Abstract
SARS-CoV-2 testing data in North Carolina during the first three months of the state's COVID-19 pandemic were analyzed to determine if there were disparities among intersecting axes of identity including race, Latinx ethnicity, age, urban-rural residence, and residence in a medically underserved area. Demographic and residential data were used to reconstruct patterns of testing metrics (including tests per capita, positive tests per capita, and test positivity rate which is an indicator of sufficient testing) across race-ethnicity groups and urban-rural populations separately. Across the entire sample, 13.1% (38,750 of 295,642) of tests were positive. Within racial-ethnic groups, 11.5% of all tests were positive among non-Latinx (NL) Whites, 22.0% for NL Blacks, and 66.5% for people of Latinx ethnicity. The test positivity rate was higher among people living in rural areas across all racial-ethnic groups. These results suggest that in the first three months of the COVID-19 pandemic, access to COVID-19 testing in North Carolina was not evenly distributed across racial-ethnic groups, especially in Latinx, NL Black and other historically marginalized populations, and further disparities existed within these groups by gender, age, urban-rural status, and residence in a medically underserved area.
Keywords: SARS-CoV-2, COVID-19, Public health surveillance, Healthcare disparity, Rural communities, North Carolina
1. Introduction and background
The COVID-19 pandemic is one of the most serious global public health threats in over a century. As of April 2021, more than 30 million Americans have been infected, resulting in more than 550,000 deaths (Johns Hopkins University, 2020a). Yet these statistics, which are largely based on routine reporting from state and territorial health departments, likely underestimate the true incidence of infection and burden of disease (Sun and Achenbach, 2020).
Infection with SARS-CoV-2, the virus that causes COVID-19 disease, is persistently underestimated because a relatively large proportion of infections are asymptomatic or mild, and therefore do not prompt care seeking, testing, and reporting (Bai et al., 2020; Chan et al., 2020; Danis et al., 2020). In addition, the well-documented technical and regulatory missteps associated with implementing a novel diagnostic testing infrastructure early in the pandemic response limited the number of symptomatic individuals tested (Shear et al., 2020). Yet even today, shortages of personal protective equipment, specimen collection kits, and test reagents limit test availability and access, especially in historically marginalized communities (Food and Drug Administ, 2021). Understanding the course of surveillance infrastructure early in the pandemic may provide insights into whether there were systematic disparities in testing especially in marginalized populations at higher risk of contracting COVID-19.
Evaluating testing patterns is critical to interpreting the available surveillance estimates and developing an effective and equitable public health response (Levesque et al., 2013). Understanding the rate of testing in different populations allows public health practitioners and researchers to contextualize whether the COVID-19 case rates and percent positivity rates that are measured by the available testing infrastructure are more likely to be overestimating or underestimating the actual disease incidence within a given population at a particular time in the pandemic (Goldstein and Burstyn, 2020; Johns Hopkins University, 2020b). This information is increasingly important as more evidence emerges highlighting the stark disparities in COVID-19 disease incidence and outcomes among historically marginalized communities (Gold et al., 2020; Menachemi et al., 2020) due to high representation in occupations with high COVID-19 incidence (Wortham et al., 2020; Bureau of Labor Statistics, 2019; Gennetian and Johnson, 2020; Hawkins, 2020a), barriers to accessing primary care (CDC, 2020), and other structural factors (Webb Hooper et al., 2020; Cowger et al., 2020; Sewell, 2016; Gee and Ford, 2011). There are higher COVID-19 prevalence rates and higher mortality rates among Black and Latinx populations in the United States (Gold et al., 2020; Menachemi et al., 2020; Webb Hooper et al., 2020; Cowger et al., 2020). Additionally, there is evidence that while urban areas generally have shown higher documented rates of COVID-19 incidence, the disease spread rapidly from urban to rural areas, which may have a weaker public health infrastructure and lower healthcare capacity (Paul et al., 2020; Peters, 2020). While previous studies have documented demographic and urban-rural disparities in COVID-19 disease outcomes (i.e. mortality) (Paul et al., 2020; Peters, 2020), there is less known about disparities in the underlying testing rates that inherently shape metrics of COVID-19 disease dynamics, such as percent test positivity and cases per capita.
Insights about the spatial and demographic patterns in testing and test positivity within North Carolina during the pandemic are likely generalizable outside the state. North Carolina is the ninth most populous state in the United States (US), with characteristics that are relevant to other states and regions of the country (American Community Survey, 2019). For example, while the state is home to rapidly growing urban centers such as Charlotte and Raleigh, it also has a large rural population (Tippett, 2016) with more people living in rural areas than any state besides Texas (American Community Survey, 2019). Rural health systems in North Carolina and nation-wide have suffered from hospital closures (Tochik et al., 2020) and persistence of medically underserved area (MUA) designation (Tochik et al., 2020; HRSA, 2021). North Carolina also has the second-largest meat and food processing industry in the US (National Agriculture Statistics Service, 2019), occupations that have been linked with focal COVID-19 outbreaks (Waltenburg et al., 2020; Dyal et al., 2020). Lastly, while the majority of the North Carolina population is White, the state ranks sixth and eleventh among all US states in Black and Latinx population size, respectively (American Community Survey, 2019). Because many published studies of disparities in COVID-19 testing have focused on urban centers in Northeastern areas of the US (Lieberman-Cribbin et al., 2020; Hawkins, 2020b; Pflugeisen and Mou, 2021), this study's state-wide analysis of a large, diverse state in the south is an important contribution to understanding the public health response throughout the country.
Given the importance of testing in addressing the COVID-19 pandemic, the primary objective of this study was to describe the spatial and temporal distribution of testing and to determine whether racial, ethnic, and urban-rural disparities in testing were apparent during the first three months of the pandemic: March to June 2020. In North Carolina, this time period covers the most restrictive public health orders, including the governor's stay-at-home order (NC Executive Order No. 121, 2020) and Phase 1 reopening for businesses (NC Executive Order No. 138, 2020); therefore, this period is characterized by the greatest departure from “business-as-usual” for population movement and social interaction. By focusing on disparities in testing during the on-ramp period to full testing capacity, we establish a baseline of systematic differences in testing access for different populations which can inform our understanding of the pandemic's infection trajectory and underscores how such disparities arise from systematic influences beyond the scope of public health infrastructure alone. Additionally, we examine intersectional differences within race-ethnic groups by age, gender, urban-rural status, and medically underserved status in order to understand further disparities within race-ethnicity groups across overlapping demographic and geographic categories. Finally, we examine overall spatial patterns of testing and test positivity in North Carolina at the finest available spatial resolution, the ZIP code tabulation area (ZCTA), and explore how these spatial patterns differ by race and ethnicity.
2. Data and methods
2.1. Data
2.1.1. COVID-19 testing and case data
The data used in this study are the official testing and case counts of COVID-19 from January 1st to June 1st, 2020 reported to the North Carolina Department of Health and Human Services (NCDHHS). The testing and case data include information about SARS-CoV-2 real-time reverse transcription polymerase chain reaction (RT-PCR) testing including the date of test administration, date of test result, and test result. Each RT-PCR test identified the presence or absence of the SARS-CoV-2 virus; however, since this process is commonly called COVID-19 testing, we will refer to all tests as “COVID-19 tests” and all tests that were positive for SARS-CoV-2 virus as “COVID-19 cases” moving forward. Additionally, each test observation included self-reported information about the age, gender, race, Hispanic ethnicity, ZIP code, and county of residence of the individual tested.
The raw data set for COVID-19 tests through June 1st contained 311,712 observations. The first test was conducted on January 2nd, the first test with a positive result was conducted on March 2nd, and that first positive result was reported on March 3rd. There were only 16 tests conducted from January 2nd to March 1st. Records that were missing ZIP code information or reported ZIP codes outside of North Carolina were excluded (N = 14,385, 4.6%). We also excluded observations that were missing the test collection date or were coded as COVID-19 deaths in the absence of any performed tests, which excluded another 1528 observations (0.5%) from the analysis. In total, 295,642 (94.7%) test observations were used in this analysis, representing 262,215 unique individuals. In analyses, cumulative incidence measures are calculated using the unique individual data only, while temporal trends in rates of testing are calculated using all test observations to capture disparities in access to repeated testing during the study period.
The NCDHHS categories for race and ethnicity correspond to the United States Census Bureau race and ethnicity categories (race: Black, White, American Indian, Asian, Native Hawaiian or Pacific Islander, Other (which refers to some other race not listed as an option for selection); ethnicity: Hispanic, Non-Hispanic), which are limited proxy categories for racial and ethnic identification (Williams et al., 2010). We use the term Latinx throughout to refer to individuals who indicated “Hispanic” in the NCDHHS testing database because the most common countries of origin for individuals who identify as either Hispanic or Latinx in North Carolina are Mexico or Central American countries (Tippett, 2019). We re-categorized race and ethnicity from the NCDHHS data into a collapsed race-ethnicity variable containing the following mutually exclusive categories: Latinx, Non-Latinx (NL) Black, NL White, NL American Indian, NL Asian, and NL Other. We examined these race and ethnic categories because the social construct of race relates to social history, social stratification, structural racism, and marginalization, which have been cited as factors that have contributed to the unequal burden of exposure to SARS-CoV-2, access to healthcare, and medical treatment inequities by race in the US. (Boyd et al., 2020; Bailey and Moon, 2020; McClure et al., 2020) Latinx ethnicity refers to factors such as shared language, religion, cultural characteristics, history, and other sociocultural measures (Meer, 2014). The Latinx category in this study included individuals that could have self-identified as any one of the available race categories described above or no race category in the NCDHHS data. Of those self-identifying as Latinx, 87.5% also reported race (45.9% White, 4.5% Black, 0.2% American Indian, 0.2% Asian, and 36.5% Other), while 12.5% (1914/15,341) of those with Latinx ethnicity had missing data for race.
