Abstract
Broad-spectrum antivirals are more needed than ever to provide treatment options for novel emerging viruses and for viruses that lack therapeutic options or have developed resistance. A large number of viruses rely on charge-dependent non-specific interactions with heparan sulfate (HS), a highly sulfated glycosaminoglycan (GAG), for attachment to cell surfaces to initiate cell entry. As such, inhibitors targeting virion-HS interactions have potential to have broad-spectrum antiviral activity. Previous research has explored organic and inorganic small molecules, peptides, and GAG mimetics to disrupt virion-HS interactions. Here we report antiviral activities against both enveloped (the herpesvirus human cytomegalovirus) and non-enveloped (adenovirus) DNA viruses for four defined marine sulfated glycans: a sulfated galactan from the red alga Botryocladia occidentalis; a sulfated fucan from the sea urchin Lytechinus variegatus, and a sulfated fucan and a fucosylated chondroitin sulfate from the sea cucumber Isostichopus badionotus. As evidenced by gene expression, time of addition, and treatment/removal assays, all four novel glycans inhibited viral attachment and entry, most likely through interactions with virions. The sulfated fucans, which both lack anticoagulant activity, had similar antiviral profiles, suggesting that their activities are not only due to sulfation content or negative charge density but also due to other physicochemical factors such as the potential conformational shapes of these carbohydrates in solution and upon interaction with virion proteins. The structural and chemical properties of these marine sulfated glycans provide unique opportunities to explore relationships between glycan structure and their antiviral activities.
Keywords: Cytomegalovirus, adenovirus, virion attachment, sulfated marine glycans, antiviral, broad-spectrum
1. Introduction
Glycosaminoglycans (GAGs) are linear polysaccharides of repeating disaccharide units containing an amino sugar (either N-acetylglucosamine or N-acetylgalactosamine) and a uronic acid (either glucuronic acid and/or iduronic acid) or the neutral sugar, galactose. As a result of their structural diversity, GAGs have a variety of biological roles, including cell signaling, growth, and wound repair (Gandhi and Mancera, 2008). GAGs are ubiquitous on cell surfaces where they serve as receptors and signals for cellular and pathogenic processes. The sulfation of GAGs presents a net negative charge at cell surfaces that viral or other pathogens can bind electrostatically to initiate entry either by membrane fusion or endocytosis (Aquino and Park, 2016). Virion attachment through GAG binding and subsequent entry are often independent events in which attachment is thought to be largely nonspecific and charge-dependent while entry is mediated by highly specific protein-protein interactions. In particular, interactions with heparan sulfate (HS), a highly sulfated GAG, is a common requirement for infection by many viruses, and consequently, inhibitors targeting virion-HS interactions have potential as broad-spectrum antivirals that could exert a major impact on global health.
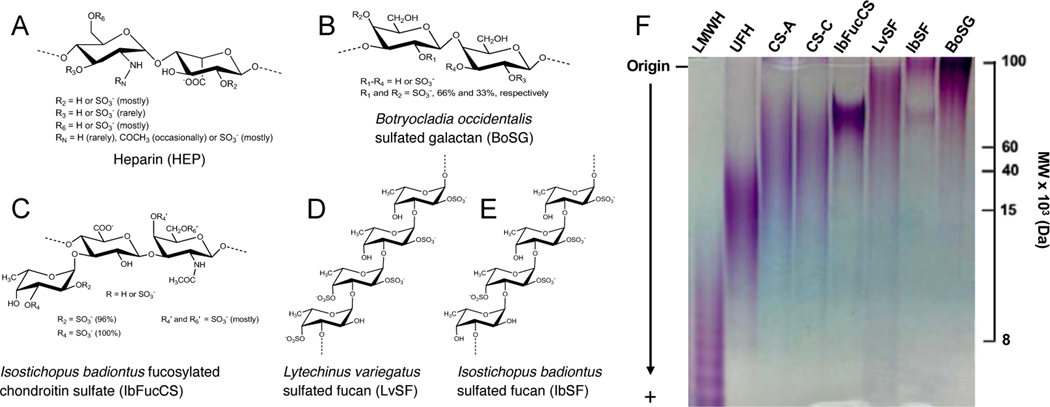
A variety of compounds have been used to bind to HS and thereby shield cells from viral attachment, including polycationic peptides, small organic compounds, and inorganic molecules (Badani et al., 2014; de Paiva et al., 2020; Hao et al., 2019; Muller et al., 2013). Alternatively, inhibitors may bind virion components to sequester and inactivate virions in a manner similar to neutralizing antibodies (Xiao et al., 2002). The majority of the latter are HS mimetics and many have broad spectrum antiviral activity. Here we describe the antiviral activities of four sulfated marine glycans and compare them to heparin, a commonly used HS-mimetic composed primarily of disaccharide repeating units of [−4)-N,6-disulfated-glucosamine-(α1–4)-2-sulfated-iduronic acid-(α1-] (Figure 1A). The marine sugars include: (i) a sulfated galactan isolated from the red alga Botryocladia occidentalis (BoSG) composed of the disaccharide repeating unit [−3)-2,4-disulfated-galactose-(α1–4)-2,3-disulfated-galactose-(β1-] in which sulfation patterns may vary in percentage but never in position (Figure 1B) (Pomin and Mourão, 2014); (ii) a fucosylated chondroitin sulfate isolated from the sea cucumber Isostichopus badionotus (IbFucCS) composed of the trisaccharide-repeating unit {−4)-[ fucose-(α1–3)]-glucuronic acid-(β1–3)-N-acetylgalactosamine-(β1-} in which sulfation patterns may vary in percentage but never in position (Figure 1C) (Chen et al., 2011); (iii) a sulfated fucan isolated from the sea urchin Lytechinus variegatus (LvSF) composed of the tetrasaccharide-repeating unit of [−3)-4-sulfated-fucose-(α1–3)-2,4-disulfated-fucose-(α1–3)-2-sulfated-fucose-(α1–3)-2-sulfated-fucose-(α1] (Figure 1D) (Pomin and Mourão, 2014); and (iv) a sulfated fucan also isolated from Isostichopus badionotus (IbSF) composed of the tetrasaccharide-repeating unit of [−3)-fucose-(α1–3)-2,4-disulfated-fucose-(α1–3)-2-sulfated-fucose-(α1–3)-2-sulfated-fucose-(α1] (Figure 1E) (Chen et al., 2012).
Fig. 1. Structures and size characterization of the sulfated marine glycans.
(A-E) Structures are shown for heparin and the four sulfated marine glycans (counterions omitted for clarity). (F) The indicated sulfated glycans (10 μg) were separated by polyacrylamide gel electrophoresis and stained with toluidine blue. To the right are indicated MWs of standard compounds LMWH (7.5 kDa), UFH (15 kDa), CS-A, (40 kDa), and CS-C (60 kDa).
In contrast to heparin and the red alga-derived BoSG, which are heterogeneous in terms of sulfation pattern (although BoSG is a homopolymer of galactose, while heparin is composed of more than one sugar in the backbone), the other three invertebrate-derived sulfated glycans exhibit chemically defined structures with more uniform distribution of sulfation and sugar composition (Pomin, 2017, 2015, 2012a). In addition, and again in contrast to heparin and other GAG mimetics that are highly hemorrhagic, no bleeding effects have been reported for the marine sulfated sugars BoSG, LvSF, and IbSF (Quindere et al., 2013; Vasconcelos et al., 2018). Therefore, this set of well defined and structurally distinct sulfated glycans provides a unique opportunity to identify through structure-activity studies those structure(s) associated with optimal antiviral activities or undesirable biological effects. These unique chemical and biological properties make the marine sugars promising molecular tools in antiviral research.
