Abstract
Purpose:
Noninvasive imaging with hyperpolarized pyruvate can capture in vivo cardiac metabolism. For proper quantification of the metabolites and optimization of imaging parameters, understanding MR characteristics such as T2*s of the hyperpolarized signals is critical. This study is to measure in vivo cardiac T2*s of hyperpolarized [1-13C]pyruvate and the products in rodents and humans.
Methods:
A dynamic 13C multi-echo spiral imaging sequence that acquires [13C]bicarbonate, [1-13C]lactate, and [1-13C]pyruvate images in an interleaved manner was implemented for a clinical 3T system. T2* of each metabolite was calculated from the multi-echo images by fitting the signal decay of each region of interest mono-exponentially. The performance of measuring T2* using the sequence was validated using a 13C phantom, then with rodents following a bolus injection of hyperpolarized [1-13C]pyruvate. In humans, T2* of each metabolite was calculated for left ventricle (LV), right ventricle (RV) and myocardium.
Results:
Cardiac T2*s of hyperpolarized [1-13C]pyruvate, [1-13C]lactate and [13C]bicarbonate in rodents were measured as 24.9 ± 5.0, 16.4 ± 4.7, and 16.9 ± 3.4 ms, respectively. In humans, T2* of [1-13C]pyruvate was 108.7 ± 22.6 ms in LV and 129.4 ± 8.9 ms in RV. T2* of [1-13C]lactate was 40.9 ± 8.3, 44.2 ± 5.5, and 43.7 ± 9.0 ms in LV, RV and myocardium, respectively. T2* of [13C]bicarbonate in myocardium was 64.4 ± 2.5 ms. The measurements were reproducible and consistent over time after the pyruvate injection.
Conclusions:
The proposed metabolite-selective multi-echo spiral imaging sequence reliably measures in vivo cardiac T2*s of hyperpolarized [1-13C]pyruvate and products.
Keywords: T2*, multi-echo imaging, dynamic nuclear polarization, hyperpolarized pyruvate, heart
Introduction
MRI or MRS with hyperpolarized (HP) [1-13C]pyruvate can assess metabolic state of the heart. In particular, detection of HP [13C]bicarbonate is a direct measure of pyruvate dehydrogenase (PDH) activity and thus an important indicator of cardiac metabolism and function. For instance, myocardial [13C]bicarbonate production is sensitive to several pathophysiological states, including myocardial infarction,1 diabetic cardiomyopathy,2 and heart failure.3,4 Consequently, cardiac imaging with HP [1-13C]pyruvate is being translated to humans.5–7 Nonetheless, quantitative analysis of HP signals in the heart need to be established.
Cardiac imaging of HP signals is particularly challenging due to the cardiac motion and the rapid circulation of blood through the heart as well as the dynamic and transient nature of HP signals. To achieve rapid k-space sampling within a specific cardiac phase (e.g., end diastole), gradient recalled-echo (GRE)-based imaging sequences with spectral-spatial (spsp) radiofrequency (RF) pulses that excite one metabolite at a time are commonly used rather than spectroscopic imaging methods. Both spiral5,8 or echo-planar imaging (EPI)9,10 readouts were proposed for cardiac imaging previously.
However, cardiac HP acquisition schemes are still considered suboptimal,11 and the HP 13C images are sensitive to acquisition parameters such as echo times, B0 field inhomogeneity12,13 and blood flow.14 For proper assessment of HP metabolites, in vivo MR characteristics such as the T2*s of the HP 13C-metabolites need to be understood. Moreover, since T2*s play critical roles in determining acquisition parameters, the knowledge of the T2* of HP [1-13C]pyruvate and its products can be used in designing the k-space sampling trajectories for optimal signal-to-noise ratio (SNR) and spatial resolution. Previous studies estimated T2*s of in vivo HP metabolites from the spectral linewidths using MR spectroscopy.13 However, this approach measures aggregated T2* information without assessing spatial and compartmental heterogeneity. A spectroscopic imaging approach, such as chemical shift imaging (CSI) or echo-planar spectroscopic imaging (EPSI), can be used to obtain spatial T2* information of metabolites of interest by mapping the spectral linewidths,15 but is not adequate for HP 13C cardiac imaging due to the transient characteristics of HP signals and the relatively long acquisition time.
In this study, we propose a dynamic metabolite-selective multi-echo spiral imaging (MESI) 13C sequence for imaging cardiac T2*s of HP [1-13C]pyruvate and the products in vivo. The feasibility and robustness of the method were validated using a 13C phantom and tested with rodents in vivo. The sequence was further applied to healthy volunteers, and T2* relaxation times of HP pyruvate, lactate, and bicarbonate were measured in left ventricle (LV), right ventricle (RV) and myocardium (Myo).
Methods
MR Scanner and RF coils
All the MR studies were performed using a clinical 3T wide-bore (diameter ⊘ = 70 cm) MRI scanner (750w Discovery, GE Healthcare, Waukesha, WI, USA). A 1H/13C dual-tuned quadrature transmit/receive birdcage RF coil (inner diameter ⊘inner = 60 mm, GE Healthcare) was used for phantom and rat studies. For human studies, a two-loop 13C transmit-receive Helmholtz coil was used (⊘ = 20 cm; PulseTeq Limited, Chobham, Surrey, UK). One loop was positioned over the anterior chest and the other was placed below the scapula of the human subject.
Hyperpolarization
A SPINlab™ system (GE Healthcare) that operates at ~0.8 K in a 5T magnet was used for the dynamic nuclear polarization (DNP) of [1-13C]pyruvate. For animal studies, a 35-μL sample of 14-M [1-13C]pyruvic acid mixed with OX063 trityl (15 mM) was prepared in a research fluid path for each dissolution. After 3 – 4 hrs of polarization, the sample was dissolved with hot solvent (16 mL saline with 0.1-g/L Na2EDTA) then mixed with pH-neutralization media (750 μL with 0.72-M NaOH). The final HP solution (~6.5 mL) that contained ~70-mM of HP [1-13C]pyruvate was injected through the tail vein up to 4.0 mL (0.875 mmol/kg body weight) with an injection rate of 0.25 mL/s.
For human studies, [1-13C]pyruvic acid (Sigma Aldrich, St. Louis, MO), produced in accordance with Good Manufacturing Practice (GMP) regulations, was prepared in clinical fluid paths as described previously (0.40 mL/kg body weight of 250-mM HP [1-13C]pyruvate solution).16 HP [1-13C]pyruvate was dissolved after 3 – 4 hrs of polarization. Pyruvate concentration, pH, temperature, volume and radical concentration of the HP solution was examined by a dedicated quality control (QC) device (GE Healthcare). Immediately after passing the QC, sterility of the sample was confirmed prior to injection as previously described.17 The HP solution was transported to the magnet and administered intravenously to subjects (injection rate = 5 mL/s), followed by a 25-mL saline flush.
RF Pulse Design and Metabolite-Interleaved Multi-Echo Spiral Imaging Sequence
A spsp RF pulse was designed to have a full width at half maximum (FWHM) of 134.4 Hz using the Spectral-Spatial RF Pulse Design Package18 to selectively excite HP [13C]bicarbonate, [1-13C]lactate, or [1-13C]pyruvate, depending on the acquisition frequency, Fig. 2. Unlike other spsp RF pulses used in previous HP 13C cardiac studies,5,8,9 this RF pulse was specifically designed and tested for the wide-bore system (GE 750w), which has limited gradient performance (maximum gradient [Gmax] = 33 mT/m, maximum slew rate [Slewmax] = 120 T/m/s), and operates with minimum gradient requirements (Gmax < 25 mT/m and Slewmax < 100 T/m/s). The spsp excitation profiles, simulated using MATLAB (MathWorks, Natick, MA, USA) and tested in the scanner, are shown in Fig. 2(B). The simulated transverse signal in log scale at the center of the image slice (horizontal dotted white line) showed 90° excitation in the passband and less than 1% excitation in the stopband. Metabolite-selective MESI scheme was implemented to sequentially acquire metabolite maps using the spsp RF pulse in an interleaved manner by shifting the transmit and receive frequencies.