There was some missingness in the data for several demographic characteristics. Race and Latinx ethnicity data have the highest rates of missingness. For race data, 138,228 (46.8%) observations have missing information; for Latinx ethnicity, 185,430 (59.4%) of observations have missing information. Observations with Unknown/Missing in both the race and ethnicity variables are counted as unknown/missing in our collapsed race-ethnicity variable (N = 136,314; 46.1%). Observations with known race data that were recorded either as non-Latinx ethnicity or Missing/Unknown ethnicity were recorded as non-Latinx and their self-identified race. Any observation with known Latinx ethnicity was recorded as Latinx. Other demographic characteristics have lower rates of missingness: 1989 (0.67%) of observations are missing gender information, and only 59 observations are missing age information. People with missing race-ethnicity information were younger, with a median age of 46 compared to 51 years for those with race-ethnicity data. Those with unknown race-ethnicity were also more likely to live in rural areas: 32.5% of observations with missing race-ethnicity were rural, compared to 25.3% for observations in urban areas.
2.1.2. Ancillary data
ZIP codes were the finest resolution geographic unit available in the NCDHHS data and were used to facilitate geographic analyses. Based on the ZIP code, each test observation was matched to a ZIP code tabulation area (ZCTA), which are generalized areal units used by the US Census Bureau to tabulate population data in the decennial census and intercensal American Community Survey (ACS) data (American Community Survey, 2019; ZIP Code to ZCTA Crosswalk, 2020; ZIP Code Tabulation Areas, 2020). The US Census Bureau reports population estimates by ZCTA and provides geographic data about the proportion of the population within each ZCTA that is classified as urban and rural. We classified each ZCTA in North Carolina as rural if 50.0% or more of the population of a given ZCTA lived in an area designated as rural by the U.S. Census Bureau; ZCTAs were classified as urban if less than 50.0% of the population lived in a rural area. This threshold was selected based on the US Census Bureau standard for defining administrative areas as either urban or rural (Ratcliffe et al., 2016). We then used the ZCTA urban-rural designation to assign urban or rural status to each testing observation based on the ZIP code of residence reported in the NCDHHS database.
ZIP code of residence was also used to identify observations that were located in a medically underserved area (MUA). MUAs are characterized by a lack of access to primary care and are federally designated based on a composite index of four criteria: the ratio of primary care physicians to residents, infant mortality rate, high poverty, and large elderly population (HRSA, 2021). While there is much overlap between the designated MUAs and many rural health systems, as rural health systems are strained from a shortage of personnel and an aging patient population in recent years (Tochik et al., 2020; HRSA, 2021), several urban areas are also designated as MUAs. For this reason, we include residence in an MUA as a separate stratification in this analysis to investigate the potential association between COVID-19 testing access, and residence in an MUA in both urban and rural areas. Observations were defined as being located in an MUA if the majority (>50%) of the ZCTA of residence was within the boundaries of an MUA based on a spatial overlay operation.
The 2014–2018 ACS 5-year estimates population estimates were used as population denominators for all analyses; 2014–2018 ACS 5-year estimates were the most up-to-date data available at the time of analysis (American Community Survey, 2019). When available, data on demographic intersections (i.e., race by age, gender) were also obtained from the 2014–2018 ACS 5-year estimates (Table B03002 for race by ethnicity, and Table B01001 including sub-tables for each race and ethnicity by age). 2018 US Census Bureau Tiger files were used for standardized areal units for mapping (American Community Survey, 2019).
2.2. Analytical approach
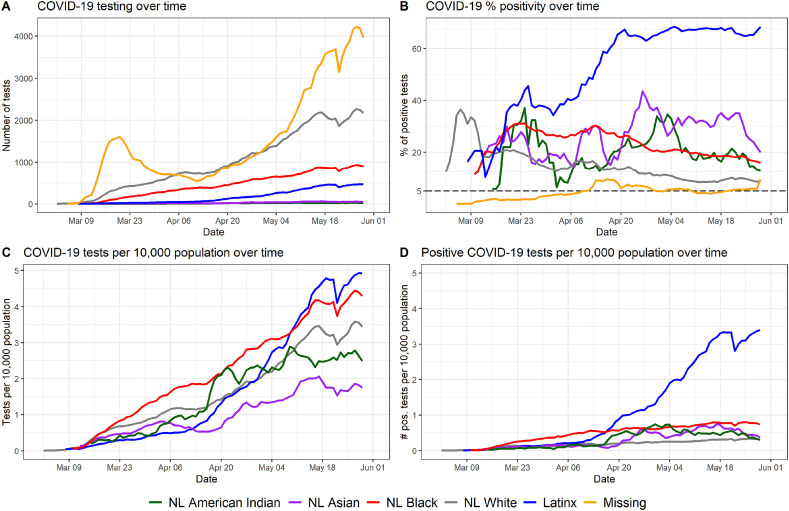
To examine disparities in testing during the first three months of the COVID-19 pandemic, we created time series of testing by race-ethnicity, focusing on four interrelated metrics: the number of tests, number of tests per capita, positive tests per capita, and percentage of positive tests (also referred to as the test percent positivity rate). We used these metrics to demonstrate the trajectory of testing in different ways; first, the volume of testing trajectory as a simple number to illustrate testing infrastructure is shown in a way commonly visualized on COVID-19 data dashboards that are widely available to the public (Johns Hopkins University, 2020b; NCDHHS, 2021). The volume of tests is then normalized by population to compare testing access by population (i.e., rate of testing per capita). Finally, we compare disparities in test detection to disparities in test positivity by calculating the rate of positive tests per capita. We calculated the test percent positivity rate over time for each race-ethnicity group as this is a key metric for understanding community spread of COVID-19 (WHO, 2020). We use all test observations for all four rolling metrics because it illustrates how certain race-ethnicity groups may have more access to tests and get repeat testing in the three month period (NCDHHS, 2021). For number of tests, number of tests per capita, and positive tests per capita, we calculated a centered rolling 7-day average to examine temporal trends. For the test percent positivity rate, we calculated the daily percentages based on the rolling average of positive tests divided by the number of tests for each day. We use this rolling-average approach to account for variation in testing availability and test result reporting between weekdays and weekends. We also compare the daily test positivity rate against a threshold of 5% as a sustained test positivity rate of 5% or lower is recommended to ensure community spread is being detected (WHO, 2020) (Fig. 1, Fig. 2 ). We assessed statistical differences between race-ethnicity groups for each of the four metrics for the entire study period using Pearson's Chi-square tests.
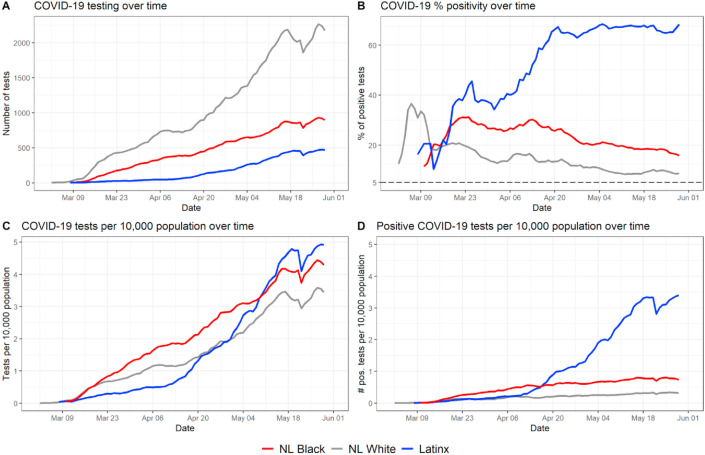
Fig. 1.
Temporal trend in testing and percent positive tests by race-ethnicity. The three largest race-ethnicity groups in North Carolina are represented here (NL White, NL Black, and Latinx). Fig. S1 in the Supplement shows the same metrics with all race-ethnicity categories represented in the data.
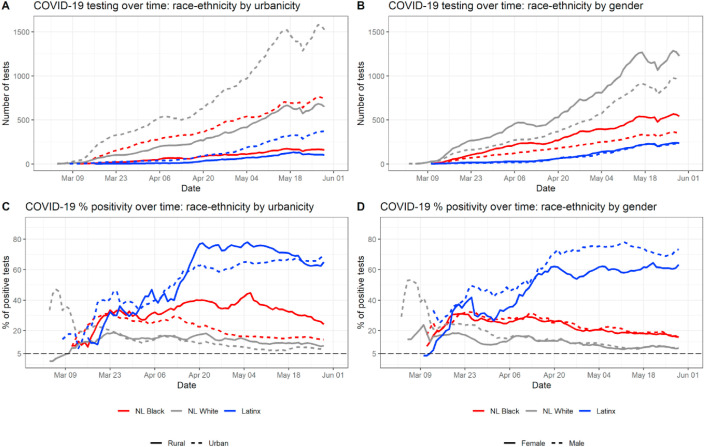
Fig. 2.
Temporal trend in testing and percent positive tests within race-ethnicity groups by urban-rural status and gender. These are broken down by urban-rural (left column) and by gender (right column) subgroups.
