SUMMARY
We focused on the relationship of 0.5% tetracaine- and 0.4% oxybuprocaine-induced corneal anesthesia in rats, and pentadecapeptide BPC 157 (0.4 µg/eye), along with nitric oxide synthase (NOS) inhibitor N(gamma)-nitro-L-arginine methyl ester (L-NAME) (0.1 mg/eye) and/or NOS substrate L-arginine (2 mg/eye), applied in the form of eye drops. We assessed corneal sensitivity recovery (Cochet-Bonnet esthesiometer), corneal lesion elimination (staining with 10% fluorescein) and decrease in tear volume (Schirmer test). BPC 157 administration had a full counteracting effect. Recovery also occurred in the presence of NOS blockade and NOS substrate application. L-arginine eventually shortened duration of corneal insensitivity and exerted corneal lesion counteraction (and counteraction of tetracaine-induced decrease of tear volume) only in earlier but not in later period. L-NAME application led to longer duration of corneal insensitivity, increase in corneal lesions and decrease in tear volume. When L-NAME and L-arginine were applied together, they antagonized each other’s effect. These distinctions may indicate particular NOS involvement (corneal insensitivity vs. corneal lesion along with tear production), distinctively affected by the administration of NO agents. However, additional BPC 157 co-administration would re-establish counteraction over topical ophthalmic anesthetic-induced effect, be it in its early or late course. We suggest BPC 157 as an antidote to topical ophthalmic anesthetics.
Key words: BPC 157, Tetracaine, Oxybuprocaine, Corneal anesthesia, NOS, Rats
Introduction
Our study was focused on counteracting corneal anesthesia using the stable gastric pentadecapeptide BPC 157, N(gamma)-nitro-L-arginine methyl ester (L-NAME) and/or L-arginine in rats (1-13). After the stable gastric pentadecapeptide BPC 157 (1-13) has been demonstrated to act as a potential antidote to bupivacaine cardiotoxicity, thereby interacting with local anesthetics (14), we focused on the potential counteracting effect of the stable gastric pentadecapeptide BPC 157 and tetracaine as a topical ophthalmic anesthetic applied onto the eye surface. That counteracting effect may be related to the nitric oxide synthase (NOS) (1-13), as follows from the BPC 157 application with NO agents, NOS blocker L-NAME and NOS substrate L-arginine, administered alone and/or in combination. Such a counteracting effect may also have a more general significance, providing additional antagonizing the oxybuprocaine-induced corneal anesthesia, and the corresponding effect of the NO agents, L-NAME and L-arginine, administered alone and/or in combination.
Indicatively, counteraction of bupivacaine arrhythmias (14) may be seen as part of the larger beneficial effect providing that BPC 157 counteracts various arrhythmias (14-19). Likewise, with BPC 157 application, counteraction of tetracaine- and oxybuprocaine-induced corneal anesthesia may appear along with healing of perforating corneal injury and total debridement of corneal epithelium, thus maintaining corneal transparency (20, 21). BPC 157 counteracts the damaging effects of lacrimal gland extirpation and dry eye syndrome in rats (22). We also revealed NOS dependence of atropine-induced mydriasis and L-NAME- and L-arginine-induced miosis and reversal by the pentadecapeptide BPC 157 in both rats and guinea pigs (23). This may be particularly important considering corneal lesions that may be induced by local anesthetic application (24-27).
As an original antiulcer peptide, BPC 157 has virtually no known toxicity of its own, the lethal dose (LD) value has not yet been established, and there were no side effects in clinical trials such as ulcerative colitis and multiple sclerosis (1-13). Also indicative of special relation, BPC 157 caused significant antagonism of general anesthesia produced by thiopental with a parallel shift of the dose-response curve to the right, manipulating in both ways with NOS activity modulation (especially, the potentiating effects of L-NAME were abolished) (28). This counteraction was observed, along with the evidence that BPC 157 largely interacts with NOS in different models and species (1-13).
On the other hand, BPC 157 produced analgesia in the MgSO4 and acetic acid test in mice, a model of prolonged pain associated with tissue injury (29). This indicates that BPC 157 may have local anesthetic activity of its own.
Thus, to counteract tetracaine- and oxybuprocaine-induced corneal anesthesia, the previously reported effective dose regimens of the pentadecapeptide BPC 157 (14-19), along with NOS inhibitor L-NAME and NOS substrate L-arginine, were administered after tetracaine and oxybuprocaine application in rats.
Methods
Animals
Wistar Albino male rats (200 g b.w.) were randomly assigned to the experiments (6 animals at least per experimental group and interval), all of which were approved by the local Ethics Committee of the School of Medicine, University of Zagreb, Zagreb, Croatia (No. 380-59-10106-19-111/100; 641-01/19-02/01). Furthermore, all experiments were carried out under blind protocol and the effect was assessed by examiners who were completely unaware of the given protocol. The research and experiments also followed the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Drugs and experimental protocol
Pentadecapeptide BPC 157 (GEPPPGKPADDAGLV, M.W. 1419, Diagen, Ljubljana, Slovenia) dissolved in saline was used in all experiments. The BPC 157 peptide is part of the sequence of human gastric juice BPC protein and is freely soluble in water at pH 7.0 and saline. It was prepared as described previously with 99% high-pressure liquid chromatography (HPLC) purity, expressing 1-des-Gly peptide as an impurity (14-19). At 15 minutes after diazepam (5 mg/kg intraperitoneally), two drops of 0.5% tetracaine (Tetrakain 0.5%, Gradska ljekarna, Zagreb, Croatia) or oxybuprocaine 0.4% (Novesine 0.4%, OmniVision, Munich, Germany) were administered into each eye. At 1 minute, rats received pentadecapeptide BPC 157 (0.4 µg/eye), L-NAME (0.1 mg/eye) and L-arginine (2 mg/eye), administered alone and in combination. The agents were administered locally (two drops/eye) (Sigma Aldrich, St. Louis, USA), while controls received a respective saline volume.
Corneal sensitivity measurement
Using the Cochet-Bonnet esthesiometer (Luneau Ophtalmologie, Chartres, France), a corneal sensitivity measurement device (24), corneal sensitivity was measured in rats administered 0.5% tetracaine or 0.4% oxybuprocaine before corneal anesthesia, then after tetracaine or oxybuprocaine application, before therapy application, at 1 min, and with therapy application, at 5-minute intervals until full sensitivity was restored.