2. Materials and Methods
2.1. Cell and viral culture
Human MRC-5 fetal lung fibroblasts (ATCC CCL-171) and human APRE-19 epithelial cells (derived from retinal pigment epithelium) (ATCC CRL-2302) were purchased from American Type Culture Collection. MRC-5 and APRE-19 cells were cultured at 37°C in a 5% CO2 atmosphere using Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum, 50 U/mL penicillin, 50 mg/mL streptomycin, and 29.2 mg/mL L-glutamine (DMEM, all from Life Technologies).
Human cytomegalovirus (HCMV) BADrUL131-Y4 (BADr), a gift from Dai Wang and Thomas Shenk, is a variant of HCMV strain AD169 that is epithelial tropic due to repair of a mutation in UL131A and contains a green-fluorescent protein (GFP) reporter cassette (Wang and Shenk, 2005). Virus RC2626 is a variant of HCMV strain Towne that contains an expression cassette for firefly luciferase (McVoy and Mocarski, 1999). RC2626 and BADr were propagated in MRC-5 and ARPE-19 cells, respectively, and stocks were derived from infected cell culture supernatants, adjusted to 0.2 M sucrose, and stored in liquid nitrogen. Infectious titers of viral stocks were determined as plaque forming units (PFU)/mL using MRC-5s or ARPE-19s as described (Cui et al., 2012). Stocks of GFP-tagged adenovirus, provided by Dr. Daniel Conway at Virginia Commonwealth University, were produced using the pAdeasy adenovirus-packaging system as described previously (Xiao et al., 2003)(He et al., 1998).
2.2. Compounds and compound isolation
Purification of BoSG and LvSF were conducted as previously described (Farias et al., 2000; Mulloy et al., 1994). Briefly, 20 mg of crude polysaccharides obtained after papain digestion were extracted from the respective tissues (red algal body wall and sea urchin egg jelly) and applied to a 2.5 × 20 cm DEAE-cellulose column equilibrated with 50 mM sodium acetate buffer (pH 5.0) and washed with 300 mL of the same buffer containing 0.2 M NaCl and 10 mM EDTA. The column was eluted with a linear gradient prepared by mixing 2000 mL of 50 mM sodium acetate buffer (pH 5.0) containing 0.2 M NaCl and 10 mM EDTA with 200 mL of 1.2 M NaCl in the same buffer. The flow rate of the column was 12 mL/h. Fractions of 1.5 mL were collected and checked by metachromatic properties (Farndale et al., 1986). The chromatograms of the two materials resulted in three major fractions and both BoSG and LvSF were associated with the third fraction. Purity and structural integrity of BoSG and LvSF were confirmed by 1D 1H (400 MHz) NMR spectra in acquired with 32 scans at the 400 MHz Bruker Avance III HD equipped with 5 mm BBFO RT probe, indicating > 98% purity, no residual solvent contamination, and NMR signal patterns consistent with those of previous publications (Farias et al., 2000; Mulloy et al., 1994). For NMR analysis, around 5 mg of sugar samples were individually dissolved in 550 μL of deuterium oxide (D2O) “100%” (D 99,96%), purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA), and transferred to a 3 mm NMR tube for data acquisition.
Sea cucumber Isostichopus badionotus was obtained from Gulf Specimen Lab (Gulf of Mexico, Florida keys). Papain (≥10Units/mg), Sephadex G15 medium, and DEAE cellulose resin (0.98meq/mg) were purchased from Sigma (St. Louis, MO, USA). Polysaccharides IbSF and IbFucCS were isolated from Isostichopus badionotus following a slightly modified protocol reported earlier (Chen et al., 2012, 2011). The dried body wall was digested using papain (0.1 mg/100 mg of dry tissue), 5 mM cysteine and 5 mM EDTA in 0.1 M NaOAc buffer, pH 6.0 (2 mL/100 mg of dry tissue) at 60°C for 24 h. The digested mixture was centrifuged (4000 rpm for 30 min) and the supernatant was precipitated using two volumes of 95% ethanol at −20°C. After 24 h the precipitate was obtained by centrifugation at 4000 rpm for 30 min., dissolved in water, and dialyzed three times against distilled water prior to lyophilization. This dry crude extract was purified by anion exchange chromatography on a 2.5 × 20 cm DEAE-cellulose column. IbSF and IbFucCS were eluted and separated using a linear gradient of NaCl (in 0.1 M NaOAc, pH 6.0) increasing from 0 to 3 M at a flow rate of 18 mL/hr. The obtained fractions were characterized for sugar by 1,9–dimethylmethylene blue (DMB) assay (Farndale et al., 1982). The polysaccharide fractions were pooled and dialyzed three times against water and lyophilized. The dialyzed sugars were further purified on a 1 × 20 cm Sephadex G15 size exclusion column. Purity and structural integrity of all five sulfated glycans studied in this work were confirmed by 1D 1H NMR spectra acquired with 32 scans at the 400 MHz Bruker Avance III HD equipped with 5 mm BBFO RT probe. For NMR analysis, around 5 mg of sugar samples were individually dissolved in 550 μL of deuterium oxide (D2O) “100%” (D 99,96%), purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA), and transferred to a 5 mm NMR tube for data acquisition.
BoSG, LvSF, IbSF, and IbFucCS were dissolved in water at a stock concentration of 3 mg/mL. Heparin sodium was purchased from Acros Organics, USA (Lot # B0146868; percent purity 150 IU/mg min.) and dissolved in water at a stock concentration of 1 mg/mL.
2.3. Molecular weight (MW) analysis of sulfated glycans
Ten μg of each sulfated glycan were electrophoresed for 30 min at 100 V on a 1-mm-thick 12% native polyacrylamide gel in 0.02 M sodium barbital (pH 8.6) then stained with 0.1% toluidine blue in 1% acetic acid and washed for 4 h in 1% acetic acid. MW were estimated by comparison with the migration of known standards low molecular weight heparin (LMWH, ∼7.5 kDa), unfractionated heparin (UFH, ∼15 kDa), chondroitin 4-sulfate from bovine trachea (CS-A, ∼40 kDa), and the chondroitin 6-sulfate from shark cartilage (CS-C, ∼60 kDa), as previously described (Pomin et al., 2005b, 2005a; Queiroz et al., 2016).
2.4. Measurement of antiviral potencies
Antiviral potency was quantitated using GFP reporter assays as previously described (Shoup et al., 2020). Briefly, confluent monolayers of MRC-5 or ARPE-19 cells in 96-well plates were treated for one h with concentrations of BoSG, LvSF, IbSF, IbFucCS, or HEP ranging from 400 μg/mL to 6.8 ng/mL, then infected with 100 PFU/well virus BADr or GFP-adenovirus. On day six post infection relative fluorescence units (RFU) of GFP fluorescence were quantified using a BioTek Synergy HT Multi-Mode Microplate reader and 50% effective concentration (EC50) values were determined as inflection points of four-parameter curves of RFU (means of triplicate data) versus log inhibitor concentration fitted using Prism 5 software (Graphpad). Graphical representations were normalized to % maximum RFU.
2.5. Cytotoxicity
Replicate cell cultures were prepared simultaneously with those described in 2.4 but were not infected. After incubation of five days cell viability was determined using the luciferase-based CellTiter-Glo® (Promega) assay as previously described (Shoup et al., 2020). Relative light units (RLU) were measured using a BioTek Synergy HT Multi-Mode Microplate reader and where possible fitted to four-parameter curves as described in 2.4. As cell viability with all compounds remained over 50% at concentrations up to and including the highest tested (400 μg/mL), 50% cytotoxicity concentrations (TC50) were reported as > 400 μg/mL. Graphical representations were normalized to % maximum RLU.
