Abstract
Background.
In HIV-1-exposed infants, nucleic acid testing (NAT) is required to diagnose infection since passively transferred maternal antibodies preclude antibody testing. The sensitivity of clinical NAT assays is lowered with infant antiretroviral prophylaxis and, with empiric very early antiretroviral treatment of high-risk infants, thereby impacting early infant diagnosis. Similarly, adult HIV-1 infections acquired under pre-exposure prophylaxis may occur at low levels, with undetectable plasma viremia and indeterminate antibody tests, for which HIV-1 DNA testing maybe a useful adjunct. Cell-associated HIV-1 DNA concentrations are also used to monitor HIV-1 persistence in viral reservoirs with relevance to HIV-1 cure therapeutics, particularly in perinatal infections.
Objectives.
We clinically validated an HIV-1 DNA quantitative assay using droplet digital PCR (ddPCR), across different HIV-1 subtypes.
Study Design.
The analytical sensitivity and specificity of an HIV-1 DNA ddPCR assay was determined using serial dilutions of a plasmid containing HIV-1 LTR-gag spiked into peripheral blood mononuclear cells (PBMCs), with MOLT-4 cells or PBMCs infected with different HIV-1 subtypes (A, B and C), and U1 cells spiked into PBMCs. Inter- and intra-run variability were used to determine assay precision.
Results.
The HIV-1 LTR-gag ddPCR assay was reliable and reproducible, and exhibited high analytical specificity with sensitivity to near single copy level, across multiple HIV-1 subtypes, and a limit of detection of 4.09 copies/million PBMCs.
Conclusions.
This assay has applications for detecting occult HIV-1-infection in the setting of combination and long-acting regimens used for HIV-1 prevention, across different HIV-1 subtypes, in infants and adults, and in HIV-1 cure interventions.
Keywords: HIV-1, ddPCR, methods
Background
In HIV-1-exposed infants younger than age 18 months, HIV-1 antibody testing cannot distinguish between infant and maternally-derived antibodies to allow use for infant HIV-1 diagnosis (1, 2). Quantitative or qualitative real-time polymerase chain reaction (RT-PCR) assays that detect HIV-1 RNA in plasma or DNA in peripheral blood mononuclear cells (PBMCs) are therefore required (3, 4). Early antiretroviral treatment (ART) of perinatal HIV-1 infection can lead to low concentrations of circulating HIV-1 infected cells in association with negative HIV-1 antibody and DNA tests by clinical diagnostic assays (5–11), possibly leading to unnecessary ART interruption in later childhood (12). Furthermore, the use of combination antiretroviral (ARV) prophylaxis in high-risk perinatal exposures, along with very early ART within days of age or directly transitioning from prophylaxis to ART, may lead to low infected cell concentrations (7, 13–16) that require more sensitive HIV-1 DNA assays to confirm infection on ART. HIV-1 DNA concentrations in peripheral blood are also used to determine therapeutic efficacy of cure strategies aimed at eradicating reservoir cells in ART-free remission and cure (17, 18). Sensitive and clinically validated HIV-1 DNA assays may become useful in these specialized settings, in both perinatal and adult infections.
Objectives
We optimized and clinically validated an HIV-1 DNA quantitative assay using the more sensitive and precise approach of droplet digital PCR (ddPCR), across different HIV-1 subtypes, with potential applications for use clinically and in HIV-1 prevention and cure trials (19).
Study Design
A detailed description of the DNA isolation and ddPCR is provided in the supplemental materials.
Preparation of cell pellets and plasmid standards.
PBMCs were isolated from leukopaks (New York City Blood Center, New York NY) using Ficoll-Hypaque centrifugation (GE Healthcare, Chicago IL), treated with Red Blood Cell Lysis Buffer (eBiosciences, San Diego CA) and aliquoted into 5-million cell pellets for storage at −80° C until further use.
Plasmid standards.
A plasmid containing a pMK-RQ-Bs backbone (GeneArt Gene Synthesis Technology; Thermo Scientific, Waltham MA) with a 160-bp region of the HIV-1–5’ LTR and gag region (positions 626 – 786, HXB2) (20), as well as a fragment of the housekeeping gene Ribonuclease Protein Subunit P30 (RPP30) (21), was constructed (Supplemental Figure 1). Prior to testing, an aliquot of plasmid stock was serially diluted and spiked into 5-million PBMC pellets to reach final concentrations ranging from 1,000 to 1.25 copies per 106 PBMCs. A second high-dilution series (10, 000 to 100 copies per 106 PBMCs) was tested to assess linearity at higher concentrations.
DNA Isolation.
Genomic DNA was isolated from the combined plasmid-cell mixtures using QIAamp DNA Blood Midi Kit (Qiagen, Germantown MD) with the addition of an overnight ethanol precipitation step to obtain highly concentrated and pure genomic DNA for downstream ddPCR applications (21).
Primers, Probes and PCR conditions.
The primers and probes for HIV-1 LTR-gag (20) and RPP30 (21) (Supplemental Table 1) were obtained from Integrated DNA Technologies, Coralville IA), reconstituted in DNase/RNase-free water, and stored at concentrations of 18 μM primers and 5 μM probes in 50 μL aliquots for single use only.
Genomic DNA preparation and ddPCR.
Genomic DNA (sufficient to perform 8 replicates at 1000 ng input per well) was subjected to restriction digest with XbaI (New England BioLabs, Ipswich MA). Negative control wells containing genomic DNA without plasmid were performed in four replicates, along with a single control well without genomic DNA as a no template control (NTC). To directly quantify the number of cells analyzed based on copies of RPP30, PBMC genomic DNA was diluted 1:10 (100 ng DNA per well) since RPP30 is present in high copy number at two copies per cell. This allowed the results to be expressed as HIV-1 DNA copies per cells analyzed with subsequent conversion to copies per 106 cells.
The reaction mixtures containing primers, probes, Supermix, and sample were loaded into the QX-200 Droplet Generator along with Droplet Generation Oil and consumables according to manufacturer instructions. PCR reactions were carried out using the conditions summarized in the Supplemental Methods and analyzed on the QX200 Droplet Digital PCR Reader (Bio-Rad, Hercules CA) that assesses individual droplet fluorescence.