Figure 2. Spectral-spatial RF pulse and profiles.
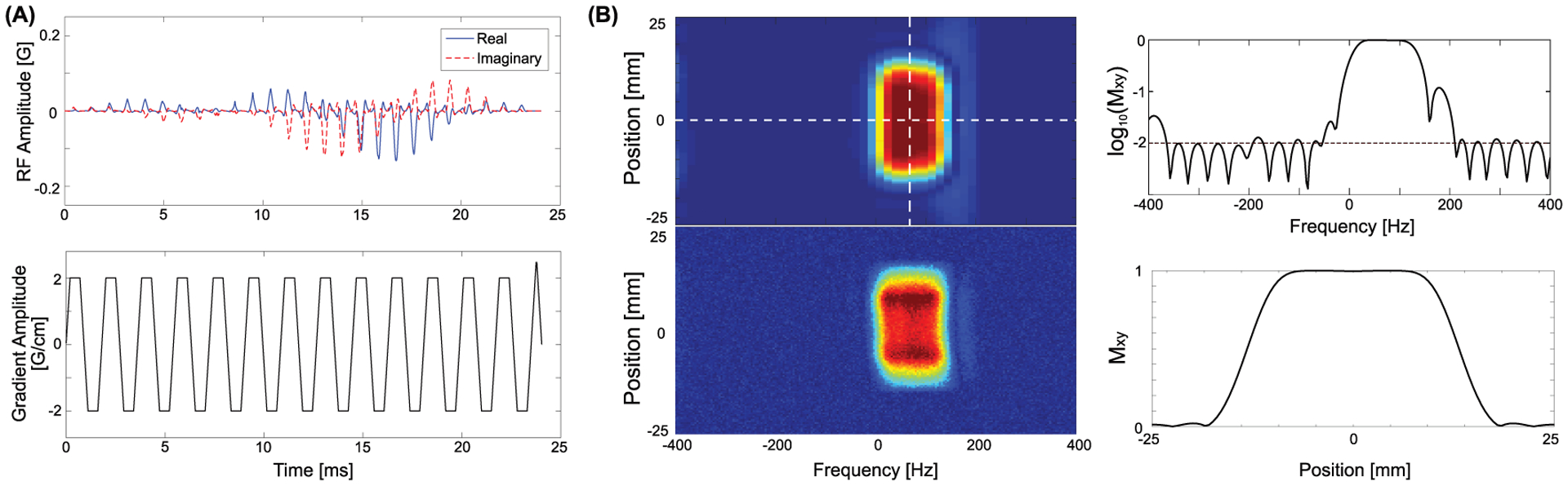
(A) Spectral-spatial RF pulse and slice-selective gradient waveforms used for bicarbonate, lactate, and pyruvate excitation, and (B) the simulated (top left) and tested (bottom left) excitation profile. The simulated spectral profile at the center of the slice (horizontal dotted white line in the top left figure) in log scale is shown at the top right, and the simulated slice profile at the center of the passing band (vertical dotted white line in the top left figure) is shown at the bottom right.
Phantom Validation
The sequence was tested with a gadolinium-doped (1-mM) spherical [13C]bicarbonate phantom (1.0 M, ⊘ = 3 cm). 1H MRI was acquired with two-dimensional (2D) T1-weighted fast spin echo (FSE) sequence (repetition time [TR] = 700 ms, echo time [TE] = 12 ms, flip angle [FA] = 90°, field of view [FOV] = 60 mm × 60 mm, slice thickness [ST] = 4 mm, spatial resolution = 0.23 mm × 0.23 mm). For 13C MRI, five-echo images of each metabolite were acquired following the spsp RF excitation with two-shot spiral readouts (FOV = 60 mm × 60 mm, spatial resolution = 3 mm × 3 mm, FA = 90°, ST = 25 mm, TR = 3 s, TE of 1st echo = 18.01 ms, ΔTE = 11.62 ms, readout length = 58.10 ms/arm, receiver spectral bandwidth = 85.06 Hz, #averages = 128). The decay of mean 13C signal within the phantom was fitted to a mono-exponential function to measure the T2* of [13C]bicarbonate. From the same phantom, 13C spectrum was acquired using a pulse-and-acquire sequence (FA = 90°, ST = 25 mm, TR = 3 s, receive spectral bandwidth = 2000 Hz, total #spectral points = 2048, #averages = 128). The T2* from the MESI was compared to the linewidth from the spectroscopic data. In the frequency domain, the ideal MRS signal can be described by the Lorentzian line shape,15 which is in the form as below in magnitude:
| #(1) |
where H is the maximum of the signal in magnitude, F0 is the center frequency, and W is the signal’s FWHM (). T2* of the phantom was calculated by fitting the spectrum to Equation (1).
T2* Measurement of Rat Heart
Cardiac T2*s of HP 13C-metabolites were measured from healthy male Wistar rats (n = 3, body weight = 556.7 ± 64.7 g). First, 2D axial T2-weighted FSE 1H images were acquired for anatomical reference (TR = 5000 ms, TE = 63 ms, FA = 160°, FOV = 80 mm × 80 mm, ST = 2 mm, spatial resolution = 0.63 mm × 0.63 mm). Each rat was imaged twice using the 13C MESI sequence (single shot, FOV = 80 mm × 80 mm, spatial resolution = 8 mm × 8 mm, ST = 25 mm, TR = 213 ms, #echo = 10, TE of 1st echo = 15.19 ms, ΔTE = 5.98 ms, readout length = 59.76 ms/arm, receive spectral bandwidth = 167.34 Hz, FA = 90° for bicarbonate, 90° for lactate, and 5° for pyruvate, #timepoint = 16) with two consecutive injections of HP [1-13C]pyruvate solution. Images of [13C]bicarbonate, [1-13C]lactate, and [1-13C]pyruvate were acquired from an axial plane containing the heart after a 15-s delay from the start of each injection. The minimum time interval between the injections was 20 min. The acquisition scheme is summarized in Fig. 1. Note that the ECG gating was used only for humans. All procedures were approved by the local Institutional Animal Care and Use Committee.
Figure 1. Metabolite-interleaved 13C multi-echo spiral imaging sequence.
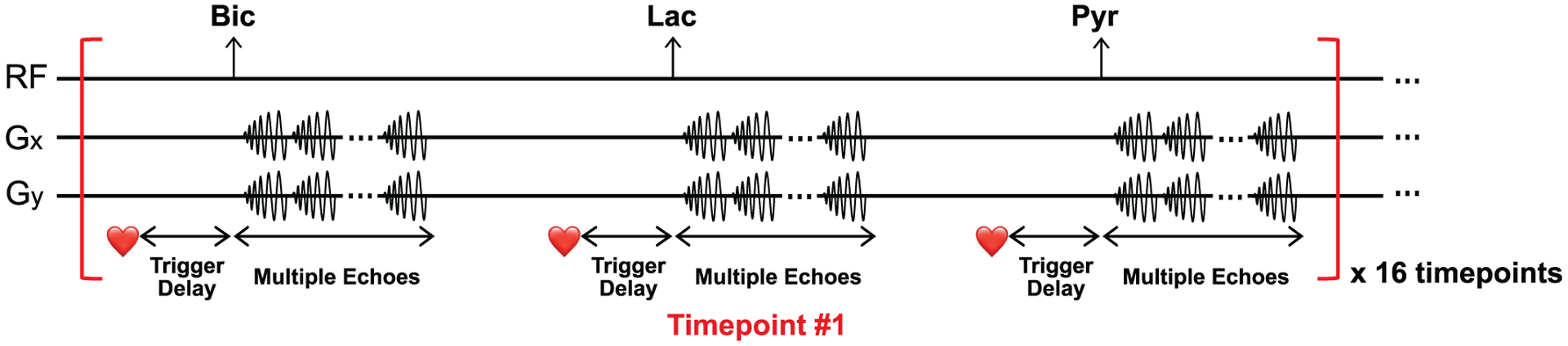
[13C]Bicarbonate, [1-13C]lactate and [1-13C]pyruvate are sequentially excited using a spectral-spatial RF pulse and imaged with multiple single-shot spiral readouts.