2.6. Time of addition, treatment/removal, and dilution studies
For time of addition studies MRC-5 or ARPE-19 cultures were infected with 100 PFU/well BADr or GFP-tagged adenovirus, respectively. Each compound was added to a final concentration of 150 μg/mL 1 h before, at the time of, and 1, 3, 6, 12, or 24 h post infection. GFP fluorescence was quantified six days after infection and plotted versus time of compound addition (relative to infection). For treatment/removal studies MRC-5 or ARPE-19 cultures were treated with 150 μg/mL of each compound for one h then washed three times with DMEM prior to infection with 100 PFU/well BADr or GFP-tagged adenovirus. Six days after infection representative micrographs were taken with a Nikon Eclipse TS100 Inverted UV microscope. For dilution studies BADr or adenovirus was incubated with 150 μg/mL of each compound for one h then the mixture was diluted with culture medium to 15 ng/mL and added to MRC-5 or ARPE-19 cultures. Six days after infection representative micrographs were taken with a Nikon Eclipse TS100 Inverted UV microscope.
2.7. Immunofluorescence microscopy
The HCMV virion-associated tegument protein pp65 and immediate early 1 and 2 (IE1/2) proteins were detected as described previously (Shoup et al., 2020). Briefly, MRC-5 cultures were pretreated with 150 μg/mL of each compound for 1 h. For IE1/2 staining cultures were infected with 125 PFU/well HCMV RC2626 and 48 h after infection fixed and stained using a monoclonal antibody specific for IE1/2. For pp65 staining similarly treated cultures were infected for one h at 4°C with 200 PFU/well HCMV BADr, then shifted to 37°C and incubated for six h before fixing and staining with a monoclonal antibody specific for pp65.
3. Results
3.1. Structures and size characterization of marine sulfated glycans
Structures for heparin and the four marine sulfated glycans are shown in figures 1A–1E. While most sulfated glycans have a polydisperse nature, each exhibits a characteristic size range and anverage MW. To better define the average and range of MWs for the four marine sulfated glycans, IbSF, IbFucCS, BoSG and LvSF were analyzed by polyacrylamide gel electrophoresis and compared with known standards (Figure 1F). Approximate average MWs were 75, 90, and 100 kDa for IbFucCS, LvSF, and IbSF, respectively, and >100 kDa for BoSG. To assess purity and integrity, all five sulfated glycans studied in this work were assessed by 1D 1H NMR. The resulting spectra, shown in supplemental Figure S1, were consistent with prior work (see Santos et al. (Santos et al., 2014) for heparin, Farias et al. (Farias et al., 2000) for BoSG, Chen et al. 2012 (Chen et al., 2012) for IbSF, Pomin et al. (Pomin et al., 2005a) and Chen et al. (Chen et al., 2011) for IbFucCS) and indicated greater than 98% purity for all five sugars with no residual solvent contamination.
3.2. Marine sulfated glycans inhibit HCMV and adenovirus reporter gene expression
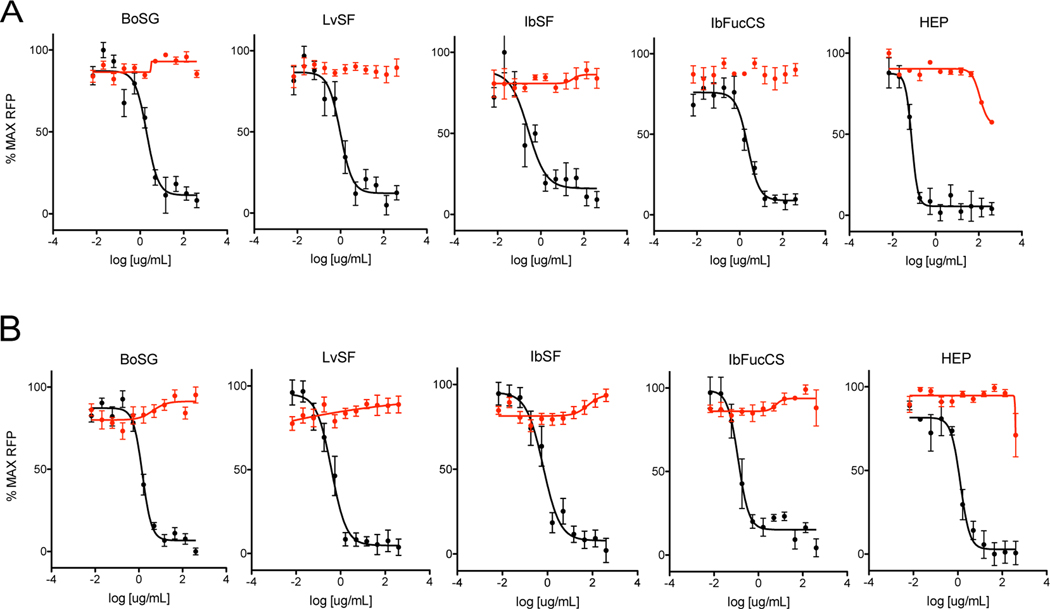
Our hypothesis predicts that marine sulfated glycans may act as HS-mimetics to inhibit viral infection by disrupting virion-HS interactions. If so, they should block entry and subsequent gene expression of viruses that enter cells through HS-dependent mechanisms. To test this hypothesis we evaluated the four marnine glycans for inhibition two HS-dependent DNA viruses: human cytomegalovirus (HCMV), an enveloped herpesvirus, and human adenovirus serotype 5 (Ad5), a non-enveloped virus. In both cases genetically modified viruses containing GFP reporter cassettes were used so that successful infection could be detected and quantitated using GFP. Human MRC-5 fibroblasts or ARPE-19 epithelial cells were pretreated for one h with increasing concentrations of BoSG, LvSF, IbSF, or IbFucCS and heparin was used as a positive control as it is known to block infection of both HCMV and Ad5 (Compton et al., 1993; Dechecchi et al., 2001). GFP-tagged HCMV variant BADr was then added to MRC-5 cultures and GFP-tagged Ad5 was added to ARPE-19 cultures and after six days total GFP fluoresence was measured. As shown in Figure 2, heparin and all four marine glycans reduced GFP expression in HCMV- and Ad5-infected cultures in a dose-dependent manner, while all compounds were non-toxic up to the highest concentration tested (400 μg/mL).
Fig. 2. HCMV and Ad5 antiviral activities and cytotoxicities of marine sulfated glycans.
(A) Anti-HCMV activity (black) was measured by incubating MRC-5 fibroblast monolayers in 96-well plates with BoSG, LvSF, IbSF, IbFucCS, or heparin (HEP) for one h, then infecting with GFP-tagged HCMV BADr (100 PFU/well). (B) Anti-adenovirus activity (black) was measured by incubating ARPE-19 epithelial cell cultures as above then infecting with GFP-tagged Ad5 (100 PFU/well). After six days GFP activities in cultures were measured. Cell viability (red) was measured in replicate uninfected cultures treated for five days using the CellTiter-Glo® assay. Data are means of triplicate experiments ± standard deviations.
To compare relative antiviral potencies the 50% effective concentration (EC50) for each compound was determined as the concentration at which GFP levels were reduced by half. Cytotoxicities were determined by measuring cell viability in replicate uninfected cultures. As cell viability with all compounds remained over 50% at concentrations up to and including the hightest tested (400 μg/mL), 50% cytotoxicity concentrations (TC50) were reported as > 400 μg/mL. A high TC50 to EC50 ratio, or selectivity index (SI), suggests a favorable safety and efficacy profile. Tables 1 and 2 summarize these quantitative data as derived from the experiments shown in Figure 2. As toxicity of all four marine glycans was > 400 μg/mL, SIs were approximated by assigning TC50 400 μg/mL. The resulting high SIs imply potentially favorable safety profiles.
Table 1.
