Abstract
The study was conducted to clarify how early high plane of nutrition related to metabolic imprinting affected growth, carcass characteristics, and meat quality of grass-fed Wagyu (Japanese Black cattle). Wagyu steers were allocated randomly into 2 dietary groups: (1) steers fed milk replacer (crude protein 26.0%, crude fat 25.5%; maximum intake 0.6 kg/d) until 3 mo of age and then fed roughage (orchard grass hay) ad libitum from 4 to 10 mo of age (roughage group, RG; n = 11); (2) steers fed milk replacer (maximum intake of 1.8 kg/d) until 3 mo of age and then fed a high-concentrate diet from 4 to 10 mo of age (early high nutrition, EHN; n = 12). After 11 mo of age, all steers were fed roughage ad libitum until 31 mo of age and then slaughtered. Growth performance, carcass traits, longissimus muscle (LM) meat quality and intramuscular fat (IMF) content, plasma insulin-like growth factor I (IGF-I) concentration, and bone mineral density were measured. Body weight was greater in EHN steers (571 kg) than RG steers (520 kg; P < 0.01). Plasma IGF-I levels were higher in EHN steers than in RG steers at 3, 10, and 14 mo of age (P < 0.01, P < 0.005, P < 0.001, respectively); however, plasma IGF-I levels were lower in EHN steers compared with RG steers at 30 mo of age (P < 0.01). The total weight of the muscles and bones of the left half of the carcass was not different between the 2 groups (P = 0.065). Five of the 19 muscles investigated (semimembranosus, P = 0.036; infraspinatus, P = 0.024; supraspinatus, P = 0.0019; serratus ventralis cervicis, P = 0.032; serratus ventralis thoracis, P = 0.027) were heavier in EHN steers. Total fat weight in the left half of the carcass was 30% greater (P = 0.025) in HNE carcasses. Subcutaneous and perirenal fat weights were 53% and 84% greater (P = 0.008, P = 0.002, respectively) in EHN carcasses. The LM IMF content was greater in EHN loins (13.2%) compared with RG loins (9.4%) at 31 mo of age (P = 0.038); however, no differences were found for shear force, tenderness, and cook loss. These results suggested early high-nutrition affected the growth and meat quality of livestock.
Keywords: carcass characteristics, grass-fattened cattle, neonatal programming, Wagyu
Introduction
Marbled beef with increased intramuscular fat (IMF) is considered valuable in Wagyu (Japanese Black cattle) raised in Japan and requires feeding a large amount of cereal grain to cause its deposition. Similar to Japan, the feedlot system in the United States also utilizes considerable amounts of concentrates during the finishing phase to produce grain-fed beef. The main alternative to grain-fed beef, grass-fed beef, is produced on pastures and minimizes environmental impacts (Provenza et al., 2019). Moreover, grass-fed beef contains a higher proportion of unsaturated fatty acids, which contribute to human health (Provenza et al., 2019). While consumer interest in grass-fed beef is increasing in developed countries, problems associated with grass-fed beef including productivity (final carcass weight), meat quality (marbling), flavor (Mandell et al., 1998; Sitz et al., 2005), and toughness are issues needing to be addressed.
Nutritional stimuli during human fetal and neonatal periods affect later-life metabolism and consequently modify phenotype under the concept of developmental origins of health and disease ( Waterland and Gorza, 1999, Barker et al., 2002; Du et al., 2010a, b). In cattle, early life events during the suckling period and months after weaning significantly affect long-term performance and productivity (Heinrichs and Heinrichs, 2010; Khan et al, 2010; Van Amburgh et al., 2013). Diet and feeding strategies in calves may represent a cost-effective means of altering carcass composition in Wagyu beef.
When compared with calves weaned at 8 mo of age and backgrounded, early-weaned calves fed high-concentrate feed for 148 d, followed by grazing and feedlot entry, did not improve feedlot performance; however, this management scheme did improve marbling score and increased hot carcass weight (Scheffler et al., 2014). Wertz et al. (2002) reported early weaning (at 142 d of age), followed by feeding with high concentrates until 9 mo of age, accelerated the accumulation of IMF in crossbred Wagyu compared with those reared by grazing until 22 mo, followed by concentrate feeding. Similarly, feeding Holstein bulls milk replacer over an extended period (to 200 d of age) shortened the fattening period by increasing growth rate per unit time, but did not affect IMF (Abdelsamei et al., 2005). These results suggest quality and quantity of milk replacer fed during the suckling stage and the diet fed during the rearing stage may significantly influence long-term growth. The objective of this study was to evaluate the influence of early and intensive high-plane of nutrient feeding on growth, carcass characteristics, and meat quality of grass-fattened Japanese Black cattle.
Materials and Methods
Animals and sampling
Animals used in this study were treated in accordance with the Guidelines for Animal Experiments of the Faculty of Agriculture of Kyushu University (Fukuoka, Japan) and with Japanese law (Law No. 105, notification No. 6).
Twenty-three male Japanese Black steers were obtained by crossing a Yasushigekatsu bull (a sire from the Livestock Improvement Association of Japan, Inc.) with cows from the National Cattle Breeding Center of Japan. The bull was from one of the popular Japanese Black cattle lines, “Kedaka,” and was a good representative of the Japanese Black breed. Steers were castrated at ~4 mo of age. Calves were randomly allocated into 2 dietary groups: (1) early high nutrition (EHN; n = 12) and (2) roughage-fed (RG; n = 11) group (Figure 1). Until 14 d of age, calves were kept in conditions similar to those of calves held in a free-stall barn. After 14 d of age, calves in both groups were fed the same quality of milk replacer (ME 4.6 M cal/kg, crude protein 26.0%, crude fat 25.5%) by an automaticfeeder (Calf-Feeders TAP5-SA2-30-P, KFA3-MA3; Forster Technik, Engen, Germany).
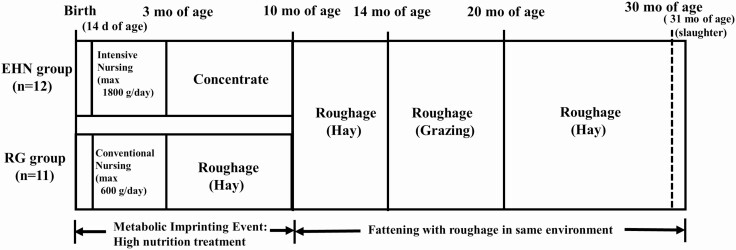
Figure 1.
