Abstract
Context
There are concerns that a higher fat mass in the early life of preterm infants is associated with adverse cardiometabolic outcomes in young adulthood.
Objective
To investigate the role of IGF-I and growth in determining body composition of preterm infants at term equivalent age.
Methods
An observational study was conducted from August 2015 to August 2018. From birth to term equivalent age, IGF-I levels were measured bi-weekly and growth was assessed weekly. At term equivalent age, body composition was assessed through air displacement plethysmography; 65 infants with a gestational age of 24 to 32 weeks were assessed at term equivalent age, of whom 58 completed body composition measurement. The main outcome measures were fat (free) mass (g) and fat (free) mass percentage at term equivalent age.
Results
In the first month of life, each 0.1 nmol/L per week increase in IGF-I was associated with a 465 g (SE 125 g) increase in fat free mass. A greater increase in weight SDS in the first month of life was associated with a higher fat free mass percentage (B 200.9; 95% CI, 12.1-389.6). A higher head circumference SDS was associated with more fat free mass (r = 0.46; 95% CI, 0.21-0.65). However, a greater increase in weight SDS up to term equivalent age was associated with a lower fat free mass percentage (B −55.7, SE 9.4).
Conclusion
These findings suggest that impaired growth in the first month of life is associated with a less favorable body composition at term equivalent age.
Keywords: IGF-I, growth, body composition, preterm infants
Preterm birth abruptly interferes with a prime stage of development. Not surprisingly, almost half of infants born preterm show postnatal growth restriction by the time they are discharged from the hospital [1, 2]. In addition, preterm infants, compared with infants born at term, have been reported to have a higher fat mass percentage at term equivalent age [3]. Postnatal growth restriction, as well as increased fat mass in infancy, have been linked to impaired neurodevelopmental outcome [4, 5]. In addition, there are concerns that higher fat mass in early life is associated with adverse cardiometabolic outcomes in young adulthood [6, 7]. Therefore, it would be of interest to gain more insight in factors determining growth and body composition in early life, as a means to ameliorate later health outcomes in infants born preterm.
Insulin-like growth factor I (IGF-I) plays a key role in the regulation of growth and body composition in early life [8]. Interestingly, the relationship between IGF-I and body composition has been reported to vary, depending on the timing of IGF-I and body composition measurement. Based on previous studies, it could be speculated that high IGF-I levels between preterm birth and term equivalent age increase fat free mass, while from term age onwards higher IGF-I could be associated with more fat mass [9-11]. Nevertheless, only a few studies report on IGF-I in relation to body composition in preterm infants. Therefore, definitive conclusions on the relationship between IGF-I and body composition are yet to be drawn.
This study aimed to explore the relationship between IGF-I, growth, and body composition in preterm infants up to term equivalent age. To account for the possible impact of timing, IGF-I levels were assessed in different time frames between birth and term equivalent age and associations between changes in IGF-I, growth, and body composition were explored.
Methods
Study Population
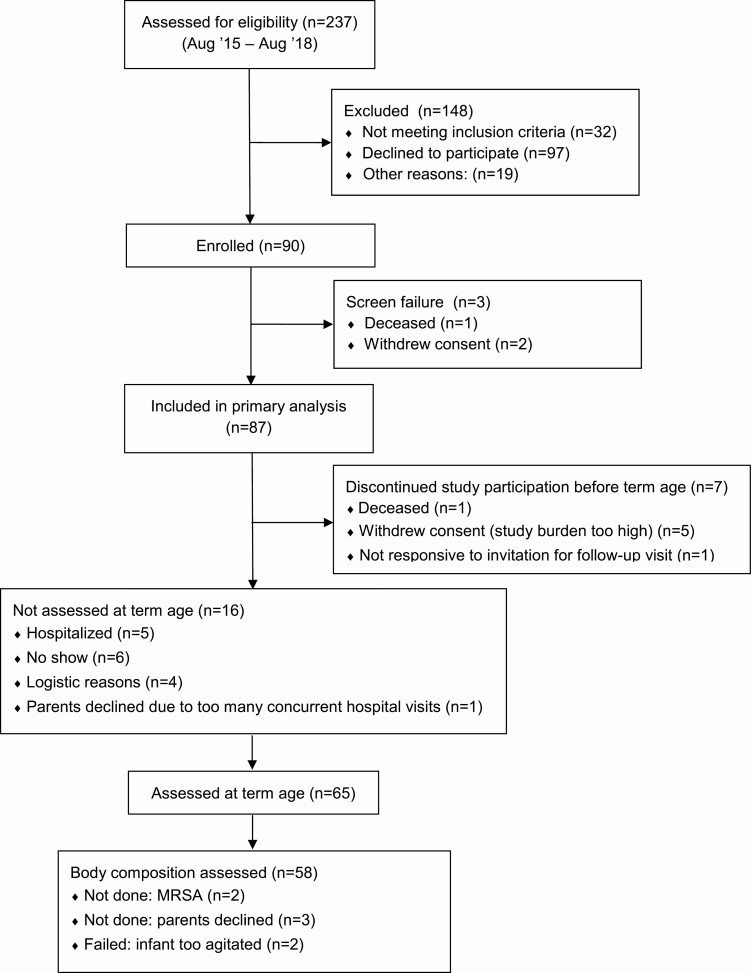