Briefly, the Cochet-Bonnet esthesiometer consists of nylon monofilament that can be pulled out and produces a pressure force at its top, which is inversely proportional to the length of the elongated portion of the monofilament. The nylon filament is 0.12 mm in diameter and is 6 cm long. Corneal sensitivity testing started with gentle touch of the fully extended tip of the esthesiometer perpendicular to the cornea and applying a small force to finely distort the filament. If the rat blinked, it was recorded as a positive response. If the blinking response was absent, filament length was reduced by 1 centimeter and the procedure was repeated until a positive response was obtained. The absence of positive response when the filament length is 1 centimeter is defined as complete block. The length of filament in centimeters that caused positive response was recorded. In the case of complete block, the value 0 was recorded. As mentioned, after initial corneal sensitivity testing, each eye was tested at 5-min intervals until full sensitivity was restored.
Corneal epithelium defect assessment
In rats administered 0.5% tetracaine that received saline or BPC 157, L-NAME and/or L-arginine eye drops as described above, corneal epithelium defects considering long-term exposure were assessed at 30, 60 and 150 minutes of the experiment. Corneal epithelium defects were monitored by slit lamp (PSL Portable Slit Lamp, Reichert, Buffalo, USA) with blue cobalt filter and by photographing the lesions after staining with standard 10% fluorescein (Alcon, Geneva, Switzerland), according to the experimental protocol. The test was positive if there was an epithelial defect and negative if there was no epithelial defect. Scoring is performed by assessing the area affected with corneal defects divided in quarters, from no defects (0/4) to the whole area of the cornea being affected with epithelium defects (4/4).
Schirmer test assessment
Schirmer test was performed at 30, 60 and 120 min in 0.5% tetracaine rats that received saline or BPC 157, L-NAME and/or L-arginine eye drops as described above (30). Standard Schirmer tracks using 2-mm wide test strips cut from Schirmer test paper to fit the size of the rat eye were inserted 1 mm into the inferior conjunctival fornix and placed for 5 minutes, after which results in millimeters were recorded (the values of 5.5±1.0 mm obtained in healthy rats were considered normal).
Statistical analysis
Data were processed by use of descriptive statistics according to their distribution (Kolmogorov-Smirnov test for normal distribution). The duration of complete corneal anesthesia (T1) and time interval until corneal sensitivity returned to baseline values (T4) for two different local anesthetic drugs (tetracaine and oxybuprocaine) in regard to different adjuvants (saline, BPC, L-arginine, L-NAME, and their combinations) were explored by the analysis of variance (ANOVA). Differences in the effect of individual adjuvants and their combination on the duration of tetracaine/oxybuprocaine induced corneal anesthesia were analyzed in detail using the Mann-Whitney test for independent samples, followed by Benjamini and Hochberg procedure to control false discovery rate (FDR). All statistical analyses were performed using MedCalc 9.5.1.0 (MedCalc Software, Mariakerke, Belgium). The values of p<0.05 were considered statistically significant.
Results
We focused on therapy relation between the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine, applied alone and/or in combination, and corneal anesthesia in rats (Tables 1-3, Figs. 1-4).
Table 1. 0.5% tetracaine induced corneal anesthesia in rats and effect of pentadecapeptide BPC 157 (0.4 µg/eye), L-NAME (0.1 mg/eye) and/or L-arginine (2 mg/eye) administered alone or in combination after complete corneal anesthesia was established, at 1 min after 0.5% tetracaine.
| Pentadecapeptide BPC 157 (0.4 µg/eye), L-NAME (0.1 mg/eye), L-arginine (2 mg/eye), administered alone and in combination after complete corneal anesthesia was established, at 1 min after 0.5% tetracaine | Duration of 0.5% tetracaine corneal anesthesia | |
|---|---|---|
| T1 (min), mean ± SD | T4 (min), mean ± SD | |
| Saline | 59±5.2 | 109±12.5 |
| BPC 157 | 45±4.6* | 94±7.0* |
| L-arginine | 51±8.5* | 90±5.3* |
| L-arginine + BPC 157 | 34±5.8*# | 72±5.8*# |
| L-NAME | 84±7.7* | 126±8.4* |
| L-NAME + BPC 157 | 65±7.1# | 100±9.4# |
| L-arginine + L-NAME | 73±8.6*+ | 123±8.2*+ |
| L-arginine + L-NAME + BPC 157 | 44±3.9*# | 91±8.4*# |
T1 = time point when blink response to maximal corneal stimulation appeared again; T4 = time point when corneal sensitivity returned to baseline values; T1-T4 = complete corneal anesthesia duration (minutes, mean ± SD); *p<0.05 vs. saline (control); #p<0.05 vs. corresponding ‘non-BPC 157 group’; +p<0.05 vs. corresponding ‘L-NAME-group’; Mann-Whitney test for independent samples followed by Benjamini and Hochberg procedure to control the false discovery rate (FDR)
Table 2. 0.4% oxybuprocaine induced corneal anesthesia in rats and effect of pentadecapeptide BPC 157 (0.4 µg/eye), L-NAME (0.1 mg/eye) and/or L-arginine (2 mg/eye) administered alone and in combination after complete corneal anesthesia was established, at 1 min after 0.4% oxybuprocaine.
| Pentadecapeptide BPC 157 (0.4 µg/eye), L-NAME (0.1 mg/eye), L-arginine (2 mg/eye), administered alone and in combination after complete corneal anesthesia was established, at 1 min after 0.4% oxybuprocaine corneal anesthesia | Duration of 0.4% oxybuprocaine corneal anesthesia | |
|---|---|---|
| T1 (min), mean ± SD | T4 (min), mean ± SD | |
| Saline | 64±5.7 | 126±3.9 |
| BPC 157 | 51±5.0* | 106±7.4* |
| L-arginine | 56±6.9* | 112±5.9* |
| L-arginine + BPC 157 | 48±5.9*# | 96±5.5*# |
| L-NAME | 89±5.7* | 137±7.9* |
| L-NAME + BPC 157 | 71±7.6# | 104±7.0*# |
| L-arginine + L-NAME | 77±6.7*+ | 131±8.0 |
| L-arginine + L-NAME + BPC 157 | 60±7.2# | 98±6.7*# |
T1 = time point when blink response to maximal corneal stimulation appeared again; T4 = time point when corneal sensitivity returned to baseline values; T1-T4 = complete corneal anesthesia duration (minutes, mean ± SD); *p<0.05 vs. saline (control); #p<0.05 vs. corresponding ‘non-BPC 157 group’; +p<0.05 vs. corresponding ‘L-NAME-group’; Mann-Whitney test for independent samples followed by Benjamini and Hochberg procedure to control the false discovery rate (FDR)
Table 3. 0.5% tetracaine corneal anesthesia in rats and effect of pentadecapeptide BPC 157 (0.4 µg/eye), L-NAME (0.1 mg/eye) and/or L-arginine (2 mg/eye) administered alone and in combination after complete corneal anesthesia was established, at 1 min after 0.5% tetracaine.