Experimental design. Two different diets were fed during the early growth stage. The high-energy (metabolic imprinting, EHN) group was intensively fed milk replacer (maximum intake of 1.8 kg (fresh matter) per day) with calf starter until 3 mo of age and then fed concentrate (with roughage available ad libitum). The roughage (RG) group was given milk replacer at the Japanese standard level (maximum intake of 0.6 kg (fresh matter) per day) with calf starter until 3 mo of age and then fed with only hay ad libitum until 10 mo of age. Thereafter, the 2 groups were fed the same roughage diets during the fattening period (11 to 31 mo of age).
As a control, the RG group was given milk replacer (Calftop ET; Zenrakuren, Tokyo, Japan) at the standard Japanese level [maximum intake of 0.6 kg (fresh matter) per day] from 14 d until 3 mo of age and fed a constant amount of dry calf starter (1.2% of body weight, ME 3.2 M cal/kg, crude protein 25.6%, crude fat 4.3%; Table 1) and hay (ME 1.2 to 1.6 Mcal/kg, crude protein 4.0% to 8.4%). The RG steers were then fed only roughage (orchard grass hay) ad libitum from 4 to 10 mo of age. The EHN group was intensively fed milk replacer [maximum intake of 1.8 kg (fresh matter) per day] from 14 d until 3 mo of age followed by a high-concentrate diet from 4 to 10 mo of age (Table 1). All calves were allowed free access to water and were provided with a mineralized salt block that contained minerals, salt, and a diuretic (Cowstone A; Nippon Zenyaku Kogyo, Japan) from the weaning stage onwards. After 11 mo of age, both groups were fed only roughage [orchard grass (Dactylis glomerata) hay, fresh matter ME 1.3 Mcal/kg, crude protein 9.8%] ad libitum in the paddock until 14 mo of age. All animals were then put onto the same pasture [perennial ryegrass (Lolium perenne) and white clover (Trifolium repens)] and grazed until 20 mo of age, fed only roughage (orchard grass hay) ad libitum in the paddock from 21 to 31 mo, and slaughtered at 31 mo of age at Fukushima Meat Factory Co.,Ltd. under the government oversight (Figure 1).
Table 1.
Diet compositions and dietary and nutrient intake during the intervention period from 0 to 10 mo of age1
| EHN | RG | P-value | |
|---|---|---|---|
| Composition of diet | |||
| Milk replacer, 0 to 3 mo of age | |||
| Dry matter, % | 97.0 | 97.0 | |
| Metabolizable energy, Mcal/kg | 4.6 | 4.6 | |
| Crude protein, % | 26.0 | 26.0 | |
| Crude fat, % | 25.5 | 25.5 | |
| Dry calf starter, 0 to 3 mo of age | |||
| Dry matter, % | 87.0 | 87.0 | |
| Metabolizable energy, Mcal/kg | 3.2 | 3.2 | |
| Crude protein, % | 25.6 | 25.6 | |
| Crude fat, % | 4.3 | 4.3 | |
| Concentrate of diet, 4 to 10 mo of age | |||
| Dry matter, % | 87.3 | 87.3 | |
| Metabolizable energy, Mcal/kg | 2.7 | 2.7 | |
| Crude protein, % | 16.0 | 16.0 | |
| Crude fat, % | 2.0 | 2.0 | |
| Dietary intake (fresh matter, kg/head), 0 to 3 mo of age | |||
| Replacer milk | 101.5 ± 5.4 | 42.4 ± 0.3 | <0.01 |
| Dry calf starter | 23.8 ± 11.2 | 42.7 ± 9.0 | <0.01 |
| Roughage | 24.9 ± 5.2 | 32.4 ± 15.9 | |
| Total | 150.1 ± 19.2 | 117.6 ± 24.5 | |
| Nutritional intake (fresh matter, kg/head), 0 to 3 mo of age | |||
| Metabolizable energy, Mcal/kg | 5.8 ± 0.3 | 3.6 ± 0.2 | <0.01 |
| Crude protein | 32.8 ± 3.4 | 21.2 ± 2.6 | <0.01 |
| Crude fat | 26.8 ± 1.6 | 12.1 ± 0.5 | <0.01 |
| Dietary intake (fresh matter, kg/head), 4 to 10 mo of age | |||
| Concentrate | 1175.7 ± 110.7 | ||
| Roughage | 437.5 ± 13.7 | 923.4 ± 61.4 | <0.01 |
| Nutritient intake (fresh matter, kg/head), 4 to 10 mo of age | |||
| Metabolizable energy, Mcal/kg | 43.6 ± 3.0 | 16.8 ± 1.0 | <0.01 |
| Crude protein | 224.9 ± 17 | 77.0 ± 6.5 | <0.01 |
| Crude fat | 31.4 ± 2.1 | 16.2 ± 1.4 | <0.01 |
1EHN, metabolic imprinting group, n = 12; RG, roughage group, n = 11. Values are means ± SD.
Carcass traits and grade
Body weight was recorded just before slaughter after fasting for 24 hr. Hot carcass, muscles, bones, and adipose tissue weights were measured as basic carcass traits. The Japan Meat Grading Association (JMGA, 1988) measured meat yield, ribeye area, rib thickness, and subcutaneous fat thickness and evaluated the carcass grade, beef marbling score (BMS), beef color standard (BCS), and beef fat standard (BFS) of all carcasses. Subcutaneous adipose tissue was dissected from the surface of the carcass and the inside of the skin. Total weights of the muscles, bones, adipose tissues of the thoracic and visceral cavities, and perirenal fat were measured after separating them by dissection from the cold carcass 24 hr after slaughter. Five randomly chosen carcass left sides from each group were dissected and weights of muscles, fat, and bones were measured.
Physical and chemical properties of the longissimus muscle (LM)
Water holding capacity was measured by drip, thawing, cooking, and grilling loss. Drip loss was quantified as described by Honikel (1987). Thawing and cooking losses were determined in 2.5-cm-thick longissimus steaks frozen in polyethylene bags at −20 °C. The percentage of thawing loss was evaluated based on the weight difference between before and after thawing at 4 °C for 24 hr. Before cooking, meat pH was determined in the LM by a pH meter (iQ170; IQ Scientific Instruments). Samples were cooked in plastic bags in a water bath at 80 °C until a meat core temperature of 70 °C was reached. The temperature was monitored by a thermocoupler. Samples were cooled to ambient temperature and weighed after their surfaces had been dried with tissue paper. For determination of the grilling loss, 2.5-cm-thick steaks were grilled in a convection oven at 150 °C until a meat core temperature of 70 °C was reached.
Broiled samples were used to determine shear force after cooling and drying with soft paper. Six 1.27-cm cores were collected parallel to the muscle fiber orientation and sheared perpendicular to the fiber direction using Instron model 5542 (Instron Co., Norwood, MA). Calibration distances were taken and the mean of the maximum forces was used for data analysis.