Ninety infants were enrolled between August 2015 and August 2018 (Fig. 1) The study participants were part of the NUTRIE study (Nutrition in relation to the endocrine regulation of preterm growth study). This longitudinal observational study collected data on nutritional intake, linear growth, body composition, and IGF-I levels between preterm birth and 2 years corrected age. Infants admitted to the neonatal intensive care unit (NICU) of the VU University Medical Center were assessed for eligibility if they were born at a gestational age of 24 weeks + 0/7 days up to and including 31 weeks + 6/7 days. Infants who had substantial congenital abnormalities were excluded. Informed consent was obtained within the first week of life.
Figure 1.
Enrollment and follow-up of study participants.
The NUTRIE study was powered to detect a medium size effect (r = 0.35) of IGF-I on fat mass percentage. To that end, a sample size of at least 62 infants was required (power 80%, significance 5%). With an expected dropout rate of 10%, the aim was to include 70 infants.
The study was approved by the medical research ethics committee of the VU University Medical Center and was registered in the Dutch trial register (www.trialregister.nl; NTR5311).
Study Procedures
All participants were admitted to the NICU of the VU University medical center within 24 hours of birth. Infants were discharged to step-down clinics in other hospitals when the clinical condition permitted to do so and the infants had reached a postmenstrual age (PMA) of at least 30 weeks and a weight of at least 1000 grams. Clinical data was collected up to 36 weeks PMA or discharge home, whichever came first. Infants were invited for follow-up at term equivalent age.
Growth
From birth to hospital discharge, weight, length, and head circumference were measured weekly by the nursing staff. Weight was measured to the nearest gram on an electronic scale. Length was measured with a length board to the nearest 0.5 cm. Occipital-frontal head circumference was measured to the nearest 0.1 cm with a nonstretchable measuring tape. At the follow-up visit, growth was measured in the same way by 2 investigators. Fenton growth charts were used to calculate weight standard deviation score (SDS) [12]. Growth restriction was defined as a weight SDS under the tenth percentile (−1.3 SD) [1].
Body Composition
Fat mass and fat free mass were measured at term equivalent age through air displacement plethysmography, using the PeaPod. Infants were measured nude and hair was smoothened using hair oil. Movement was allowed, but in case of excessive crying the measurement was stopped. All measurements were done by 2 investigators. In line with the manufacturer’s guideline, quality control checks were done daily and the PeaPod scale was calibrated every 2 weeks. A more detailed description of the PeaPod measurements can be found elsewhere [13]. The coefficient of variation for repeated measurements has been reported to lie between 0.02% and 0.09% [13].
IGF-I
IGF-I was sampled from umbilical cord blood at birth, followed by venous or capillary blood sampling from the infant every other week up to 36 weeks PMA and once at term equivalent age. A chemiluminescence immunoassay (LIAISON, DiaSorin, Italy) was used for the analysis (coefficients of variation: intra-assay, 8%; interassay, 7%).
Potential Critical Windows
Based on previous studies investigating the influence of IGF-I on body composition, the following time frames were assessed to analyze the predictive value of (changes in) IGF-I and weight, length, and head circumference SDS on body composition at term equivalent age:
Potential Confounders
Nutrition
Actual daily macronutrient intake was calculated from the intake documented in hospital records between birth and 36 weeks PMA and total macronutrient intake was assessed as a potential confounder.
