ABSTRACT
Point-of-care ultrasound (POCUS) has become an essential part of the evaluation of vision loss among emergency physicians in the emergency department (ED). It is frequently used to evaluate for vitreous hemorrhage, foreign bodies, retinal detachment, optic neuritis and posterior vitreous detachment; however, it can also be used to evaluate for a central retinal artery occlusion (CRAO). A POCUS can reveal a hyperechoic density in the optic nerve sheath just proximal to the retinal surface, and this is referred to as a retrobulbar ‘spot sign’ (RBSS). We present the case of an 88-year-old male that presented to our community ED with a painless loss of vision to his right eye. A POCUS revealed an RBSS of the central retinal artery and he was subsequently diagnosed with a CRAO. At his 1-month follow-up, he has regained light perception and 15% of his vision, however, remains with significant visual impairment.
INTRODUCTION
A central retinal artery occlusion (CRAO) is an ophthalmologic emergency with a poor overall prognosis. Research has shown that within 90–100 min of infarction, the retina begins to develop permanent damage [1, 2, 5]. Some case reports show that the visual recovery beyond this time frame is related to the collateral flow, intact cilioretinal artery and incomplete occlusion of the central retinal artery [1, 3]. The incidence is 8.5 per 100 000 patients [5]. This type of cerebral vascular accident has a mean age of 60–65 years, with over 90% of the cases involving patients over the age of 40 [2].
CASE REPORT
An 88-year-old male presented to our emergency department (ED) with an acute onset of a painless loss of vision to his right eye. His past medical history was significant for hypertension, coronary artery disease and atrial fibrillation (on apixaban). He stated he was lying in bed reading when he had a sudden painless loss of vision to his right eye, described as a curtain closing, for which he presented to our ED ~24 h later. He denied any other symptoms including headache, scalp pain, jaw claudication, focal weakness, numbness, angina or shortness of breath.
Vitals on arrival to the ED were as follows: 36.9°C, heart rate of 62 beats/min, respiratory rate of 16 breaths/min, blood pressure of 123/53 mmHg and pulse oximetry of 99% on room air. His cardiopulmonary exam was unremarkable. His neurologic exam was notable for a National Institute of Health Stroke Scale score of +2 for complete unilateral vision loss to the right eye. Intraocular pressures were 14 mmHg bilaterally. His remaining physical exam was notable for a right afferent pupillary defect (APD). To further assess the eye and its structures, a point-of-care ultrasound (POCUS) was then performed. Utilizing the high-frequency linear probe to the right eye, a transverse view demonstrated a retrobulbar spot sign (RBSS) in the distal portion of the central retinal artery within the optic nerve sheath (Fig. 1, red arrow). He had normal blood flow in his left eye (Fig. 2a), and no blood flow was seen to the central retinal artery of the right eye, with the yellow arrow pointing to the RBSS (Fig. 2b). The patient was then transferred to our tertiary referral center (TRC) for specialist consultation.
Figure 1 .
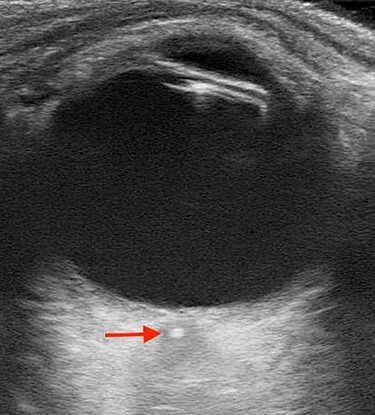
Transverse view using the high-frequency linear probe of the right eye; arrow demonstrates RBSS within the optic nerve sheath.
Figure 2 .
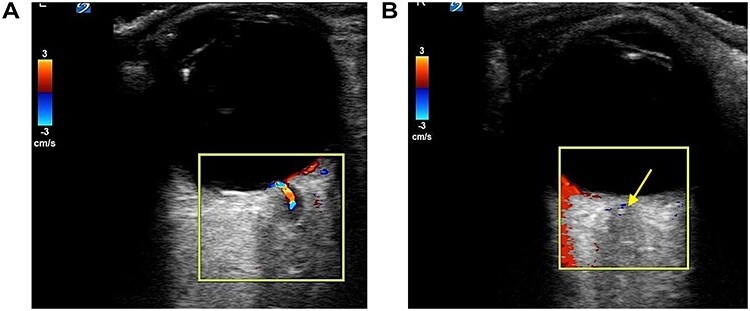
(a) Normal retinal blood flow in left eye; (b) CRAO with no blood flow seen to the central retinal artery or retina in right eye, yellow arrow highlightsRBSS.