Analysis of ddPCR data.
Analysis of HIV-1 DNA copies per sample was completed in QuantaSoft v1.7.4.0917 (Bio-rad, Hercules CA) according to manufacturer’s directions. The fluorescence threshold was pre-set to 2000 RFUs. Reaction wells with <6,000 droplets were excluded. The HIV-1 LTR-gag concentration was estimated in copies per μL as the average of all evaluable wells, then multiplied by the well volume of 20 μL. The RPP30 concentration was corrected for dilution (1:10) and copies per cell (two per cell) to obtain number of cells per well and total number of cells analyzed. HIV-1 LTR-gag copies per well was normalized to cells per well and expressed as HIV-1 LTR-gag copies per 106 cells.
Determining assay performance.
Analytic sensitivity and limit of detection (LoD), analytic specificity, precision and accuracy were determined (22) using diluted plasmids at the varying concentrations and across two different blood donors in three independent runs. The LoD was determined with a second set of experiments across 20 independent runs at the lower concentrations (5, 2.5 and 1.25 copies per 106 cells). Validation panels were obtained from several sources. External validation for subtypes AE, AG, and C were provided by the Duke University External Quality Assurance Program Oversight Laboratory (EQAPOL); blinded samples (seven- three-fold dilutions of U1 cells (0 to 14,580 HIV-1 copies per 106 cells spiked into PBMC pellets) from the NIAID Virology Quality Assurance (VQA) program for which four independent assay runs were conducted. The U1 cell line harbors two copies of HIV-1 genomic DNA per cell (23, 24), which was accounted for in the analysis. Serially diluted standards of HIV-1 subtypes A1, B and C diluted in one million uninfected cells) were provided by the National Institute for Biological Standard and Control (Potters Bar, United Kingdom). An additional dilution series was created with pellets of 5 million A3.01 cells spiked with increasing quantity of a mixture of two HIV-1 subtype A infectious molecular clones quantified by Nanodrop to examine lower sensitivity with subtype A standards spiked into one million cells.
Statistical Analyses.
A least squares regression model was fit to the data using a simple linear model. The coefficient of determination, R2, was calculated to assess the linearity of this relationship overall and individually for each donor. Additionally, a second and third-order polynomial model was applied to the data. These models were then compared using an F-test to determine the model of best fit. A probit model, which uses maximum likelihood estimates to analyze a concentration-response relationship with a binomial response variable (detection or no detection by HIV-1 LTR-gag assay) was also performed to further assess the LoD (25). Inter-assay variation was assessed by calculating the average measured HIV-LTR-gag copies per 106 cells detected at each dilution level across the six independent assay runs, across two donors. Intra-assay variation was determined by assessing the variation across all six independent runs of the HIV-LTR-gag assay, across the two donors.
Study Approval.
This study and use of Leukopaks from the NY Blood Center was approved by the Johns Hopkins Medicine Office of Human Subjects Research Institutional Review Boards as study number NA_00087629.
Results
Reportable Range and Linearity.
A statistically significant linear relationship between expected plasmid concentration and measured HIV-1 LTR-gag copies (R2 =0.927 and p-value <0.001) was observed (Figure 1). With polynomial regression modeling and comparisons using F-tests, the data appeared nonlinear at the high end (1,000 copies per 106 cells and above). Eliminating the highest analyte concentration (≥1000) showed that the data followed a linear model, with a slope significantly different from zero and a y-intercept not significantly different from zero (data not shown). The linearity of the assay at the highest concentrations across the range of 10,000, 1,000, 100 and 0 plasmid copies per 106 PBMCs were re-tested across three independent runs. A statistically significant linear relationship between expected plasmid concentration per 106 cells and measured HIV-1 LTR-gag copies (R2 =0.9968 and p-value <0.0001, Supplemental Figure 2) was found. With eight replicates of a 1000 ng of DNA, a median of 1,012,800 cells were analyzed (IQR 880,950 – 1,084,550).
Figure 1.
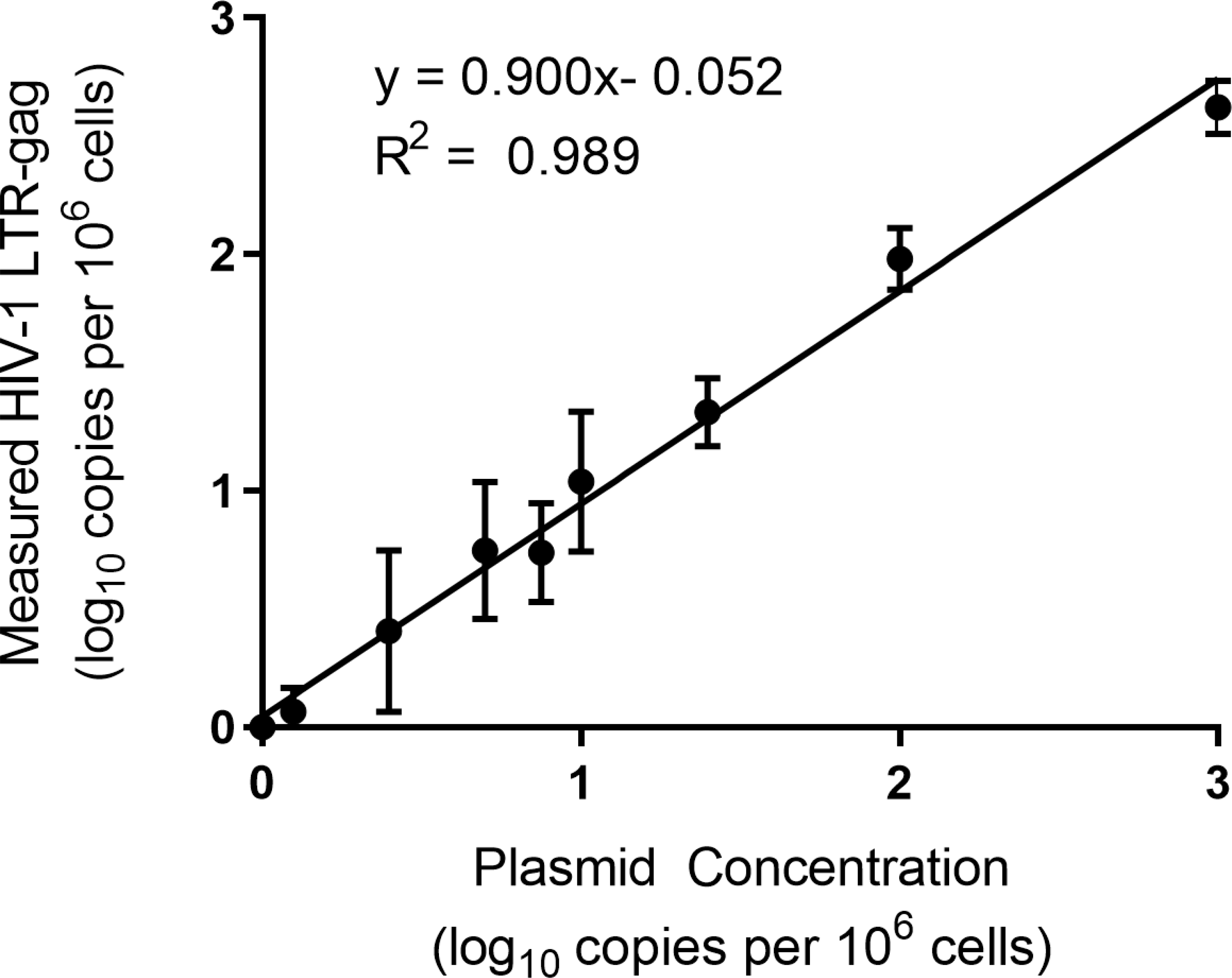
Measured HIV-1 LTR-gag copies (log10) from six replicate experiments with PBMCs from two different donors plotted against expected plasmid copies per 106 cells (log10).