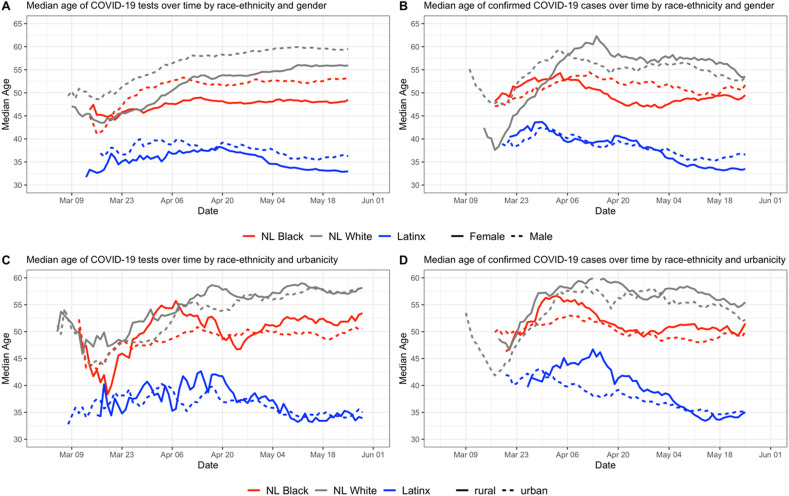
Additionally, we conducted within-group analyses to examine intersectional relationships between race-ethnicity and age, gender, urban-rural status, and residence in a medically underserved area (MUA). For these within-group analyses, we focus on the three largest race-ethnic groups in North Carolina, which account for over 95% of the population. According to the most current available estimates from the US Census Bureau (American Community Survey, 2019), the population of North Carolina is 64.8% NL White, 21.6% NL Black, and 9.4% Latinx; all other race-ethnic groups account for 4.2% of the population. For the three largest race-ethnicity groups, within-group differences in testing and test positivity by gender, age, urban-rural status, and residence in an MUA were assessed using Chi-square tests and visualized using time series graphs. To describe age differences across time both between and within race-ethnicity groups, we created a panel of time-series graphs and related bar graphs. The time series graphs were created using a 14-day centered rolling average of median age (we chose 14 days instead of 7 days due to greater variability in age values) and highlight the changes in median age distribution of individuals tested and individuals who tested positive across each race-ethnicity group as the pandemic progressed and testing capacity increased. The bar graphs show distribution of individuals tested and those who tested positive per capita based on age distributions available in the 2014-18 ACS 5-year estimates survey Table B03002 (American Community Survey, 2019).
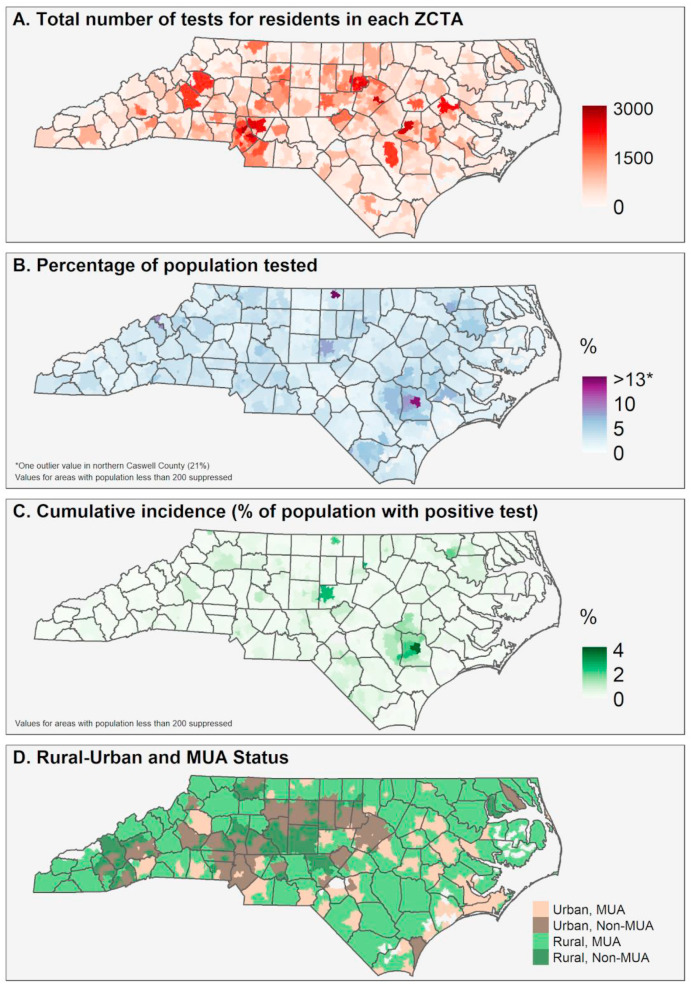
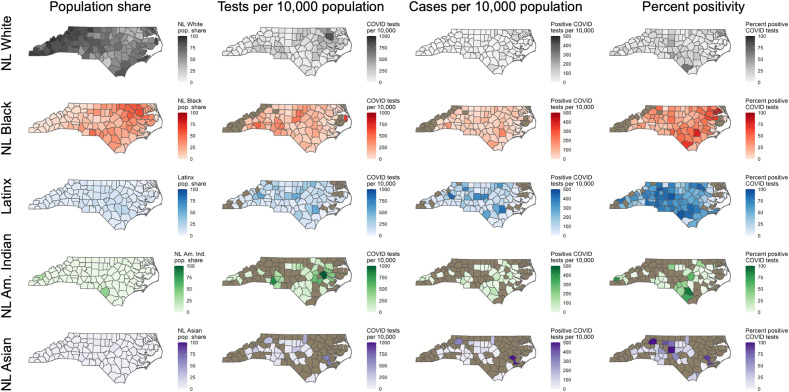
Maps were created at the finest available spatial resolution, the ZCTA level, of the total number of tests, percent of population ever tested, and cumulative incidence of COVID-19. To further examine geographic variation in COVID-19 testing and cases, we examined spatial patterns in tests per capita, cases per capita, and percent positivity by race-ethnicity. Testing metrics calculated at the ZCTA level included total tests for residents in the ZCTA, number of residents tested at least once, and cumulative incidence of COVID-19 for residents of the ZCTA. Because of identifiability concerns due to small numbers in some ZCTAs and variance instability concerns associated with small denominators, Empirical Bayesian smoothed values were calculated for total number ever tested and cumulative incidence; additionally, values in these maps (Fig. 4 B and C) were suppressed for ZCTAs with populations less than 200. Global Moran's I was calculated using first-order queen contiguity weights with an Empirical Bayes standardization adjustment was calculated to determine clustering of key testing metrics for the overall population at the ZCTA resolution. Because of small numbers in some jurisdictions, additional race-ethnicity stratified spatial patterns in testing and test positivity are presented at the county level, and data was suppressed for counties that had fewer than five tests among a given race-ethnic group and/or fewer than 100 residents of a given race-ethnicity group. All analyses use the dataset representing total test observations (N = 295,642) except the cumulative incidence of COVID-19 and cumulative incidence of individuals ever tested, which uses the dataset of unique individuals tested (N = 262,215). Global Moran's I was calculated using GeoDa 1.14 (Anselin et al., 2006) while all other analyses were conducted using R 4.0.1 (R Core Team, 2020).
Fig. 4.
Spatial patterns in testing and confirmed cases in North Carolina through June 1. Note: Empirical Bayesian smoothed values are mapped for Fig. 1B and C, to account for rates with small population denominators.
3. Results
3.1. Population characteristics
Table 1 shows the relative distribution of observations (total number of tests) and relative distribution of positive tests by sub-groups. Within each category (gender, age, race-ethnicity, urban-rural, and MUA residence), reading the columns vertically shows the relative share of tests or positive tests and percentage of observations (in parentheses) belonging to that subgroup. Table 2 compares test positivity rates, per capita testing rates, and positive test per capita rates for different age, urban-rural, gender, and MUA variables for entire study period stratified by race-ethnicity groups, and the right-hand column includes results of Chi-square tests for significance of difference between groups.
Table 1.
Characteristics of North Carolina residents in the NCDHHS database who were tested for COVID-19. Data includes tests in North Carolina from January 1 through June 1, 2020. Figures are numbers (percentages within each category) unless stated otherwise.
| Total number of tests (%) |
Positive tests (%) |
|
|---|---|---|
| N | 295,642 | 38,750 |
| Gender | ||
| Female | 172,529 (58.75) | 20,218 (52.72) |
| Male | 121,124 (41.25) | 18,134 (47.28) |
| Age | ||
| Under 18 | 19,408 (6.56) | 2339 (6.04) |
| 18-49 | 132,880 (44.95) | 20,366 (52.52) |
| 50-65 | 77,090 (26.08) | 9436 (24.37) |
| Over 65 | 66,264 (22.41) | 6609 (17.07) |
| Race-ethnicity | ||
| NL American Indian | 1338 (0.45) | 268 (0.69) |
| NL Asian | 2517 (0.85) | 743 (1.92) |
| NL Black | 40,640 (13.75) | 8939 (23.07) |
| NL Native Hawaiian/Pac. Islander | 191 (0.06) | 81 (0.21) |
| NL White | 93,045 (31.47) | 10,732 (27.69) |
| NL Other | 6256 (2.12) | 883 (2.28) |
| Latinx | 15,341 (5.19) | 10,207 (26.34) |
| Unknown/Missing | 136,314 (46.11) | 6897 (17.80) |
| Urban-rural | ||
| Urban | 211,218 (71.44) | 27,346 (70.57) |
| Rural | 84,424 (28.56) | 11,404 (29.43) |
| MUA Status | ||
| MUA | 132,804 (44.92) | 18,023 (46.51) |
| Non-MUA | 162,838 (55.08) | 20,727 (53.49) |
Table 2.