Infants were started off with minimal enteral feeding and total parenteral nutrition shortly after birth. Full enteral feeding (160 mL.kg-1.day-1) was aimed to be achieved within 7 to 10 days after birth. In case of poor growth, as assessed by the clinician in charge, enteral nutrition was supplemented with protein fortifier (Nutrilon Nenatal Protein Fortifier, Nutricia, Wageningen, the Netherlands) or fat emulsion (Calogen, Nutricia, Wageningen, the Netherlands).
Infants were primarily fed their own mother’s milk. The average intake of own mother’s milk was 90% of the total enteral intake, supplemented with donor human milk or preterm starters formula, if parents declined donor human milk use. Donor human milk was only administered up to 32 weeks PMA, thereafter preterm starters formula was given if own mother’s milk did not suffice. During hospitalization no infants were exclusively formula fed.
Comorbidities
Data were collected on the occurrence of bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), late-onset sepsis (LOS), intraventricular hemorrhage (IVH), patent ductus arteriosus (PDA) and retinopathy of prematurity (ROP). The occurrence and severity of these conditions were assessed as potential confounders.
Other Confounders
Lastly, gender and ethnicity were assessed as a potential confounders.
Statistical Analysis
Continuous variables were presented as mean and SD. Dichotomous variables were presented as count and percentages.
Mixed models were used to predict the change in IGF-I and the SDS of weight, length, and head circumference for every individual in the previously mentioned time frames.
Partial correlations, controlling for gestational age at birth and postmenstrual age at the time of body composition measurements, were reported for associations between the (change in) SD scores for weight, length, and head circumference and fat mass, and fat free mass. Likewise, partial correlations were reported for IGF-I and the aforementioned outcomes.
Due to the nonnormal distribution of fat free mass percentage the association between the (change in) SD scores for weight, length, and head circumference and fat free mass percentage was explored using linear regression modeling. Gestational age at birth and PMA at the time of body composition measurement were entered in the regression model as covariates.
Potential confounders were evaluated with stepwise (backward) regression analysis.
All statistical analyses were done using IBM SPSS Statistics 24 for Windows (IBM Corp., Armonk, NY, USA). Two-sided statistical significance was assumed at P values less than 0.05.
Results
Eighty-seven infants were included in the primary analysis for growth and IGF-I. Sixty-five infants were assessed at term equivalent age, of whom 58 completed body composition measurement (Fig. 1). The sample size for IGF-I measurements per postmenstrual age is shown in Table 1. Baseline characteristics are shown in Table 2.
Table 1.
IGF-I sample size per postmenstrual age
| Postmenstrual age (weeks) | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | TEA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Postnatal samples, n | 0 | 0 | 1 | 3 | 6 | 12 | 16 | 22 | 25 | 33 | 25 | 26 | 29 | 40 |
| Number of infants born at respective gestational ages | 2 | 3 | 6 | 14 | 15 | 21 | 14 | 12 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total number of participants | 2 | 5 | 10 | 24 | 39 | 60 | 74 | 86 | 81 | 81 | 81 | 81 | 81 | 65 |
Postnatal samples numbers reflect the number of samples excluding umbilical cord blood. Mean (SD) postmenstrual age at the term equivalent visit was 43.3 (2.6) weeks.
Abbreviations: TEA, term equivalent age.
Table 2.
Baseline characteristics
| All (n = 87) | |
|---|---|
| Gender, n (%) | |
| Male | 44 (50.6) |
| Female | 43 (49.4) |
| Ethnicity, n (%) | |
| White | 66 (75.9) |
| Other | 21 (24.1) |
| Gestational age (weeks), mean (SD) | 29.0 (1.8) |
| Birthweight (g), mean (SD) | 1210 (216) |
| Birthweight SDS, mean (SD) | 0.0 (0.7) |
| Birthweight SDS < −1.3, n (%) | 3 (3.4) |
| Birth length (cm), mean (SD) | 37.4 (3.2) |
| Birth length SDS, mean (SD) | 0.0 (0.9) |
| Birth head circumference (cm), mean (SD) | 26.7 (2.2) |
| Birth head circumference SDS, mean (SD) | 0.3 (1.0) |
| BPD, n (%) | |
| Yes | 30 (34.4) |
| No | 57 (65.5) |
| NEC, n (%) | |
| Yes | 8 (9.2) |
| No | 79 (90.8) |
| LOS, n (%) | |
| Yes | 30 (34.5) |
| No | 57 (65.5) |
| PDA, n (%) | |
| No PDA | 68 (78.2) |
| Hemodynamically insignificant PDA | 11 (12.6) |
| Hemodynamically significant PDA | 8 (9.2) |
| ROP, n (%) | |
| No ROP | 82 (94.3) |
| ROP stage I | 4 (4.6) |
| ROP stage III | 1 (1.1) |
| IVH, n (%) | |
| No IVH | 64 (73.6) |
| IVH grade I | 8 (9.2) |
| IVH grade II | 11 (12.6) |
| IVH grade III | 4 (4.6) |
Abbreviations: BPD, bronchopulmonary dysplasia; IRDS, infant respiratory stress syndrome; IVH, intraventricular hemorrhage; LOS, late-onset sepsis; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PHVD, post-hemorrhagic ventricular dilatation; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity.