Analytic Sensitivity.
The assay was highly sensitive, detecting the presence of HIV-1 LTR-gag copies 100% of the time at concentrations of 1,000 to 5 copies per 106 cells. The analytic sensitivity of the assay declined at 2.5 or lower HIV-1 DNA copies per 106 cells, with approximately 83.33% and 33.33% detection at 2.5 and 1.25 copies per 106 cells, respectively. At the lower concentrations across 20 independent replicates, sensitivity of the assay was 100%, 60%, and 30% at 5, 2.5, and 1.25 copies per million cells, respectively. The LoD, the lowest concentration at which the assay can reliably detect 95% of the time, was estimated with the predicted probability line of 95% at 4.09 copies per 106 cells assessed by the probit regression (Figure 2).
Figure 2.
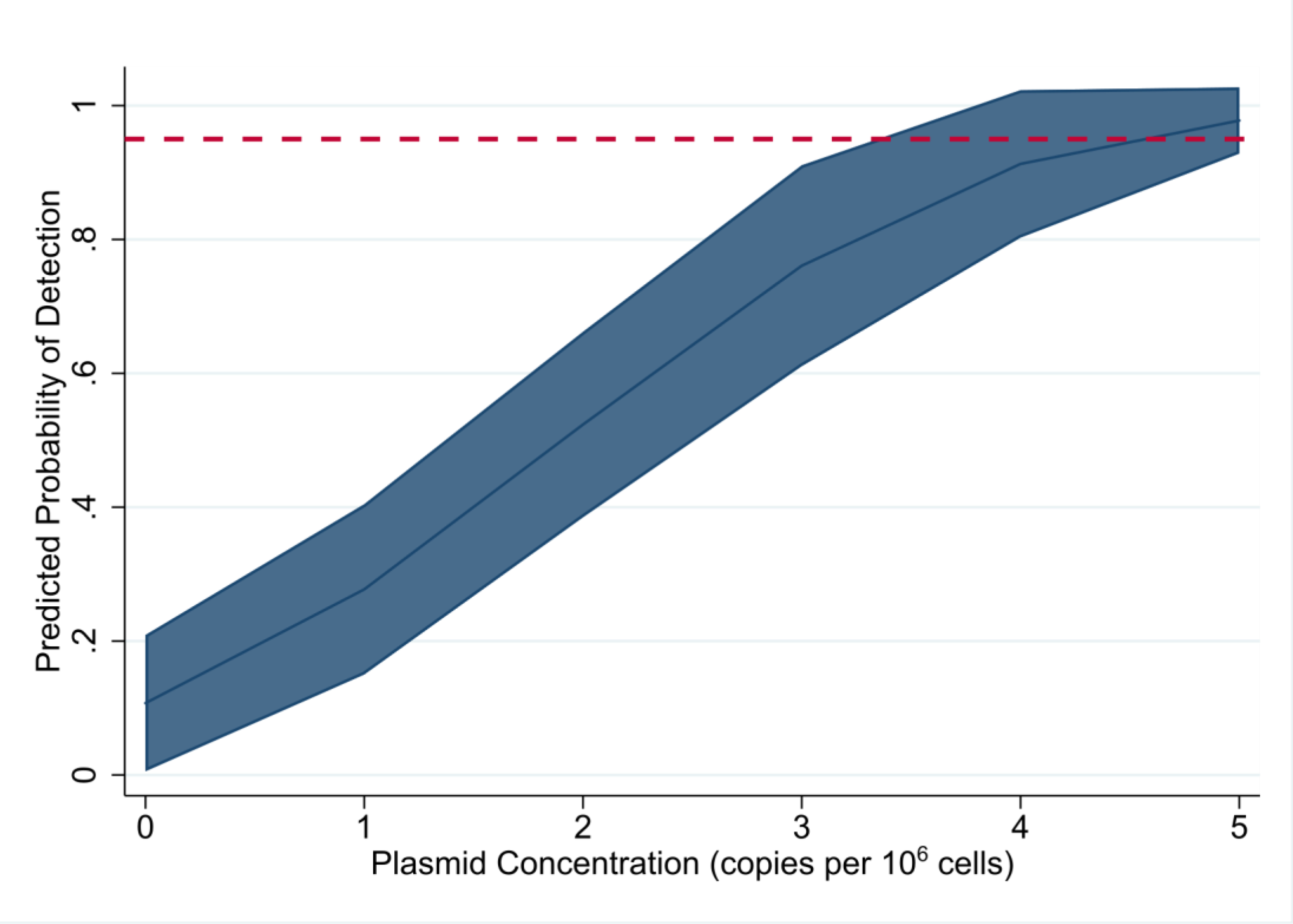
Predicted probability of detection by HIV-1 LTR-gag assay, as determined by probit analysis, at increasing plasmid copies per million cells with 95% confidence interval. Red dotted line depicts 95% probability of detection.