The IMF content of the LM was measured in triplicate via the Soxhlet extraction method. Petroleum ether was used as the solvent and fat content was determined gravimetrically after evaporating the extracted solvent (AOAC, 2000).
Muscle and bone sample collection and analysis of bone mineral density
Eighteen muscles were then removed and weighed from 5 carcasses per group: longissimus thoracis, psoas major, trapezius, latissimus dorsi, spinalis and semispinalis thoracis and cervices, infraspinatus, supraspinatus, serratus ventralis cervicis, serratus ventralis thoracis, semispinalis capitis, tensor fascia latae, biceps femoris, gluteus medius, vastus lateralis, rectus femoris, semitendinosus, semimembranosus, and adductor muscles.
Bones including the cervical vertebrae, thoracic vertebrae, lumbar vertebrae, ribs, scapula, humerus, radius, carpal bones, femur, tibia, patella, tuber coxae, and other small bones were collected after muscle removal. After the adhering connective tissues and muscles had been carefully removed, bones were weighed and mineral density of the left femur of each animal was measured using dual-energy X-ray absorptiometry (QDR-2000; Hologic, Waltham, MA).
Measurements of plasma insulin-like growth factor-I (IGF-I) levels
To establish the influence of intensified milk replacer intake on the secretion of growth factors in Japanese Black calves, change in plasma IGF-I over the study period was investigated. Before morning feeding, blood samples were drawn from the jugular vein into heparin-sodium tubes at 3, 10, 14, 20, and 30 mo of age. Plasma was separated by centrifugation at 1,752 × g for 20 min at a temperature of 4 °C and stored at −40 °C until measurement. After the removal of binding proteins, the level of IGF-I in the plasma was determined by radioimmunoassay (IGF-I IRMA, SRL, Inc., Tokyo, Japan). IGF-I intra- and interassay CV were 1.8% and 2.4 %, respectively
Statistical analysis
Statistical analyses were performed using SAS statistical software (SAS University Edition; SAS Inst. Inc. 2014, Cary, NC). Between-group comparisons were made by ANOVA using the Mixed procedure with a group as a fixed effect. Data are presented as the means ± SD for all animals in each experimental group (EHN, n = 12; and RG, n = 11), while data on carcass composition and the weights of dissected muscles, fat depots, and bones are from subsets of animals (EHN, n = 5; and RG, n = 5). The analyses of change of IGF-I concentration during growth were performed using repeated measures ANOVA of EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics (Kanda, 2013). Differences were considered statistically significant at P ≤ 0.05. Different superscript capital letters (A, B) indicate differences between treatments (P < 0.05 and P < 0.01, respectively).
Results
Feed efficiency and growth performance
Steers in the EHN group consumed 1.5 times more crude protein (P < 0.01) and 2.2 times more crude fat (P < 0.01) than those in the RG group from week 2 until 3 mo of age. Afterward, EHN steers consumed 2.9 times more crude protein (P < 0.01) and 1.9 times more crude fat (P < 0.01) until 10 mo of age (Table 1). Intensive feeding of milk replacer and concentrate increased (P < 0.001) EHN average daily gain (ADG) during the metabolic imprinting intervention (0 to 10 mo of age; Table 2).
Table 2.
Growth performance of cattle in EHN and RG groups1
| EHN | RG | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P-value | |
| Growth performance | |||||
| Birth weight, kg | 34 | 5.3 | 35 | 4.2 | 0.703 |
| ADG from 0 to 90 d2, kg/d | 1.02 | 0.09 | 0.75 | 0.12 | <0.001 |
| BW at 90 d, kg | 126 | 11.2 | 103 | 11.2 | <0.001 |
| ADG from 90 to 300 d3, kg/d | 1.06 | 0.07 | 0.45 | 0.08 | <0.001 |
| BW at 300 d, kg | 370 | 23.7 | 205 | 23.6 | <0.001 |
| ADG from 300 to 450 d4, kg/d | 0.16 | 0.08 | 0.38 | 0.10 | <0.001 |
| BW at 450 d, kg | 394 | 26.9 | 262 | 36.3 | <0.001 |
| ADG from 450 to 600 d5, kg/d | 0.50 | 0.19 | 0.91 | 0.13 | <0.001 |
| BW at 600 d, kg | 470 | 23.7 | 399 | 33 | <0.001 |
| ADG from 600 to 900 d6, kg/d | 0.21 | 0.05 | 0.28 | 0.07 | <0.001 |
| Final BW, kg | 571 | 36.3 | 520 | 39.2 | 0.0049 |
1EHN, metabolic imprinting group, n = 12; RG, roughage group, n = 11.
2The EHN group was intensively fed high-protein and high-fat milk replacer (maximum 1.8 kg/d), and the RG group was received conventional feeding during the suckling period (maximum 0.6 kg/d).
3The EHN group was fed concentrates (2.5% of body weight) and hay, and the RG group was fed only hay ad libitum.
4The 2 groups were fed the same hay during fattening.
5The 2 groups grazed on the same pasture.
6The 2 groups were fed the same hay during finishing.
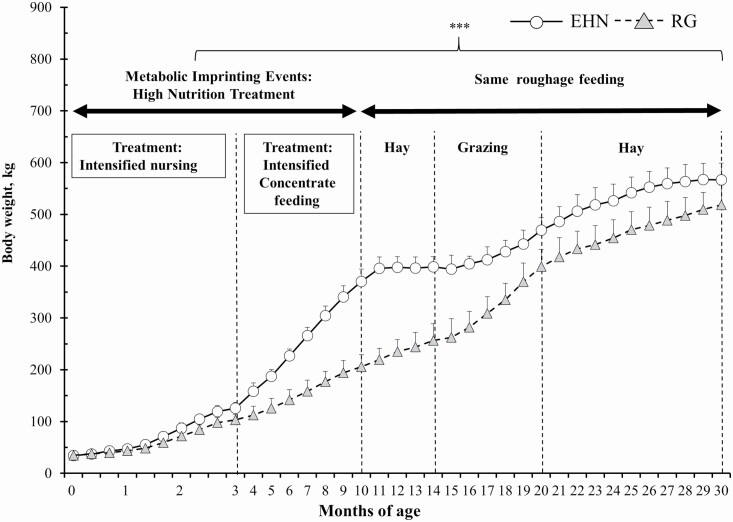
Upon entering the roughage fattening phase, EHN steers were 165-kg heavier (P < 0.01) than those in the RG group; however, EHN steers did not gain any additional body weight for the first 5 mo after the switch from a concentrate- to a hay-based diet (Figure 2).