Cardiac T2* Measurement of Healthy Human Subjects
Three healthy subjects were recruited for the study (2 female, 1 male, age = 38 – 48 years, Table 1). After positioning the subject in the magnet, horizontal long-axis (HLA), vertical long-axis (VLA) and short-axis (SA) images were acquired using a 1H balanced steady-state free precession (bSSFP) sequence, which was triggered to mid-diastole during expiration breath-hold. B0 inhomogeneity was inspected in a mid LV SA plane using a 1H GRE (FOV = 400 mm × 400 mm, spatial resolution = 6.25 mm × 6.25 mm, 2 echoes with ΔTE = 3.30 ms, TE of 1st echo = 2.80 ms) with breath-hold (no cardiac triggering). The SA plane and the shim currents were retained for 13C imaging. The HP 13C images were acquired with ECG gating. The scanning sequence began after a 25-s delay from the start of pyruvate injection using an automated breath-hold voice instruction given immediately before the scan. Subjects were instructed to hold their breath in expiration for ~20 s, followed by a shallow breathing to minimize the respiratory motion. The center frequency was set to the in vivo HP [13C]bicarbonate resonance, which was predetermined by the in vivo HP [1-13C]lactate frequency, and the center frequency of water protons was used to calculate the frequency of [1-13C]lactate with an empirically-derived scaling factor of 0.2514949. The multi-echo images of [13C]bicarbonate, [1-13C]lactate and [1-13C]pyruvate were acquired in an interleaved manner every 2 R-R intervals (single shot, FOV = 400 mm × 400 mm, spatial resolution = 16 mm × 16 mm, ST = 30 mm, #echo = 6, TE of 1st echo = 16.99 ms, ΔTE = 9.59 ms, readout length = 57.53 ms/arm, receive spectral bandwidth = 104.4 Hz, FA = 60° for bicarbonate, 60° for lactate, and 20° for pyruvate, #timepoint = 16). The imaging protocol was approved by the local Institutional Review Board (IRB#: STU-2018–0227).
Table 1.
Subject characteristics and compartmentalized time-averaged T2*s of HP [1-13C]pyruvate, [1-13C]lactate and [13C]bicarbonate.
| Subject Demographics | Time-Averaged T2* [ms] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject ID | Sex | Age [years] | Height [cm] | Heart Rate [bpm] | Weight [kg] | [1-13C]Pyr | [1-13C]Lac | [13C]Bic | |||
| LV | RV | LV | RV | Myo | Myo | ||||||
| #1 | F | 38 | 172.7 | 72 | 55.0 | 83.3 | 133.0 | 47.9 | 48.2 | 46.2 | 61.5 |
| #2 | M | 45 | 188.0 | 60 | 83.0 | 126.7 | 136.0 | 43.1 | 38.0 | 51.2 | 66.1 |
| #3 | F | 48 | 152.4 | 64 | 61.1 | 116.1 | 119.3 | 31.7 | 46.5 | 33.7 | 65.5 |
| Mean ± Standard Deviation | 108.7 ± 22.6 | 129.4 ± 8.9 | 40.9 ± 8.3 | 44.2 ± 5.5 | 43.7 ± 9.0 | 64.4 ± 2.5 | |||||
Data Reconstruction and Statistical Analysis
All the acquired HP 13C data were reconstructed and analyzed using MATLAB. Images from different echoes were reconstructed by inverse Fourier transform after gridding to Cartesian coordinates, apodization with a Gaussian function, sample density compensation, and a four-fold zero-filling in k-space. To combine the multi-echo data, images from all echoes were added with equal weights.
Off-resonance correction for the HP 13C images was conducted with a frequency-segmented method as described in.19 The data was demodulated over a range of off-resonance frequencies from −50 Hz to 50 Hz in 5 Hz step. From each of the resultant images, pixels whose field map values most closely match the reconstruction frequency were selected to form the final image. The 13C field map was estimated from the 1H field map, which firstly passed a median filter (3 × 3) to eliminate noise spikes and was then resampled to the resolution of 13C image and scaled by the ratio of .
For the animal data, regions of interest (ROIs) that contain hyperintense 13C signals were drawn in the 13C images, and the mean 13C signal within each ROI was fitted to an exponential decaying function along the echo times to calculate the T2*s of HP [13C]bicarbonate, [1-13C]lactate and [1-13C]pyruvate. Paired Student’s t-test (α = 0.05, two-tailed) was performed between the consecutively measured T2*s of HP metabolites to verify the reproducibility of the proposed method. Dynamic change of the T2* measurements along the timepoints was evaluated by the coefficient of variation (CV).
For the human 13C data, LV, RV, and Myo were manually segmented based on the 1H SA MRI, as shown in Fig. 7(A). The spatially averaged 13C signals over the compartments at each echo were used to calculate the compartmentalized T2*s in the heart. To ensure the accuracy of T2* measurement, signals from the first m echoes (m = 3 or 4, depending on the SNR) were used for linear regression with the premise that coefficients of determination (r2) for the linear regressions of first m and m+1 echoes are both larger than 0.95.
Figure 7. Hyperpolarized 13C imaging from a healthy human subject.
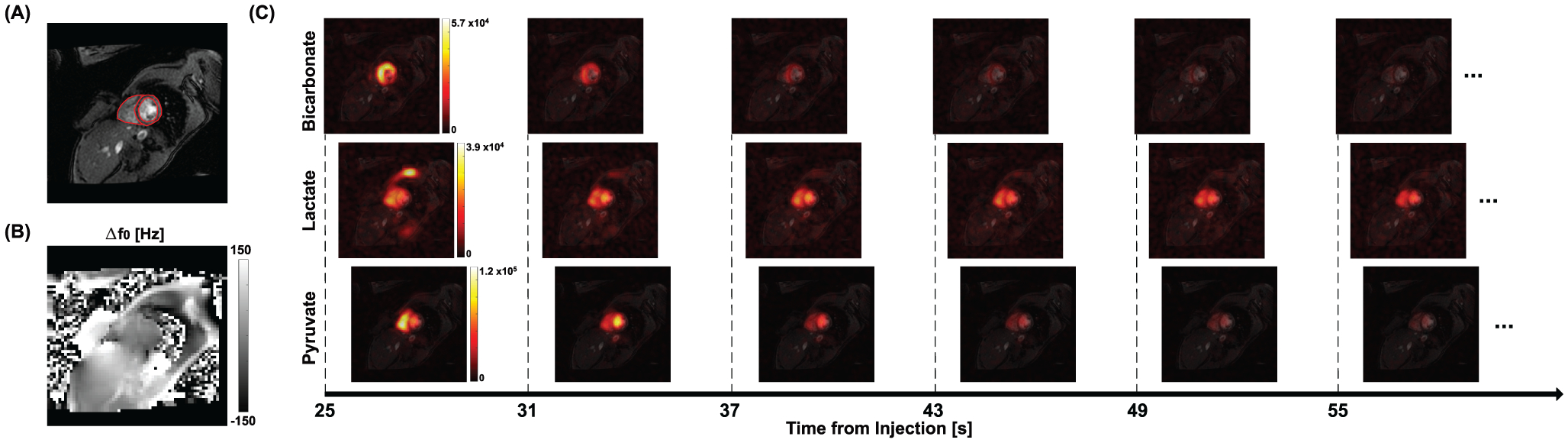
(A) The prescribed SA plane for 13C imaging (shown in 1H MRI). (B) Δf0 map of the corresponding slice. (C) Dynamic 13C images (combination of 6 echoes) of HP [1-13C]pyruvate, [1-13C]lactate and [13C]bicarbonate, acquired after an injection of HP [1-13C]pyruvate.