Analytic Specificity.
The diagnostic specificity of the HIV-1 LTR-gag assay was 100% with all six samples (three from each donor in three independent assay runs) without the plasmid DNA detected. Interference with hemoglobin was tested through analysis of blood samples saved as dried blood spots, no interference was detected (data not shown). Cross-reactivity to assess for the primer/probe to cross-react with genetically similar organisms was not performed.
Inter-assay and intra-assay precision.
The inter-assay standard deviation (SD) increased with the decreasing average concentration. At log10 3 to 1.4 copies per 106 cells, the SD range between 0.11 and 0.14; variation at the lower concentrations were higher at 0.30, 0.21 and 0.29 at log10 1, 0.88 and 0.70 copies per 106 cells, respectively (Table 1). The inter- and intra-assay %CV revealed a similar trend, with the lowest %CV across the two donors at 1,000 – 25 copies per 106 cells (Supplemental Tables 2–3).
Table 1.
Inter-assay precision
| LOG10 COPIES PER MILLION CELLS | LOG10 AVERAGE MEASURED HIV-1 LTR-GAG PER 106 CELLS | STANDARD DEVIATION |
|---|---|---|
| 3 | 2.62 (2.42 – 2.77) | 0.11 |
| 2 | 1.98 (1.83 – 2.13) | 0.13 |
| 1.40 | 1.33 (1.15 – 1.56) | 0.14 |
| 1 | 1.04 (0.64 – 1.31) | 0.30 |
| 0.88 | 0.74 (0.39 – 1.04) | 0.21 |
| 0.70 | 0.75 (0.21 – 0.98) | 0.29 |
| 0.40 | 0.41 (0 – 0.83) | 0.34 |
| 0.10 | 0.07 (0 – 0.20) | 0.10 |
| 0 | N/A | N/A |
Accuracy.
This analysis showed that the assay can accurately measure HIV-1 DNA at known input concentrations (Figure 3) with detection of approximately 92.82% of the expected copies per 106 cells at each concentration level.
Figure 3.
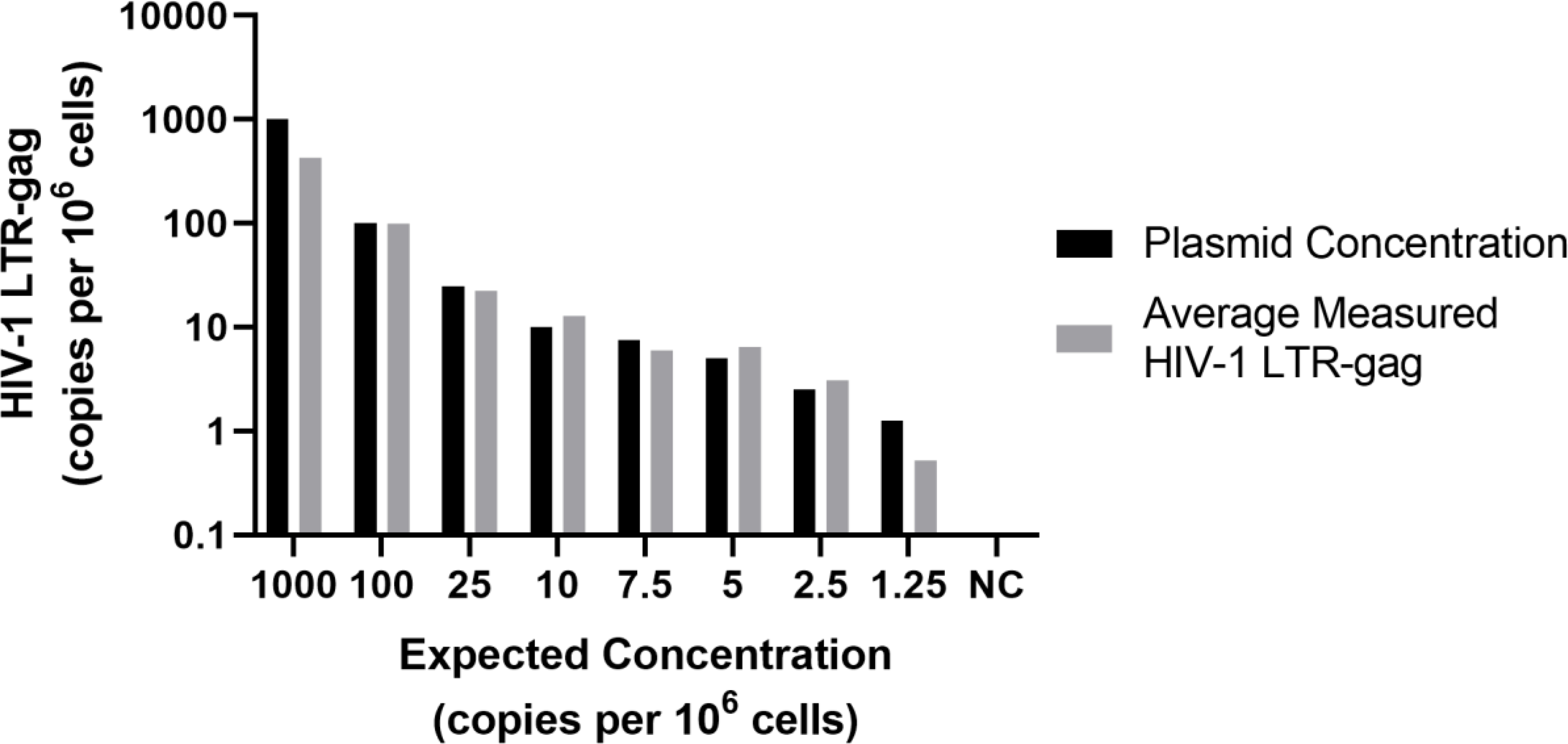
Comparison of expected plasmid (copies/million cells) and measured HIV-1 LTR-gag (copies per 106 cells) to assess accuracy.