Figure 2.
Changes in mean body weight over the study period in EHN and RG groups. EHN metabolic imprinting group, n = 12. RG: roughage group, n = 11. ***: P < 0.01.
From 11 to 14 mo of age, RG steers had greater (P < 0.001) ADG than EHN steers (Table 2). Nonetheless, EHN steers remained heavier (P < 0.001) at 15 to 20 mo of age, when they entered the next grazing phase. Steers in the EHN group began gaining body weight after 16 mo of age, while in the transition-to-grazing phase.
Growth performance and serum IGF-I level
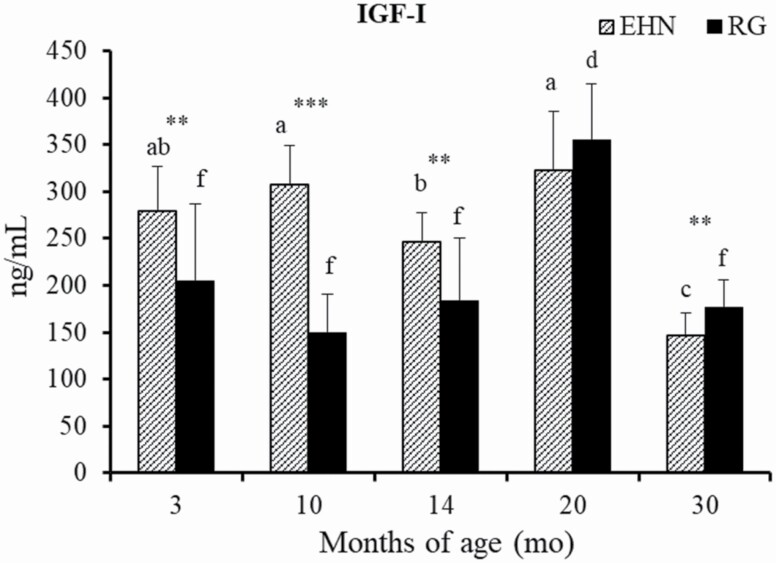
Serum IGF-I levels were greater in EHN steers at 3, 10, and 14 mo of age (P < 0.01, P < 0.005, and P < 0.01, respectively; Figure3). Serum IGF-I levels were less in EHN steers at 30 mo of age (P < 0.05). The change in IGF-I levels with growth seen in this study was consistent with the change in ADG during the study. Body weight was always higher in EHN steers (P < 0.01, Figure 2); however, its relationship with ADG changed after 10 mo of age (Table 2). The IGF-I levels in EHN steers decreased after the switch from a concentrate based to a roughage-based diet at 11 mo of age.
Figure 3.
Comparison of serum IGF-I level during growth between EHN and RG groups. EHN: metabolic imprinting group, n = 12. RG: roughage group, n = 11. Values are means ± SD. ** P < 0.01. *** P < 0.005. a,b,cSignificant differences among months of age in EHN group (P < 0.05). e,fSignificant differences among months of age in RG group (P < 0.05).
Carcass characteristics
At 30 mo of age, after the steers had been fed hay from 21 to 30 mo, differences (P < 0.001) in body weight were found between the groups (Table 2). Steers in the EHN group finished an average of 51 kg heavier (P < 0.005) than RG steers (Table 2), with a corresponding increase in hot carcass weight (P = 0.0001; Table 3). When the ratio of each fat depot to hot carcass weight was calculated, EHN steers had greater subcutaneous fat (9.8% vs. 7.2%, P = 0.018) and perirenal fat (3.1% vs. 1.8%, P = 0.003) ratios. The hot carcass weight was greater at different subcutaneous fat and rib thicknesses for EHN steers, which suggests that they deposited subcutaneous fat as readily as the steers in the RG group relative to their changes in body weight.
Table 3.
Carcass characteristics and physical and chemical properties of the LM in EHN and RG groups at slaughter1
| EHN | RG | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P-value | |
| Carcass characteristics and meat quality | |||||
| HCW, kg | 293.63 | 17.27 | 256 | 20.57 | 0.0001 |
| Meat yield,2% | 71.5 | 0.4 | 71.7 | 0.7 | 0.4900 |
| Rib eye area,3 cm2 | 32.67 | 2.5 | 29.36 | 6.7 | 0.1483 |
| Rib thickness,4 cm | 4.28 | 0.32 | 3.64 | 0.22 | <0.0001 |
| Subcutaneous fat thickness,5 cm | 1.45 | 0.3 | 0.83 | 0.4 | 0.0004 |
| Carcass grade6 | 2 | 0.0 | 1.36 | 0.5 | <0.0001 |
| BMS No7 | 2.41 | 0.66 | 1.54 | 0.52 | 0.0022 |
| BCS No8 | 4.91 | 0.67 | 5.73 | 0.90 | 0.0257 |
| BFS No9 | 4.25 | 0.87 | 5.55 | 0.69 | 0.0007 |
| Physical and chemical properties of LM | |||||
| Shear force after CL10 | 3.13 | 0.61 | 3.26 | 0.82 | 0.6734 |
| Tenderness,10 kgw/cm2 | 63 | 21.2 | 67 | 30.3 | 0.7223 |
| Moisture, % | 66.54 | 2.69 | 69.66 | 2.19 | 0.0279 |
| Lipid, % | 13.24 | 3.68 | 9.35 | 2.80 | 0.0388 |
| Protein, % | 19.26 | 1.46 | 19.95 | 0.89 | 0.1869 |
| pH | 5.58 | 0.09 | 5.7 | 0.10 | 0.0327 |
| Cookinloss, % | 25.1 | 1.9 | 25.7 | 2.2 | 0.5100 |
| Water holding capacity of LM | |||||
| Press method | 80.22 | 2.08 | 80.31 | 1.48 | 0.9049 |
| Centrifuge method | 70.84 | 1.79 | 71.78 | 2.10 | 0.2667 |
1EHN, metabolic imprinting, n = 12; RG, roughage, n = 11.
2Meat yield percentage was calculated using the following equation: dressing percentage value = 67.37+[0.130 × cross-sectional area of longissimus thoracis muscle at thoracic vertebrae 6 to 7 (cm2)] + [0.667 × thickness of ribs including meat (cm)]−[0.025 × half-carcass weight (kg)] − [0.896 × subcutaneous fat thickness (cm)] (Japan Meat Grading Association (JMGA), 1988; Gotoh et al., 2014).
3Cross-sectional area of longissimus thoracis at thoracic vertebrae 6 to 7 (JMGA, 1988).
4Thickness of specific region of the ribs including meat, measured by the Japan Beef Grading Standard (JMGA, 1988; Gotoh et al., 2014).