Growth and Body Composition
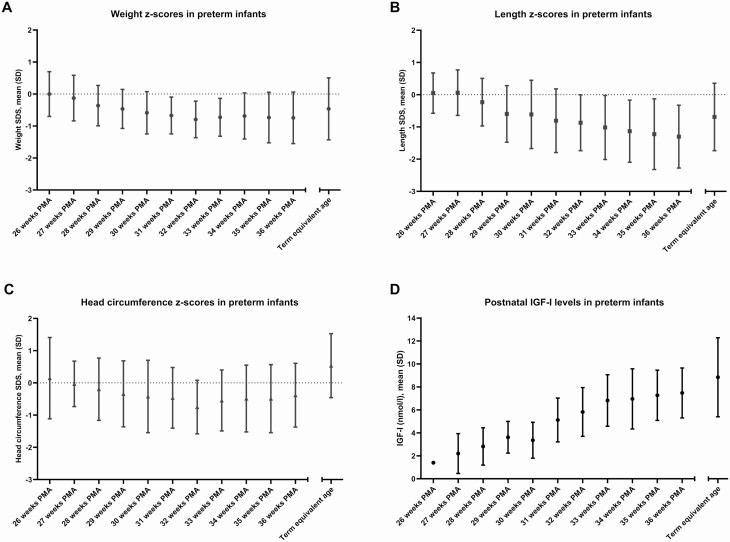
At birth, mean (SD) weight SDS was 0.0 (0.7) and 3 infants were growth restricted (3.4%). Between birth and 36 weeks PMA, 25 infants (28.7%) showed more than 1 SD decline in weight SDS. At 36 weeks PMA, 17 infants (19.5%) were growth restricted (SDS < −1.3). Fig. 2 depicts the SDS for weight, length, and head circumference over time. While the SDS for weight and head circumference increased after 32 weeks PMA, the SDS for length showed a continued decline up to 36 weeks PMA.
Figure 2.
Postnatal growth and IGF-I levels in preterm infants. A, Mean weight z-scores (Fenton 2013) up to term equivalent age. B, Mean length z-scores (Fenton 2013) up to term equivalent age. C, Mean head circumference z-scores (Fenton 2013) up to term equivalent age. D, Mean IGF-I levels up to term equivalent age. Umbilical cord blood samples were excluded from this graph. Abbreviations: PMA, postmenstrual age.
At the term equivalent age visit, infants had a mean (SD) PMA of 43.3 (2.6) weeks. The mean (SD) SDS for weight, length, and head circumference at term equivalent age were −0.5 (1.0), −0.7 (1.0), and 0.5 (1.0) respectively. The mean (SD) fat free mass and percentage at the term age visit were 3301 (448) g and 79.5 (4.0)% respectively. The 17 infants who were growth restricted at 36 weeks PMA had a comparable fat free mass percentage compared to those who were not growth restricted: median (interquartile range) 80% (79%-82%) and 78% (77%-82%), respectively, P = 0.147.