| Pentadecapeptide BPC 157 (0.4 µg/eye), L-NAME (0.1 mg/eye), L-arginine (2 mg/eye) administered alone and in combination after complete corneal anesthesia was established, at 1 min after 0.5% tetracaine | At 30 min | At 60 min | At 150 min |
|---|---|---|---|
| Saline | 2.5±0.5 | 2.0±0.6 | 2.0±0.6 |
| BPC 157 | 6.5±0.5* | 5.0±0.6* | 5.5±0.5* |
| L-arginine | 5.5±0.5* | 3.5±0.5* | 3.0±0.9 |
| L-arginine + BPC 157 | 5.5±0.5* | 6.0±0.6* | 6.0±0.6* |
| L-NAME | 3.5±0.5* | 2.5±0.5 | 1.0±0.6* |
| L-NAME + BPC 157 | 4.0±0.6* | 4.5±0.5* | 5.5±0.5* |
| L-arginine + L-NAME | 4.0±0.6* | 4.0±0.9* | 5.0±0.9* |
| L-arginine + L-NAME + BPC 157 | 4.5±0.8* | 4.5±0.5* | 5.5±0.5* |
Standard Schirmer tracks using 2 mm-wide test strips cut from Schirmer test paper to fit the size of the rat’s eye were inserted 1 mm into the inferior conjunctival fornix and placed for 5 minutes after which results in millimeters (mean ± SD) were read; *p<0.05 vs. saline (control); Mann-Whitney test for independent samples followed by Benjamini and Hochberg procedure to control false discovery rate (FDR)
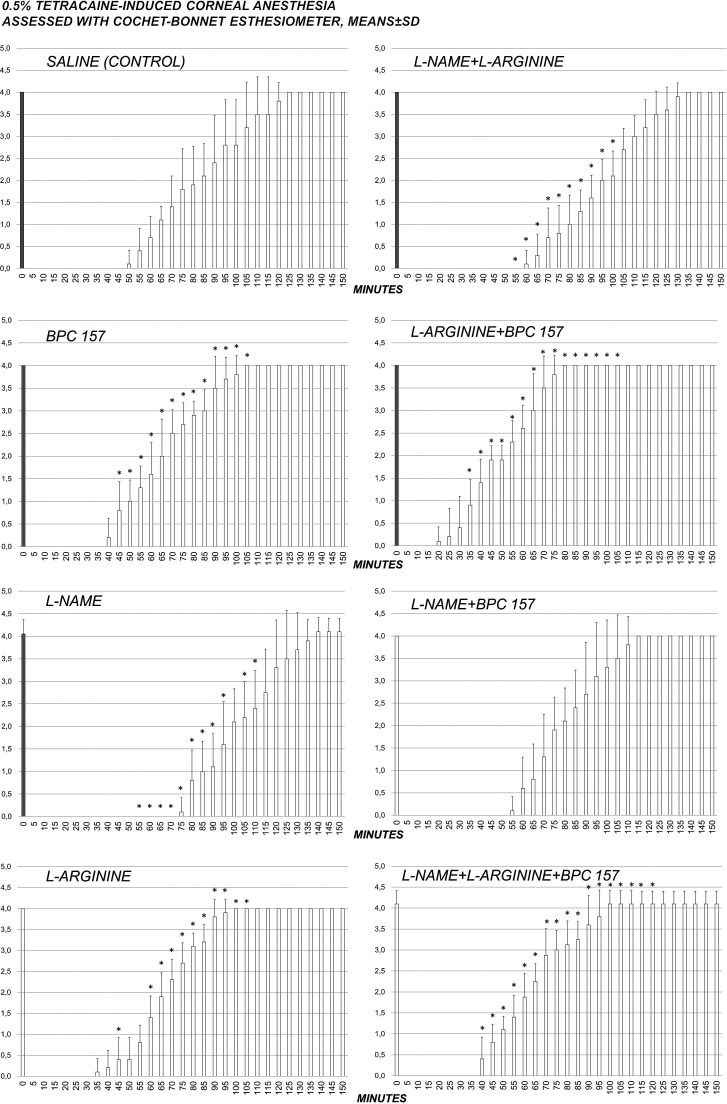
Fig. 1.
Complete course of 0.5% tetracaine corneal anesthesia in rats and effect of pentadecapeptide BPC 157 (0.4 µg/eye), L-NAME (0.1 mg/eye) and/or L-arginine (2 mg/eye) administered alone and in combination after complete corneal anesthesia was established, at 1 min after 0.5% tetracaine. After initial corneal sensitivity testing, each eye was tested at 5-minute intervals until full sensitivity was restored; *p<0.05 vs. saline (control).
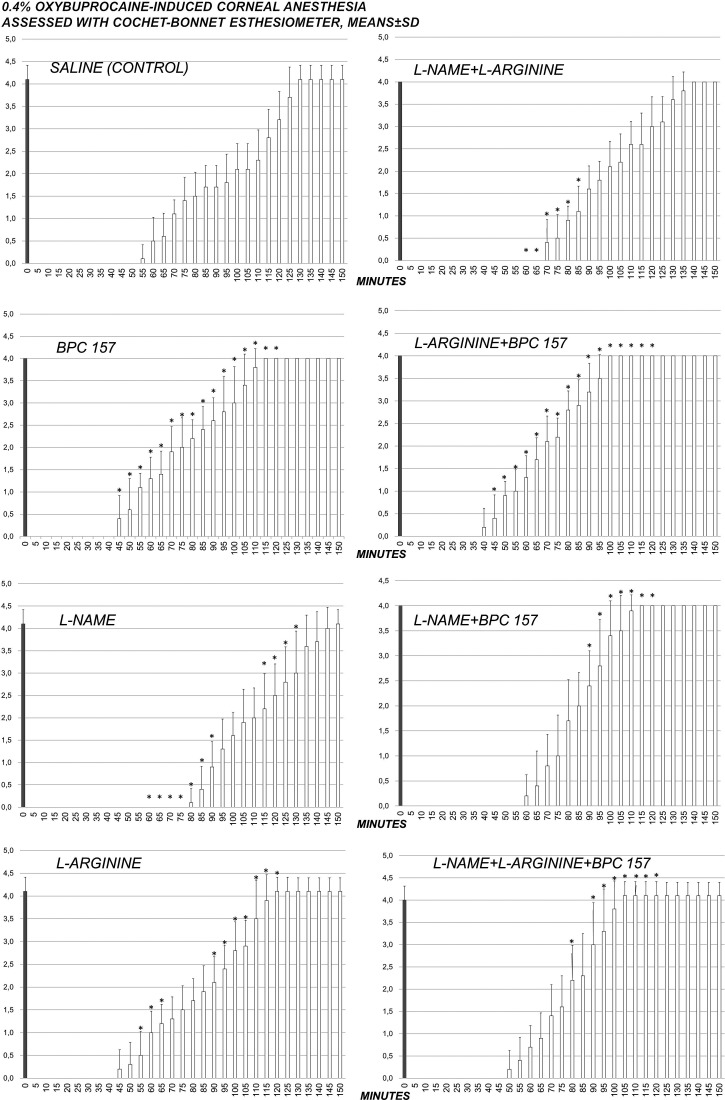
Fig. 2.