To evaluate the improvement of SNR with the sequence, peak SNRs from the first-echo and echo-combined HP 13C images were calculated by:
| #(2) |
where smax is the maximum signal of the image and n is the background noise. For human images, SNR maps were also reported for both first-echo and echo-combined images.
Results
1H MRI and 13C images of the [13C]bicarbonate phantom are shown in Fig. 3(A). The T2* calculated from the multi-echo images was 54.5 ms (r2 = 0.99), Fig. 3(B). T2* of the phantom was estimated as 54.0 ms using the Lorentzian fitting (red dotted line) of the acquired spectrum (blue solid line) in magnitude as in Fig. 3(C).
Figure 3. Phantom evaluation.
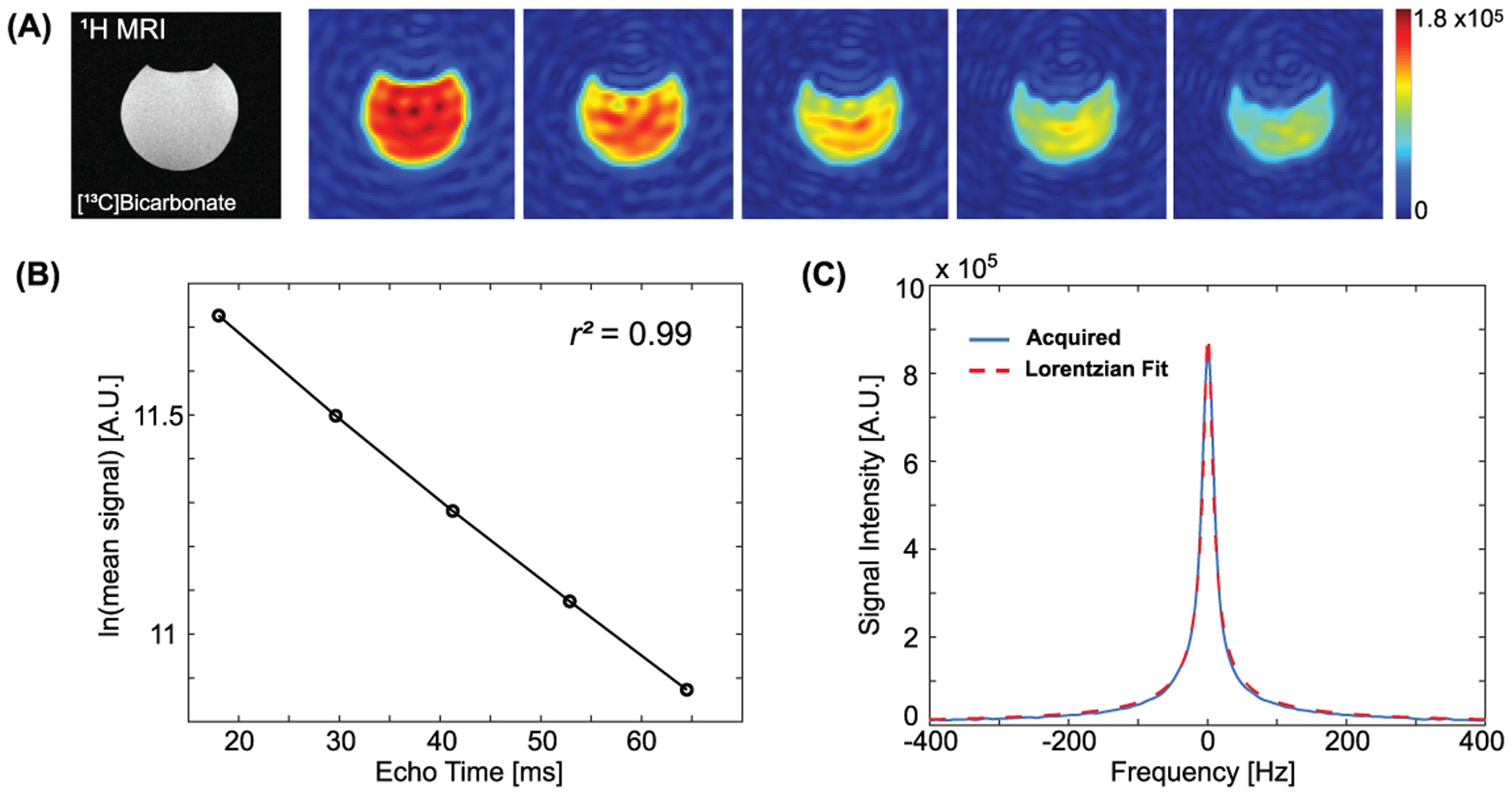
(A) Performance evaluation of the pulse sequence using a [13C]bicarbonate phantom. The T2* from the MESI sequence (B) was compared to that from the Lorentzian fit of 13C spectrum (C).
Time-resolved (15 – 40 s from the start of injection) cardiac 13C images of HP [1-13C]pyruvate, [1-13C]lactate and [13C]bicarbonate from a representative rat are shown in Fig. 4. The echo-combined 13C images are overlaid on top of the corresponding 1H image (Fig. 4(A)). 1H Δf0 map of the imaging slice is shown in Fig. 4(B). Strong pyruvate and lactate signals were detected from the LV, and a large bicarbonate signal was observed in the left anterior Myo, as previously reported.9,10 The cardiac T2* of HP [1-13C]pyruvate was measured as 24.9 ± 5.0 ms. T2*s of [1-13C]lactate and [13C]bicarbonate were 16.4 ± 4.7 and 16.9 ± 3.4 ms, respectively.
Figure 4. Hyperpolarized 13C imaging from a representative rat.
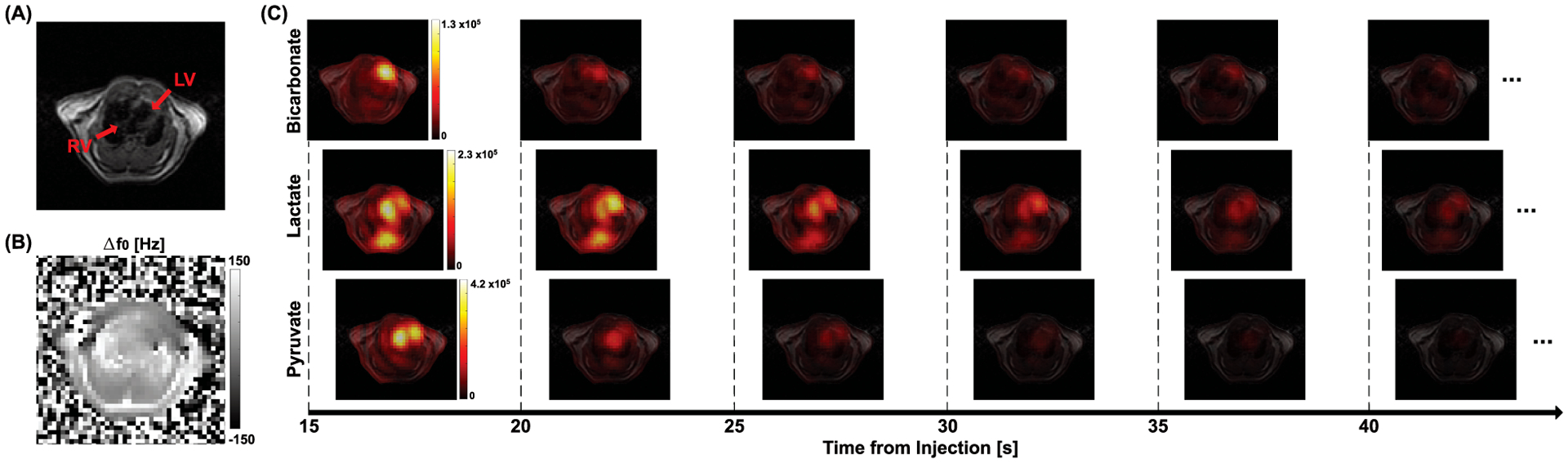
An axial slice that includes the heart was prescribed for both 1H and 13C MRI. (A) T2-weighted image and (B) Δf0 map were acquired in 1H. (C) Multi-echo images (10 echoes) of [1-13C]pyruvate, [1-13C]lactate and [13C]bicarbonate were acquired every 5 s after an injection of HP [1-13C]pyruvate, and combined at each timepoint.
