations. Knowing the overall benefit of cancer screening versus the risk of acquiring COVID-19, utilizing at-home screening tests and keeping the COVID-19–induced delay in screening to a minimum might slow the growth of disparity.
Racial and ethnic minorities and other medically underserved populations continue to experience substantial healthcare disparities in the United States. One factor that contributes to these disparities is low uptake of cancer screening that detects precancerous or early cancer lesions when they can be better treated and cured. The U.S. healthcare inequities have drawn renewed attention and concern in light of the COVID-19 pandemic, which has also disproportionately impacted communities of color. Coinciding with the COVID-19 pandemic, there has been a sharp decrease in cancer screening rates. We are concerned that these sharp declines, if sustained, could result in a disproportionate negative impact for the most vulnerable populations and reverse the gains on early cancer detection in African American, Latinx, and Native Americans. There is an urgent need for rigorous studies investigating how the COVID-19 pandemic is affecting the uptake of cancer screening, especially among the medically underserved, so that effective mitigation strategies could be designed and implemented.
The benefit of cancer screening and identifying cancers early has been rigorously demonstrated to reduce cancer-related deaths, and is endorsed by multiple medical societies and insurers. Several randomized control trials regarding screening for specific cancers have demonstrated enormous benefits in reducing patient mortality from cancer when screened (1–3); earlier cancer detection by screening drives substantial improvements in 5-year survival rates among those diagnosed with localized instead of metastatic disease (90% vs. 14% survival for colon and 99% vs. 28% survival for breast cancer; ref. 4; https://seer.cancer.gov/csr/1975_2017/). These screening benefits are clear, but screening does have harms as well, including false positives or overdiagnosis as some advanced sensitive screening methods detect not-as-specific biomarkers and/or smaller lesions without a reduction in incidence of invasive cancers, and can lead to additional unnecessary follow-up testing (5–7).
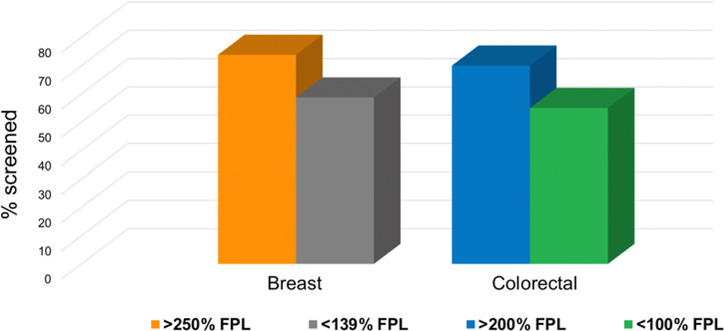
Many professional societies have evidence-based recommendations for the use of the screening tests for breast, cervical, and colorectal cancer among average-risk individuals. Even though the benefits of cancer screening outweigh the potential risks, a substantial percentage of individuals who are eligible are not up to date with their screening regimen. For example, in 2018, 31% of adults ages 50–75 years were not up to date with colorectal cancer screening (8). Notably, individuals who are not up to date with cancer screening recommendations are disproportionately found in medically underserved segments of the U.S. population, including racial and ethnic minorities. For example, 69% of Whites ages 50–75 years are up to date on colorectal cancer screening compared with 66% of African Americans, 59% of Hispanics, and only 56% of American Indian/Alaska Natives (9). In addition, according to recent estimates, women in the lowest income bracket are significantly less likely to be up to date with breast cancer screening than women in the highest income bracket; the same income differential is observed for colorectal cancer screening, with those in the lowest income bracket significantly less likely to be up to date with colorectal cancer screening than adults who are in higher income brackets (Fig. 1; ref. 10).
Figure 1.
Rates for screening for breast cancer and colorectal cancer based on level of income. Data obtained from Siegel and colleagues (9) and White and colleagues (10). FPL, federal poverty level.