Comparison of percent test positivity, tests per capita, and positive tests per capita among the three main racial and ethnic groups in North Carolina. Data includes tests in North Carolina from January 1 through June 1, 2020, and percent of positive tests for each available demographic characteristic. Significance tests are calculated using Chi-Square tests to assess whether the observed differences among the three groups are different than expected.
| NL White | NL Black | Latinx | P-value | |
|---|---|---|---|---|
| Test percent positivity | ||||
| Overall | 11.53 | 22.00 | 66.54 | <.001 |
| Male | 12.01 | 22.76 | 72.57 | <.001 |
| Female | 11.10 | 21.43 | 60.49 | <.001 |
| 0-9 | 4.01 | 12.63 | 53.46 | <.001 |
| 10-19 | 10.02 | 21.99 | 68.69 | <.001 |
| 20-29 | 13.75 | 20.74 | 65.15 | <.001 |
| 30-44 | 11.94 | 22.51 | 70.64 | <.001 |
| 45-64 | 12.17 | 23.13 | 69.57 | <.001 |
| 65-84 | 9.73 | 21.19 | 48.29 | <.001 |
| 85+ | 16.36 | 31.29 | 38.89 | <.001 |
| Urban | 10.71 | 19.42 | 65.21 | <.001 |
| Rural | 13.51 | 34.00 | 70.45 | <.001 |
| MUA | 11.79 | 29.25 | 64.78 | <.001 |
| Non-MUA | 11.29 | 17.13 | 67.53 | <.001 |
| Tests per 10,000 | ||||
| Overall | 147.36 | 193.93 | 159.37 | <.001 |
| 85+ | 404.89 | 432.97 | 289.86 | <.001 |
| 65-84 | 242.12 | 341.14 | 261.41 | <.001 |
| 45-64 | 158.99 | 263.77 | 268.68 | <.001 |
| 30-44 | 143.83 | 218.85 | 222.88 | <.001 |
| 20-29 | 111.48 | 154.03 | 227.97 | <.001 |
| 10-19 | 36.39 | 41.09 | 78.71 | <.001 |
| 0-9 | 42.45 | 50.26 | 48.64 | <.001 |
| Positive tests per 10,000 | ||||
| Overall | 17.00 | 42.66 | 106.03 | <.001 |
| 85+ | 66.24 | 135.48 | 112.72 | <.001 |
| 65-84 | 23.56 | 72.30 | 126.23 | <.001 |
| 45-64 | 19.35 | 61.00 | 186.92 | <.001 |
| 30-44 | 17.17 | 49.26 | 157.45 | <.001 |
| 20-29 | 15.32 | 31.95 | 148.53 | <.001 |
| 10-19 | 3.64 | 9.04 | 54.06 | <.001 |
| 0-9 | 1.70 | 6.35 | 26.00 | <.001 |
Across the entire dataset, 13.1% (38,750 of 295,642) of tests were positive (Table 1). Women represent a higher share of the test observations compared to men (58.75%–41.25%) and a higher proportion of positive test results though the distribution of positive tests results is more even (52.72%–47.28%). Working aged adults (18–49) represent a higher share of positive tests than tests overall (52.2% of positives to 44.95% of tests), and all other age groups represent a smaller share of positive tests compared to their share of tests overall. Among race-ethnicity groups, NL Whites and those with missing/unknown race-ethnicity data are the only groups to represent a smaller share of positive tests than the overall share of tests. Notably, NL Black people represent nearly 10% higher share of positive tests than tests overall (23.07% of positive tests to 13.75% of tests overall), and the share of Latinx positive tests is over five times greater than the share of tests overall (26.34% of positive tests to 5.16% of tests overall). Both NL Black and Latinx people are underrepresented in their share of tests overall and overrepresented in their share of positive tests when compared to make-up of state demographics (21.6% NL Black, and 9.4% Latinx). Rural residents represent a much lower share of tests overall, and a slightly higher share of positive tests (29.43%) than their share of tests overall (28.56%). Distribution of tests for residents in an MUA versus area not classified as an MUA is more even than urban-rural distribution (44.92% in MUA, 55.08% non-MUA), though MUA residents represent a higher share of positive tests than tests overall (46.51%).
Table 2 shows different testing metrics (test positivity rate, tests per capita, and positive tests per capita) for the overall study period for NL Black, NL White, and Latinx populations (data for other race-ethnicity groups not shown). Differences in test positivity rate, tests per capita, and positive tests per capita were statistically significant across race-ethnicity groups overall and across gender, age, rural-urban residence, and MUA residence sub-groups. There are large differences in test positivity rates between the three major racial-ethnic groups: 11.5% of all tests were positive among NL Whites, 22.0% among NL Blacks, 66.5% among Latinx people. Though we do not further analyze and discuss test positivity rates among other racial-ethnic groups throughout the main text of this article due to small numbers, here we list the test positivity rates of the other groups listed in Table 1 for comparison: 20.0% among NL American Indians, 29.5% among NL Asians, 42.4% among NL Native Hawaiian/Pac. Islanders, and 5.1% among those with missing information about their race or ethnicity in the NCDHHS database. Among the three major race-ethnicity groups shown in Table 2, the positivity rate was higher among Latinx people than the overall rate and for any sub-group. Test positivity rates were higher across all race-ethnicity groups for rural men and rural residents. By age, people over 85 had the highest positivity rate among NL Whites (16.36%) and NL Blacks (31.29%) but was highest for Latinx people aged 30–44 (70.64%). Test positivity was slightly higher for NL Whites living in MUAs compared to non-MUAs (less than 1% difference) and was much higher for NL Black people living in MUAs (29.25%) compared to those not living in MUAs (17.13%). However, test positivity was modestly higher for Latinx people living in non-MUAs (67.53%) compared to those living in MUAs (64.78%).
The test per capita rate for people over 65 was highest among NL Blacks; for people aged 10–64, the test per capita rate was highest among Latinx people. Similarly, the rates of positive tests per capita were highest among Latinx for people under 85 years old; for those over 85, the positive tests per capita rate was highest among NL Blacks.
3.2. Temporal trends in key testing metrics
Fig. 1 shows the temporal trends in testing and percent positive tests by race-ethnicity. Fig. 1A shows the absolute number of COVID-19 tests performed on a given day for each of the three major race-ethnicity groups in North Carolina. Fig. 1B shows the percentage of positive COVID-19 by race-ethnicity group. Fig. 1C shows the rate of testing per 10,000 population for each race-ethnicity group, and Fig. 1D shows the rate of positive tests per 10,000 population for each race-ethnicity group. In the time period between March 1st and mid-April, NL Blacks in North Carolina received the most tests per capita; however, by mid-May, Latinx people were undergoing the most tests per capita in a typical daily or weekly reporting period (Fig. 1C). This coincides with a sharp increase in Latinx test positivity (Fig. 1B) fluctuating near 40%–70%; this contrasts with contemporaneous, but steadily decreasing trajectories of test positivity for NL White and NL Black populations (though NL Black positivity rate remained roughly 10% higher than NL White positivity rate for the entire period). In general, per capita positive tests remained constant through the three-month period among NL Whites and NL Blacks, with NL Blacks showing a consistently higher per-capita rate of positivity. However, the per capita positivity rate in the Latinx population increased nearly monotonically throughout the study period.
Throughout the study period, the percentage of positive tests did not fall below the recommended 5% threshold to prevent community spread (WHO, 2020) at any time for any race-ethnic group. However, after initial increases during March, the percentage of positive tests decreased steadily throughout April and May in both NL Whites and NL Blacks. In contrast, the percentage of positive tests increased steadily in the Latinx population during this time period.
To better understand disparities in COVID-19 testing and test positivity rate, we examined intersecting demographic characteristics by analyzing differences within race-ethnicity groups by urban-rural status and by gender (Fig. 2). The left-hand column of Fig. 2 shows temporal trends in testing and in percent positivity, by the intersection of race and urban-rural status. While more tests were conducted among people living in urban areas of North Carolina across all race-ethnicity groups (Fig. 2A), the percentage of positive tests was higher among people living in rural areas of North Carolina across all race-ethnicity groups for the duration of the study period, with the exception of the Latinx population (Fig. 2C). The difference between rural and urban populations’ positivity rates is much more pronounced among NL Black people compared to NL White and Latinx populations, and continues to be consistently higher for the rural NL Black population than the urban NL Black population from mid-April to June (Fig. 2C). Test positivity rates before mid-April should be interpreted with caution among the urban and rural Latinx populations and rural NL Black population due to small daily numbers of total tests (Fig. 2A). While test positivity for Latinx people is consistently higher among rural observations beginning mid-April, the positivity rates converge mid-May (Fig. 2C). The right-hand column of Fig. 2 shows temporal trends in testing and test positivity rate, by the intersection of race-ethnicity and gender. Among both NL White and NL Black residents of North Carolina, more women received tests than men (Fig. 2B), and the percentage of positive tests over time was similar between men and women (Fig. 2D). Among Latinx people, men and women received similar numbers of tests throughout the study period (Fig. 2B); however, the percentage of positive tests was consistently higher in men, with Latino men showing a rate of 70–75% positive tests throughout late April and the month of May (Fig. 2D). With the exception of the first weeks of the epidemic in North Carolina, percent positivity never fell below the 5% threshold (represented by the dashed line), for any race-ethnicity group or subgroup during the study period.