5Subcutaneous fat thickness of the specific region indicated by the Japan Beef Grading Standard (JMGA, 1988; Gotoh et al., 2014).
61 = the lowest grade, 5 = the highest grade.
7Beef Marbling Standard No. evaluated by Graders based on the revised Japan Beef Grading Standard (JMGA, 2014) at thoracic vertebrae 6 to 7 of the LM. 1 = the least marbled, 12 = the most marbled beef.
8Beef Color Standard No. evaluated by Graders based on the Japan Beef Grading Standard (JMGA, 1988). 1 = pale red, 7 = dark red.
9Beef fat (Color) Standard No. evaluated by Graders based on the Japan Beef Grading Standard (JMGA, 1988). 1 = white, 7 = yellow.
10Measurements using a tensipresser before cooking loss (CL) analysis (Tsuji, 1982).
The beef marbling standard (BMS, JMGA, 2014; 1 = the least marbled, 12 = the most marbled beef), beef color standards (BCS, JMGA, 1988; 1 = pale red, 7 = dark red), and beef fat standard (BFS, JMGA, 1988; 1 = white, 7 = yellow.) were measured at 6th and 7th thoracic vertebrae junction by professional graders. Results indicated beef from EHN carcasses were better marbled, more pale-red in lean meat color, and whiter in fat color (P = 0.0022, P = 0.0257, and P = 0.0007, respectively; Table 3).
No between-group differences were found in shear force, tenderness, and cooking loss in the LM (Table 3). However, the LM from EHN cattle had a higher lipid percentage and lower moisture percentage (P = 0.0388 and P = 0.0279, respectively; Table 3), although LM weight did not differ between the groups (Table 4). The pH of the EHN LM was lower (P = 0.0327).
Table 4.
Body composition of cattle in EHN and RG groups1
| Weight | Ratio, % | |||||
|---|---|---|---|---|---|---|
| EHN | RG | P-value | EHN | RG | P-value | |
| Half-carcass weight2, g | 147,240 | 130,336 | 0.028 | ― | ― | ― |
| Muscle3, g | ||||||
| Total muscle (18 muscles with all other muscles) | 86,795 | 79,574 | 0.065 | ― | ― | ― |
| Tensor fasciae latae | 1,019 | 1,007 | 0.914 | 1.17 | 1.26 | 0.384 |
| Biceps femoris | 6,047 | 5,619 | 0.186 | 6.96 | 7.06 | 0.445 |
| Gluteus medius | 3,163 | 2,914 | 0.148 | 3.64 | 3.66 | 0.877 |
| Vastus lateralis | 2,133 | 1,918 | 0.147 | 2.46 | 2.41 | 0.730 |
| Rectus femoris | 1,828 | 1,677 | 0.221 | 2.11 | 2.10 | 0.987 |
| Semitendinosus | 1,799 | 1,844 | 0.691 | 2.07 | 2.32 | 0.019 |
| Semimembranosus | 4,140 | 3,685 | 0.036 | 4.77 | 4.63 | 0.170 |
| Adductor | 1,484 | 1,363 | 0.157 | 1.71 | 1.71 | 0.988 |
| Psoas major | 1,599 | 1,503 | 0.161 | 1.85 | 1.89 | 0.634 |
| Latissmus dorsi | 1,614 | 1,569 | 0.634 | 1.86 | 1.97 | 0.118 |
| Trapezius | 7,97 | 800 | 0.980 | 0.93 | 1.00 | 0.635 |
| Longissimus thoracis | 5,575 | 5,095 | 0.178 | 6.43 | 6.39 | 0.899 |
| Spinalis et semispinalis thoracis et cervicis |
874 | 708 | 0.055 | 1.01 | 0.89 | 0.196 |
| Infraspinatus | 2,081 | 1,798 | 0.024 | 2.39 | 2.26 | 0.125 |
| Supuraspinatus | 1,424 | 1,254 | 0.019 | 1.64 | 1.58 | 0.158 |
| Serratus ventralis cervicis | 2,967 | 2,642 | 0.032 | 3.42 | 3.32 | 0.209 |
| Serratus ventralis thoracis | 901 | 674 | 0.027 | 1.04 | 0.84 | 0.041 |
| Semispinalis capitis | 1,401 | 1,374 | 0.797 | 1.62 | 1.72 | 0.352 |
| Fat, g | ||||||
| Total fat | 34,715 | 26,385 | 0.025 | ― | ― | ― |
| Subcutaneous fat | 14,464 | 9,473 | 0.008 | 41.6 | 35.8 | 0.025 |
| Intermuscular fat | 13,872 | 12,109 | 0.256 | 40.0 | 45.9 | 0.014 |
| Thoracic cavity fat | 615 | 554 | 0.805 | 1.75 | 2.12 | 0.598 |
| Visceral cavity fat | 1,309 | 1,830 | 0.092 | 3.78 | 6.94 | 0.003 |
| Perirenal fat | 4,455 | 2,419 | 0.002 | 12.82 | 9.23 | 0.006 |
| Bone, g | ||||||
| Total bone | 22,975 | 21,675 | 0.244 | ― | ― | ― |
| Vertebrae cervicales | 1,517 | 1,494 | 0.813 | 6.60 | 6.93 | 0.191 |
| Vertebrae thoracicae | 2,173 | 2,063 | 0.643 | 9.43 | 9.59 | 0.859 |
| Vertebrae lumbales | 1,194 | 1,039 | 0.109 | 5.20 | 4.81 | 0.184 |
| Coastae | 4,345 | 4,004 | 0.134 | 18.94 | 19.02 | 0.852 |
| Scapula | 1,259 | 1198 | 0.303 | 5.48 | 5.67 | 0.209 |
| Humerus | 2,121 | 1,971 | 0.137 | 9.24 | 9.30 | 0.769 |
| Radius | 1,535 | 1,512 | 0.785 | 6.69 | 6.80 | 0.358 |
| Ossa carpi | 299 | 279 | 0.390 | 1.30 | 1.29 | 0.925 |
| Os femoris | 2,820 | 2,637 | 0.381 | 12.25 | 12.63 | 0.403 |
| Tibia | 1,752 | 1,671 | 0.375 | 7.62 | 7.88 | 0.079 |
| Patella | 186 | 300 | 0.399 | 0.81 | 1.42 | 0.356 |
| Os coxae | 2,956 | 2,718 | 0.105 | 12.89 | 12.93 | 0.925 |
| Others | 818 | 504 | 0.083 | 3.56 | 1.73 | 0.066 |
| Bone mineral density4,g/cm2 | 0.701 | 0.705 | 0.830 | - | - | - |
1EHN, metabolic imprinting group, n = 5. RG, roughage group: n = 5. 2Left half-carcass.