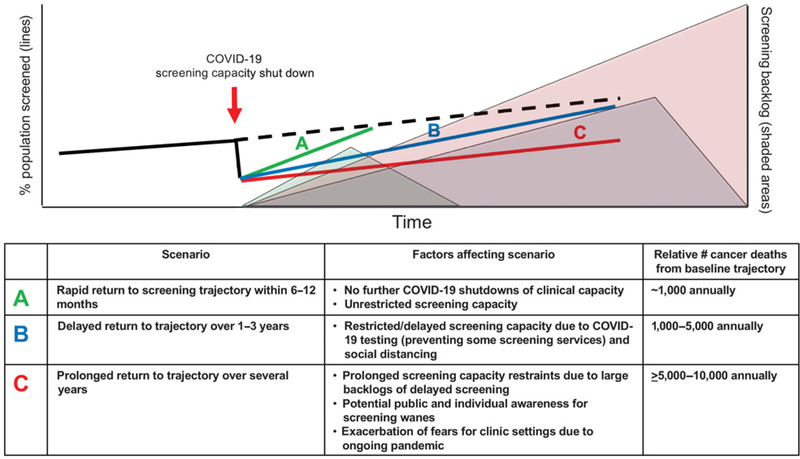
The COVID-19 pandemic that commenced in March 2020 in the United States caused a stark shut down of preventive clinical services including cancer screening to prevent spread of infection and preserve personal protective equipment. Compared with 2019, screening tests for breast and colorectal cancer were decreased by 89.2% and 84.5%, respectively, by April 2020 (11). The level and vigor of rebound from the COVID-19–induced delay for cancer screening (Fig. 2) will determine the degree and extent of missed preventive tests for all cancers, and will drive the long-term consequence of projected increased cancer-related deaths (12). For medically underserved populations, such as African Americans, who have the highest rates of colorectal cancer incidence and mortality, screening through the use of systemized patient navigation has been shown to reduce and eliminate colorectal cancer disparities (13); the actual attainment of elimination of disparities in clinical practice has not yet been achieved. In the face of the COVID-19, the potential reduction or elimination of colorectal cancer disparities is further threatened, and could reverse the years of steady progress toward equity in screening (14).
Figure 2.
Scenarios for cancer prevention after COVID-19 surges. Scenario A indicates a rapid recovery under 1 year with fairly rapid clearance of the backlog. Scenario B depicts a delayed return to historical screening trends over 1–3 years, with a much longer clearance of backlog over several years. Scenario C depicts a situation in which we never get back to historical trends, creating a public health crisis. Delays in returning to baseline or higher levels of screening with reverse gains, causing additional preventable deaths from cancer. Estimates for excess deaths are from a baseline of delay of 6-months for breast and colorectal cancers as modeled in Sharpless (12).
Medically underserved populations have long demonstrated structural socioeconomic disadvantages along with numerous barriers to attaining medical access and use. Disadvantages such as low income, living in neighborhoods with food deserts, and possessing little or no insurance all hinder use of preventive services such as cancer screening. In addition, there is often higher prevalence of cancer risk factors such as tobacco and alcohol use and ingestion of high-fat, high-caloric diets that predispose these populations to a higher risk of cancer formation (15). In addition, certain medically underserved populations have long-standing fear of “going to the doctor” for cultural and/or historical exploitation reasons (16).
COVID-19's arrival into the human population has disproportionately affected vulnerable and medically underserved populations (17), exacerbating preexisting fears and creating new ones in these populations that may extend beyond the length of the pandemic to have disproportional consequences for preventive care. Fear of COVID-19 exposure at a medical site (even when clinical precautions may have made risk lower than community exposure) postpones preventive care. For others without fear of coming to clinic, access may be limited by other COVID-19–related changes such as changes in local transportation, work schedule issues, and lack of childcare, which may disproportionally impact communities which are already medically underserved. Effects of stay-at-home policies and travel restrictions, loss of or lack of financial savings, and loss of any employment (due to loss of job or getting sick on the job as an essential employee in a high COVID-19 risk situation) all undoubtedly worsen the ability to obtain preventive care.