Anti-HCMV activities of Sulfated GAGs
| Structure | HCMV Antivirala (EC50) | Cytotoxicity (TC50)b | Selectivity index (SI)c |
|---|---|---|---|
| BoSG | 1.719 ± 1.028 | > 400 | 232.7 |
| LvSF | 1.369 ± 0.477 | > 400 | 292.1 |
| IbSF | 0.662 ± 0.450 | > 400 | 603.6 |
| IbFucCS | 0.422 ± 0.349 | > 400 | 947.5 |
| Heparin | 0.331 ± 0.106 | > 400 | 1211.1 |
GFP-based assay
CellTiter-Glo® assay
μg/mL; means of three independent experiments ± standard deviations
SI = TC50/EC50 (TC50 = 400 for these calculations)
Table 2.
Anti-Adenovirus activities of Sulfated GAGs
| Structure | Adenovirus Antivirala (EC50) | Cytotoxicity (TC50)b | Selectivity index (SI)c |
|---|---|---|---|
| BoSG | 1.738 ± 0.201 | > 400 | 230.1 |
| LvSF | 0.229 ± 0.129 | > 400 | 1742.4 |
| IbSF | 0.237 ± 0.116 | > 400 | 1685.6 |
| IbFucCS | 0.124 ± 0.034 | > 400 | 3238.9 |
| Heparin | 1.815 ± 0.719 | > 400 | 220.4 |
GFP-based assay
CellTiter-Glo® assay
μg/mL; means of three independent experiments ± standard deviations
SI = TC50/EC50 (TC50 = 400 for these calculations)
3.3. Time of addition studies indicate an early-acting mechanism of action
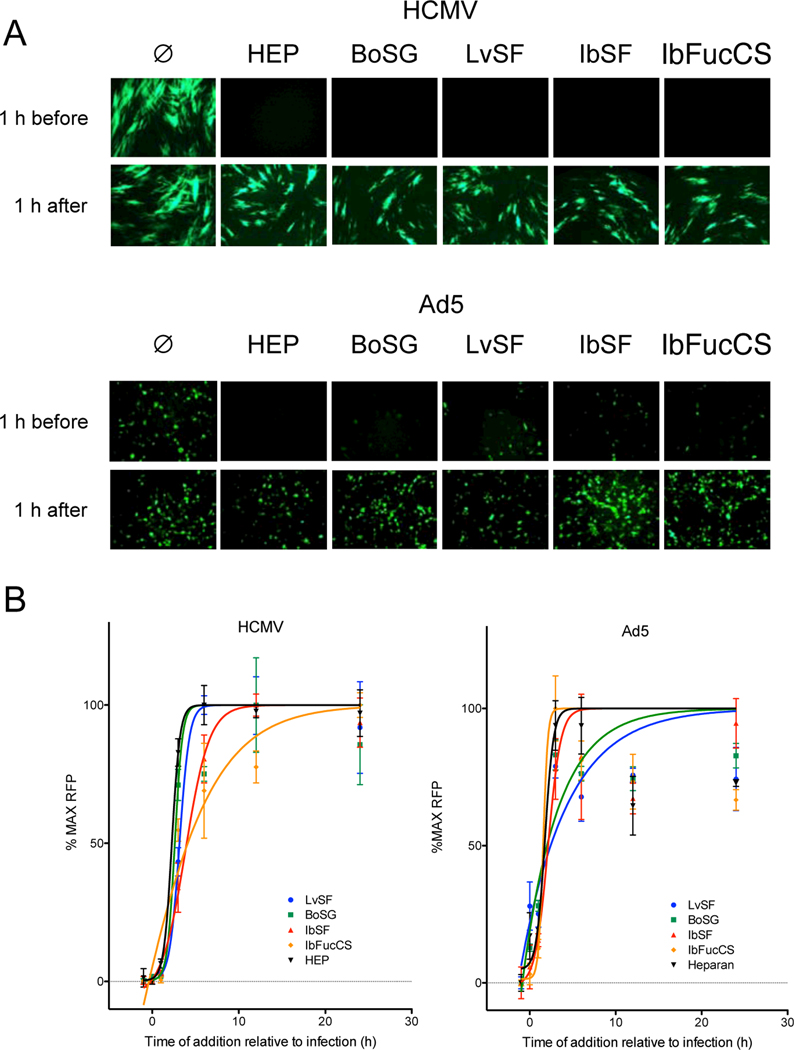
If marine glycans act by blocking virion attachment or entry, they should be ineffective if added after viral entry is complete. To address this question a single inhibitory concentration (150 μg/mL) of BoSG, LvSF, IbSF, IbFucCS, or heparin was added to cells one h before or one h after infection with GFP-tagged HCMV or Ad5 and GFP expression was visualized by fluoresence microscopy. When added to cells prior to adding the virus heparin and all four marine glycans greatly reduced or eliminated GFP expression in both HCMV- and Ad5-infected cultures. In contrast, no inhibition of GFP expression was observed if heparin or the four marine glycans were added 1 h after cells were exposed to either virus (Figure 3A).
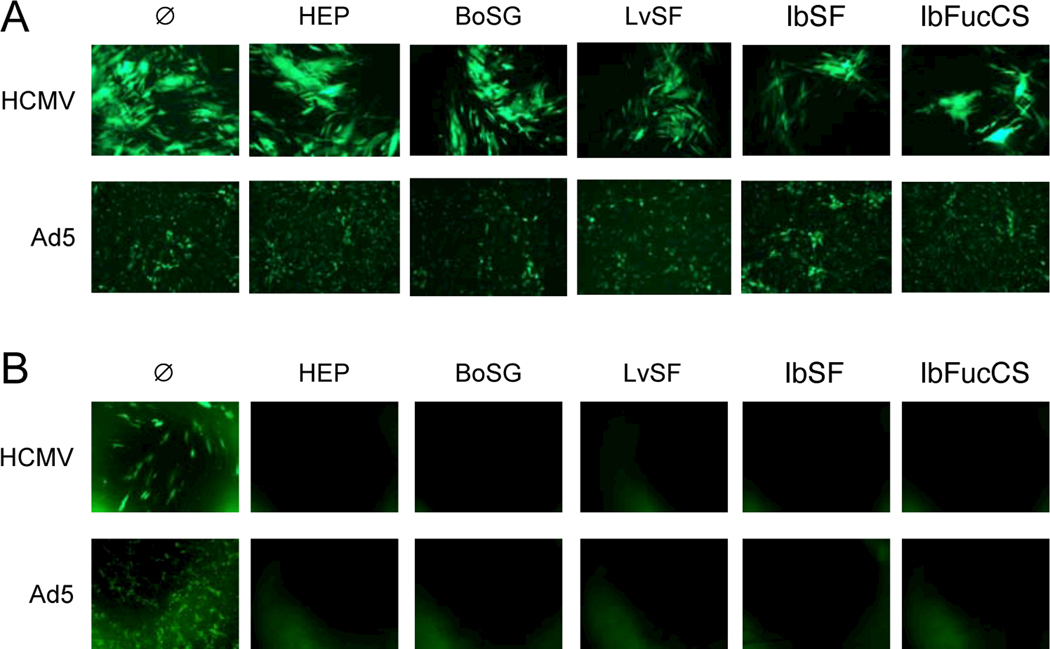
Fig. 3. Marine sulfated glycans inhibit viral-encoded GFP expression when present prior to HCMV or Ad5 infection.
(A) Confluent monolayers of MRC-5 fibroblasts or ARPE-19 epithelial cells in 96-well plates were treated with medium (Ø) or with 150 μg/mL BoSG, LvSF, IbSF, IbFucCS, or heparin (HEP) either one h before or one h after infection with GFP-tagged HCMV BADr (100 PFU/well) or GFP-tagged Ad5 (100 PFU/well). Representative fluorescent micrographs were taken six days post infection. (B) Confluent monolayers of MRC-5 fibroblasts or ARPE-19 epithelial cells were treated as above either one h before, concurrent with, or 1, 3, 6, 12, or 24 h after infection with GFP-tagged HCMV BADr (100 PFU/well) or GFP-tagged Ad5 (100 PFU/well). GFP expression was quantified on day six post infection. Data are means of triplicate wells ± standard deviations.