Complete course of 0.4% oxybuprocaine corneal anesthesia in rats and effect of pentadecapeptide BPC 157 (0.4 µg/eye), L-NAME (0.1 mg/eye) and/or L-arginine (2 mg/eye) administered alone and in combination after complete corneal anesthesia was established, at 1 min after 0.4% oxybuprocaine corneal anesthesia. After initial corneal sensitivity testing, each eye was tested at 5-minute intervals until full sensitivity was restored; *p<0.05 vs. saline (control).
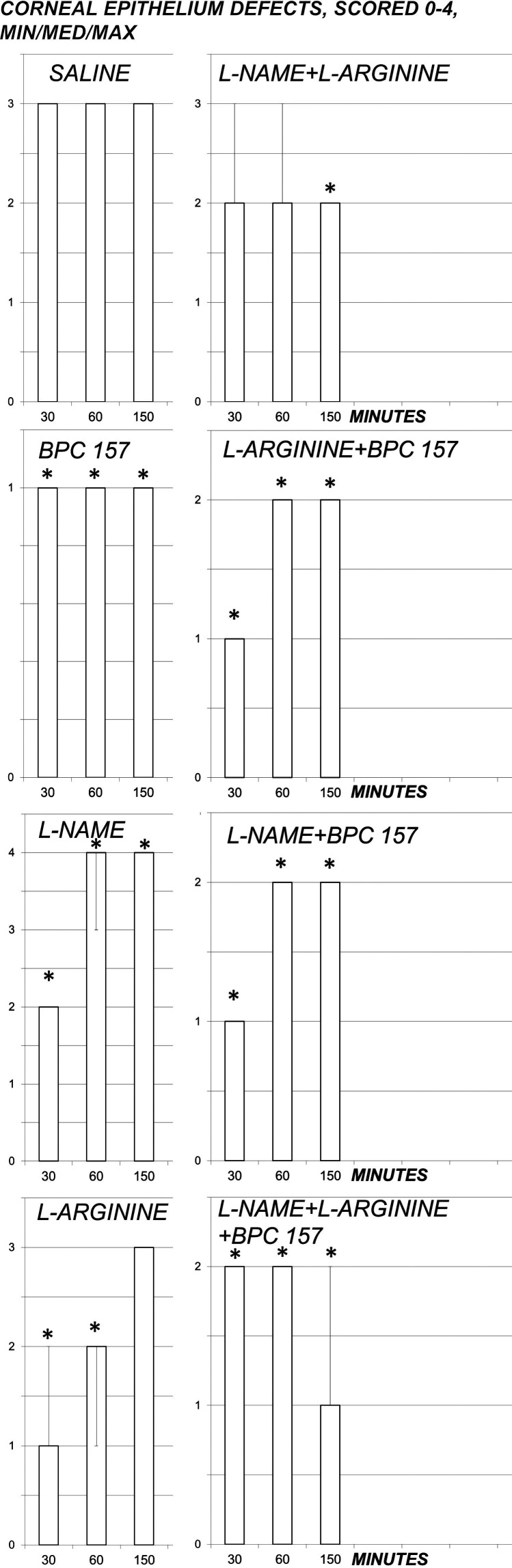
Fig. 3.
Corneal epithelium defects, scored 0-4 (Min/Med/Max), course with 0.5% tetracaine corneal anesthesia in rats and effect of pentadecapeptide BPC 157 (0.4 µg/eye), L-NAME (0.1 mg/eye) and/or L-arginine (2 mg/eye) administered alone and in combination after complete corneal anesthesia was established, at 1 min after 0.5% tetracaine corneal anesthesia. After initial corneal sensitivity testing, each eye was tested at 5-minute intervals until full sensitivity was restored; *p<0.05 vs. saline (control).
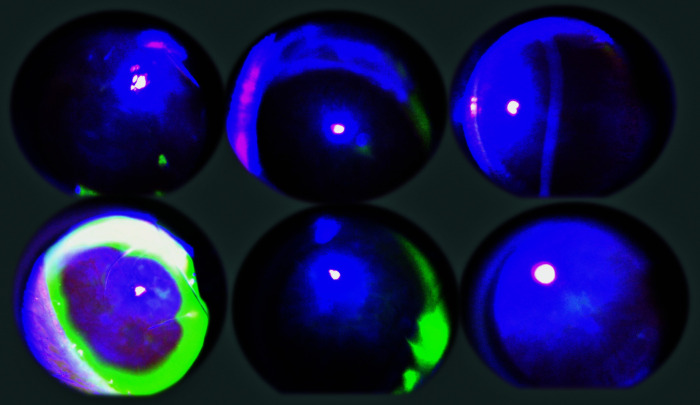
Fig. 4.
Illustrative presentation of the course of corneal defects with 0.5% tetracaine corneal anesthesia in BPC 157 treated rats (upper) and control rats (lower), at 30 (left), 60 (middle) and 150 (right) minutes after 0.5% tetracaine. Corneal epithelium defects were monitored by slit lamp (PSL Portable Slit Lamp, Reichert, Buffalo, USA) with blue cobalt filter and by photographing the lesions after staining with standard 10% fluorescein (Alcon, Geneva, Switzerland) according to the experimental protocol.
Corneal sensitivity measurement
A quite long-lasting corneal insensitivity was induced by 0.5% tetracaine or 0.4% oxybuprocaine in each eye and measured using the Cochet-Bonnet esthesiometer (Tables 1-2, Figs. 1-2). At 1 minute after two drops of 0.5% tetracaine or 0.4% oxybuprocaine in each eye, rats received locally (two drops/eye) pentadecapeptide BPC 157, L-NAME, L-arginine, administered alone and in combination. BPC 157 applied as eye drops consistently recovered corneal sensitivity in both tetracaine- and oxybuprocaine-rats. In tetracaine-rats and oxybuprocaine-rats, an indicative point was that the full counteracting effect of the BPC 157 administration also occurred in the presence of NOS-blockade (L-NAME+BPC 157), as well as in NOS-substrate application (L-arginine+BPC 157). L-arginine eventually shortened the duration of corneal insensitivity. Lengthening of corneal insensitivity appeared after L-NAME. When L-NAME and L-arginine were given together (L-NAME+L-arginine), they antagonized each other’s effect (i.e. NOS specific effect), but these rats still exhibited lengthening of corneal insensitivity. Additional BPC 157 co-application (L-NAME+L-arginine+BPC 157) would have re-established counteraction over topical ophthalmic anesthetic-induced corneal insensitivity.