Early during the pandemic, academic and community practices ceased cancer screenings and other elective procedures at the bequest of the U.S. Surgeon General (18); for example, it is estimated that between March and May 2020 there were 95,000 missed screens for colorectal cancer in the United States (https://ehrn.org/delayed-cancer-screenings-a-second-look/) among the estimated 67% of eligible Americans utilizing colorectal cancer screening. With reestablishment of colonoscopic screening, the backlog will take time to be reduced, depending on practice pace, physician availability, and patient utilization (Fig. 2). The other 33% of eligible Americans who have never utilized colorectal cancer screening for a variety of reasons are disproportionately from medically underserved populations (19). COVID-19 has the potential to shrink the number of medically underserved that were already in the screening utilization group, and expand the nonutilization group. Professional gastroenterology organizations have called for increase in use of noninvasive stool screening tests that are inexpensive and sensitive that can ensure appropriate and broad screening uptake throughout medically underserved populations (https://www.medscape.com/viewarticle/935288). Noninvasive testing that is positive needs to be followed by a diagnostic colonoscopy which can create a financial challenge if the colonoscopy is not covered by insurance or unavailable in the area.
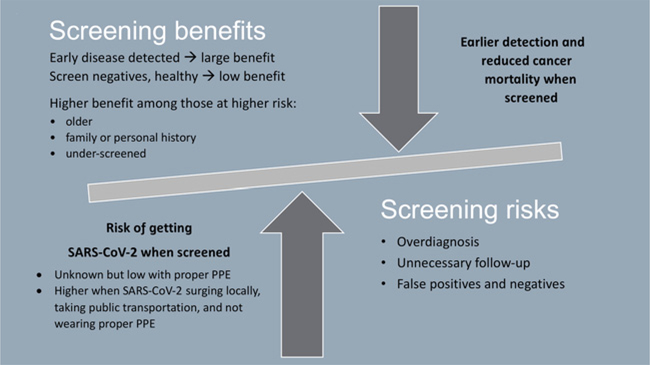
The immediate risk of acquiring COVID-19 may seem much more pressing than the potential benefit of cancer screening. Weighing the risks and benefits of elective procedures like cancer screening during the COVID-19 pandemic can be unclear (Fig. 3). Short-term delays in screening (e.g., 6–12 months) among asymptomatic individuals might be reasonable and were universally recommended and enacted because of the immediate risks associated with the COVID-19 surge. However, as the pandemic timeline extends, and the delay in screening lengthens, so does the cancer risk. Modeling studies of the impact of a 12-month delay in cancer diagnosis increase deaths rates (5 years after diagnosis) by approximately 8% for breast cancer and approximately 16% for colorectal cancer (20). Thus, while short-term interruptions in screening may be low risk, longer intervals extend the risk of delayed detection of cancers.
Figure 3.
Benefits versus risks of cancer screening during the COVID-19 pandemic.
Cancer prevention by screening is a proven approach to reduce risk from dying from cancer; if utilized universally to the identical extent in all racial and ethnic populations, screening can greatly reduce disparities. COVID-19 has caused delay of population screening, and we fear it will exacerbate currently observed disparities in screening. Some professional societies aptly recommend noninvasive approaches to screening, several of which may be originated in the home setting. For example, self-sampling has been proven to be highly effective both for cervical cancer screening, using human papillomavirus DNA sampling (21), and for colorectal cancer screening, using fecal immunochemical testing or fecal DNA sampling (1, 22). Some at-home tests, such as fecal DNA sampling for colorectal cancer, may be as sensitive as other tests administered in the clinical setting (22). At-home testing may improve uptake of screening for medically underserved patients in conjunction with patient education and availability of the test to the patient in the home; however, screening tests administered in the home setting, if found positive, often require a follow-up test in the clinical setting that may be difficult to execute in this population. We must be vigilant to: (i) keep the COVID-19–induced delay in cancer screening to a minimum of time, (ii) utilize services such as navigation to continue inroads into reducing disparities in screening rates between populations, (iii) increase population screening rates nationally even in the face of a pandemic for the long-term health of our nation, and (iv) continue to improve upon methods used to detect clinically impactful precursors and presence of cancers at its earliest stage for cancer prevention and patient cure.