External Validation.
Testing of VQA program panel showed 100% detection of HIV-1 DNA at all concentrations (0, 60, 180, 540, 1,620, 4,860 and 14,580 copies per 106 cells) in each of four independent runs and with no detection at zero copies HIV-1 DNA. The average measured HIV-LTR-gag log10 copies per 106 cells detected and the degree of variation between all independent runs (%CV) with the imprecision around the four independent runs varying between 23.55% at the lowest concentration (60 copies per million cells) and 9.97% at the highest concentration (14,580 copies per 106 cells). On a log scale, total imprecision (% CV) varied from 0.99% to 1.37%, with increased imprecision at lower concentrations with a 95% confidence interval of 1.21 to 1.58-fold change in measured HIV-LTR-gag (Table 2). The average percent detection at each concentration was higher with an overall quantification of the HIV-1-LTRGAG approximately 129.23% of the expected copies per 106 cells, likely reflective of cell line standards harboring two proviral genomes per cell.
Table 2.
Evaluation of precision in external validation samples by log %CV and 95% confidence interval of measured HIV-1-LTR-gag
| DILUTION | LOG10 COPIES PER 106 CELLS | LOG10 AVERAGE HIV-1 LTR-GAG COPY PER 106 CELLS | STANDARD DEVIATION | 95% CI (LOG10 HIV-1 LTR-GAG COPY PER 106 CELLS) | LOG CHANGE 95% CI | FOLD CHANGE 95% CI |
|---|---|---|---|---|---|---|
| VQA428 A | 0 | 0 | 0 | N/A | N/A | N/A |
| VQA428 B | 1.78 | 1.99 | 1.36 | 1.88–2.08 | 0.20 | 1.58 |
| VQA428 C | 2.26 | 2.43 | 1.64 | 2.35–2.50 | 0.19 | 1.41 |
| VQA428 D | 2.73 | 2.84 | 2.14 | 2.74–2.92 | 0.18 | 1.51 |
| VQA428 E | 3.21 | 3.31 | 2.38 | 3.26–3.35 | 0.10 | 1.25 |
| VQA428 F | 3.69 | 3.74 | 2.80 | 3.69–3.78 | 0.10 | 1.25 |
| VQA428 G | 4.16 | 4.27 | 3.27 | 4.22–4.31 | 0.08 | 1.21 |
HIV-1 Subtype analyses.
HIV-1 subtypes AE and AG were readily detected using this assay, and displayed expected similar linear detection of at least 3-logs when tested on 10-fold serial dilutions of HIV-1 DNA from subtypes B and C spiked into 1 ×106 PBMCs, but not for subtype A (data not shown). However, when subtype A was reassessed with a preparation of 5 × 106 million pellet that yielded sufficient genomic DNA to allow input of 8000 ng of genomic DNA, the performance and detection of subtype A was consistent with previous results for subtypes B and C (Figure 4A), and highly reproducible by two different operators (p <0.0001; Figure 4B).
Fig. 4.
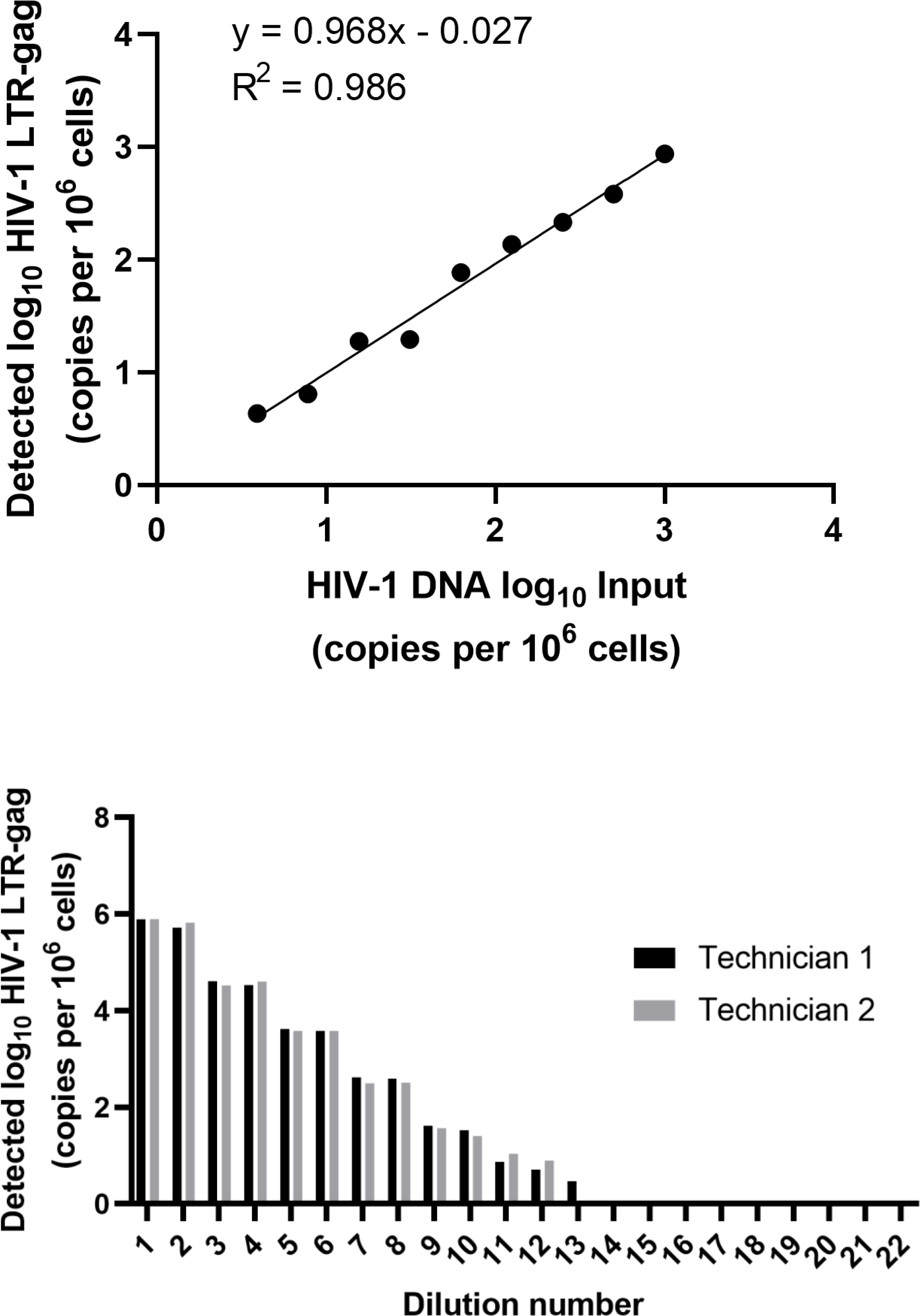
A) Measured copies of HIV-1 subtype A plotted against DNA spiked into uninfected cells. B) Variations in quantitation between two technicians processing samples in parallel.