3Eighteen muscles weight in left half-carcass.
4Evaluated from Os femoris. Ratio of each weight to the total weight of half-carcass.
Comparison of individual muscle, fat depots, and bone weight between EHN and RG groups
The total weight of the muscles of the left half of the carcass did not differ between EHN and RG carcasses (P = 0.065, Table 4). Only 5 of the 19 muscles investigated (semimembranosus, P = 0.036; infraspinatus, P = 0.024; supraspinatus, P = 0.0019; serratus ventralis cervicis, P = 0.032; serratus ventralis thoracis, P =0.027) were heavier in EHN carcasses (Table 4). When individual muscle weight was normalized to total muscle weight, just 2 muscles (semitendinosus and serratus ventralis thoracis) were heavier in the EHN carcasses (P = 0.019 and P = 0.041, respectively; Table 4).
Total fat weight in the left half of the carcass was 30% greater in the EHN group (P = 0.025, Table 4). The weights of the subcutaneous and perirenal fat were 53% (P = 0.008) and 84% (P = 0.002) greater, respectively, in EHN carcasses. Calculated subcutaneous:total fat and perirenal:total fat weights were greater in EHN carcasses (P = 0.025 and P = 0.006, respectively) and lower for intermuscular: total fat and visceral cavity:total fat (P = 0.014 and P = 0.003, respectively). By contrast, no differences in bone weight (P = 0.244) or bone mineral density (P =0.830) were found (Table 4). Thus, there was a higher fat percentage in subcutaneous and perirenal fat of EHN carcasses with the tendency of a smaller percentage of the visceral cavity (P = 0.092) in EHN carcasses.
General carcass composition
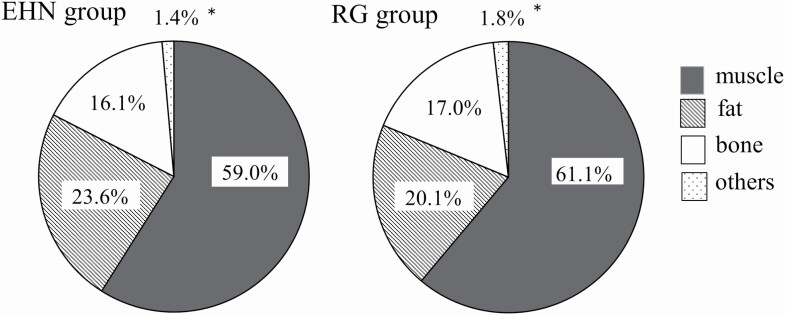
Regarding general carcass composition (Figure 4), no significant differences in the percentages of muscle, fat, and bone were observed; however, the absolute amount of fat in the carcass (Table 4) was larger (P = 0.025) in EHN carcasses.
Figure 4.
Carcass composition at slaughter. EHN: metabolic imprinting group, n = 5. RG: roughage group, n = 5. These data were obtained using half-carcasses. *Significant difference of percentage of others (like tendons and larger blood vessels, etc. except for muscle, fat, and bone in half carcass) between among EHN and RG groups (P < 0.05).
Discussion
In this study, we compared the performance of calves that were intensively fed milk replacer for 3 mo and then fed a high-concentrate diet for 7 mo with that of calves raised in a grass-based feeding system. This schedule was selected because the effects of neonatal metabolic imprinting events are thought to continue until weaning (Vickers et al., 2008). Metabolic programming (or imprinting) is characterized by (1) a susceptibility limited to a critical ontogenic window early in development; (2) a persistent effect lasting through to adulthood; (3) a specific and measurable phenotypic outcome that may differ quantitatively among individuals; and (4) a dose–response or threshold relationship between a specific exposure and outcome (Waterland and Garza 1999). In this study, a different plane of nutrition from birth to weaning affected body weight and IMF of the LM in Wagyu steers fattened with only grass. An absence of EHN growth, as measured by body weight, occurred from 11 mo to 14 mo of age revealing a shortcoming with the management system. It is likely that the EHN steers did not adapt readily to their change in diet, which was more extreme than for the steers in the RG group. Depressed growth in the EHN steers likely reflects a change in the ruminal microbe populations that allows for digestion of the roughage-based diet (Brown et al., 2006; Chen et al., 2011). Potentially, this could be alleviated by gradually decreasing the level of concentrate being fed during the transition period back to a hay-based diet.
In this study, serum IGF-I levels were greater in the EHN group than in the RG group until 14 mo of age followed by a decline in serum content. IGF-I is the primary mediator of many of the responses regulated by growth hormone (GH) in tissues throughout the body, and is generally secreted by the liver as a result of stimulation by GH from the pituitary. It affects most cells, but functions especially in the muscle and bone (Cohen et al., 1991). Matsuzaki et al. (2001) reported that the crude protein intake from an experimental diet influenced the plasma IGF-I level in Holstein cattle. In this study, the lower protein level of roughage compared with that of milk replacer and concentrate during the suckling period may have resulted in the reduced level of IGF-I during the fattening period.
Adipose tissue is not only an energy storage site but also an endocrine organ. Adipocytes secrete adipokines (Avram et al., 2007) that control energy homeostasis (Trujillo and Scherer, 2006). Japanese Black cattle have a greater ability to accumulate intramuscular adipocytes than European cattle (Gotoh et al., 2009). Most adipocytes form during the fetal and early postnatal stages of development, and adipocyte hyperplasia in perirenal fat is complete at birth (Du et al., 2010b). Du et al. (2013) suggested that the marbling process occurs from late gestation until about 250 d of age in beef cattle. Goessling et al. (2009) suggested that the total number of adipocytes is set by adolescence. However, a visceral depot of adipocytes forms from mid-gestation to the early postnatal period (Robelin, 1981). Several studies have identified the time from early weaning until approximately 250 d of age as the “marbling window.” In this study, early higher fat and protein nutrition with milk replacer strongly affected the accretion of IMF in the longissimus, along with higher levels of accumulation of subcutaneous and perirenal fat.