To more precisely and quantitatively define the kinetics of inhibition, a time of addition experiment was conducted in which cells were treated with compounds before, during, or after infection and GFP was measured on day six post infection. Consistent with the qualitative results described above, heparin and the four marine glycans were only active in inhibiting HCMV or Ad5 GFP expression if present prior to or within the first 1 h post infection, while addition of compounds three or more h after infection had no effect on GFP levels (Figure 3B).
3.4. Marine sulfated glycans inhibit HCMV immediate early gene expression and cellular deposition of virion-associated tegument protein pp65
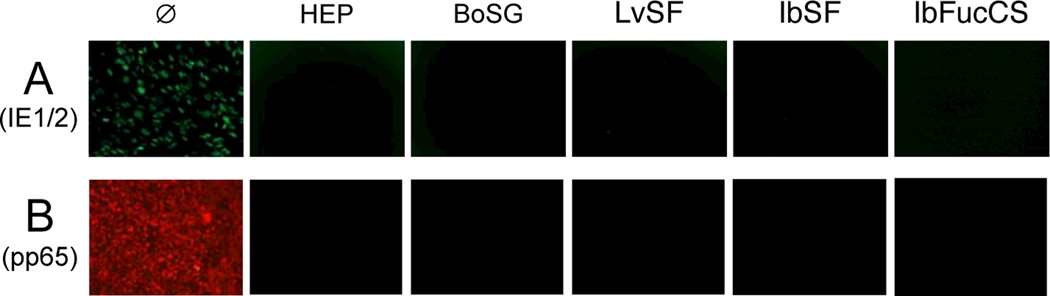
The earliest de novo gene expression events during HCMV replication result in production of the viral Immediate Early 1 and 2 (IE1/2) proteins. To determine if marine glycans block IE1/2 expression, cells were pretreated with heparin or the four marine glycans prior to infection with HCMV variant RC2626 (which does not express GFP), and the IE1/2 proteins were detected by immunofluorescence 48 h after infection. Again consistent with inhibition of virion attachment or entry, IE1/2-positive cells were undetectable in cultures pretreated with heparin, BoSG, LvSF, IbSF, or IbFucCS (Figure 4A).
Fig. 4. Marine sulfated glycans inhibit expression of HCMV IE1/2 proteins and deposition of tegument protein pp65.
(A) MRC-5 fibroblast monolayers were treated for one h with medium (Ø) or 150 μg/mL heparin (HEP), BoSG, LvSF, IbSF, or IbFucCS, then infected with HCMV RC2626 (125 PFU/well). Cultures were fixed and fluorescently stained for HCMV IE1/2 proteins 48 h post infection. (B) MRC-5 fibroblast monolayers were treated as above but incubated with HCMV BADr (200 PFU/well) for one h at 4°C. Cultures were then shifted to 37°C and incubated for six h before being fixed and fluorescently stained for the HCMV tegument protein pp65.
The HCMV pp65 protein is an abundant component of the virion tegument (Varnum et al., 2004) that is deposited into the cytoplasm upon infection and subsequently localizes to the nucleus (Duan et al., 2012). Consequently, detection of pp65 shortly after infection indicates virion attachment (Ibig-rehm et al., 2011). To determine if marine glycans block attachment of HCMV virions to cells, deposition of pp65 was similarly assessed by immunofluorescence 6 h after HCMV infection. Again, heparin and the four marine glycans eliminated pp65 deposition onto cells, consistent with inhibition of virion attachment (Figure 4B).
3.5. Sulfated marine glycans appear to target virion rather than cellular factors
To further define the potential virion target(s) of BoSG, LvSF, IbSF, and IbFucCS, cells were pretreated with each glycan for 1 h and then washed three times with media prior to infection with GFP-tagged HCMV or Ad5 viruses. Detection of GFP by fluorescence microscopy six days after infection demonstrated that cells exposed to heparin or the four marine glycans remained susceptible to HCMV or Ad5 infection as pretreatment and removal failed to significantly inhibit GFP expression (Figure 5A). However, when the marine glycans were incubated with HCMV or Ad5 and diluted 10,000-fold to a non-inhibitory concentration before being added to cell cultures, GFP expression remained fully inhibited (Figure 5B). These results indicate that heparin and the marine glycans do not act by binding to cellular components, but more likely interact with virion components and thereby disrupt the ability of virions to attach to cells.
Fig. 5. Treatment/removal studies.
(A) Confluent monolayers of MRC-5 fibroblasts (top) or ARPE-19 epithelial cells (bottom) in 96-well plates were treated with medium (Ø) or 150 μg/mL heparin (HEP), BoSG, LvSF, IbSF, or IbFucCS for one h then cells were washed three times with medium and infected with GFP-tagged HCMV BADr (100 PFU/well) or GFP-tagged Ad5 (100 PFU/well). (B) HCMV BADr or adenovirus virions were incubated with sulfated glycans as in (A) for 1 h, then diluted 10,000-fold with culture medium to a non-inhibitory concentration (15 ng/mL). Virions with sulfated glycans were then added to MRC-5 fibroblasts (top) or ARPE-19 epithelial cells (bottom) in 96-well plates. Representative fluorescent micrographs were taken six days post infection.
4. Discussion
In 2018 the World Health Organization released a list of priority pathogens to direct research and development. These pathogens were chosen based on their epidemic potential and/or lack of effective countermeasures (i.e., antivirals or vaccines). As of Fall 2020 there are eleven viruses on the list, namely SARS-CoV-1, SARS-CoV-2, MERS, Crimean-Congo hemorrhagic fever virus, Ebola virus, Marburg virus, Lassa fever virus, Nipah virus, henipavirus, and Rift Valley fever virus. Of these, seven are HS-dependent and one is speculated to be HS-dependent (Cagno et al., 2019; Liu et al., 2020). An inhibitor targeting virion-HS interactions could potentially prevent infections by many or all of these viruses.
Compounds targeting virion-HS interactions have seen moderate success as broad spectrum antivirals in vitro. Polycationic peptides, like polyarginine, associate with HS and have broad spectrum antiviral activity (Badani et al., 2014; Qureshi et al., 2014) but are subject to inactivation by proteolytic cleavage. Organic small molecules such as N,N’-bisheteryl derivatives of dispirotripiperazine inhibit the attachment of several viruses, including strains resistant to traditional specifically-targeted treatments (Paeschke et al., 2014; Schmidtke et al., 2003; Selinka et al., 2007). Inorganic compounds take advantage of the natural affinity between metal cations and GAGs, which in vivo are associated with physiologically relevant cations (Stevic et al., 2011). Inorganic polymers and small molecules have both demonstrated broad-spectrum antiviral activity though none have progressed beyond the clinical evaluation phase or been licensed (de Paiva et al., 2020; Muller et al., 2013; Shoup et al., 2020).
Alternatively, compounds that disrupt virion-HS interactions by targeting viral glycoproteins can also be broad-spectrum but have a number of issues that preclude their general use as antivirals. Although polystyrene sulfonate demonstrated activity against HIV-1, HSV-1, HSV-2, and influenza A virus, high concentrations are needed, which increases the likelihood of non-specific binding and reduces effectiveness (Anderson et al., 2000; Herold et al., 2000; Mauck et al., 2004; Neurath et al., 2002; Zaneveld et al., 2002). Suramin inhibits viral attachment but is also bound by plasma proteins, which reduces its efficacy in vivo (De Clercq, 1987, 1979; Yao et al., 1991).