Discussion
In contrast to qPCR, ddPCR technology separates PCR mastermix into thousands of partitions and this, combined with Poisson statistics, provides for precise and absolute quantification of a molecular target while minimizing interference from PCR inhibitors (19). By avoiding the need for a standard curve to infer target copy number as in qPCR, ddPCR leads to more reproducible and precise quantitation (22). However, discrepancies can occur with ddPCR when setting a threshold fluorescence at which a droplet is considered positive or negative. Variations in droplet fluorescence can stem from differences in primer/probe concentration, baseline autofluorescence, and intermediate fluorescence, referred to as rain, which can complicate interpretation (26–28). Here, we found that setting a fixed threshold of fluorescence, we obtained highly reproducible, precise and accurate quantification of HIV-1 DNA present in samples with known input concentrations and across different HIV-1 subtypes.
The HIV-1 DNA assay reported here exhibits a statistically significant linear relationship between expected and measured HIV-1 LTR-gag copies with high diagnostic specificity and reproducibility. The assay detects concentrations of HIV-1 DNA as low as 1.25 copies per million cells with a predicted probability of LoD of 4.09 copies per million cells. This assay can effectively detect reproducibly across HIV-1 subtypes A, B, and C with equivalent performance characteristics and with consistency in subtypes AE and AG. The eight replicates of 1,000 ng of genomic DNA input allowed an average of 1,034,258 cells to be analyzed, which likely contributed to the increased the sensitivity and accuracy.
Detecting and quantifying HIV-1infected cell concentrations is relevant to the fields of HIV-1 prevention and cure therapeutics (15, 17). Important insights into the immunopathogenesis of HIV-1 infection have been gained from studies of cell-associated infection during ART in infected individuals (29–33). Early treatment of perinatal infection is important for monitoring reservoir size and requires a highly sensitive HIV-1 DNA assay. However, quantifying total HIV DNA with the ddPCR assay reported here is limited by its capacity to not distinguish intact from defective genomes as with the recently described intact proviral HIV-1 DNA assay (IPDA), which allows quantification of proviral genomes with potential to refuel infection (34, 35), although the sensitivity of the IPDA across a broad range of HIV subtypes has not been fully determined. Nevertheless, our HIV-1 LTR-gag assay has been shown to be sensitive and accurate across multiple HIV-1 subtypes allowing for assessment of low copy number samples without a priori knowledge of HIV subtype.
With enhanced HIV-1 prophylactic regimens that include combination antiretrovirals to prevent and empirically treat perinatal HIV-1 infection in high- risk exposure settings, access to more sensitive and precise HIV-1 DNA assays across various HIV-1 subtypes is becoming important. (16, 36). Similarly, the use of long-acting, potent antiretrovirals such as with long-acting injectables, including broadly neutralizing antibodies, to prevent adult infection, may alter the sensitivity of RNA and antibody testing. Ultrasensitive HIV-1 DNA testing may provide unique opportunities to detect occult, low level infection, enabling early transition to combination ART regimens to limit HIV-1 spread into reservoirs that preclude cure. In summary, this assay has potential applications for assessing therapeutic efficacy in the emerging fields of HIV-1 prevention, where occult HIV-1 infection may become more prevalent (37). Standard HIV-1 RNA and antibody testing methods are insufficiently sensitive in the field of HIV-1 cure and perinatal infections, where HIV-1 infected cell concentrations can reach exceedingly low levels.
Supplementary Material
Highlights.
Sensitive detection of HIV-1 DNA is required for HIV-1 cure therapeutics.
Quantitative HIV-1 DNA ddPCR assay was validated across different HIV-1 subtypes.
HIV-1 ddPCR assay exhibited high analytical specificity and sensitivity.
Acknowledgements
We thank EQAPOL, the DAIDS VQA (HHSN272201200023C), the National Institute for Biological Standard and Control (Potters Bar, United Kingdom) for providing blinded samples for this study. U1 cells was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: U1/HIV-1 from Dr. Thomas Folks.
Sources of Funding
This research was supported by the National Institutes of Health (NIH) (R01 HD080474 (DP), PO1 AI131365 (DP)); the BELIEVE Collaboratory (1UM1AI26617); EPIICAL (16108367); the IMPAACT Center subspecialty laboratory (5UM1AI106716); UO1 (1U01AI135941) and the Johns Hopkins Center for AIDS Research (P30 AI094189).