In cattle, early nutrition influences carcass composition and meat quality. The growth performance and carcass quality of Holstein bulls fed varying amounts of milk replacer over an extended period (to 200 d of age) were investigated by Abdelsamei et al. (2005), which reported that feeding increased amounts of milk replacer shortened the fattening period to achieve the same body weight by accelerating growth, and as a final result IMF content did not decrease at slaughter. These results suggest that the quality and quantity of milk replacer during the suckling stage, and the diet during the rearing stage, significantly influence growth. Scheffler et al. (2014) reported that early weaning and high-concentrate feeding for 148 d, followed by grazing and feedlot entry, did not improve feedlot performance. However, this management scheme did improve the marbling score and increased the hot carcass weights compared with those of conventionally weaned (around 253 ± 6 d of age) and backgrounded calves, although no significant differences in lipid content were found between the conventionally weaned (4.5%) and early weaned-imprinted groups (6.0%). In this study, neonatal metabolic imprinting events, including feeding a greater amount of high-protein and -fat milk replacer until 3 mo of age, had strong impacts on body weight, carcass weight, muscle composition of the carcass, adipose tissue weight, and IMF content in Japanese Black cattle fattened on a roughage diet. Our results mimic those reported in mice (Waterland and Graza, 1999; Gluckman et al., 2008), fed a high-fat diet during suckling that subsequently develop obesity as adults.
Daily body weight gain of crossbred steers (Angus × Simmental) fed a 50% grain diet ad libitum was significantly higher than that of steers fed a restricted amount of 50% grain diets or a large amount of forage during a specific stage of early development (119–218 d) (Schoonmaker et al., 2003; 2004). Consequently, the former carcasses contained larger subcutaneous fat layers and greater IMF content. In contrast, the steers with restricted feed intake during a specific stage of early development had leaner carcasses with less IMF, as well as poorer growth performance (Myers et al., 1999a,b; Schoonmaker et al., 2002). Although these previous reports suggest that nutritional conditions during early development influence growth performance during fattening and skeletal muscle characteristics at the time of slaughter, our results indicate that nutritional influences during the suckling period may have even greater effects on later growth in beef cattle.
Conclusion
The results of this study suggest that an early high plane of nutrition significantly affects the growth and body composition of Wagyu steers. Additionally, these findings indicate that nutritional conditions during the suckling period can strongly affect growth performance during later development stages. The imprinting phenomenon suggests that there is a unique biological mechanism that allows early-life nutrition to have long-lasting effects on phenotype.
Acknowledgements
This work was supported by Kakenhi (grant nos. 20380150, 25292162, 26310312, and 19KT0013) from Japan Society for the Promotion of Science, Young Researcher Overseas Visit Program for Vitalizing Brain Circulation (grant no. S2305) from Japan Society for the Promotion of Science, and Kyushu University P&P grant. The authors wish to thank Tetsuji Etoh, Yuji Shiotsuka, Shuichi Kaneda, and Hiroyuki Hasebe for their technical assistance. The authors also thank Edanz (https://en-author-services.edanzgroup.com/ac) for editing the English text of a draft of this manuscript.
Glossary
Abbreviations
- ADG
average daily gain
- BCS
beef color standard
- BFS
beef fat standard
- BMS
beef marbling score
- BW
body weight
- GH
growth hormone
- HCW
hot carcass weight
- IGF-I
insulin-like growth factor-I
- IMF
intramuscular fat
- LM
longissimus muscle
- TDN
total digestible nutrients
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abdelsamei, A. H., Fox D. G., Tedeschi L. O., Thonney M. L., Ketchen D. J., and Stouffer J. R.. . 2005. The effect of milk intake on forage intake and growth of nursing calves. J. Anim. Sci. 83: 940– 947. doi: 10.2527/2005.834940x. [DOI] [PubMed] [Google Scholar]
- AOAC. 2000. Official methods of analysis (17th ed.). Washington, DC: Association of Official Agricultural Chemists. [Google Scholar]
- Avram, M. M., Avram A. S., and James W. D.. . 2007. Subcutaneous fat in normal and diseased states 3. Adipogenesis: From stem cell to fat cell. JAAD. 56: 472–492. doi: 10.1016/j.jaad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Barker, D. J., Eriksson J. G., Forsen T., and Osmond C.. . 2002. Fetal origins of adult disease: strength of effects and biological basis. Int. J. Epidemiol. 31: 1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Brown, M. S., Ponce C. H., and Pulikanti R.. . 2006. Adaptation of beef cattle to high-concentrate diets: Performance and ruminal metabolism. J. Anim. Sci. 84: E25–E33. doi: 10.2527/2006.8413_supple25x. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Penner G. B., Li M., Oba M., and Guan L. L.. . 2011. Changes in bacterial diversity associated with epithelial tissue in the beef cow rumen during the transition to a high-grain diet. Appl. Environ. Microbiol. 77: 5770–5781. doi: 10.1128/AEM.00375-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M., Figueroa A. A., Haviv Y., Schafer M. E., and Aduss H.. . 1991. Iliac versus cranial bone for secondary grafting of residual alveolar clefts. Plast. Reconstr. Surg. 87: 423–427. [PubMed] [Google Scholar]
- Du, M., Huang Y., Das A. K., Yang Q., Duarte M. S., Dodson M. V., and Zhu M. J.. . 2013. Meat science and muscle biology symposium: Manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle. J. Anim. Sci. 91: 1419–1427. doi: 10.2527/jas.2012-5670. [DOI] [PubMed] [Google Scholar]
- Du, M., Tong J., Zhao J., Underwood K. R., Zhu M., Ford S. P., Nathanielsz P. W.. . 2010a. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 88: E51–E60. doi: 10.2527/jas.2009-2311. [DOI] [PubMed] [Google Scholar]