Corneal epithelium defect assessment
A quite long-lasting 0.5% tetracaine corneal insensitivity was soon associated with development of corneal lesions, sustained consistently until the end of the experiment, which was markedly influenced by pentadecapeptide BPC 157, L-NAME and L-arginine administered alone and in combination (Figs. 3-4). Much like corneal insensitivity, BPC 157 applied as eye drops consistently reduced development of corneal lesions. Likewise, the full counteracting effect of the BPC 157 administration also occurred in the presence of NOS-blockade (L-NAME+BPC 157), as well as in NOS-substrate application (L-arginine+BPC 157). L-arginine that markedly shortened the duration of corneal insensitivity had only temporary effect on corneal lesions, unable to counteract final lesions. Consistent lengthening of corneal insensitivity with L-NAME was associated with initial protection reversed to subsequent aggravation. When L-NAME and L-arginine were given together (L-NAME+L-arginine), their mutual antagonizing ensured that these rats, which exhibited lengthening of corneal insensitivity, consistently had fewer lesions. Additional BPC 157 co-application (L-NAME+L-arginine+BPC 157) resulted consistently in fewer lesions.
Schirmer test assessment
Schirmer test showed that tetracaine application consistently decreased tear volume (Table 3). BPC 157 application completely counteracted tetracaine-induced decrease of tear volume and maintained normal healthy values. L-arginine counteracted tetracaine-induced decrease of tear volume only at the earliest interval. L-NAME initially counteracted but later even augmented tetracaine-induced decrease of tear volume. When given together, L-NAME+L-arginine, they consistently counteracted tetracaine-induced decrease of tear volume. Additional BPC 157 co-application (L-NAME+BPC 157, L-arginine+BPC 157, L-NAME+L-arginine+BPC 157) consistently counteracted tetracaine-induced decrease of tear volume.
Discussion
With the investigated topical ophthalmic anesthetics applied on the surface of the eye and known to act by blocking sodium channels in neuronal axons, thus preventing conduction along the axons and keeping the brain from detecting painful stimuli (26), BPC 157 applied as eye drops has a particular counteracting action. It recovers corneal sensitivity, markedly eliminates corneal lesions, and counteracts decrease of tear volume by interacting with NOS. Likely, this particular action may be consequential to the interference with the essential action of local anesthetics.
Of note, we should appreciate the BPC 157 actions responsible for healing of the perforating corneal injury and total debridement of corneal epithelium and maintained corneal transparency, or BPC 157 counteraction of the damaging effects of lacrimal gland extirpation and dry eye syndrome in rats (20-22). These actions against topical ophthalmic anesthetics may be sustained, thus preventing the otherwise inescapable direct epithelial toxicity that would lead to corneal drying due to the loss of corneal sensation, causing subsequent decrease in blink rate and tear production.
Thus, we recorded lesion counteraction and eventually less lesion formation in both tetracaine-rats and oxybuprocaine-rats administered BPC 157 therapy. In addition, BPC 157 markedly interferes with atropine effects, reduces atropine-induced mydriasis, and thereby it may increase tear volume once it has been decreased with the application of topical ophthalmic anesthetics on the surface of the eye.
As mentioned above, BPC 157 largely interacts with NOS in different models and species, with both L-NAME and L-arginine effects (1-13). In tetracaine-rats and oxybuprocaine-rats, an indicative point is that the full counteracting effect of BPC 157 administration also occurs in the presence of NOS blockade (L-NAME+BPC 157) and NOS substrate application (L-arginine+BPC 157). Moreover, it occurs in the circumstances of particular NOS involvement. While both antagonize atropine-induced mydriasis (23), after topical ophthalmic anesthetics L-NAME and L-arginine they have a distinctive effect on corneal insensitivity duration, rate of corneal lesions and tear volume. L-arginine eventually shortens duration of corneal insensitivity, shows counteraction to corneal lesions (and counteraction of tetracaine-induced decrease in tear volume), however, only in the early but not in later period. L-NAME application was associated with prolonged corneal insensitivity, higher rate of corneal lesions and reduction of tear volume. When L-NAME and L-arginine were given together (L-NAME+L-arginine), they antagonized each other’s effect (i.e. NOS specific effect), and these rats exhibited lengthening of corneal insensitivity, but had less corneal lesions. They also counteracted tetracaine-induced decrease of tear volume. Thus, with corneal insensitivity, L-NAME effect prevailed over L-arginine effect; with less corneal lesions, L-arginine effect prevailed over L-NAME effect. This distinctive effect (corneal insensitivity, corneal lesion, tear formation) may indicate particular NOS involvement (corneal insensitivity vs. corneal lesion along with tear formation) that may be distinctively affected by the application of NO agents. Anyway, be it in the early or late course, additional BPC 157 co-application (L-NAME+L-arginine+BPC 157) would have re-established counteraction over topical ophthalmic anesthetic-induced effects (i.e. particular BPC 157 to NOS relations).
Of note, distinctive NOS presentation was also recorded in other studies with various models (23, 31-34). This provides a common model (23, 31-34) that supports particular NOS involvement also beyond the topical ophthalmic anesthetic-induced adverse effects. On the other hand, this combined point observed with BPC 157 administration implicates its particular relations with topical ophthalmic anesthetics and possible counteraction of their adverse effects. Unlike topical ophthalmic anesthetic inhibition, with the open eye, there are light-evoked increases in tear volume (35), thus BPC 157 can permanently (L-arginine only temporarily) evoke protective reflexes such as lacrimation.
The noted counteraction of severe cardiac disturbances (bradycardia, prolongation of all of the observed waves and intervals, AV-block, ventricular ectopy, ventricular tachycardia, T-wave elevation and asystole) after bupivacaine (and counteraction of other various arrhythmias) (14-19) appears much like counteraction of adverse effects of tetracaine and oxybuprocaine topical ophthalmic anesthetics applied on the surface of the eye. Greater bupivacaine potential of direct cardiac toxicity than other agents, greater affinity for inactive and resting sodium channel configurations and slower dissociation from these channels (36) together support an even more effective counteraction of all tetracaine- and oxybuprocaine-induced adverse effects, as recorded in our study. Moreover, these findings approach the BPC 157 counteracting effect on the essential chain of the events following local anesthetic application (36, 37). These may be essential for penetration of both the epineurium and neuronal membrane, duration of the action by the time that local anesthetic remains in close proximity to neural fibers, and tetracaine lipid solubility and neuronal protein binding resulting in a local anesthetic that is more potent with an extended duration of action (38).