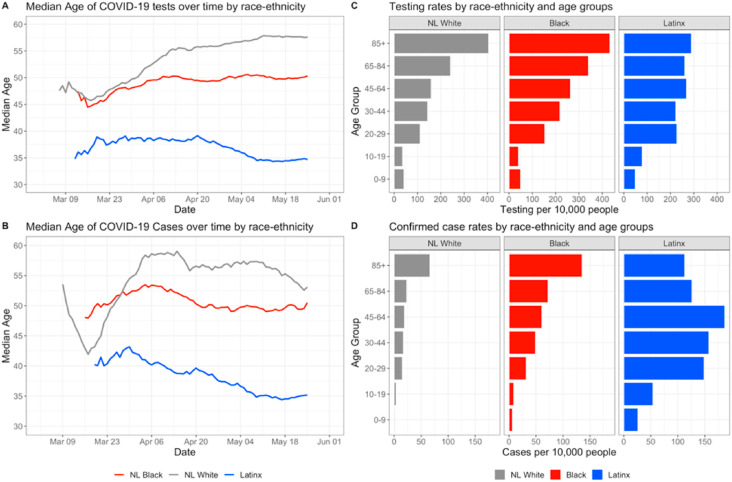
Fig. 3 displays the temporal trends in within-group differences by race-ethnicity and age. Fig. 3A shows the median age of residents tested for COVID-19 on a given day for the three major race-ethnicity groups in North Carolina. Fig. 3B shows the median age of residents having a confirmed positive test for COVID-19 on a given day by race-ethnicity group. Fig. 3C shows the rate of testing per 10,000 population for each race-ethnicity group stratified by age groups, and Fig. 4D shows the rate of positive tests per 10,000 population for each race-ethnic and age group. Since the ACS data does not provide age distributions for NL Blacks, testing and confirmed case rates were calculated using data for those who identified as Black or African American (coded as Black) regardless of ethnicity; the population of NL Black people in North Carolina represent a large (98.47%) percentage of the overall Black population in the state (American Community Survey, 2019), so this data limitation is not likely to affect results. Median age of tests and confirmed cases by urban-rural status and gender are shown in supplemental Figure S2.
Fig. 3.
Intersections between race and age in COVID-19 testing and positivity. Fig. S2 in the Supplement extends the findings of Fig. 3A and B by showing median ages of individuals tested and individuals testing positive among sub-groups stratified by race-ethnicity and rural-urban status or gender.
Overall, Latinx residents who were tested were younger compared to NL Black and NL White residents in North Carolina (Fig. 3A). As testing capacity increased, the median age for testing for Latinx decreased (never reaching 40) and for NL Blacks remained steady, hovering near 50. Median age among NL Whites steadily increased across the testing period, reaching a peak of 57 years. Temporal trends in median age of confirmed cases by race-ethnicity (Fig. 3B) are similar to the median age of testing trajectory for all groups and suggest over time, younger populations constituted a larger proportion of COVID-19 cases. Within-group differences however remain similar, where Latinx residents who tested positive were notably younger, followed by NL Black and NL White residents. For people 30 years and older, cumulative population-adjusted rates of testing were highest among Black residents compared to NL Whites and Latinx counterparts, while we observed slightly higher testing rates for Latinx residents below 30 years old compared to similarly aged NL Black and NL White residents. Additionally, number of tests per capita generally increased with age among NL White and Black populations, but the rate of testing is similar across all age groups 20 and older for the Latinx population. Cumulative population-adjusted case rates show that across every age group, case rates among Black and Latinx populations were higher compared to NL White residents, and highest for Latinx people, except those aged 85 and above. Across all race-ethnicity groups and age groups, case rates were highest among working aged Latinx residents (20–29 years, 30–44 years and 45–64 years) and exceeded more than 150 confirmed cases per 10,000. This is in contrast to NL Whites and Blacks, in which the oldest age groups have the highest confirmed case rates (approximately 60 and 135 per 10,000 respectively).
3.3. Spatial patterns in key testing metrics
Data for all 295,642 tests and their results with ZIP code information were geocoded and mapped (Fig. 4). Fig. 4A shows the total number of tests performed for residents by ZCTA in the study period; this was created using all test observations. Fig. 4B shows the percentage of residents by ZCTA that were tested at least once during the study period; this was calculated using unique individual observations who were tested at least once over ZCTA population. Fig. 4C shows the percentage of residents that had ever tested positive for COVID-19 as of June 1, 2020; this was calculated using unique individual observations who ever tested positive over ZCTA population. Fig. 4D shows the ZCTAs by status as an MUA or non-MUA, and urban or rural. In Fig. 4A, we observe greater testing concentration mostly in urban areas, which is expected given patterns of population density. However, there are some ZCTAs with a high volume of tests in rural areas; these highly concentrated testing distributions are even more apparent Fig. 4B, where volume of tests is normalized by population. Higher relative percentage of the population ever tested appears in the eastern part of the state and rural ZCTAs. Additionally, we observe higher cumulative incidence of COVID-19 in these same areas where there were high testing rates. Fig. 4D shows that large swaths of the state are characterized as MUAs, including rural and urban areas.
From Global Moran's I analysis, a coefficient of 0.2310 for the percentage of the population ever tested (p < 0.01 based on 99,999 Monte-Carlo simulations) indicates a weak clustering pattern of testing concentration over the study period. A more strongly clustered pattern was detected for cumulative incidence by ZCTA with a coefficient of 0.4233 (p < 0.01 based on 99,999 Monte Carlo simulations).
Table 3 shows the distribution of tests and test positivity rate by race-ethnicity and MUA status for the entire study period. Comparing the relative distribution of tests from each of the urban-rural and medically underserved sub-strata demonstrates that there are disparities in what race-ethnicity populations were tested by geographical markers of access to healthcare. Tests from residents living in MUAs represented a higher share of the population's total tests among NL Whites compared to both NL Black and Latinx residents. However, NL Black share of tests in urban MUAs (24.15% was similar to the NL White proportion (24.82%), while Latinx proportion was much lower (15.24%). Conversely, Latinx share of tests in rural MUAs (20.77%) was similar to NL White share of tests in rural MUAs (23.81%) when compared to NL Blacks' share of tests in rural MUAs (16.03%). Across all race-ethnicity groups, tests for residents of MUAs in rural areas represented a higher proportion of tests than non-MUAs in rural areas. In contrast, tests in urban areas were more highly represented for all race-ethnicity groups in non-MUAs compared to urban MUAs.
Table 3.
Distribution of tests and positivity by MUA and urban-rural status.
| NL White |
NL Black |
Latinx |
P-value |
|
|---|---|---|---|---|
| N | 93,045 | 40,640 | 15,341 | |
| Number of tests (%) | ||||
| Rural, MUA | 22,150 (23.81) | 6514 (16.03) | 3186 (20.77) | |
| Rural, Non-MUA | 5126 (5.51) | 668 (1.64) | 686 (4.47) | |
| Urban, MUA | 23,093 (24.82) | 9813 (24.15) | 2338 (15.24) | |
| Urban, Non-MUA | 42,979 (45.87) | 23,645 (58.18) | 9131 (59.52) | |
| Percent positive | ||||
| Rural, MUA | 13.42 | 35.16 | 68.61 | <.001 |
| Rural, Non-MUA | 13.93 | 22.75 | 79.01 | <.001 |
| Urban, MUA | 10.23 | 25.32 | 59.54 | <.001 |
| Urban, Non-MUA | 10.98 | 16.97 | 66.66 | <.001 |
Comparison of test positivity rates by MUA stratification reveals even greater disparities. Among NL Whites, positivity is slightly higher (a less than 1% difference) in non-MUA areas for both urban and rural areas. Among NL Blacks, positivity in MUAs is 12.4% higher in rural areas and 8.35% higher in urban areas. However, among Latinx residents in MUAs, the positivity is lower: 10.4% lower in rural and 7.12% lower in urban compared to non-MUA counterparts. Chi-square tests were used to test for significant differences in test positivity between MUA and non-MUA residents across race-ethnicity groups. Across all groups, the differences are significant (Table 2).
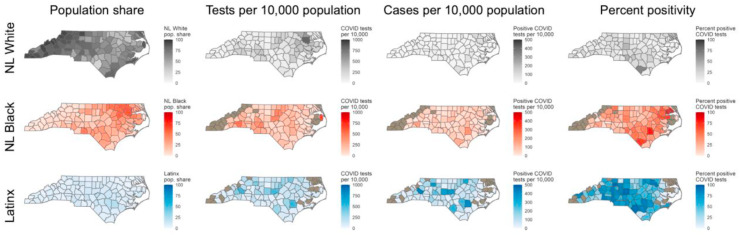
Fig. 5 shows county-level metrics for each race-ethnicity group. Each row represents one of the major race-ethnicity groups: NL White (gray, first row), NL Black (red, second row), and Latinx (blue, third row). Columns represent the following metrics for each race-ethnicity group over the study period: percent share of county population (1), total tests per 10,000 population (2), total number of positive tests per population (3), and percent of positive tests (4). Rates were calculated at the county level due to small numbers in some demographic strata at the ZCTA level.
Fig. 5.
Spatial patterns in tests per capita, positive tests per capita, and percent positive at the county level, stratified by race-ethnicity. Fig. S3 in the Supplement shows these metrics for NL American Indian and NL Asian populations. The tan color indicates that data in that county was suppressed due to identifiability concerns for a given race-ethnic group.
Within race-ethnicity strata, there were notably different spatial patterns between tests per capita and the two metrics of case positivity (positive tests per capita and percentage of positive tests). Rates of testing per 10,000 were highest in the eastern part of the state for NL Whites, in the central and western counties for NL Blacks, and in the rural counties adjacent to central, urban counties among Latinx people. It is notable that among the NL Black and the NL American Indian populations, the counties with the highest per capita rates of testing did not correspond to the counties with the highest rates of cases per capita and highest rates of test positivity, indicating a spatial mismatch between geographic areas where marginalized race-ethnic groups had the greatest access to COVID testing and the areas where those groups experienced the greatest burden of disease (Figure S3 contains county-level maps with results for NL American Indian and NL Asian). Positive tests per capita also varies widely throughout the state by race-ethnic groups. Among NL Whites, there is little variation in county-level positive tests per capita and there are uniformly low-value observations. Among NL Blacks, the spatial pattern of number of positive tests per capita does not reflect the spatial pattern of tests per capita. The counties with highest positive tests per capita values were rural, echoing that there may have been a lack of access to testing for NL Black populations in rural areas as demonstrated in Fig. 2A. The pattern of positive tests per capita is spatially similar to the pattern of tests per capita among the Latinx population, and there are notably more counties with higher rates of positive tests per capita.