- Du, M., Yan X., Tong J. F., Zhao J. X., and Zhu M. J.. . 2010b. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol. Reprod. 82: 4–12. doi: 10.1095/biolreprod.109.077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman, P. D., Hanson M. A., Cooper C., and Thornburg K. L.. . 2008. Effect of in utero and early-life conditions on adult health and disease. N. Eng. J. Med. 359: 61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling, W., T. E. North, S. Loewer, A. M. Lord, S. Lee, C. L. Stoick-Cooper, G. Weidinger, M. Puder, G. Q. Daley, R. T. Moon, et al. 2009. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 136(6): 1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh, T., Albrecht E., Teuscher F., Hawabata K., Sakashita K., Iwamoto H., and Wegner J.. . 2009. Differences in muscle and fat accretion in Japanese Black and European cattle. Meat.Sci. 82: 300–308. doi: 10.1016/j.meatsci.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Gotoh, T., Takahashi H., Nishimura T., Kuchida K., and Mannen H.. . 2014. Meat produced by Japanese Black cattle and Wagyu. Anim. Front. 4: 46–54. doi: 10.2527/af.2014-0033. [DOI] [Google Scholar]
- Heinrichs, A. J., and Heinrichs B. S.. . 2010. A prospective study of calf factors affecting first-lactation and lifetime milk production and age of cows when removed from the herd. J. Dairy.Sci. 94: 336–341. doi: 10.3168/jds.2010-3170. [DOI] [PubMed] [Google Scholar]
- Honikel, K. O. 1987. How to measure the water-holding capacity of meat? recommendation of standardized methods. In: Tarrant, P. V., G. Eikelenboom, and G. Monin, editors. Evaluation and control of meat quality in pigs. Dordrecht: Springer. (Current Topics in Veterinary Medicine and Animal Science; vol. 38). [Google Scholar]
- Japan Meat Grading Association (JMGA). 1988. Beef Carcass Trading Standards (approved by the Ministry of Agriculture, Forestry, and Fisheries: No. 747, Animal Products 1988-A). Tokyo, JMGA. [Google Scholar]
- Japan Meat Grading Association. 2014. Beef Carcass Trading Standards (revised version). Tokyo, JMGA. [Google Scholar]
- Kanda, Y. 2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. BMT. 48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. A., Weary D. M., and von Keyserlingk M. A. G.. . 2010. Invited review: Effects of milk ration on solid feed intake, weaning, and performance in dairy heifers. J. Dairy Sci. 94:1071–1081. doi: 10.3168/jds.2010-3733. [DOI] [PubMed] [Google Scholar]
- Mandell, I. B., Buchanan-Smith J. G., and Campbell C. P.. . 1998. Effects of forage vs grain feeding on carcass characteristics, fatty acid composition, and beef quality in Limousin-cross steers when time on feed is controlled. J. Anim. Sci. 76: 2619–2630. doi: 10.2527/1998.76102619x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki, M., Sato T., Morita S., Shiba N., Tsuneishi E., Hara S., Ozutsumi K., and Yamaguchi T.. . 2001. Pulsatile growth hormone secretion, circulating insulin-like growth factor-1 concentration and cellular density of somatotrophs differ between Wagyu and Holstein steers. Tohoku J. Animal Sci. Technol. 48: 7–13. doi: 10.1017/S1357729800058392. [DOI] [Google Scholar]
- Myers, S. E., Faulkner D. B., Ireland F. A., Berger L. L., and Parrett D. F.. . 1999b. Production systems comparing early weaning to normal weaning with or without creep feeding for beef steers. J. Anim. Sci. 77: 300–310. doi: 10.2527/1999.772323x. [DOI] [PubMed] [Google Scholar]
- Myers, S. E., Faulkner D. B., Ireland F. A., and Parrett D. F.. . 1999a. Comparison of three weaning ages on cow–calf performance andsteer carcass traits. J. Anim. Sci. 77:323–329. doi: 10.2527/1999.772300x. [DOI] [PubMed] [Google Scholar]
- Provenza, F.D., Kronberg S.L. and Gregorini P.. . 2019. Is grassfed meat and dairy better for human and environmental health? Front. Nutr. 6:1–7. doi: 10.3389/fnut.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robelin, J. 1981. Cellularity of bovine adipose tissues: developmental changes from 15 to 65 percent mature weight. J. Lipid. Res. 22: 452–457. doi: 10.1016/S0022-2275(20)34959-2. [DOI] [PubMed] [Google Scholar]
- Schoonmaker, J.P., S. C. Loerch, F. L. Fluharty, H. N. Zerby, and T. B. Turner. 2002. Effect of age at feedlot entry on performance and carcass characteristics of bulls and steers. J. Anim. Sci. 80(9): 2247–2254. [DOI] [PubMed] [Google Scholar]
- Schoonmaker, J. P., Cecava M. J., Faulkner D. B., Fluharty F. L., Zerby H. N. and Loerch S. C.. . 2003. Effect of source of energy and rate of growth on performance, carcass characteristics, ruminal fermentation, and serum glucose and insulin of early-weaned steers. J. Anim. Sci. 81: 843–855. doi: 10.2527/2003.814843x. [DOI] [PubMed] [Google Scholar]
- Schoonmaker, J. P., Fluharty F. L. and Loerch S. C.. . 2004. Effect of source and amount of energy and rate of growth in the growing phase on adipocyte cellularity and lipogenic enzyme activity in the intramuscular and subcutaneous fat depots of Holstein steers. J. Anim. Sci. 82: 137–148. doi: 10.2527/2004.821137x. [DOI] [PubMed] [Google Scholar]
- Scheffler, J. M., McCann M. A., Greiner S. P., Jiang H., Hanigan M. D., Bridges G. A. and Gerrard D. E.. . 2014. Early metabolic imprinting events increase marbling scores in fed cattle. J. Anim. Sci. 92: 320–324. doi: 10.2527/jas.2012-6209. [DOI] [PubMed] [Google Scholar]
- Sitz, B. M., Calkins C. R., Feuz D. M., Umberger W. J., and Eskridge K. M.. . 2005. Consumer sensory acceptance and value of domestic, Canadian, and Australian grass-fed beef steaks. J. Anim. Sci. 83: 2863–2868. doi: 10.2527/2005.83122863x. [DOI] [PubMed] [Google Scholar]
- Tsuji, S. 1982. Texture profile analysis of processed foods using the Tensipresser and the multi-point mensuration method. J. Texture Study. 13:135–186. doi: 10.1111/j.1745-4603.1982.tb01393x. [DOI] [Google Scholar]
- Trujillo, M. E., and Scherer P. E.. . 2006. Adipose tissue-derived factors: impact on health and disease. Endocr. Rev. 27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- Van Amburgh, M., and Lopez D. J.. . 2013. A brief review of the developmental role of colostrums in neonates. Paper presented at The Minnesota Dairy Health Conference.University of Minnesota, Minneapolis, Minnesota, 21–23 May; p. 12– 18. https://hdl.handle.net/11299/141760. [Google Scholar]
- Vickers, M. H., Gluckman P. D., Coveny A. H., Hofman P. L., Cutfield W. S., Gertler A., Breier B. H., and Harris M.. . 2008. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology 149:1906–1913. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- Waterland, R. A., and Garza C.. . 1999. Potential mechanisms of metabolic imprinting that lead to chronic disease. Am. J. Clin. Nutr. 69: 179–197. doi: 10.1093/ajcn/69.2.179. [DOI] [PubMed] [Google Scholar]
- Wertz, A. E., Berger L. L., Walker P. M., Faulkner D. B., McKeith F. K., and Rodriguez-Zas S. L.. . 2002. Early-weaning and postweaning nutritional management affect feedlot performance, carcass merit, and the relationship of 12th-rib fat, marbling score, and feed efficiency among Angus and Wagyu heifers. J. Anim. Sci. 80: 28–37. doi: 10.2527/2002.80128x. [DOI] [PubMed] [Google Scholar]