Of note, in corneal innervation studies (39-41), mechanical threshold assessed by Belmonte esthesiometer provides structural and functional correlation, and thus corneal nerve function maintenance and recovery. In systemic terms, this may explain why BPC 157 produced analgesia in the MgSO4 and acetic acid intraperitoneal test (29), and counteracted peritonitis in several models (31-33, 42).
Likewise, in rats with severed sciatic nerve, BPC 157 markedly improves nerve healing and counteracts autotomy (43) that reflects chronic neuropathic pain, neuroma at the proximal nerve stump, regenerative nerve sprouts growing into all directions, as prevents or, at least, significantly attenuates the chain of events otherwise leading to the painful sensation referred to as denervated region (44). A corresponding autotomy counteraction appears in L2-L3 spinal compression model when the stable gastric pentadecapeptide BPC 157 improves the healing course of the spinal cord injury and leads to functional recovery in rats (45). As mentioned above, much like topical ophthalmic anesthetic-induced adverse effects, BPC 157 counteracts morphine-induced analgesia (46) and counteracts NSAID-induced toxicity (7, 47-55), also known to decrease corneal sensitivity (56). Therefore, the stable gastric pentadecapeptide BPC 157 can be a quite distinctive ‘healing antidote’ for adverse effects of topical ophthalmic anesthetics.
Considering the combined vasoconstrictive and local anesthetic properties, BPC 157 may also have a particular effect on vasculature that may influence the duration of topical ophthalmic anesthetic effect. This may be rapid activation of the bypassing loop that occurs in the rat with infrarenal occlusion of the inferior vena cava (and thereby, resolved Virchow), much like in the rats with ischemic/reperfusion colitis, duodenal venous congestion lesions, perforated cecum, bile duct ligation induced liver cirrhosis and portal hypertension (31-33, 42, 57). Accordingly, BPC 157 interacts with several molecular pathways (1, 42, 58-64). In particular, BPC 157 increased expression and internalization of VEGFR2, activation of the VEGFR2-Akt-eNOS signaling pathway without the need of other known ligands or shear stress (61).
Finally, to counteract topical ophthalmic anesthetic-induced disturbances, it may be that BPC 157 has an effect of its own on membrane potential. Inhibition of depolarization of HEK293 cells by bupivacaine in vitro fully supports that BPC 157 may, through direct action, successfully counteract the effect of topical ophthalmic anesthetics, much like the effect of bupivacaine (14).
Likewise, cell depolarization due to increasing magnesium concentration was inhibited in the presence of BPC 157 (1 µM) in vitro (34). Furthermore, BPC 157 reduced depolarization caused by hyperkalemia in HEK293 cells (15). Depolarization caused by BPC 157 (1 µm) was inhibited by the application of the nonspecific potassium blocker BaCl2 (1 mM) (15). In conclusion, we suggest BPC 157 as an antidote of topical ophthalmic anesthetics.
Acknowledgments
This work was supported by the Ministry of Science, Education and Sports, Republic of Croatia (grant number 108-1083570-3635) and by the Osijek Faculty of Medicine, Josip Juraj Strossmayer University of Osijek (grant code VIF2017-MEFOS-17).
References
- 1.Kang EA, Han YM, An JM, Park YJ, Sikiric P, Kim DH, et al. BPC157 as potential agent rescuing from cancer cachexia. Curr Pharm Des. 2018;24:1947–56. 10.2174/1381612824666180614082950 [DOI] [PubMed] [Google Scholar]
- 2.Seiwerth S, Brcic L, Vuletic LB, Kolenc D, Aralica G, Misic M, et al. BPC 157 and blood vessels. Curr Pharm Des. 2014;20:1121–5. 10.2174/13816128113199990421 [DOI] [PubMed] [Google Scholar]
- 3.Seiwerth S, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, et al. BPC 157 and standard angiogenic growth factors, gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr Pharm Des. 2018;24:1972–89. 10.2174/1381612824666180712110447 [DOI] [PubMed] [Google Scholar]
- 4.Sikiric P, Seiwerth S, Brcic L, Sever M, Klicek R, Radic B, et al. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des. 2010;16:1224–34. 10.2174/138161210790945977 [DOI] [PubMed] [Google Scholar]
- 5.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17:1612–32. 10.2174/138161211796196954 [DOI] [PubMed] [Google Scholar]
- 6.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Curr Med Chem. 2012;19:126–32. 10.2174/092986712803414015 [DOI] [PubMed] [Google Scholar]
- 7.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des. 2013;19:76–83. [DOI] [PubMed] [Google Scholar]
- 8.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014;20:1126–35. 10.2174/13816128113190990411 [DOI] [PubMed] [Google Scholar]
- 9.Sikiric P, Seiwerth S, Rucman R, Kolenc D, Vuletic LB, Drmic D, et al. Brain-gut axis and pentadecapeptide BPC 157: theoretical and practical implications. Curr Neuropharmacol. 2016;14:857–65. 10.2174/1570159X13666160502153022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikiric P, Seiwerth S, Rucman R, Drmic D, Stupnisek M, Kokot A, et al. Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr Pharm Des. 2017;23:4012–28. 10.2174/1381612823666170220163219 [DOI] [PubMed] [Google Scholar]
- 11.Sikiric P, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr Pharm Des. 2018;24:1990–2001. 10.2174/1381612824666180608101119 [DOI] [PubMed] [Google Scholar]
- 12.Sikiric P, Hahm KB, Blagaic AB, Tvrdeic A, Pavlov KH, Petrovic A, et al. Stable gastric pentadecapeptide BPC 157, Robert’s stomach cytoprotection/adaptive cytoprotection/organoprotection, Selye’s stress coping response: progress, achievement, future. Gut Liver. 2020. June 4;•••: [Epub ahead of print] 10.5009/gnl18490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwyer D, Wragg NM, Wilson SL. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res. 2019. August;377(2):153–9. 10.1007/s00441-019-03016-8 [DOI] [PubMed] [Google Scholar]
- 14.Zivanovic-Posilovic G, Balenovic D, Barisic I, Strinic D, Stambolija V, Udovicic M, et al. Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur J Pharmacol. 2016;793:56–65. 10.1016/j.ejphar.2016.10.035 [DOI] [PubMed] [Google Scholar]
- 15.Barisic I, Balenovic D, Klicek R, Radic B, Nikitovic B, Drmic D, et al. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul Pept. 2013;181:50–66. 10.1016/j.regpep.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 16.Balenovic D, Bencic ML, Udovicic M, Simonji K, Hanzevacki JS, Barisic I, et al. Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: a relation with NO-system. Regul Pept. 2009;156:83–9. 10.1016/j.regpep.2009.05.008 [DOI] [PubMed] [Google Scholar]
- 17.Bosnjak ZJ, Graf BM, Sikiric P, Stowe DF. Protective effects of newly isolated gastric peptide following hypoxic and reoxygenation injury in the isolated guinea pig heart. FASEB J. 1994;8(4):A129. [Google Scholar]
- 18.Stambolija V, Stambolija TP, Holjevac JK, Murselovic T, Radonic J, Duzel V, et al. BPC 157. The counteraction of succinylcholine, hyperkalemia, and arrhythmias. Eur J Pharmacol. 2016;781:83–91. 10.1016/j.ejphar.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 19.Strinic D, Belosic Halle Z, Luetic K, Nedic A, Petrovic I, Sucic M, et al. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life Sci. 2017;186:66–79. 10.1016/j.lfs.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 20.Masnec S, Kokot A, Zlatar M, Kalauz M, Kunjko K, Radic B, et al. Perforating corneal injury in rat and pentadecapeptide BPC 157. Exp Eye Res. 2015. July;136:9–15. 10.1016/j.exer.2015.04.016 [DOI] [PubMed] [Google Scholar]
- 21.Lazić R, Gabrić N, Dekaris I, Bosnar D, Boban-Blagaić A, Sikirić P. Gastric pentadecapeptide BPC 157 promotes corneal epithelial defects healing in rats. Coll Antropol. 2005. June;29(1):321–5. [PubMed] [Google Scholar]
- 22.Kralj T, Kokot A, Kasnik K, Drmic D, Zlatar M, Seiwerth S, Sikiric P. Effects of pentadecapeptide BPC 157 on experimental rat model of dry eye. FASEB J. 2017;31(Suppl 1):993.3.