Ophthalmology visualized a plaque on the dilated fundoscopic exam with associated retinal ischemia. Given the onset of ~24 h prior to arrival, the risks of tissue plasminogen activator (tPA) outweighed the benefits, as such, the patient was medically managed. His head computed tomography (CT) was unremarkable for acute changes. A magnetic resonance imaging MRI could not be done, secondary to his pacemaker not being compatible. His carotid duplex was without severe stenosis or thrombus. His echocardiography showed left ventricular diastolic dysfunction with an ejection fraction of 40%, which was similar to previous studies.
Overall, his workup was without evidence of carotid artery disease or vasculitis and it was suspected to be cardioembolic in origin related to his atrial fibrillation. On further questioning, it was found that the patient’s family member had passed away 2 weeks prior, leading to depression and multiple missed doses of his anticoagulation. Compliance of his anticoagulation was stressed on discharge. At his 1-month follow-up after his hospital admission, he has regained light perception and 15% of his vision.
DISCUSSION
A CRAO is a rare presentation in the ED; however, it is an ophthalmologic emergency with a poor overall prognosis which requires a high index of clinical suspicion. The clinical presentation of a CRAO in the ED typically involves a patient with an acute, painless unilateral loss of vision, with the possibility of temporal sparing [6]. The pertinent differential diagnoses for acute painless vision loss include optic neuritis, central retinal artery/vein occlusion, retinal detachment, temporal arteritis and atypical/complex migraines, to name a few. Optic neuritis may present with painful eye movements and temporal arteritis may have associated craniofacial pain despite the neuropathy being painless.
The ophthalmic artery is the first branch of the internal carotid artery and supplies the central retinal artery. The central retinal artery provides blood supply to the inner retina [1]. An acute occlusion of the central retinal artery results in ischemia to the inner retina, which may appear pale and edematous.
On the physical exam, patients may have an APD. A fundoscopic exam may show a ‘cherry red spot’, with a pale retina that is seen in ~90% of patients, or ‘box car segmentation’ that is seen in ~15% of patients [5]. For many causes of acute painless loss of vision in the ED, a dilated fundoscopic exam has been considered the gold standard. However, not many emergency physicians feel comfortable performing this, especially in a setting without ophthalmology coverage and with potentially limited resource settings. Alternatively, ultrasound (US) can provide a rapid and useful diagnostictool.
A US of the eye can be performed at the bedside to evaluate for blood flow and for a hyperechoic density in the optic nerve sheath just proximal to the retinal surface. When this density is seen, this is referred to as the RBSS and can indicate the type of embolism responsible for clinical symptoms. This RBSS is the actual clot responsible for the loss of vision; it is likely a cholesterol and fibrin clot that occludes the ophthalmic artery and retinal blood supply [7]. RBSS on US been seen in up to 51% of CRAOs and is found to have 100% specificity for thromboembolic CRAO [7–9]. When present, RBSS has been shown to have a poor long-term prognosis, and visual symptoms are poorly responsive to thrombolytics or other therapeutic interventions [7, 8]. If an RBSS is not visualized, other etiologies for CRAO should be considered and evaluated. An ocular US can also be used to assess for blood flow in the central retinal artery and vein using color Doppler imaging. In the setting of CRAO, there will be reduced or no blood flow in the central retinal artery in the affected eye [9]. CRAO is considered a stroke-equivalent, so patients in the ED should undergo further evaluation and be admitted for risk factor modification. Table 1 lists the potential causes of a CRAO [6, 7, 10].
Table 1.