Individual echo images could be also resolved at each timepoint. The first six echo images at the second timepoint (20 s post-injection), for instance, are shown in Fig. 5(A) from the representative rat. Changes of spatially-averaged 13C signals along the echo time are displayed in Fig. 5(B) for selected ROIs (solid black squares in Fig. 5(A)). For [1-13C]pyruvate and [1-13C]lactate, the mean 13C signals in the first four echoes were used for linear regression. For HP [13C]bicarbonate, the first three echoes were used due to the limited SNR in the images with longer echoes. The T2*s of [1-13C]pyruvate, [1-13C]lactate and [13C]bicarbonate were 27.3 ms (r2 = 0.99), 9.5 ms (r2 = 0.99) and 13.9 ms (r2 = 0.99), respectively, at 20 s post-injection (Fig. 5B) from the representative rat.
Figure 5. In vivo cardiac T2* measurement from the representative rat.
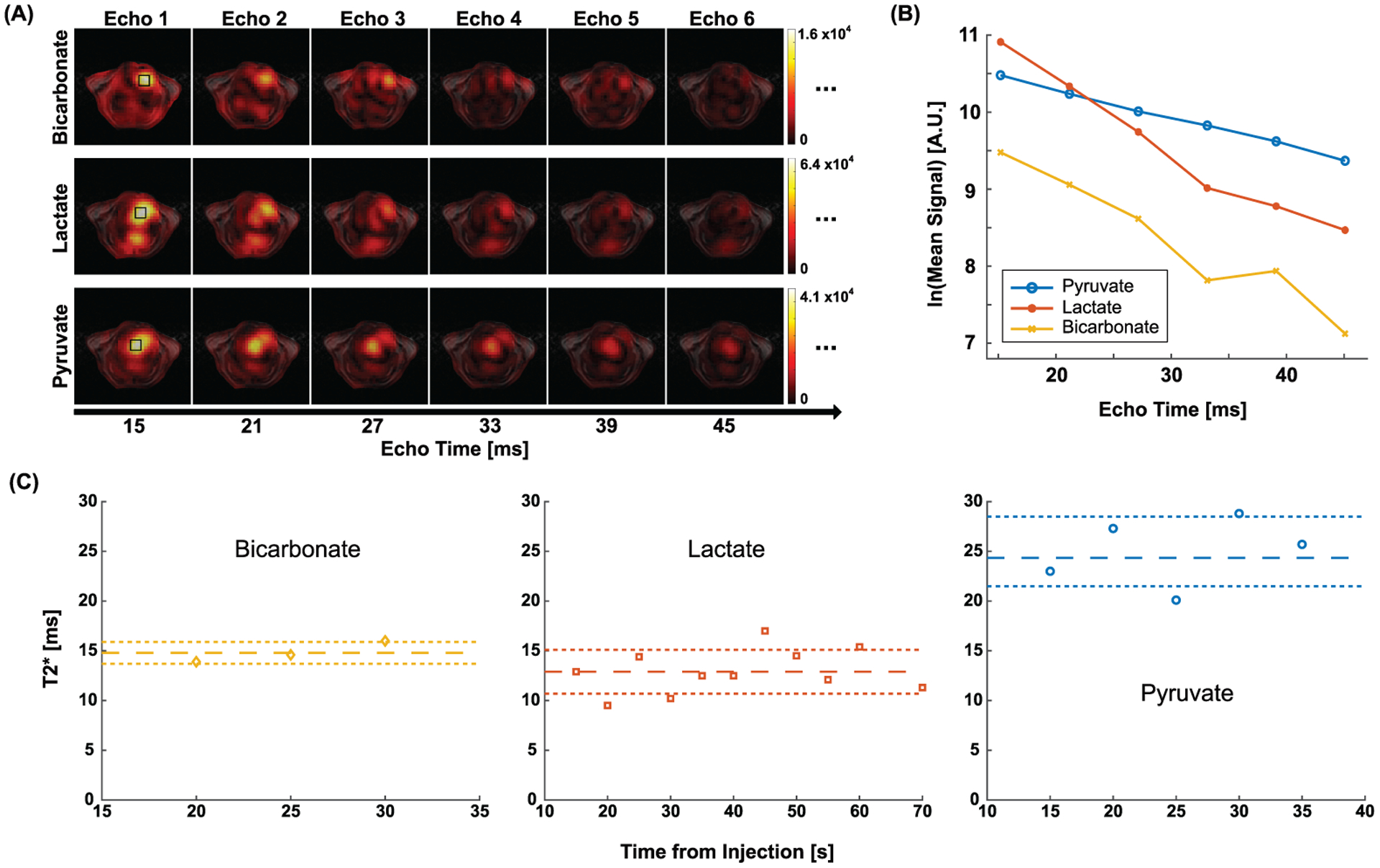
(A) The first six echo images of [1-13C]pyruvate, [1-13C]lactate and [13C]bicarbonate at 20 s. (B) Signal decay of HP 13C metabolites in the ROI (black squares in (C)) along echo time. (C) T2*s of HP signals at different timepoints. The reference lines denote mean ± standard deviation.
T2*s were consistent over time (CV < 0.23). From the representative rat, the time-averaged T2*s of [1-13C]pyruvate, [1-13C]lactate, and [13C]bicarbonate were measured as 25.0 ± 3.5, 12.9 ± 2.2, and 14.8 ± 1.1 ms, respectively, as shown in Fig. 5(C). No significant difference was found from the two consecutive measurements of time-averaged T2*s, as summarized in Fig. 6.
Figure 6. Reproducibility of T2* measurements in rats.