Footnotes
Declarations of Interests: Deborah Persaud, MD has served as a Scientific Advisor for Merck &Co.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Simpson BJ, Andiman WA. Difficulties in assigning human immunodeficiency virus-1 infection and seroreversion status in a cohort of HIV-exposed in children using serologic criteria established by the Centers for Disease Control and Prevention. Pediatrics. 1994;93(5):840–2. [PubMed] [Google Scholar]
- 2.Sison AV, Campos JM. Laboratory methods for early detection of human immunodeficiency virus type 1 in newborns and infants. Clin Microbiol Rev. 1992;5(3):238–47. Epub 1992/07/01. doi: 10.1128/cmr.5.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mofenson LM, Cohn J, Sacks E. Challenges in the Early Infant HIV Diagnosis and Treatment Cascade. Journal of acquired immune deficiency syndromes (1999). 2020;84 Suppl 1:S1–S4. Epub 2020/06/11. doi: 10.1097/QAI.0000000000002366. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Diagnosis of HIV infection in infants and children. World Health Organization, 2010. [PubMed] [Google Scholar]
- 5.Persaud D, Patel K, Karalius B, Rainwater-Lovett K, Ziemniak C, Ellis A, Chen YH, Richman D, Siberry GK, Van Dyke RB, Burchett S, Seage GR, 3rd, Luzuriaga K, Pediatric HIVACS. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr. 2014;168(12):1138–46. Epub 2014/10/07. doi: 10.1001/jamapediatrics.2014.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma P, McManus M, Cotugno N, Rocca S, Rossi P, Luzuriaga K. The HIV-1 antibody response: a footprint of the viral reservoir in children vertically infected with HIV. The lancet HIV. 2020;7(5):e359–e65. Epub 2020/05/11. doi: 10.1016/S2352-3018(20)30100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M Jr., Chun TW, Strain M, Richman D, Luzuriaga K Absence of detectable HIV-1 viremia after treatment cessation in an infant. The New England journal of medicine. 2013;369(19):1828–35. Epub 2013/10/25. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocca S, Zangari P, Cotugno N, De Rossi A, Ferns B, Petricone D, Rinaldi S, Giaquinto C, Bernardi S, Rojo P, Rossi P, Pahwa S, Nastouli E, Palma P, Consortium E. Human Immunodeficiency Virus (HIV)-Antibody Repertoire Estimates Reservoir Size and Time of Antiretroviral Therapy Initiation in Virally Suppressed Perinatally HIV-Infected Children. J Pediatric Infect Dis Soc. 2018. Epub 2018/09/01. doi: 10.1093/jpids/piy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler KM, Gavin P, Coughlan S, Rochford A, Mc Donagh S, Cunningham O, Poulsom H, Watters SA, Klein N. Rapid viral rebound after 4 years of suppressive therapy in a seronegative HIV-1 infected infant treated from birth. Pediatr Infect Dis J. 2015;34(3):e48–51. doi: 10.1097/INF.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 10.Ananworanich J, Puthanakit T, Suntarattiwong P, Chokephaibulkit K, Kerr SJ, Fromentin R, Bakeman W, Intasan J, Mahanontharit A, Sirivichayakul S, Chomont N, Group H-NS. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS (London, England). 2014;28(7):1015–20. Epub 2014/01/05. doi: 10.1097/QAD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 11.Veldsman KA, Maritz J, Isaacs S, Katusiime MG, Janse van Rensburg A, Laughton B, Mellors JW, Cotton MF, van Zyl GU. Rapid decline of HIV-1 DNA and RNA in infants starting very early antiretroviral therapy may pose a diagnostic challenge. AIDS (London, England). 2018;32(5):629–34. Epub 2018/01/16. doi: 10.1097/QAD.0000000000001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Prats AJ, Draper HR, Sanders JE, Agrawal AK, Mohapi EQ, Schutze GE. False-negative post-18-month confirmatory HIV tests in HIV DNA PCR-positive children: a retrospective analysis. AIDS (London, England). 2012;26(15):1927–34. Epub 2012/06/29. doi: 10.1097/QAD.0b013e32835705bf. [DOI] [PubMed] [Google Scholar]
- 13.Transmission PoToPWwHIaPoP. Recommendations for Use of Antiretroviral Drugs in Transmission in the United States. Department of Human Health and Services; 2020. [Google Scholar]
- 14.Garcia-Broncano P, Maddali S, Einkauf KB, Jiang C, Gao C, Chevalier J, Chowdhury FZ, Maswabi K, Ajibola G, Moyo S, Mohammed T, Ncube T, Makhema J, Jean-Philippe P, Yu XG, Powis KM, Lockman S, Kuritzkes DR, Shapiro R, Lichterfeld M. Early antiretroviral therapy in neonates with HIV-1 infection restricts viral reservoir size and induces a distinct innate immune profile. Science translational medicine. 2019;11(520). doi: 10.1126/scitranslmed.aax7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henrich TJ, Hatano H, Bacon O, Hogan LE, Rutishauser R, Hill A, Kearney MF, Anderson EM, Buchbinder SP, Cohen SE, Abdel-Mohsen M, Pohlmeyer CW, Fromentin R, Hoh R, Liu AY, McCune JM, Spindler J, Metcalf-Pate K, Hobbs KS, Thanh C, Gibson EA, Kuritzkes DR, Siliciano RF, Price RW, Richman DD, Chomont N, Siliciano JD, Mellors JW, Yukl SA, Blankson JN, Liegler T, Deeks SG. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med. 2017;14(11):e1002417. Epub 2017/11/08. doi: 10.1371/journal.pmed.1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massanella M, Puthanakit T, Leyre L, Jupimai T, Sawangsinth P, de Souza M, Suntarattiwong P, Kosalarksa P, Borkird T, Kanjanavanit S, Chokephaibulkit K, Hansudewechakul R, Petdachai W, Mitchell JL, Robb ML, Trautmann L, Ananworanich J, Chomont N. Continuous prophylactic ARV/ART since birth reduces seeding and persistence of the viral reservoir in vertically HIV-infected children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020. Epub 2020/06/07. doi: 10.1093/cid/ciaa718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Mohsen M, Richman D, Siliciano RF, Nussenzweig MC, Howell BJ, Martinez-Picado J, Chomont N, Bar KJ, Yu XG, Lichterfeld M, Alcami J, Hazuda D, Bushman F, Siliciano JD, Betts MR, Spivak AM, Planelles V, Hahn BH, Smith DM, Ho YC, Buzon MJ, Gaebler C, Paiardini M, Li Q, Estes JD, Hope TJ, Kostman J, Mounzer K, Caskey M, Fox L, Frank I, Riley JL, Tebas P, Montaner LJ, infection B-HDCtCH-. Recommendations for measuring HIV reservoir size in cure-directed clinical trials. Nature medicine. 