Supplementary Material
Acknowledgments
The following authors were supported by the United States Public Health Service: R35DE026631 (to G. D’Souza), R01CA206010 (to J.M. Carethers), and R35 CA197633 (to A. Ribas). J.M. Carethers also received support from the A. Alfred Taubman Medical Research Institute of the University of Michigan.
Disclosure of Potential Conflicts of Interest
A. Ribas reports grants from Agilent and Bristol-Myers Squibb (research funding), personal fees from Amgen, AstraZeneca, Checkmate, Merck, Novartis, Sanofi, and Vedanta (honoraria from consulting) and 4C Biomed, Apricity, Arcus, Highlight, Compugen, ImaginAb, MapKure, Merus, Rgenix, Lutris, PACT Pharma, and Tango (SAB member and stock holder) outside the submitted work. G.D’Souza reports grants from NIH during the conduct of the study.
Footnotes
No potential conflicts of interest were disclosed by the other authors.
References
- 1.Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Eng J Med 2013;369:1106–14. [DOI] [PubMed] [Google Scholar]
- 2.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Eng J Med 2007;357:1589–97. [DOI] [PubMed] [Google Scholar]
- 3.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Eng J Med 2020;382:503–13. [DOI] [PubMed] [Google Scholar]
- 4.Duffy SW, Tabar L, Yen AM-F, Dean PB, Smith RA, Johsson H, et al. Mammography screening reduces rates of advanced and fata breast cancers: results in 549,091 women. Cancer 2020;126:2971–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch HG, Fisher E. Income and cancer overdiagnosis - when too much care is harmful. N Eng J Med 2017;2208–09. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava S, Koay EJ, Borowsky AD, De Marzo AM, Ghosh S, Wagner PD, et al. Cancer overdiagnosis: a biological challenge and clinical dilemma. Nat Rev Cancer 2019;19:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brawley OW, Paller CJ. Overdiagnosis in the age of digital cancer screening. J Natl Cancer Inst 2020. doi. 10.1093/jnci/djaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph DA, King JB, Dowling NF, Thomas CC, Richardson LC. Vital signs: colorectal cancer screening test use — United States, 2018. MMWR Morb Mortal Wkly Rep 2020;69:253–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70:145–64. [DOI] [PubMed] [Google Scholar]
- 10.White A, Thompson TD, White MC, Sabatino SA, de Moor J, Doria-Rose PV, et al. Cancer screening test use - United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effect of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform 2020;4:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharpless NE. COVID-19 and cancer. Science 2020;368:1290. [DOI] [PubMed] [Google Scholar]
- 13.Grubbs SS, Polite BN, Carney J Jr, Bowser W, Rogers J, Katurakes N, et al. Elimination racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol 2013;31:1928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci 2015;60:711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology 2020;158:354–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kupfer SS, Carr RM, Carethers JM. Reducing colorectal cancer risk among African Americans. Gastroenterology 2015;149:1302–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price-Haywood EG, Burton J, Fort D, Seone L. Hospitalization and mortality among black patients and white patients with COVID-19. N Eng J Med 2020;382:2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balzora S, Issaka R, Anyane-Yeboa A, Gray DM, May FP. Impact of COVID-19 on colorectal cancer disparities and the way forward. Gastrointest Endosc 2020;20:S0016–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carethers JM. Clinical and genetic factors to inform reducing colorectal cancer disparities in African Americans. Front Oncol 2018;8:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020;21:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aarnio R, Ostensson E, Olovsson M, Gustavsson I, Gyllensten U. Cost-effectiveness analysis of repeated self-sampling for HPV testing in primary cervical screening: a randomized study. BMC Cancer 2020; 20:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carethers JM. Fecal DNA testing for colorectal cancer screening. Annu Rev Med 2020;71:59–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.