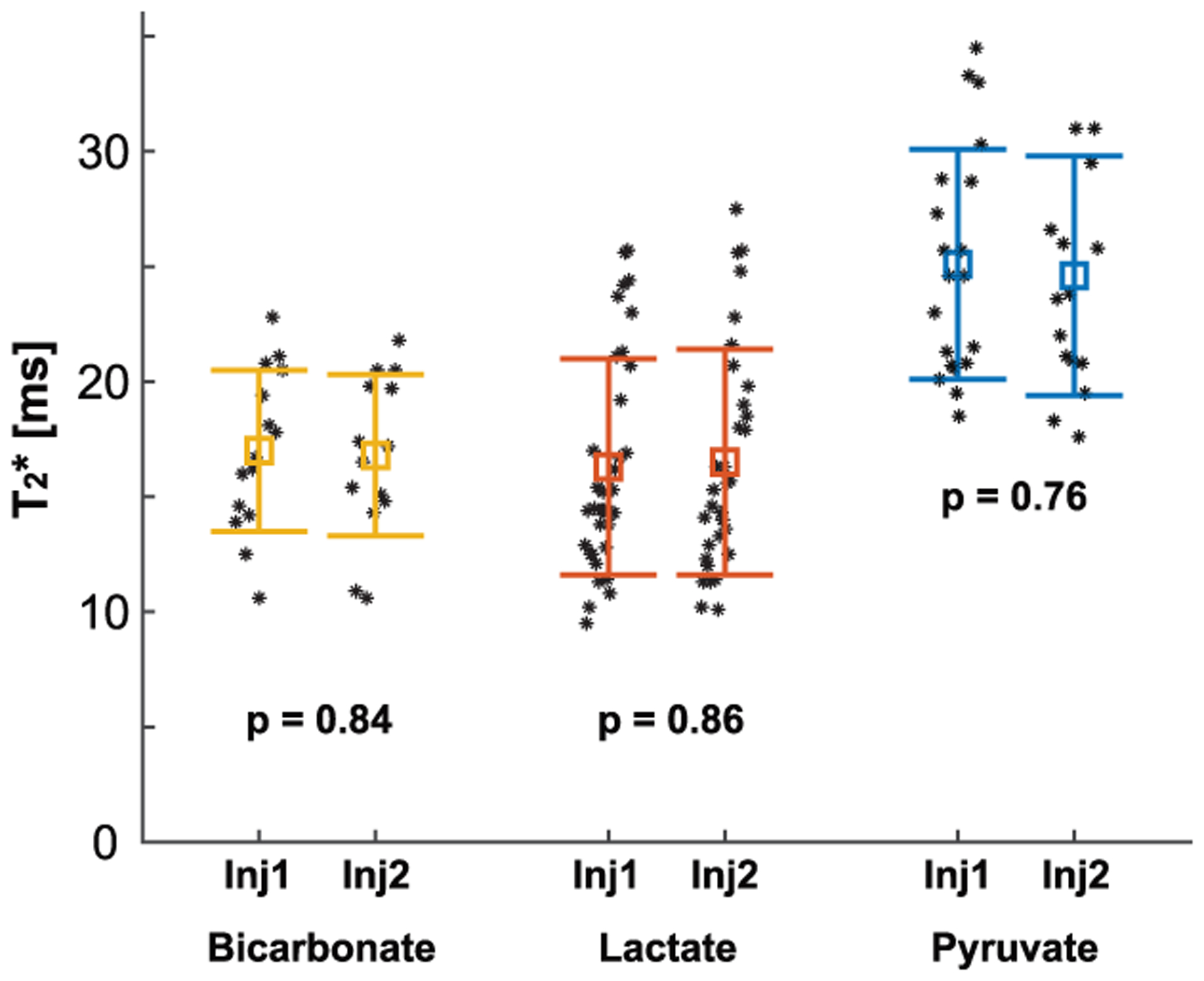
T2*s of the HP 13C-metabolites measured from two consecutive HP [1-13C]pyruvate injections were consistent (p > 0.05). Each marker represents a T2* measurement from a single timepoint and the error bars denote the mean ± standard deviation.
For HP 13C cardiac imaging of healthy subjects, pyruvate and lactate signals were primarily detected in LV and RV while bicarbonate was preserved in Myo, which is consistent with a previous report.5 T2* of HP [1-13C]pyruvate was 108.7 ± 22.6 ms in LV and 129.4 ± 8.9 ms in RV. T2* of [1-13C]pyruvate in Myo was not calculated due to the low SNR. [1-13C]Lactate T2* was measured as 40.9 ± 8.3 ms in LV, 44.2 ± 5.5 ms in RV, and 43.7 ± 9.0 ms in Myo. T2* of [13C]bicarbonate was reliable only in Myo and measured as 64.4 ± 2.5 ms, which is within the range of previous measurement from human heart (T2* = 58 ± 20 ms) using the spectral linewidth of HP [13C]bicarbonate.13 Time-averaged T2*s from individual subjects are summarized in Table 1. Fig. 7 shows HP 13C metabolite maps from a representative subject (subject 1). As shown in Fig. 7(C), HP [1-13C]pyruvate, [1-13C]lactate, and [13C]bicarbonate images were acquired at multiple timepoints, and multi-echo images at each timepoint were examined for T2* estimation (Fig. 8). From the subject, T2*s of [1-13C]pyruvate and [1-13C]lactate were 85.7 ms (r2 = 0.99) and 56.6 ms (r2 = 0.98), respectively, in LV at 25 s post-injection. In RV, the T2*s of [1-13C]pyruvate and [1-13C]lactate were 164.2 ms (r2 = 0.98) and 42.9 ms (r2 = 0.99), respectively. T2*s of [1-13C]lactate and [13C]bicarbonate in Myo was 49.2 ms (r2 = 0.95) and 63.6 ms(r2 = 0.97), respectively. T2*s were consistent over the timepoints for all the HP metabolites (CV < 0.21), as shown in Fig. 8(C). The QC measured that the pyruvate concentration in the final solution was 248.3 ± 11.2 mM with 0.4 ± 0.3 μM residual radical. The liquid-state polarization level was measured as 33.5% ± 9.6%. The dissolution to injection time was 64.7 ± 3.8 s.
Figure 8. In vivo cardiac T2* measurement from the representative human subject.
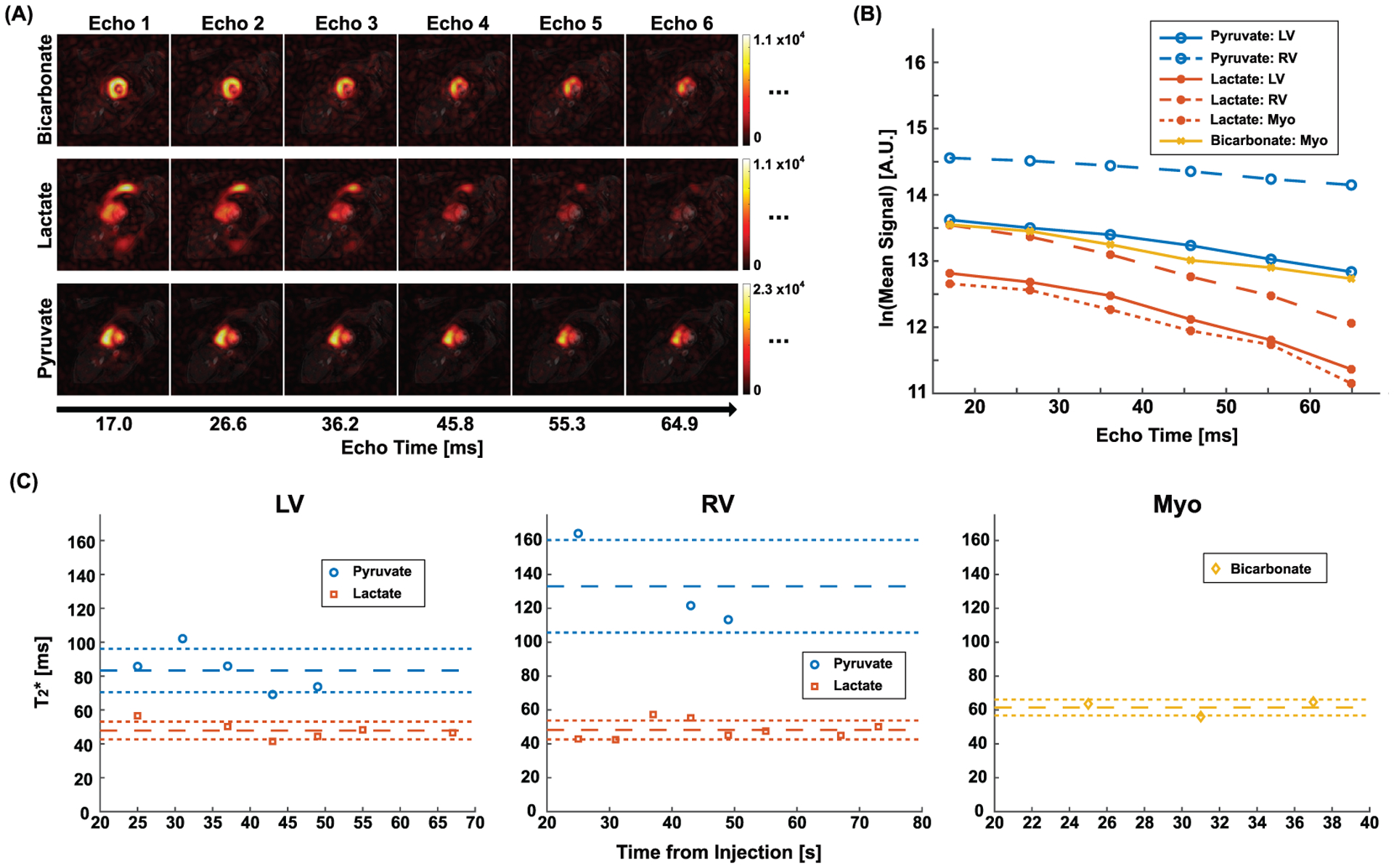
(A) Multiple echo images of HP [1-13C]pyruvate, [1-13C]lactate and [13C]bicarbonate at 25 s post-injection. (B) Signal decays of HP signals in LV, RV, and Myo (ROIs are depicted as red contours in Fig. 7 (A)) along the echo time. (C) Dynamic changes of the T2*s in the ROIs. The reference lines denote mean ± standard deviation.