- 23.Kokot A, Zlatar M, Stupnisek M, Drmic D, Radic R, Vcev A, et al. NO system dependence of atropine-induced mydriasis and L-NAME- and L-arginine-induced miosis: reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur J Pharmacol. 2016. January 15;771:211–9. 10.1016/j.ejphar.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 24.McAlvin JB, Zhan C, Dohlman JC, Kolovou PE, Salvador-Culla B, Kohane DS. Corneal anesthesia with site 1 sodium channel blockers and dexmedetomidine. Invest Ophthalmol Vis Sci. 2015. June;56(6):3820–6. 10.1167/iovs.15-16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brewitt H, Bonatz E, Honegger H. Morphological changes of the corneal epithelium after application of topical anaesthetic ointments. Ophthalmologica. 1980;180(4):198–206. 10.1159/000308974 [DOI] [PubMed] [Google Scholar]
- 26.McGee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007. November;6(6):637–40. 10.1517/14740338.6.6.637 [DOI] [PubMed] [Google Scholar]
- 27.Judge AJ, Najafi K, Lee DA, Miller KM. Corneal endothelial toxicity of topical anesthesia. Ophthalmology. 1997. September;104(9):1373–9. 10.1016/S0161-6420(97)30128-6 [DOI] [PubMed] [Google Scholar]
- 28.Zemba M, Cilic AZ, Balenovic I, Cilic M, Radic B, Suran J, et al. BPC 157 antagonized the general anaesthetic potency of thiopental and reduced prolongation of anaesthesia induced by L-NAME/thiopental combination. Inflammopharmacology. 2015. December;23(6):329–36. 10.1007/s10787-015-0249-9 [DOI] [PubMed] [Google Scholar]
- 29.Sikiric P, Gyires K, Seiwerth S, Grabarevic Z, Rucman R, Petek M, et al. The effect of pentadecapeptide BPC 157 on inflammatory non-inflammatory direct and indirect pain and capsaicin neurotoxicity. Inflammopharmacology. 1993;2:121–7. 10.1007/BF02659088 [DOI] [Google Scholar]
- 30.Nemet A, Belkin M, Rosner M. Transplantation of newborn lacrimal gland cells in a rat model of reduced tear secretion. Isr Med Assoc J. 2007. February;9(2):94–8. [PubMed] [Google Scholar]
- 31.Duzel A, Vlainic J, Antunovic M, Malekinusic D, Vrdoljak B, Samara M, et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: new insights. World J Gastroenterol. 2017;23:8465–88. 10.3748/wjg.v23.i48.8465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drmic D, Samara M, Vidovic T, Malekinusic D, Antunovic M, Vrdoljak B, et al. Counteraction of perforated cecum lesions in rats: effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J Gastroenterol. 2018;24:5462–76. 10.3748/wjg.v24.i48.5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amic F, Drmic D, Bilic Z, Krezic I, Zizek H, Peklic M, et al. Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. World J Gastroenterol. 2018;24:5366–78. 10.3748/wjg.v24.i47.5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medvidovic-Grubisic M, Stambolija V, Kolenc D, Katancic J, Murselovic T, Plestina-Borjan I, et al. Hypermagnesemia disturbances in rats, NO-related: pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology. 2017;25:439–49. 10.1007/s10787-017-0323-6 [DOI] [PubMed] [Google Scholar]
- 35.Okamoto K, Tashiro A, Thompson R, Nishida Y, Bereiter DA. Trigeminal interpolaris/caudalis transition neurons mediate reflex lacrimation evoked by bright light in the rat. Eur J Neurosci. 2012. December;36(11):3492–9. 10.1111/j.1460-9568.2012.08272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012;59:90–101. 10.2344/0003-3006-59.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cummins TR. Setting up for the block: the mechanism underlying lidocaine’s use-dependent inhibition of sodium channels. J Physiol. 2007;582(Pt 1):11. 10.1113/jphysiol.2007.136671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hersh EV, Saraghi M, Moore PA. Intranasal tetracaine and oxymetazoline: a newly approved drug formulation that provides maxillary dental anesthesia without needles. Curr Med Res Opin. 2016. November;32(11):1919–25. 10.1080/03007995.2016.1238352 [DOI] [PubMed] [Google Scholar]
- 39.Erkan Turan K, Kocabeyoglu S, Bekircan-Kurt CE, Bezci F, Erdem-Ozdamar S, Irkec M. Ocular surface alterations and in vivo confocal microscopic characteristics of corneas in patients with myasthenia gravis. Eur J Ophthalmol. 2018. September;28(5):541–6. 10.1177/1120672117753688 [DOI] [PubMed] [Google Scholar]
- 40.Aykut V, Elbay A, Çigdem Uçar I, Esen F, Durmus A, Karadag R, et al. Corneal sensitivity and subjective complaints of ocular pain in patients with fibromyalgia. Eye (Lond). 2018. April;32(4):763–7. 10.1038/eye.2017.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcos-Fernández MÁ, Tabernero SS, Herreras JM, Galarreta DJ. Impact of herpetic stromal immune keratitis in corneal biomechanics and innervation. Graefes Arch Clin Exp Ophthalmol. 2018. January;256(1):155–61. 10.1007/s00417-017-3826-3 [DOI] [PubMed] [Google Scholar]
- 42.Vukojević J, Siroglavic M, Kasnik K, Kralj T, Stancic D, Kokot A, et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul Pharmacol. 2018;106:54–66. 10.1016/j.vph.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 43.Gjurasin M, Miklic P, Zupancic B, Perovic D, Zarkovic K, Brcic L, et al. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regul Pept. 2010;160:33–41. 10.1016/j.regpep.2009.11.005 [DOI] [PubMed] [Google Scholar]