We observed substantial geographic variation in percent positive tests by race-ethnicity. The highest percentage of positive tests was observed in the central and southern parts of the state among NL Whites, in eastern North Carolina among NL Blacks, and in the southern and western counties among the Latinx population. Additionally, while it is difficult to observe overall spatial patterns in test positivity among NL American Indians and NL Asians due to suppressed data in counties with low numbers, the counties that did have sufficient data most often displayed higher percent positivity among NL American Indians and NL Asians, compared to NL Whites in that county (Supplemental Fig. S3).
4. Discussion
Using NCDHHS SARS-CoV-2 testing data from the first three months of the COVID-19 pandemic, we identified disparities in testing and incidence of disease among historically marginalized populations in North Carolina. Foremost among these findings are the much higher test positivity and incidence rates observed in the Latinx, NL Black, and NL American Indian communities (Table 1, Table 2). Even in absolute terms, it is striking that the number of positive test results among Latinx residents nearly equals the number of positive test results among NL White residents, despite the Latinx population representing less than one-sixth of the population in the state (Table 1). Additionally, for all populations except NL White, the counties with the highest per capita rates of testing did not correspond to the counties with the highest rates of per capita positive tests and the highest percent test positivity (Fig. 5), suggesting that the areas where marginalized race-ethnic groups experienced the greatest access to testing during the first three months of the epidemic were not those areas with the highest burden of disease.
Understanding geographic disparities in testing during the first three months of the COVID-19 pandemic is important for reconstructing the progression of the social, economic, and public health burden of the disease, and for planning the response to future public health or natural disaster emergencies. Our findings not only show disparities in testing by race-ethnicity, but also highlight the fact that within the largest race-ethnic groups in North Carolina there were further differences in testing and infection rates by gender, age, urban-rural status, and medically underserved status. Additionally, in the race-ethnicity stratified county-level analysis (Fig. 5), and the analysis of differences within race-ethnicity group by urban-rural status and medically underserved status (Fig. 2, Table 3), we observed that there was a mismatch between the geographic areas where a given race-ethnic group had the highest per-capita rate of testing, and the areas where a given race-ethnic group experienced the highest percentage of positive tests, indicating undetected community spread. These findings suggest that the extent to which testing did not accurately capture the true disease dynamics varied by both race-ethnicity and geography in North Carolina during the first three months of the COVID-19 epidemic. Additionally, despite the on-ramp to increased testing capacity over this time period, (NCDHHS, 2020b) the percent positivity in tests among NL Black and Latinx populations remained high (Fig. 1), suggesting that the early geographic disparities in testing created a lagged effect of increased community spread, which was not ameliorated by increased test availability later in the study period. Not only are these insights important in understanding possible systematic undercounts in COVID-19 case data that relies on reporting of positive tests, but also how the structural and geographic factors contribute to differential testing both between and within race-ethnicity groups. Therefore, these findings are important for understanding the course of the pandemic in North Carolina and can inform public health preparedness for future events.
4.1. Disparities by race-ethnicity across all testing metrics
Structural inequities, such as a larger proportion of the Black and Latinx populations engaged in essential and high-risk occupations, have contributed to the significant disparities in COVID-19 by race and ethnicity (Wortham et al., 2020; Bureau of Labor Statistics, 2019; Gennetian and Johnson, 2020; Hawkins, 2020a). Because Latinx, Black, and American Indian groups in the US face deep and historic structural inequities in occupational opportunity, and discrimination (Krieger, 2010), these groups experience a substantially higher burden of exposure to COVID-19 (Hawkins, 2020a; McClure et al., 2020; NCDHHS, 2021; Laster Pirtle, 2020). Our results support the notion that people who are in low wage jobs account for these disparities, given the lower median age of those who tested positive (Fig. 3B), highest case rate among working-age populations (Table 2, Fig. 3D), and high incidence in rural areas where meat processing and agriculture are primary industries (Fig. 4C). Household characteristics, including larger, multi-generational households, may also affect disease transmission rates among individuals who are working in low wage occupations (CDC, 2020; Taylor et al., 2011; Carrión et al., 2020). Moreover, structural barriers, particularly with regard to access to care and thus testing (CDC, 2020), have been described previously and are also likely to play a role in these findings. For example, while population-adjusted testing rates increased over time among all groups, the rate of testing among Latinx residents did not reach parity with NL White residents for nearly two months after the first case was reported in the state (Fig. 1C). Perhaps of most concern, the sustained positivity rate above 50% among Latinx residents and the consistently higher incidence rate among NL Black populations compared to NL Whites Fig. 1B), suggests that testing was inadequate and inequitably distributed by race and ethnicity early on in the study period, allowing for undetected infections to spread rapidly within these underserved communities (WHO, 2020). Similar differences were identified in NL American Indian populations and NL Asian populations compared to NL Whites.
4.2. Disparities by race-ethnicity across geography
Many of the demographic and spatial patterns of testing and test positivity shown here suggest the potential role of living conditions or possible occupational exposure for those in high-risk occupations for rural communities in the spread of SARS-CoV-2. Both urban and rural essential worker communities are at high risk of exposure to the virus (McClure et al., 2020). However, in the early weeks of the pandemic, testing was emphasized for urban communities because its presence was known there and high-density areas emerged as the early hotspots in the US. (Shear et al., 2020; Paul et al., 2020; Taylor et al., 2011; Carrión et al., 2020) Early discussion about rural vulnerability to the pandemic was centered on high mortality rates among aging rural populations (Sommer, 2020; Cunningham, 2020; Williams et al., 2020; Ajilore, 2020) and rural access to medical facilities in light of increasing rates of hospital closures in the past ten years (Tochik et al., 2020). However, this study illustrates the importance of considering the case distribution among groups at higher risk for exposure in rural areas, not just COVID-19 related death. Additionally, a strength of this study is that we assessed urban-rural dynamics at the ZCTA level and incorporated information about medically served status; previous evidence about urban-rural disparities in COVID-19 outcomes in the United States have all been conducted at the county level. These county-level analyses overlook variation in population density within counties, which often contain both urban centers and outlying rural areas that could have notably different community composition, economic characteristics, and healthcare access (Paul et al., 2020; Peters, 2020). Furthermore, our geographic analyses were based on individual-level data, allowing us to understand the demographic variation in COVID-19 metrics within counties as well as between counties. This represents an important addition to literature focused on racial disparities in COVID-19 outcomes: most previous studies use an ecological approach to examine the relationship between race and COVID-19 cases, usually by comparing the overall per capita COVID-19 case rate at the county level to the proportion of racial minorities in that county (Millett et al., 2020; Rodriguez-Diaz et al., 2020; Karaye and Horney, 2020; Liao and De Maio, 2021). In contrast, this study uses individual-level data to understand the proportion of people of each race-ethnic group in each county that received a COVID-19 test, and accordingly the proportion that had a confirmed COVID-19 case. This allowed us to further understand intersectional dimensions of COVID-19 and allowed us to conduct spatial analyses that were stratified by race-ethnicity.
The distribution of cases between urban and rural communities is an integral part of understanding the virus's spread; we observed divergence in test volume trajectories between urban and rural populations (Fig. 2A), and concurrently a divergence in test positivity rates between rural and urban populations, especially within NL Black and Latinx populations (Fig. 2B). Taken together, these trends suggest that there was a lag in test availability for racial-ethnic minority groups in rural areas which allowed for uncontrolled community spread. The trends of test positivity by urban-rural status and age (Table 2, Fig. 2A and B, Fig. 3) illustrate how the outbreak and spread of COVID-19 has disproportionately affected the state's rural, working-age, Black and Latinx populations relative to the other groups and the overall makeup of the state's population and emphasizes the importance of residential location as another major barrier to access for health care, including testing. By further stratifying race-ethnicity groups by urban-rural and MUA status (Table 3), we observe that nearly half of tests among NL Whites are from residents of MUAs (48.63%); the shares of tests from MUAs among NL Blacks (40.18%) and Latinx (36.01%) are much lower. Despite a large representation of MUA residents in NL White test observations, test percent positivity is much lower compared to NL Black and Latinx counterparts. Additionally, there is negligible difference between test positivity in MUA and non-MUA areas for NL Whites, suggesting that NL Whites' access to testing was not affected by MUA status. However, we observe much higher test positivity rates for NL Black residents of MUAs in both urban and rural ZCTAs (Table 3), which suggests that there are structural barriers to testing for NL Black populations in MUAs that NL Whites do not face. In contrast, test positivity among Latinx populations in MUAs are lower in both urban and rural areas. This difference between Latinx residents of MUAs and non-MUAs may be partially explained by the NCDHHS targeted testing campaigns in rural and underserved communities (NCDHHS, 2020c). Still, the inconsistent magnitudes and directions in differences in test positivity within the three major race-ethnicity groups by MUA status illustrate the importance of interrogating the influence of community-level geographic information. Such geographic measures intersect with other socially constructed demographic categories, and failure to consider these intersections may ultimately obfuscate the true influence of socio-environmental processes (Bowleg, 2012; McKane et al., 2018).