Although T2*-weighted, multi-echo images of HP metabolite maps can be combined to improve the SNR, the improvements varied for different metabolites depending on the T2*s and the initial SNRs of the first-echo images. In animals, the peak SNRs of the echo-combined 13C images increased by 44 ± 22 %, 29 ± 23 %, and 28 ± 22 % compared to those of the first-echo images for [13C]bicarbonate, [1-13C]lactate and [1-13C]pyruvate, respectively. In humans, the peak SNRs increased by 135 ± 22 % for [13C]bicarbonate, 42 ± 18 % for [1-13C]lactate, and 105 ± 33 % for [1-13C]pyruvate. The echo-combined SNR improvements are summarized in Supporting Information Fig. S1.
Discussion
In this study, we implemented ECG-gated metabolite-selective 13C MESI sequence for cardiac T2* mapping of HP metabolites. The feasibility and the reproducibility of T2* measurement using the proposed method was validated in a phantom and animals. In humans, T2*s of HP 13C-metabolites could be spatially resolved for LV, RV and Myo despite the limited spatial resolution due to the short echo-spacing and the compromised gradient performance of the wide-bore system.
Contributing Factors to T2*s of HP 13C Metabolites
The T2* values were reported in the heart for three HP metabolites: [1-13C]pyruvate, [1-13C]lactate and [13C]bicarbonate. The distinct T2* values could originate from several contributing factors such as off-resonance, blood flow, and T2, in addition to acquisition parameters. The off-resonance could be from the spatial inhomogeneity of B0 or inaccurate assignment of acquisition frequencies for each metabolite. Comparing the T2*s measured from rat and human hearts, the longer T2*s of HP metabolites in human heart may arise from the more homogeneous B0 field (e.g., better shimming) due to the slower heart rate and the presence of ECG gating. (R2.9) Likewise, the inter-subject variance of B0 inhomogeneities may explain the relatively wide distribution of T2*s in rats, as shown in Fig. 6. Pyruvate had significantly longer T2*s than lactate (p < 0.0001) and bicarbonate (p < 0.001); this is likely due to the T2 difference between HP metabolites. Indeed, a previous rat study reported longer T2 for [1-13C]pyruvate compared to [1-13C]lactate in the liver.20 Moreover, it was noted that T2*s of HP [1-13C]pyruvate and [1-13C]lactate were longer in RV compared to those in LV (Table 1). The difference could be related to the higher filling velocity in LV than RV.21 Flow-induced signal decay is strong in LV, resulting in shorter T2*s for HP [1-13C]pyruvate and [1-13C]lactate in LV than RV. On the contrary, the relatively long T2* of HP [13C]bicarbonate in Myo is likely due to the absence of the blood flow effect.
Improved Assessment of Cardiac HP Signals
For most HP studies, utility of measured HP T2*s lies primarily in accurate quantification of HP signals. In particular, understanding T2* effect is crucial for appropriate assessment of HP data acquired by GRE-based pulse sequences and the design of the readout trajectories. Previous efforts to compensate the T2* effect in HP 13C images have been focused on the off-resonance effect. Reed, et al. demonstrated improved B0 shimming to mitigate the non-uniform circumferential HP [13C]bicarbonate signal from the Myo.13 Traechtler, et al. proposed a model-based reconstruction to correct the B0-induced phase offsets in HP 13C multi-echo acquisition.12 Considering the other contributing factors besides off-resonance effect, direct measurements of in vivo T2* would further benefit the cardiac HP 13C studies.
Potential Improvements and Applications
In this study, in-plane spatial resolution of in vivo HP studies was compromised due to SNR available and the echo time needed for accurate T2* measurement. The partial volume effect may cause mismatch between the segmentation from 1H MRI and actual 13C signal distribution, leading to inaccurate measurement of T2*. The low in-plane resolution could also result in sub-optimal T2* maps. Therefore, in this study, we took the spatially averaged signal for T2* fitting instead of the voxel-by-voxel analysis to insure the accurate measurement of T2*. For future work, multi-shot spiral readout can be considered with carefully designed excitation angle strategy.22 Alternatively, acceleration techniques such as parallel imaging23 and compressed sensing24 can also be integrated into the MESI sequence for improving spatial resolution.
In the rat studies, cardiac gating was not available for HP imaging, resulting in motion artifacts in the 13C images. By carefully choosing ROIs for the mean signal quantification, we were able to mitigate the impact from the artifact, while it would be beneficial to include cardiac gating or respiratory control for animal study in the future.
Several aspects of T2*s in HP metabolites need further investigation. For instance, flow contribution to the T2* can be clarified by utilizing flow-sensitive gradients.25 Moreover, it should be noted that the T2*s depend on acquisition parameters such as the size26,27 and the shape27 of the voxel, the slice thickness,28 eddy currents.29
As HP pyruvate is utilized to study metabolism in various in vivo applications,30–32 T2*s of HP 13C metabolites in other organs and tissue types will be beneficial for establishing optimized k-space sampling patterns. In particular, large variation of magnetic susceptibility is expected in pathological states such as glioblastoma33 due to blood depositions and calcifications, leading to potential underestimation of HP signals.
In vivo T2 is another under-explored feature of HP molecules.20,34,35 Yen, et al. reported nearly 1-second-long T2s of HP signals with potentially unique metabolic contrast in rat hepatocellular carcinoma.20 Joe, et al. estimated in vivo T2s of HP 13C-metabolites by combining the 1H B0 map and 13C T2* maps.35 Due to the complex T2* relaxation mechanism of HP 13C-metabolites, T2 calculation using 1H B0 map will need further verification.
Conclusions
In conclusion, we proposed a 13C multi-echo spiral imaging sequence to measure in vivo cardiac T2*s of HP [1-13C]pyruvate and its products. A phantom test and in vivo animal studies validated the method is robust and reproducible. Cardiac T2*s of HP [1-13C]pyruvate, [1-13C]lactate and [13C]bicarbonate at 8 mm × 8 mm spatial resolution in rodents were measured as 24.9 ± 5.0, 16.4 ± 4.7, and 16.9 ± 3.4 ms, respectively. T2*s of the HP 13C-metabolites at 16 mm × 16 mm spatial resolution in human heart are observed as 108.7 ± 22.6 ms (LV) and 129.4 ± 8.9 ms (RV) for [1-13C]pyruvate, 40.9 ± 8.3 ms (LV), 44.2 ± 5.5 ms (RV) and 43.7 ± 9.0 ms (Myo) for [1-13C]lactate, and 64.4 ± 2.5 ms (Myo) for [13C]bicarbonate.