A large number of compounds modeled after HS inhibit viral infection by sequestering virions. Heparin is a well-known viral entry inhibitor that electrostatically interacts with viral glycoproteins and competitively inhibits virion attachment (Astrup and Galsmar, 1944; Busso and Resnick, 1990; Cagno et al., 2019; De Clercq, 1995; Ito et al., 1987). However, heparin is also an effective anticoagulant and as such common side effects of heparin include excessive bleeding and thrombocytopenia, requiring constant patient monitoring and, if needed, use of an antidote (protamine) (Oduah et al., 2016). In an effort to circumvent these issues, a number of other GAGs or HS-mimetics have been tested for antiviral activity. Dextran sulfate inhibits viral attachment but suffers from similar issues as heparin (Astrup and Galsmar, 1944; Busso and Resnick, 1990; De Clercq, 1995; Mitsuya et al., 1988). Carrageenans (sulfated polysaccharides from red seaweeds) inhibit enveloped DNA and RNA viruses but do not inhibit non-enveloped viruses, suggesting that enveloped viruses may be more susceptible to GAG mimetic antivirals (Baba et al., 1988; Ehresmann et al., 1976; Gerber et al., 1958; Nakashima et al., 1987). Carraguard failed Phase III clinical trials due to a large number of adverse events (Skoler-karpoff et al., 2008). Sulfated derivatives of the K5 polysaccharide from E. coli have also shown broad-spectrum antiviral activity (Donalisio et al., 2014; Mercorelli et al., 2010). Cellulose sulfate is active against a number of viruses and bacteria, including HSV-1, HSV-2, HPV, HIV-1, Neisseria gonorrhoeae, and Chlamydia trachomatis, but failed to show efficacy in phase two clinical trials (Anderson et al., 2000; Astrup and Alkjaersig, 1950; Christensen et al., 2001; Halpern et al., 2008; Van Damme et al., 2008).
The anticoagulant properties of the marine sugars used in this work have been previously described. BoSG and IbFucCS exhibit strong anticoagulant activities of 93 and 183 international units (IU)/mg, respectively, as determined by the activated partial thromboplastin time method, whereas the two sulfated fucans LvSF and IbSF exhibit negligible activities of 3 and 9 IU/mg, respectively (Chen et al., 2012; Pomin, 2012b). From the current work both sulfated fucans have sub-μM EC50s against HCMV and Ad5 with no evident cytotoxicity at 400 μg/mL (Figure 2, Tables 1 and 2). These features make these sulfated fucans promising candidates for further evaluation and development as potential antivirals.
IbSF shows the lowest density of charge due to the presence of lower sulfation content as compared to the other polysaccharides tested here (Figure 1). From the structure-activity relationship standpoint achieved by exploiting the marine sugars of defined structures, it was interesting to see that LvSF and IbSF presented similar antiviral activities against both viruses (Tables 1 and 2), although slightly better for IbSF. We note that LvSF and IbSF are comparatively similar in terms of structure with regard to monosaccharide composition of glycosidic bonds, anomericity, and sulfation positions and sequencing. However, IbSF is less sulfated than LvSF as the 4-sulfated-fucose in LvSF is replaced by a non-sulfated fucose in IbSF (Figure 1). These observations suggest that a non-anticoagulant sulfated glycan can still present antiviral effects and that activity is not merely due to a consequence of sulfation content and negative charge density but also due to the potential conformational shapes of these carbohydrates in solution and upon interaction with their protein partners. Such conformational differences might lead to different affinities for the viral proteins, ultimately resulting in different antiviral actions. Recently we showed by NMR and computational simulations the 3D structure of LvSF in solution (Bezerra et al., 2019), PDB ID 7KS6. We are currently investigating through the same methods the conformational view of IbSF. Data from these two works concerned with the structural biology of the invertebrate-derived sulfated fucans may help to elucidate the antiviral activities of these two sugars as reported here.
Conclusions
Our studies demonstrate the antiviral activities of marine sulfated glycans against two very different DNA viruses, one enveloped (HCMV) and one non-enveloped (Ad5). All four compounds had μg/mL activities, suggesting that structure and sulfation of the glycans did not have a large influence on antiviral activity. Unlike previously studied GAG mimetics and heparin, BoSG, LvSF, IbSF, and IbFucCS have homogenous structures; their sulfation levels vary but the pattern of saccharide units is consistent. However, BoSG and IbFucCS have anticoagulant activities which, like other GAG mimetics, would presumably preclude their general use as antivirals. Consistent with the similarity of their structures, LvSF and IbSF have similar antiviral activities, and as both lack anticoagulant activity they merit further study as antivirals. Conformational differences, studied trough NMR and computational simulations, may help to explain differences in antiviral and anticoagulant activities of these glycans.
Supplementary Material
Supplemental Fig. S1. 1D 1H NMR spectra (expansions from δH 6.0–0.5 ppm) of all sulfated glycans analyzed in this work: heparin, BoSG, IbSF, LvSF, and IbFucCS.
Highlights.
Four sulfated marine glycans with low cytotoxicity have antiviral activity against adenovirus and human cytomegalovirus
Mechanistic studies indicate an early acting mechanism consistent with inhibition of virion/host cell attachment
In contrast to heparin, two of the sulfated marine glycans lack anticoagulant activity
Greater structural homogeneity (relative to heparin) will facilitate elucidation of structure-activity relationships
5. Acknowledgments
The authors thank Thomas Shenk and Dai Wang for the gift of virus BADr, and Daniel Conway for the gift of Ad5. This work was supported by grants to M.M from the Virginia Commonwealth University COVID-19 Rapid Research Funding program and from the Children’s Hospital Foundation Research Fund, Children’s Hospital of Richmond at VCU. V.H.P. acknowledges research funds from the University of Mississippi, the AACP 219 NIA, the NIH/NIGMS 1P20GM130460–01A1, sub-project 7936, and NIH/NINDS 1R03NS110996–01A1. V.H.P. is grateful to William Vignovich and Francisco Felipe Bezerra for their contributions on the isolation steps of BoSG and LvSF, respectively.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RA, Zaneveld LJD, Usher TC, 2000. Cellulose sulfate for use as antimicrobial and contraceptive agent. [Google Scholar]
- Aquino RS, Park PW, 2016. Glycosaminoglycans and infection. Front. Biosci 21, 1260–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup T, Alkjaersig N, 1950. Polysaccharide polysulphic acids as antihyaluronidases. Nature 166, 568–569. [DOI] [PubMed] [Google Scholar]
- Astrup T, Galsmar I, 1944. On the anticoagulant acitivty of heparin and synthetic polysaccharide sulfuric acids. Acta Physiol. Scand 8, 361–364. [Google Scholar]