- 44.Asada H, Yamaguchi Y, Tsunoda S, Fukuda Y. The role of spinal cord activation before neurectomy in the development of autotomy. Pain. 1996;64:161–7. 10.1016/0304-3959(95)00086-0 [DOI] [PubMed] [Google Scholar]
- 45.Perovic D, Kolenc D, Bilic V, Somun N, Drmic D, Elabjer E, et al. Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats. J Orthop Surg Res. 2019;14:199. 10.1186/s13018-019-1242-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boban Blagaic A, Turcic P, Blagaic V, Dubovecak M, Jelovac N, Zemba M, et al. Gastric pentadecapeptide BPC 157 counteracts morphine-induced analgesia in mice. J Physiol Pharmacol. 2009;60 Suppl 7:177–81. [PubMed] [Google Scholar]
- 47.Sikirić P, Seiwerth S, Grabarević Z, Rucman R, Petek M, Jagić V, et al. Beneficial effect of a novel pentadecapeptide BPC 157 on gastric lesions induced by restraint stress, ethanol, indomethacin, and capsaicin neurotoxicity. Dig Dis Sci. 1996;41:1604–14. 10.1007/BF02087908 [DOI] [PubMed] [Google Scholar]
- 48.Ilic S, Drmic D, Zarkovic K, Kolenc D, Coric M, Brcic L, et al. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J Physiol Pharmacol. 2010;61:241–50. [PubMed] [Google Scholar]
- 49.Ilic S, Drmic D, Franjic S, Kolenc D, Coric M, Brcic L, et al. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011;88:535–42. 10.1016/j.lfs.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 50.Ilic S, Drmic D, Zarkovic K, Kolenc D, Brcic L, Radic B, et al. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur J Pharmacol. 2011;667:322–9. 10.1016/j.ejphar.2011.05.038 [DOI] [PubMed] [Google Scholar]
- 51.Stupnisek M, Franjic S, Drmic D, Hrelec M, Kolenc D, Radic B, et al. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb Res. 2012;129:652–9. 10.1016/j.thromres.2011.07.035 [DOI] [PubMed] [Google Scholar]
- 52.Lojo N, Rasic Z, Zenko Sever A, Kolenc D, Vukusic D, Drmic D, et al. Effects of diclofenac, L-NAME, L-arginine, and pentadecapeptide BPC 157 on gastrointestinal, liver, and brain lesions, failed anastomosis, and intestinal adaptation deterioration in 24 hour-short-bowel rats. PLoS One. 2016;11(9):e0162590. 10.1371/journal.pone.0162590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drmic D, Kolenc D, Ilic S, Bauk L, Sever M, Zenko Sever A, et al. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME. World J Gastroenterol. 2017;23:5304–12. 10.3748/wjg.v23.i29.5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vitaic S, Stupnisek M, Drmic D, Bauk L, Kokot A, Klicek R, et al. Nonsteroidal anti-inflammatory drug-induced failure of lower esophageal and pyloric sphincter and counteraction of sphincter failure with stable gatric pentadecapeptide BPC 157 in rats. J Physiol Pharmacol. 2017;68:265–72. [PubMed] [Google Scholar]
- 55.Sikiric P, Seiwerth S, Grabarevic Z, Rucman R, Petek M, Jagic V, et al. Pentadecapeptide BPC 157 positively affects both non-steroidal anti-inflammatory agent-induced gastrointestinal lesions and adjuvant arthritis in rats. J Physiol Paris. 1997;91:113–22. 10.1016/S0928-4257(97)89474-0 [DOI] [PubMed] [Google Scholar]
- 56.Cantarella RA, de Oliveira JK, Dorbandt DM, Montiani-Ferreira F. Effects of topical flurbiprofen sodium, diclofenac sodium, ketorolac tromethamine and benzalkonium chloride on corneal sensitivity in normal dogs. Open Vet J. 2017;7(3):254–60. 10.4314/ovj.v7i3.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sever AZ, Sever M, Vidovic T, Lojo N, Kolenc D, Vuletic LB, et al. Stable gastric pentadecapeptide BPC 157 in the therapy of the rats with bile duct ligation. Eur J Pharmacol. 2019;847:130–42. 10.1016/j.ejphar.2019.01.030 [DOI] [PubMed] [Google Scholar]
- 58.Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol. 2011;110:774–80. 10.1152/japplphysiol.00945.2010 [DOI] [PubMed] [Google Scholar]
- 59.Chang CH, Tsai WC, Hsu YH, Pang JH. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19:19066–77. 10.3390/molecules191119066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang T, Zhang K, Sun L, Xue X, Zhang C, Shu Z, et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther. 2015;9:2485–99. 10.2147/DDDT.S82030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsieh MJ, Liu HT, Wang CN, Huang HY, Lin Y, Ko YS, et al. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med (Berl). 2017;95:323–33. 10.1007/s00109-016-1488-y [DOI] [PubMed] [Google Scholar]
- 62.Tkalcevic VI, Cuzic S, Brajsa K, Mildner B, Bokulic A, Situm K, et al. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol. 2007;570:212–21. 10.1016/j.ejphar.2007.05.072 [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Wang W, Wang H, Li Y, Shi M, Li H, et al. Rapamycin attenuates splenomegaly in both intrahepatic and prehepatic portal hypertensive rats by blocking mTOR signaling pathway. PLoS One. 2016. January 6;11(1):e0141159. 10.1371/journal.pone.0141159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cesarec V, Becejac T, Misic M, Djakovic Z, Olujic D, Drmic D, et al. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur J Pharmacol. 2013;701:203–12. 10.1016/j.ejphar.2012.11.055 [DOI] [PubMed] [Google Scholar]