Supplementary Material
Acknowledgements
Personnel Support:
We appreciate the clinical research team and the supporting staffs of the Advanced Imaging Research Center at UT Southwestern for imaging the volunteers - Jeff Liticker, PharmD, Ronald G. Hall, PharmD, Jaffar Raza, PharmD, Jeannie Baxter, RN, Kelley Derner, RN, Salvador Pena, Corey Mozingo, Maida Tai, and Richard Martin.
Funding:
National Institutes of Health of the United States (P41 EB015908, S10 RR029119, S10 OD018468, R01 NS107409); The Welch Foundation (I-2009-20190330); UT Dallas Collaborative Biomedical Research Award (UTD 1907789); The Cancer Prevention and Research Institute of Texas (RP180404). The Texas Institute for Brain Injury and Repair.
References
- 1.Golman K, Petersson JS, Magnusson P, et al. Cardiac metabolism measured noninvasively by hyperpolarized 13C MRI. Magn Reson Med. 2008;59:1005–1013. [DOI] [PubMed] [Google Scholar]
- 2.Le Page LM, Rider OJ, Lewis AJ, et al. Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Diabetes. 2015;64:2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agger P, Hyldebrandt JA, Hansen ESS, et al. Magnetic resonance hyperpolarization imaging detects early myocardial dysfunction in a porcine model of right ventricular heart failure. Eur Heart J Cardiovasc Imaging. 2020;21:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroeder MA, Lau AZ, Chen AP, et al. Hyperpolarized 13C magnetic resonance reveals early- and late-onset changes to in vivo pyruvate metabolism in the failing heart. Eur J Heart Fail. 2013;15:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham CH, Lau JYC, Chen AP, et al. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res. 2016;119:1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rider OJ, Apps A, Miller JJJJ, et al. Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized 13C MRI. Circ Res. 2020;126:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JM, Reed GD, Liticker J, et al. Effect of doxorubicin on myocardial bicarbonate production from pyruvate dehydrogenase in women with breast cancer. Circ Res. 2020;127:1568–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau AZ, Chen AP, Ghugre NR, et al. Rapid multislice imaging of hyperpolarized 13C pyruvate and bicarbonate in the heart. Magn Reson Med. 2010;64:1323–1331. [DOI] [PubMed] [Google Scholar]
- 9.Miller JJ, Lau AZ, Teh I, et al. Robust and high resolution hyperpolarized metabolic imaging of the rat heart at 7 T with 3D spectral-spatial EPI. Magn Reson Med. 2016;75:1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wespi P, Steinhauser J, Kwiatkowski G, Kozerke S. High-resolution hyperpolarized metabolic imaging of the rat heart using k-t PCA and k-t SPARSE. NMR Biomed. 2018;31:e3876. [DOI] [PubMed] [Google Scholar]
- 11.Malloy CR, Sherry AD. Biochemical specificity in human cardiac imaging by 13C magnetic resonance imaging. Circ Res. 2016;119:1146–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traechtler J, Vishnevskiy V, Fuetterer M, Dounas A, Kozerke S. Joint image and field map estimation for multi-echo hyperpolarized 13C metabolic imaging. In Proceedings of the 2020 ISMRM & SMRT Virtual Conference & Exhibition. #3016.
- 13.Reed GD, Ma J, Park JM, et al. Characterization and compensation of f0 inhomogeneity artifact in spiral hyperpolarized 13C imaging of the human heart. Magn Reson Med. 2021;00:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wespi P, Steinhauser J, Kwiatkowski G, Kozerke S. Overestimation of cardiac lactate production caused by liver metabolism of hyperpolarized [1-13C]pyruvate. Magn Reson Med. 2018;80:1882–1890. [DOI] [PubMed] [Google Scholar]
- 15.Alger JR. Quantitative proton magnetic resonance spectroscopy and spectroscopic imaging of the brain: a didactic review. Top Magn Reson Imaging. 2010;21:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate. Sci Transl Med. 2013;5:198ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park I, Larson PEZ, Gordon JW, et al. Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magn Reson Med. 2018;80:864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson PEZ, Kerr AB, Chen AP, et al. Multiband excitation pulses for hyperpolarized 13C dynamic chemical-shift imaging. J Magn Reson. 2008;194:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Man LC, Pauly JM, Macovski A. Multifrequency interpolation for fast off-resonance correction. Magn Reson Med. 1997;37:785–792. [DOI] [PubMed] [Google Scholar]
- 20.Yen Y-F, Le Roux P, Mayer D, et al. T2 relaxation times of 13C metabolites in a rat hepatocellular carcinoma model measured in vivo using 13C-MRS of hyperpolarized [1-13C]pyruvate. NMR Biomed. 2010;23:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. [DOI] [PubMed] [Google Scholar]
- 22.Walker CM, Fuentes D, Larson PEZ, Kundra V, Vigneron DB, Bankson JA. Effects of excitation angle strategy on quantitative analysis of hyperpolarized pyruvate. Magn Reson Med. 2019;81:3754–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arunachalam A, Whitt D, Fish K, et al. Accelerated spectroscopic imaging of hyperpolarized C-13 pyruvate using SENSE parallel imaging. NMR Biomed. 2009;22:867–873. [DOI] [PubMed] [Google Scholar]
- 24.Geraghty BJ, Lau JYC, Chen AP, Cunningham CH. Accelerated 3D echo-planar imaging with compressed sensing for time-resolved hyperpolarized 13C studies. Magn Reson Med. 2017;77:538–546. [DOI] [PubMed] [Google Scholar]
- 25.Gordon JW, Niles DJ, Adamson EB, Johnson KM, Fain SB. Application of flow sensitive gradients for improved measures of metabolism using hyperpolarized 13c MRI. Magn Reson Med. 2016;75:1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernndez-Seara MA, Wehrli FW. Postprocessing technique to correct for background gradients in image-based R*2 measurements. Magn Reson Med. 2000;44:358–366. [DOI] [PubMed] [Google Scholar]
- 27.Yablonskiy DA, Haacke EM. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med. 1994;32:749–763. [DOI] [PubMed] [Google Scholar]
- 28.Reeder SB, Faranesh AZ, Boxerman JL, McVeigh ER. In vivo measurement of T2* and field inhomogeneity maps in the human heart at 1.5 T. Magn Reson Med. 1998;39:988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josan S, Kaye E, Pauly JM, Daniel BL, Pauly KB. Improved half RF slice selectivity in the presence of eddy currents with out-of-slice saturation. Magn Reson Med. 2009;61:1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grist JT, McLean MA, Riemer F, et al. Quantifying normal human brain metabolism using hyperpolarized [1-13C]pyruvate and magnetic resonance imaging. Neuroimage. 2019;189:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallagher FA, Woitek R, McLean MA, et al. Imaging breast cancer using hyperpolarized carbon-13 MRI. Proc Natl Acad Sci USA. 2020;117:2092–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang S, Bok R, Qin H, et al. A metabolite-specific 3D stack-of-spiral bSSFP sequence for improved lactate imaging in hyperpolarized [1-13C]pyruvate studies on a 3 T clinical scanner. Magn Reson Med. 2020;84:1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deistung A, Schweser F, Wiestler B, et al. Quantitative susceptibility mapping differentiates between blood depositions and calcifications in patients with glioblastoma. PLoS One. 2013;8:e57924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milshteyn E, Reed GD, Gordon JW, et al. Simultaneous T1 and T2 mapping of hyperpolarized 13C compounds using the bSSFP sequence. J Magn Reson. 2020;312:106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joe E, Lee H, Lee J, et al. An indirect method for in vivo T2 mapping of [1-13C]pyruvate using hyperpolarized 13C CSI. NMR Biomed. 2017;30:e3690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