- Baba M, Snoeck R, Pauwels R, De Clercq E, 1988. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother 32, 1742–1745. 10.1128/AAC.32.11.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badani H, Garry RF, Wimley WC, 2014. Peptide entry inhibitors of enveloped viruses: The importance of interfacial hydrophobicity. Biochim. Biophys. Acta 1838, 2180–2197. 10.1016/j.bbamem.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra FF, Vignovich WP, Aderibigbe AO, Sharp JS, Doerksen RJ, Pomin VH, 2019. Conformational properties of L -fucose and the tetrasaccharide building block of the sulfated L -fucan from Lytechinus variegatus. J. Struct. Biol 107407. 10.1016/j.jsb.2019.107407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso ME, Resnick L, 1990. Anti-human immunodeficiency virus effects of dextran sulfate are strain dependent and synergistic or antagonistic when dextran sulfate is given in combination with dideoxynucleosides. Antimicrob. Agents Chemother 34, 1991–1995. 10.1128/AAC.34.10.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagno V, Tseligka ED, Jones ST, Tapparel C, 2019. Heparan sulfate proteoglycans and viral attachment: True receptors or adaptation bias? Viruses 11, 1–24. 10.3390/v11070596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Hu Y, Ye X, Li G, Yu G, Xue C, Chai W, 2012. Sequence determination and anticoagulant and antithrombotic activities of a novel sulfated fucan isolated from the sea cucumber Isostichopus badionotus. Biochim. Biophys. Acta - Gen. Subj 1820, 989–1000. 10.1016/j.bbagen.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Chen S, Xue C, Yin L, Tang Q, Yu G, Chai W, 2011. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym 83, 688–696. 10.1016/j.carbpol.2010.08.040 [DOI] [Google Scholar]
- Christensen ND, Reed CA, Culp TD, Hermonat PL, Howett MK, Anderson RA, Zaneveld LJD, 2001. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob. Agents Chemother 45, 3427–3432. 10.1128/AAC.45.12.3427-3432.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T, Nowlin DM, Cooper NR, 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193, 834–841. 10.1006/viro.1993.1192 [DOI] [PubMed] [Google Scholar]
- Cui X, Adler SP, Davison AJ, Smith L, Habib ESE, McVoy MA, 2012. Bacterial artificial chromosome clones of viruses comprising the Towne cytomegalovirus vaccine. J. Biomed. Biotechnol 2012, 428498. 10.1155/2012/428498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E, 1995. Antiviral therapy for HIV infections.pdf. Clin. Microbiol. Rev 8, 200–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E, 1987. Suramin in the treatment of AIDS - mechanism of action. Antiviral Res. 7, 1–10. [DOI] [PubMed] [Google Scholar]
- De Clercq E, 1979. Suramin - a potent inhibitor of the reverse transcriptase of RNA tumor viruses. Cancer Lett. 8, 9–22. [DOI] [PubMed] [Google Scholar]
- de Paiva RE, Neto AM, Santos IA, Jardim ACG, Corbi PP, Bergamini F, 2020. What is holding back the development of antiviral metallodrugs? A literature overview and implications for SARS-CoV-2 therapeutics and future viral outbreaks. Dalt. Trans. Accepted M, 1772. 10.1039/D0DT02478C [DOI] [PubMed] [Google Scholar]
- Dechecchi MC, Melotti P, Bonizzato A, Santacatterina M, Chilosi M, Cabrini G, 2001. Heparan Sulfate Glycosaminoglycans Are Receptors Sufficient To Mediate the Initial Binding of Adenovirus Types 2 and 5. J. Virol 75, 8772–8780. 10.1128/jvi.75.18.8772-8780.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donalisio M, Ranucci E, Cagno V, Civra A, Manfredi A, Cavalli R, Ferruti P, Lembo D, 2014. Agmatine-containing poly(amidoamine)s as a novel class of antiviral macromolecules: Structural properties and in vitro evaluation of infectivity inhibition. Antimicrob. Agents Chemother 58, 6315–6319. 10.1128/AAC.03420-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Miao L, Ye H, Yang C, Fu B, Schwartz PH, Rayner S, Fortunato EA, Luo M, 2012. A faster immunofluorescence assay for tracking infection progress of human cytomegalovirus. Acta Biochim Biophys Sin 44, 597–605. 10.1093/abbs/gms041.Advance [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann DW, Deig EF, Hatch MT, DiSalco LH, Verdos NA, 1976. Antiviral substances from California marine algae. J. Phycol 13, 37–40. [Google Scholar]
- Farias WRL, Valente A-P, Pereira MS, Mourão PAS, 2000. Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J. Biol. Chem 275, 29299–29307. 10.1074/jbc.M002422200 [DOI] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ, 1986. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 883, 173–177. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Sayers CA, Barrett AJ, 1982. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect. Tissue Res 9, 247–248. [DOI] [PubMed] [Google Scholar]
- Gandhi NS, Mancera RL, 2008. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des 72, 455–482. 10.1111/j.1747-0285.2008.00741.x [DOI] [PubMed] [Google Scholar]
- Gerber P, Dutcher JD, Adams EV, Sherman H, Sherman JH, 1958. Protective effect of seaweed extracts for chicken embryos infected with Influenza B or Mumps virus. Exp. Biol. Med 99, 590–593. [DOI] [PubMed] [Google Scholar]
- Halpern V, Ogunsola F, Obunge O, Wang CH, Onyejepu N, Oduyebo O, Taylor D, McNeil L, Mehta N, Umo-Otong J, Otusanya S, Crucitti T, Abdellati S, 2008. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: Results of a phase III trial in Nigeria. PLoS One 3, 1–7. 10.1371/journal.pone.0003784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C, Yu G, He Y, Xu C, Zhang L, Wang W, 2019. Marine glycan–based antiviral agents in clinical or preclinical trials. Rev. Med. Virol 29, e2043. 10.1002/rmv.2043 [DOI] [PubMed] [Google Scholar]
- He T-C, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B, 1998. A simplified system for generating recombinant adenoviruses. PNAS 95, 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold BC, Bourne N, Marcellino D, Kirkpatrick R, Strauss DM, Zaneveld LJD, Waller DP, Anderson RA, Chany CJ, Barham BJ, Stanberry LR, Cooper MD, 2000. Poly(sodium 4-styrene sulfonate): An effective candidate topical antimicrobial for the prevention of sexually transmitted diseases. J. Infect. Dis 181, 770–773. 10.1086/315228 [DOI] [PubMed] [Google Scholar]
- Ibig-rehm Y, Götte M, Gabriel D, Woodhall D, Shea A, Brown NE, Compton T, Feire AL, 2011. High-content screening to distinguish between attachment and post-attachment steps of human cytomegalovirus entry into fibroblasts and epithelial cells. Antiviral Res. 89, 246–256. 10.1016/j.antiviral.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Ito M, Baba M, Sato A, Pauwels R, De Clercq E, Shigeta S, 1987. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antiviral Res. 7, 361–367. [DOI] [PubMed] [Google Scholar]
- Liu L, Chopra P, Li X, Wolfert MA, Tompkins SM, Boons GJ, 2020. SARS-CoV-2 spike protein binds heparan sulfate in a length- and sequence-dependent manner. bioRxiv prepreint, 1–15. [Google Scholar]
- Mauck CK, Weiner DH, Ballagh SA, Creinin MD, Archer DF, Schwartz JL, Pymar HC, Lai J, Rencher WF, Callahan MM, 2004. Single and multiple exposure tolerance study of polystyrene sulfonate gel: a Phase I safety and colposcopy study. Contraception 70, 77–83. 10.1016/j.contraception.2004.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVoy MA, Mocarski ES, 1999. Tetracycline-mediated regulation of gene expression within the human cytomegalovirus genome. Virology 258, 295–303. 10.1006/viro.1999.9724 [DOI] [PubMed] [Google Scholar]
- Mercorelli B, Oreste P, Sinigalia E, Muratore G, Lembo D, Palù G, Loregian A, 2010. Sulfated derivatives of Escherichia coli K5 capsular polysaccharide are potent inhibitors of human cytomegalovirus. Antimicrob. Agents Chemother 54, 4561–4567. 10.1128/AAC.00721-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H, Looney DJ, Kuno S, Ueno R, Staal FW, Broder S, 1988. Dextran Sulfate Suppression of Viruses in the HIV Family: Inhibition of Virion Binding to CD4+ cells. Science (80-. ). 240, 646–649. [DOI] [PubMed] [Google Scholar]