2020. Epub 2020/09/09. doi: 10.1038/s41591-020-1022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas J, Ruggiero A, Paxton WA, Pollakis G. Measuring the Success of HIV-1 Cure Strategies. Front Cell Infect Microbiol. 2020;10:134. Epub 2020/04/23. doi: 10.3389/fcimb.2020.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY, Hiddessen AL, Legler TC, Kitano TK, Hodel MR, Petersen JF, Wyatt PW, Steenblock ER, Shah PH, Bousse LJ, Troup CB, Mellen JC, Wittmann DK, Erndt NG, Cauley TH, Koehler RT, So AP, Dube S, Rose KA, Montesclaros L, Wang S, Stumbo DP, Hodges SP, Romine S, Milanovich FP, White HE, Regan JF, Karlin-Neumann GA, Hindson CM, Saxonov S, Colston BW. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83(22):8604–10. Epub 2011/11/01. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sneller MC, Huiting ED, Clarridge KE, Seamon C, Blazkova J, Justement JS, Shi V, Whitehead EJ, Schneck RF, Proschan M, Moir S, Fauci AS, Chun TW. Kinetics of plasma HIV rebound in the era of modern antiretroviral therapy. The Journal of infectious diseases. 2020. Epub 2020/05/23. doi: 10.1093/infdis/jiaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, Spina CA, Woelk CH, Richman DD. Highly precise measurement of HIV DNA by droplet digital PCR. PloS one. 2013;8(4):e55943. Epub 2013/04/11. doi: 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor SC, Laperriere G, Germain H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: from variable nonsense to publication quality data. Scientific reports. 2017;7(1):2409. Epub 2017/05/27. doi: 10.1038/s41598-017-02217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folks TM, Clouse KA, Justement J, Rabson A, Duh E, Kehrl JH, Fauci AS. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989;86(7):2365–8. Epub 1989/04/01. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Symons J, Chopra A, Malatinkova E, De Spiegelaere W, Leary S, Cooper D, Abana CO, Rhodes A, Rezaei SD, Vandekerckhove L, Mallal S, Lewin SR, Cameron PU. HIV integration sites in latently infected cell lines: evidence of ongoing replication. Retrovirology. 2017;14(1):2. Epub 2017/01/15. doi: 10.1186/s12977-016-0325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp. Stata 16 Base Reference Manual. College Station, TX: Stata Press; 2019. [Google Scholar]
- 26.Jones M, Williams J, Gartner K, Phillips R, Hurst J, Frater J. Low copy target detection by Droplet Digital PCR through application of a novel open access bioinformatic pipeline, ‘definetherain’. J Virol Methods. 2014;202:46–53. Epub 2014/03/07. doi: 10.1016/j.jviromet.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trypsteen W, Vynck M, De Neve J, Bonczkowski P, Kiselinova M, Malatinkova E, Vervisch K, Thas O, Vandekerckhove L, De Spiegelaere W. ddpcRquant: threshold determination for single channel droplet digital PCR experiments. Anal Bioanal Chem. 2015;407(19):5827–34. Epub 2015/05/30. doi: 10.1007/s00216-015-8773-4. [DOI] [PubMed] [Google Scholar]
- 28.Dreo T, Pirc M, Ramsak Z, Pavsic J, Milavec M, Zel J, Gruden K. Optimising droplet digital PCR analysis approaches for detection and quantification of bacteria: a case study of fire blight and potato brown rot. Anal Bioanal Chem. 2014;406(26):6513–28. Epub 2014/09/01. doi: 10.1007/s00216-014-8084-1. [DOI] [PubMed] [Google Scholar]
- 29.Uprety P, Patel K, Karalius B, Ziemniak C, Chen YH, Brummel SS, Siminski S, Van Dyke RB, Seage GR, Persaud D, Pediatric HIVACS. Human Immunodeficiency Virus Type 1 DNA Decay Dynamics With Early, Long-term Virologic Control of Perinatal Infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;64(11):1471–8. Epub 2017/03/23. doi: 10.1093/cid/cix192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veldsman KA, Janse van Rensburg A, Isaacs S, Naidoo S, Laughton B, Lombard C, Cotton MF, Mellors JW, van Zyl GU. HIV-1 DNA decay is faster in children who initiate ART shortly after birth than later. Journal of the International AIDS Society. 2019;22(8):e25368. Epub 2019/08/24. doi: 10.1002/jia2.25368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayon-Gil A, Puertas MC, Urrea V, Bailon L, Moron-Lopez S, Cobarsi P, Brander C, Mothe B, Martinez-Picado J. HIV-1 DNA decay dynamics in early treated individuals: practical considerations for clinical trial design. J Antimicrob Chemother. 2020;75(8):2258–63. Epub 2020/04/27. doi: 10.1093/jac/dkaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moragas M, Distefano M, Mecikovsky D, Arazi Caillaud S, Cernadas C, Bologna R, Aulicino P, Mangano A. Impact of the time to achieve viral control on the dynamics of circulating HIV-1 reservoir in vertically infected children with long-term sustained virological suppression: A longitudinal study. PloS one. 2018;13(10):e0205579. Epub 2018/10/24. doi: 10.1371/journal.pone.0205579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besson GJ, Lalama CM, Bosch RJ, Gandhi RT, Bedison MA, Aga E, Riddler SA, McMahon DK, Hong F, Mellors JW. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(9):1312–21. Epub 2014/07/31. doi: 10.1093/cid/ciu585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, Lai J, Strain MC, Lada SM, Hoh R, Ho YC, Richman DD, Deeks SG, Siliciano JD, Siliciano RF. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nature medicine. 2016;22(9):1043–9. Epub 2016/08/09. doi: 10.1038/nm.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruner KM, Wang Z, Simonetti FR, Bender AM, Kwon KJ, Sengupta S, Fray EJ, Beg SA, Antar AAR, Jenike KM, Bertagnolli LN, Capoferri AA, Kufera JT, Timmons A, Nobles C, Gregg J, Wada N, Ho YC, Zhang H, Margolick JB, Blankson JN, Deeks SG, Bushman FD, Siliciano JD, Laird GM, Siliciano RF. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature. 2019;566(7742):120–5. Epub 2019/02/01. doi: 10.1038/s41586-019-0898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhummakupt A, Persaud D. Capitalizing on Post-exposure ARV Prophylaxis to Restrict Seeding of the HIV Reservoir. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020. Epub 2020/06/06. doi: 10.1093/cid/ciaa715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulick RM, Flexner C. Long-Acting HIV Drugs for Treatment and Prevention. Annu Rev Med. 2019;70:137–50. Epub 2018/10/26. doi: 10.1146/annurev-med-041217-013717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