4.3. Study limitations
Our analysis contributes to the understanding of the COVID-19 pandemic through use of comprehensive, individual-level statewide data on testing and cases with geographic and demographic information. Incorporating demographic and spatial variables highlights the structural inequalities experienced across race and ethnic groups in North Carolina that hinge both on occupational opportunities and risk as well as access to public health infrastructure and medical care. While these insights are important, there are some limitations in the data. First, there is the use of the Census Bureau racial and ethnic categories, which constrain self-identification and may not accurately represent all race and ethnic identification. Additionally, due to small numbers in many racial strata among those who identified as Latinx, we were unable to conduct analyses examining Latinx-race intersectionality, for example, comparisons of testing trends between those who identify as Black and Latinx versus those who identify as White and Latinx. Such assessments could add important nuance to understandings of the COVID-19 pandemic among the Latinx community, because access to testing and infection risk may be influenced by the social and structural intersection of race and ethnicity, and future studies with sufficient sample size should examine the multiplicity of Latinx racial identity to better understand heterogeneity in COVID-19 testing and test positivity within this group. Also, we do not have occupational data, and therefore we cannot distinguish between occupational and community exposure such as in the rural agricultural areas of the state.
Second, there was a high rate of missingness for the race and ethnicity information available, potentially resulting in an ascertainment bias if certain groups were more or less likely to share their demographic information. For example, if rural, NL Blacks were less likely to have their race recorded than rural NL Whites, we may observe a greater disparity in testing rates between these populations than truly exists. Further, if the recording of race-ethnicity was associated having COVID-19– for example, if an outbreak among the Latinx population was suspected in a particular geographic area and influenced more diligent recording of race-ethnicity data– our results based on the raw data may over- or under-estimate positivity in this group. While understanding missingness patterns in race-ethnicity data is interesting in itself and merits further investigation, such questions are beyond the scope of this paper. There is some evidence that even after accounting for missing race and ethnicity data in COVID-19 testing and incidence, disparities between race-ethnicity groups persist (Labgold et al., 2021). Further, missing data on race and ethnicity for COVID-19 surveillance is a persistent problem in the United States, despite national mandates to collect these data (Krieger et al., 2020). Thus, we have chosen to report and describe the patterns based on the data that are available using multiple metrics of testing for comparability across race-ethnicity strata because they provide valuable insights into disparities and raise important questions about testing access for marginalized groups.
Finally, reporting of negative test results was not mandatory in North Carolina until early July (NCDHHS, 2020a). Thus, the resulting analysis may overestimate the overall and specific racial-ethnic positivity rates while also underestimating per capita testing rates in low-incidence areas. Given that the majority of testing, especially early in the outbreak, was performed at the North Carolina State Laboratory of Public Health and a few larger referral centers and/or private companies with locally developed assays, we believe non-reporting likely represents a small proportion of tests performed.
4.4. Conclusions
This study has identified large disparities in testing and test positivity by race-ethnicity and urban-rural residence in North Carolina during the first three months of the state's COVID-19 pandemic. Our findings underscore the role that long-standing structural inequalities play in amplifying the burden of COVID-19 in marginalized communities in the US. Furthermore, the results highlight the rapid spread of COVID-19 to rural areas early in the course of the pandemic, even as much of the risk assessment at the time focused on more densely populated urban centers. We conclude that interventions should include investments in public health and social infrastructure to address the historic and present-day structural inequalities that continue to result in excess disease burden among Latinx, Black, American Indian, and rural residents who have higher exposure and more limited access to testing for SARS-CoV-2 in North Carolina.
Funding
We are grateful to the Carolina Population Center for training support (T32 HD091058) and for general support (P2C HD050924). Additionally, CK is grateful for support provided by a training grant from the National Institute of Environmental Health Sciences (T32ES007018) and VG is thankful for support from the Melinda Meade Research Award from the UNC Department of Geography.
Declaration of competing interest
The authors have no conflicts of interest to report.
Acknowledgements
We would like to acknowledge the anonymous reviewers whose comments and suggestions strengthened this work. The data in this analysis were provided by the North Carolina Department of Health and Human Services.
Footnotes
Fig. S1.
Fig. S2.
Fig. S3.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ajilore O. Rural America is starting to feel the impact of the coronavirus. Center for American Progress. April 28, 2020 https://www.americanprogress.org/issues/economy/reports/2020/04/28/484016/rural-america-starting-feel-impact-coronavirus/ [Google Scholar]
- American Community Survey . US Census Bureau; 2019. 2014-2018 American Community Survey 5-year Estimates.https://www.census.gov/data/developers/data-sets/acs-5year.html [Google Scholar]
- Anselin Luc, Syabri Ibnu, Kho Youngihn. GeoDa: an introduction to spatial data analysis. Geogr. Anal. 2006;38(1):5–22. [Google Scholar]
- Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. J. Am. Med. Assoc. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey Z.D., Moon J.R. Racism and the political economy of COVID-19: will we continue to resurrect the past? J. Health Polit. Pol. Law. 2020:8641481. doi: 10.1215/03616878-8641481. [DOI] [PubMed] [Google Scholar]
- Bowleg L. The problem with the phrase women and minorities: intersectionality—an important theoretical framework for public health. Am J Public Health. Jul. 2012;102(7):1267–1273. doi: 10.2105/AJPH.2012.300750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R.W., Lindo E.G., Weeks L.D., et al. On racism: a new standard for publishing on racial health inequities. Health Affairs Blog. 2020;10 doi: 10.1377/hblog20200630.939347. https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/ [DOI] [Google Scholar]
- Bureau of Labor Statistics . US Dept of Labor; 2019. Labor Force Characteristics by Race and Ethnicity, 2018.https://www.bls.gov/opub/reports/race-and-ethnicity/2018/home.htm [Google Scholar]
- Carrión D., Colicino E., Foppa Pedretti N., et al. Assessing capacity to social distance and neighborhood-level health disparities during the COVID-19 pandemic. medRxiv. Preprint posted online June. 2020;13 doi: 10.1101/2020.06.02.20120790. [DOI] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowger T.L., Davis B.A., Etkins O.S., et al. Comparison of weighted and unweighted population data to assess inequities in coronavirus disease 2019 deaths by race/ethnicity reported by the US centers for disease control and prevention. JAMA Netw Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis K., Epaulard O., Bénet T., et al. Cluster of coronavirus disease 2019 (Covid-19) in the French Alps, 2020 [published online ahead of print, 2020 Apr 11] Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa424. ciaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyal J.W., Grant M.P., Broadwater K., et al. COVID-19 among workers in meat and poultry processing facilities ― 19 states, April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(18):557–561. doi: 10.15585/mmwr.mm6918e3. [DOI] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration Medical device shortages during the COVID-19 public health emergency. January. 2021;21 https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/medical-device-shortages-during-covid-19-public-health-emergency [Google Scholar]
- Gee G.C., Ford C.L. Structural racism and health inequities: old issues, new Directions 1. Du bois review. Social Science Research on Race. 2011;8(1):115–132. doi: 10.1017/S1742058X11000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2020. "COVID-19 in Racial and Ethnic Minority Groups." Centers for Disease Control and Prevention. Updated 25 June 25 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html (Accessed 18 July 2020).
- Gold J.A., Wong K.K., Szablewski C.M., et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 — Georgia, March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(18):545–550. doi: 10.15585/mmwr.mm6918e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein N.D., Burstyn I. On the importance of early testing even when imperfect in a pandemic such as COVID-19. Global epidemiology. 2020;2:100031. doi: 10.1016/j.gloepi.2020.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins D. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity [published online ahead of print, 2020 Jun 15] Am. J. Ind. Med. 2020 doi: 10.1002/ajim.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins D. Social determinants of COVID-19 in Massachusetts, United States: an ecological study. J Prev Med Public Health. 2020;53:220–227. doi: 10.3961/jpmph.20.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaye I.M., Horney J.A. The impact of social vulnerability on COVID-19 in the U.S.: an analysis of spatially varying relationships. Am. J. Prev. Med. 2020 Sep;59(3):317–325. doi: 10.1016/j.amepre.2020.06.006. Epub 2020 Jun 26. PMID: 32703701; PMCID: PMC7318979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. Workers are people too: societal aspects of occupational health disparities—an ecosocial perspective. Am. J. Ind. Med. 2010;53(2):104‐115. doi: 10.1002/ajim.20759. [DOI] [PubMed] [Google Scholar]
- Krieger N., Testa C., Hanage W.P., Chen J.T. US racial and ethnic data for COVID-19 cases: Still missing in action. Lancet. 2020;396(10261):e81. doi: 10.1016/S0140-6736(20)32220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labgold K., Hamid S., Shah S., Gandhi N., Chamberlain A., Khan F., Khan S., et al. Estimating the unknown: greater racial and ethnic disparities in COVID-19 burden after accounting for missing race and ethnicity data. Epidemiology. 2021;32(2):157–161. doi: 10.1097/EDE.0000000000001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laster Pirtle W.N. Racial capitalism: a fundamental cause of novel coronavirus (COVID-19) pandemic inequities in the United States. Health Educ. Behav. 2020;47(4):504–508. doi: 10.1177/1090198120922942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J.F., Harris M.F., Russell G. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. Int. J. Equity Health. 2013;12:18. doi: 10.1186/1475-9276-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T.F., De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021 Jan 4;4(1) doi: 10.1001/jamanetworkopen.2020.34578. PMID: 33471120; PMCID: PMC7818127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman-Cribbin W., Tuminello S., Flores R.M., et al. Disparities in COVID-19 testing and positivity in New York city. Am. J. Prev. Med. 2020 doi: 10.1016/j.amepre.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure E.S., Vasudevan P., Bailey Z., et al. Racial capitalism within public health: how occupational settings drive COVID-19 disparities. Am. J. Epidemiol. 2020 doi: 10.1093/aje/kwaa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKane R.G., Satcher L.A., Houston S.L., Hess D.J. Race, class, and space: an intersectional approach to environmental justice in New York City. Env Sociol. 2018;4:79–92. doi: 10.1080/23251042.2018.1429177. [DOI] [Google Scholar]
- Meer N. Sage; 2014. Race & Ethnicity: Key Concepts. [Google Scholar]
- Menachemi N., Yiannoutsos C.T., Dixon B.E., et al. Population point prevalence of SARS-CoV-2 infection based on a statewide random sample — Indiana, April 25–29, 2020. MMWR Morb Mortal Wkly Rep. ePub. 21 July 2020:69. doi: 10.15585/mmwr.mm6929e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett G.A., Jones A.T., Benkeser D., et al. Assessing differential impacts of COVID-19 on black communities. Ann. Epidemiol. 2020 Jul;47:37–44. doi: 10.1016/j.annepidem.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Agriculture Statistics Service . US Dept of Agriculture; 2019. NC Dept of Agriculture & Consumer Services. North Carolina Agricultural Statistics.https://www.nass.usda.gov/Statistics_by_State/North_Carolina/Publications/Annual_Statistical_Bulletin/AgStat/NCAgStatBook.pdf Publication No. 220. Accessed online. [Google Scholar]
- NC Executive Order No 121 . 27 March 2020. STAY AT HOME ORDER AND STRATEGIC DIRECTIONS FOR NORTH CAROLINA IN RESPONSE TO INCREASING COVID-19 CASES.https://files.nc.gov/governor/documents/files/EO121-Stay-at-Home-Order-text.pdf Available at: [Google Scholar]
- NC Executive Order No 138 . 5 May 2020. EASING RESTRICTIONS ON TRAVEL, BUSINESS OPERATIONS, AND MASS GATHERINGS: PHASE 1.https://files.nc.gov/governor/documents/files/EO138-Phase-1.pdf Available at: [Google Scholar]
- ZIP Code Tabulation Areas (ZCTAs). United States Census Bureau. Updated April 1, 2020. Accessed June 25, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/zctas.html.