- Muller W, Wang X, Schroder H, 2013. Biomedical Inorganic Polymers. [Google Scholar]
- Mulloy B, Ribeiro AC, Alves AP, Vieira RP, Mourão PAS, 1994. Sulfated fucans from echinoderms have a regular tetrasaccharide repeating unit defined by specific patterns of sulfation at the 0–2 and 0–4 positions. J. Biol. Chem 269, 22113–22123. [PubMed] [Google Scholar]
- Nakashima H, Kido Y, Kobayashi N, Motoki Y, Neushul M, Yamamoto N, 1987. Antiretroviral activity in a marine red alga: reverse transcriptase inhibition by an aqueous extract of Schizymenia pacifica. J. Cancer Res. Clin. Oncol 113, 413–416. [DOI] [PubMed] [Google Scholar]
- Neurath AR, Strick N, Li YY, 2002. Anti-HIV-1 activity of anionic polymers: A comparative study of candidate microbicides. BMC Infect. Dis 2, 1–11. 10.1186/1471-2334-2-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduah EI, Linhardt RJ, Sharfstein ST, 2016. Heparin: Past, Present, and Future. Pharmaceuticals 9, 1–12. 10.3390/ph9030038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeschke R, Woskobojnik I, Makarov V, Schmidtke M, Bogner E, 2014. DSTP-27 prevents entry of human cytomegalovirus. Antimicrob. Agents Chemother 58, 1963–1971. 10.1128/AAC.01964-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomin VH, 2017. Antimicrobial Sulfated Glycans: Structure and Function. Curr. Top. Med. Chem 17, 319–330. 10.2174/15680266156661506051 [DOI] [PubMed] [Google Scholar]
- Pomin VH, 2015. Marine Non-Glycosaminoglycan Sulfated Glycans as Potential Pharmaceuticals. Pharmaceuticals 8, 848–864. 10.3390/ph8040848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomin VH, 2012a. Fucanomics and Galactanomics: Marine Distribution, Medicinal Impact, Conceptions, and Challenges. Mar. Drugs 10, 793–811. 10.3390/md10040793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomin VH, 2012b. Fucanomics and galactanomics: Current status in drug discovery, mechanisms of action, and role of the well-de fi ned structures. Biochim. Biophys. Acta 1820, 1971–1979. 10.1016/j.bbagen.2012.08.022 [DOI] [PubMed] [Google Scholar]
- Pomin VH, Mourão PAS, 2014. Specific sulfation and glycosylation — a structural combination for the anticoagulation of marine carbohydrates. Front. Cell. Infect. Microbiol 4, 1–8. 10.3389/fcimb.2014.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomin VH, Pereira MS, Valente AP, Tollefsen DM, Pavão MSG, Mourão PAS, 2005a. Selective cleavage and anticoagulant activity of a sulfated fucan: Stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II-dependent anticoagulant activity. Glycobiology 15, 369–381. 10.1093/glycob/cwi021 [DOI] [PubMed] [Google Scholar]
- Pomin VH, Valente AP, Pereira MS, Mourão PAS, 2005b. Mild acid hydrolysis of sulfated fucans: A selective 2-desulfation reaction and an alternative approach for preparing tailored sulfated oligosaccharides. Glycobiology 15, 1376–1385. 10.1093/glycob/cwj030 [DOI] [PubMed] [Google Scholar]
- Queiroz INL, Vilela-Silva ACES, Pomin VH, 2016. Oligosaccharides from the 3-linked 2-sulfated alpha-L-fucan and alpha-L-galactan show similar conformations but different dynamics. Glycobiology 26, 1257–1264. 10.1093/glycob/cww080 [DOI] [PubMed] [Google Scholar]
- Quindere ALG, Santos GRC, Oliveira SNMCG, Glauser BF, Fontes BP, Queiroz INL, Benevides NMB, Pomin VH, Mourao PAS, 2013. Is the antithrombotic effect of sulfated galactans independent of serpin? J. Thromb. Haemost 12, 43–53. 10.1111/jth.12448 [DOI] [PubMed] [Google Scholar]
- Qureshi A, Thakur N, Tandon H, Kumar M, 2014. AVPdb: A database of experimentally validated antiviral peptides targeting medically important viruses. Nucleic Acids Res. 42, 1147–1153. 10.1093/nar/gkt1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GRC, Tovar AMF, Capillé NVM, Pereira MS, Pomin VH, Mourão PAS, 2014. Structural and functional analyses of bovine and porcine intestinal heparins confirm they are different drugs. Drug Discov. Today 19, 1801–1807. 10.1016/j.drudis.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Schmidtke M, Karger A, Meerbach A, Egerer R, Stelzner A, Makarov V, 2003. Binding of a N,N′-bisheteryl derivative of dispirotripiperazine to heparan sulfate residues on the cell surface specifically prevents infection of viruses from different families. Virology 311, 134–143. 10.1016/S0042-6822(03)00166-1 [DOI] [PubMed] [Google Scholar]
- Selinka H-C, Florin L, Patel HD, Freitag K, Schmidtke M, Makarov VA, Sapp M, 2007. Inhibition of Transfer to Secondary Receptors by Heparan Sulfate-Binding Drug or Antibody Induces Noninfectious Uptake of Human Papillomavirus. J. Virol 81, 10970–10980. 10.1128/jvi.00998-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoup M, Ourahmane A, Ginsburg EP, Farrell NP, Mcvoy MA, 2020. Substitution-inert polynuclear platinum compounds inhibit human cytomegalovirus attachment and entry. Antiviral Res. 184, 104957. [DOI] [PubMed] [Google Scholar]
- Skoler-karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, Maguire R, Lahteenmaki P, 2008. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 372, 1977–1987. 10.1016/S0140-6736(08)61842-5 [DOI] [PubMed] [Google Scholar]
- Stevic I, Parmar N, Paredes N, Berry LR, Chan AKC, 2011. Binding of Heparin to Metals. Cell Biochem. Biophys 59, 171–178. 10.1007/s12013-010-9129-5 [DOI] [PubMed] [Google Scholar]
- Van Damme L, Govinden R, Mirembe FM, Guédou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D, 2008. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N. Engl. J. Med 359, 463–472. 10.1056/NEJMoa0707957 [DOI] [PubMed] [Google Scholar]
- Varnum SM, Streblow DN, Monroe ME, Smith P, Auberry KJ, Pas L, Wang D,Ii DGC, Rodland K, Wiley S, Britt W, Shenk T, Smith RD, Nelson JA, 2004. Identification of Proteins in Human Cytomegalovirus (HCMV) Particles: the HCMV Proteome. J. Virol 78, 10960–10966. 10.1128/JVI.78.20.10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos AA, Sucupira ID, Guedes AL, Queiroz IN, Frattani FS, Fonseca RJ, Pomin VH, 2018. Anticoagulant and Antithrombotic Properties of Three Structurally Correlated Sea Urchin Sulfated Glycans and Their Low-Molecular-Weight Derivatives. Mar. Drugs 16. 10.3390/md16090304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Shenk T, 2005. Human Cytomegalovirus UL131 Open Reading Frame Is Required for Epithelial Cell Tropism. J. Virol 79, 10330–10338. 10.1128/jvi.79.16.10330-10338.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP, 2003. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol 163, 535–545. 10.1083/jcb.200306001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Dong X, Chen Y, 2002. Neutralizing Antibodies: Mechanism of neutralization and protective activity against HIV-1. Immunol. Res 25, 193–200. [DOI] [PubMed] [Google Scholar]
- Yao XJ, Wainberg MA, Richard M, Pollak M, 1991. The ability of suramin to block CD4-gp120 binding is reversed in the presence of albumin. Antimicrob. Agents Chemother 35, 2636–2638. 10.1128/AAC.35.12.2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaneveld LJD, Waller DP, Anderson RA, Chany C, Rencher WF, Feathergill K, Diao XH, Doncel GF, Herold B, Cooper M, 2002. Efficacy and safety of a new vaginal contraceptive antimicrobial formulation containing high molecular weight poly(sodium 4-styrenesulfonate). Biol. Reprod 66, 886–894. 10.1095/biolreprod66.4.886 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1. 1D 1H NMR spectra (expansions from δH 6.0–0.5 ppm) of all sulfated glycans analyzed in this work: heparin, BoSG, IbSF, LvSF, and IbFucCS.