Common Causes for a CRAO
| Renal artery thrombosis | Cardioembolic disease (atrial fibrillation, valvular disease) | Vasculitis (polyarteritis nodosa, lupus) | Giant cell arteritis | Trauma |
| Sickle-cell disease | Aortic dissection | Glaucoma | Vasospasm (migraine) | Hypercoagulable states |
| Conditions with low retinal blood flow including hypotension and carotid stenosis |
The initial management principals include an emergent ophthalmologic consultation, reduction of intraocular pressure if elevated, dislodgement of the thromboembolism and optimization of arterial flow to the retina. Various maneuvers are often attempted for patients presenting within 24 h of symptom onset. Techniques include ocular massage (direct and intermittent pressure over a closed eyelid for 10–15 s at a time with a rapid release to facilitate a pressure gradient that could potentially dislodge any embolism) and anterior chamber paracentesis (this allows for an acute decrease in intraocular pressure that could dislodge any embolism). Medications include intraocular pressure lowering agents (topical timolol, intravenous acetazolamide or mannitol), vasodilatory agents (e.g. nitroglycerin) and corticosteroids [3, 4]. Overall, little data exist to show that any of the above methods will improve the patient outcomes compared with observation alone, with some evidence showing they may actually worsen the visual outcomes [4]. Further, no clinical trials have demonstrated any improvement compared to the observation alone [3].
Other potential treatment modalities include the use of thrombolytics. Since a CRAO is a vaso-occlusive phenomenon, there has been interest in using tPA, with some published reports showing some improvement; however, it is not considered as the standard of care and is considered as an unproved benefit [4]. Additionally, some researchers are currently evaluating the use of hyperbaric oxygen in CRAO, with some studies showing a significant improvement in vision [2].
AVAILABILITY OF DATA AND MATERIAL
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
AUTHOR’S CONTRIBUTIONS
All authors contributed significantly to the literature review, manuscript draft and editing.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
No honorarium, grant or funding was received to produce this manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not required.
CONSENT FOR PUBLICATION
Recorded verbal consent has been obtained.
REFERENCES
- 1. Brown GC, Shieflds JA. Cilioretinal arteries and retinal arterial occlusion. Arch Ophthalmol June 2014;157:6. [DOI] [PubMed] [Google Scholar]
- 2. Murphy-Lavoie H, Butler F, Hagan C. Central retinal artery occlusion treated with oxygen: a literature review and treatment algorithm. Undersea and Hyperbaric Med September-October 2012;39:943–53. [PubMed] [Google Scholar]
- 3. Rudkin AK, Lee AW, Aldrich E et al. Clinical characteristics and outcome of current standard management of central retinal artery occlusion. Clin Experiment Ophthalmol 2010;38:496. doi: 10.1111/j.1442-9071.2010.02280.x. [DOI] [PubMed] [Google Scholar]
- 4. Schrag M, Youn T, Schindler J, Kirshner H, Greer D. Intravenous fibrinoglytic therapy in central retinal artery occlusion. A patient-level meta-analysis. JAMA Neurol 2015;72:1148–54. doi: 10.1001/jamaneurol.2015.1578. [DOI] [PubMed] [Google Scholar]
- 5. Hayreh SS, Zimmerman MD. Fundus changes in central retinal artery occlusion. Retina 2007;27:276–89. doi: 10.1097/01.iae.0000238095.97104.9b. [DOI] [PubMed] [Google Scholar]
- 6. Simon A, Gibbons R, Costantino T. Point of care ultrasound assessment of acute monocular vision loss. Vis J Emerg Med 2018;13:48–9. [Google Scholar]
- 7. Nedelmann M, Graef M, Weinand F et al. Retrobulbar spot sign predicts thrombolytic treatment effects and etiology in central retinal artery occlusion. Stroke 2015;46:2322–4. doi: 10.1161/STROKEAHA.115.009839. [DOI] [PubMed] [Google Scholar]
- 8. Smith AT, Wilbert CD, Ferre RM. Using the retrobulbar spot sign to assist in diagnosis and management of central retinal artery occlusions. J Ultrasound Med 2020;39:197–202. [DOI] [PubMed] [Google Scholar]
- 9. Stoner-Duncan B, Morris SC. Early identification of central retinal artery occlusion using point-of-care ultrasound. Clin Prac Cases Emerg Med 2019;3:13–5. doi: 10.5811/cpcem.2018.11.39406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varma DD, Cugati S, Lee AW et al. A review of central retinal artery occlusion: clinical presentation and management. Eye 2013;688–97. doi: 10.1038/eye.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