- NCDHHS “North Carolina Announces statewide COVID-19 test standing order, requires reporting of all test results.”. NCDHHS Media. 2020 https://www.ncdhhs.gov/news/press-releases/north-carolina-announces-statewide-covid-19-test-standing-order-requires [Google Scholar]
- Paul R., Arif A.A., Adeyemi O., et al. Progression of COVID-19 from urban to rural areas in the United States: a spatiotemporal analysis of prevalence rates. J. Rural Health. 2020;36(4):591–601. doi: 10.1111/jrh.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters D.J. Community susceptibility and resiliency to COVID-19 across the rural-urban continuum in the United States. J. Rural Health. 2020;36(3):446–456. doi: 10.1111/jrh.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugeisen B.M., Mou J. Empiric evidence of ethnic disparities in coronavirus positivity in Washington State. Ethn Health. January. 2021;11:1–13. doi: 10.1080/13557858.2020.1863922. Epub ahead of print. PMID: 33428455. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Ratcliffe M., Burd C., Holder K., et al. Defining Rural at the U.S. Census Bureau. US census Bureau report. 2016. https://www.census.gov/library/publications/2016/acs/acsgeo-1.html Report number ACSGEO-1. Accessed.
- Rodriguez-Diaz C.E., Guilamo-Ramos V., Mena L., et al. Risk for COVID-19 infection and death among Latinos in the United States: examining heterogeneity in transmission dynamics. Ann. Epidemiol. 2020 Dec;52:46–53. doi: 10.1016/j.annepidem.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell A.A. The racism-race reification process: a mesolevel political economic framework for understanding racial health disparities. Sociology of Race and Ethnicity. 2016;2(4):402–432. doi: 10.1177/2332649215626936. [DOI] [Google Scholar]
- Shear M., Goodnough A., Kaplan S., Fink S., Thomas K. 2020. The lost month: how a failure to test blinded the U.S. to COVID-19. New York Times, 28 March.https://www.nytimes.com/2020/03/28/us/testing-coronavirus-pandemic.html Available at: (Accessed 17 July 2020) [Google Scholar]
- Sommer L. Why the warning that coronavirus was on the move in U.S. Cities came so late. National Public Radio. 2020 https://www.npr.org/sections/health-shots/2020/04/24/842025982/why-the-warning-that-coronavirus-was-on-the-move-in-u-s-cities-came-so-late [Google Scholar]
- Sun L.H., Achenbach J. CDC chief says coronavirus cases may be 10 times higher than reported. Wash. Post. 2020 https://www.washingtonpost.com/health/2020/06/25/coronavirus-cases-10-times-larger/ June 25. [Google Scholar]
- Taylor P., Kocchar R., Cohn D., et al. Pew Research Center; 2011. Fighting Poverty in a Tough Economy, Americans Move in with Their Relatives Chapter 3: Demographics of Multigenerational Households.https://www.pewsocialtrends.org/2011/10/03/chapter-3-demographics-of-multi-generational-households/ [Google Scholar]
- Tippett R. 2016. "The persistent "rurality" of North Carolina." Carolina Demography, 21 March.https://www.ncdemography.org/2016/03/21/the-persistent-rurality-of-north-carolina/ Available at: (Accessed 18 July 2020) [Google Scholar]
- Tippett R. 2019. North Carolina's Hispanic Community: 2019 Snapshot. Carolina Demography, 26 September. (Accessed 6 October 2020) [Google Scholar]
- Tochik M., Gross K., Pinette M. Chartis Center for Rural Health; 2020. The Rural Health Safety Net under Pressure: Rural Hospital Vulnerability.https://www.ivantageindex.com/rural-hospital-vulnerability-study/ [Google Scholar]
- Waltenburg M.A., Victoroff T., Rose C.E., et al. Update: COVID-19 among workers in meat and poultry processing facilities ― United States, april–may 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(27):887–892. doi: 10.15585/mmwr.mm6927e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. J. Am. Med. Assoc. 2020;323(24):2466. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., Mohammed S.A., Leavell J., et al. Race, socioeconomic status and health: complexities, ongoing challenges and Research opportunities. Ann. N. Y. Acad. Sci. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.A., Gelaye B., Broad Leib E.M. The covid-19 crisis is going to get much worse when it hits rural areas. Washington Post. 2020 https://www.washingtonpost.com/opinions/2020/04/06/covid-19-crisis-is-going-get-much-worse-when-it-hits-rural-areas/ [Google Scholar]
- Wortham J.M., Lee J.T., Althomsons S., et al. Characteristics of people who died with COVID-19 — United States, february 12–may 18, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(28):923–929. doi: 10.15585/mmwr.mm6928e1. [DOI] [PubMed] [Google Scholar]
- ZIP Code to ZCTA Crosswalk. Uniform Data System Mapper. Available at: https://www.udsmapper.org/zcta-crosswalk.cfms (Accessed 25 June 2020).
- NCDHHS . NCDHHS Media; 2020. NCDHHS Convenes Testing Surge Workgroup.https://www.ncdhhs.gov/news/press-releases/ncdhhs-convenes-testing-surge-workgroup 17 April 2020. Available at: (Accessed 30 January 2021) [Google Scholar]
- Cunningham P.W. Washington Post; 2020. The Health 202: Coronavirus is shifting from urban areas to rural ones.https://www.washingtonpost.com/news/powerpost/paloma/the-health-202/2020/05/06/the-health-202-coronavirus-is-shifting-from-the-urban-areas-to-rural-ones/5eb1913b602ff15fb00257f8/ 6 May. Available at: (Accessed: 18 July 2020) [Google Scholar]
- NCDHHS . 2021. COVID-19 Clusters in North Carolina. NCDHHS Report, 25 January 2021.https://covid19.ncdhhs.gov/media/725/open Available at: (Accessed 30 January 2021) [Google Scholar]
- Gennetian L.A., Johnson M.S. EconoFact; 2020. Work-based Risks to Latino Workers and their Families From COVID-19.https://econofact.org/work-based-risks-to-latino-workers-and-their-families-from-covid-19 26 May. Available at: (Accessed: 17 July 2020) [Google Scholar]
- HRSA . Health Resources and Services Administration; 2021. Shortage Areas.https://data.hrsa.gov/topics/health-workforce/shortage-areas Available at: (Accessed 12 January 2021) [Google Scholar]
- Johns Hopkins University, 2020a. Coronavirus Resource Center. Avalable at: https://coronavirus.jhu.edu (Accessed 1 April 2021).
- Johns Hopkins University . Coronavirus Resource Center; 2020. Differences in positivity rates.https://coronavirus.jhu.edu/testing/differences-in-positivity-rates Available at: (Accesssed 30 January 2021) [Google Scholar]
- NCDHHS . 2021. COVID-19 North Carolina Dashboard.https://covid19.ncdhhs.gov/dashboard Available at: (Accessed 30 January 2021) [Google Scholar]
- WHO . World Helath Organization Institutional Repository for Information Sharing; 2020. Public Health Criteria to Adjust Public Health and Social Measures in the Context of COVID-19.https://apps.who.int/iris/handle/10665/332073 12 May 2020. Available at: (Accessed 15 July 2020) [Google Scholar]
- NCDHHS . NCDHHS Media; 2020. NCDHHS to Deploy up to 300 Free Testing Sites in Underserved Communities.https://www.ncdhhs.gov/news/press-releases/ncdhhs-deploy-300-free-testing-sites-underserved-communities 7 July. Available at: (Accessed 30 January 2021) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.