Potential Critical Windows for Growth
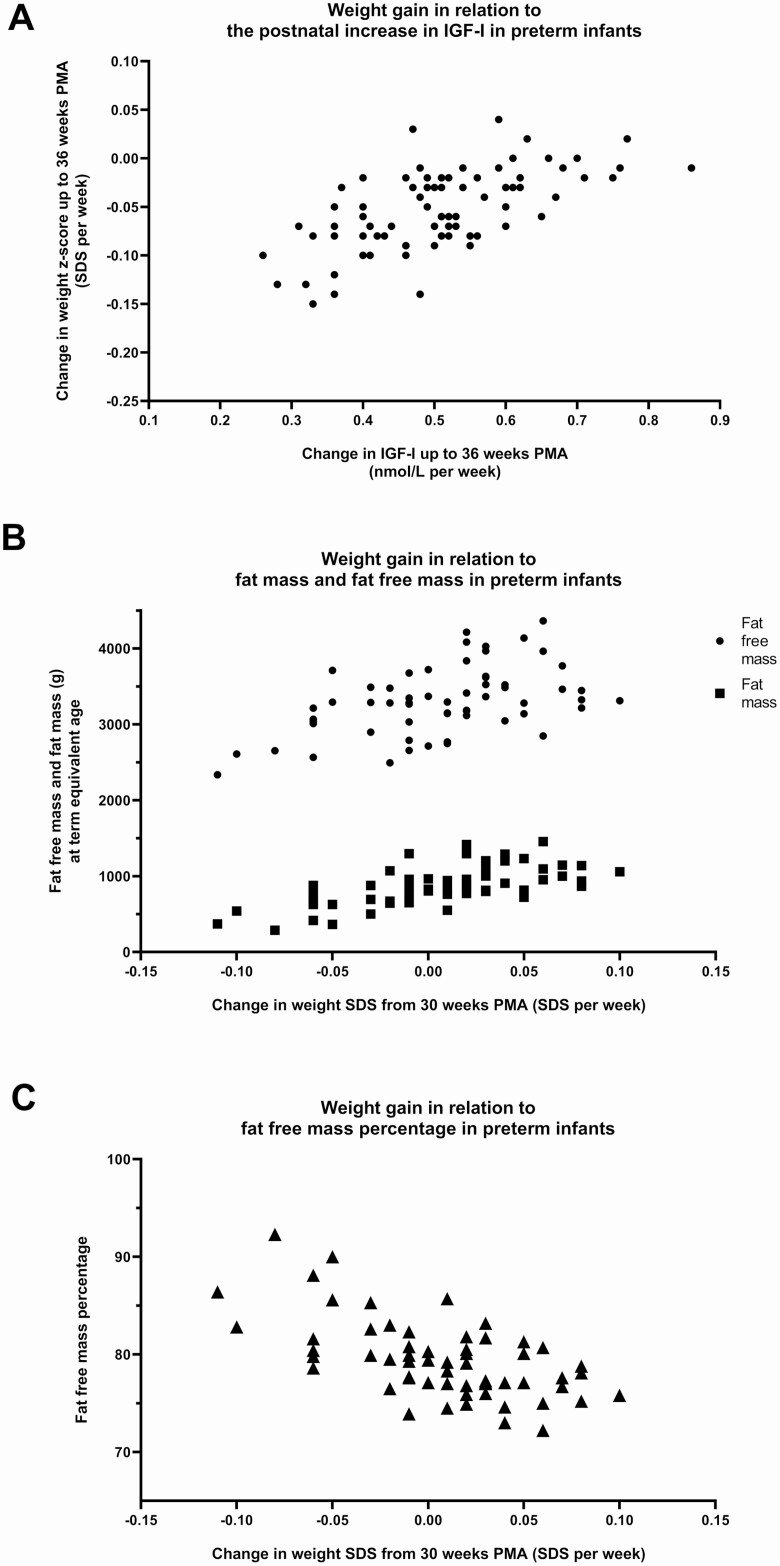
From birth to 4 weeks PNA, more gain in weight SDS was associated with a higher fat free mass percentage at term equivalent age (Table 3). On the contrary, more weight gain from birth to 36 weeks PMA and from 30 weeks PMA onwards, was associated with a lower fat free mass percentage at term equivalent age (Table 3). Of the potential critical windows for weight gain, the strongest predictor of fat free mass percentage at term equivalent age was the change of weight SDS from 30 weeks PMA onward (Table 6). More gain in weight SDS in this period was associated with more fat free mass and more fat mass, but a lower fat free mass percentage at term equivalent age (Fig. 3).
Table 3.
Associations between weight gain and body composition at term age
| Fat mass term age, r (95% CI)1 | Fat free mass term age, r (95% CI)1 | Fat mass percentage term age, B (95% CI)2 | Fat free mass percentage term age, B (95% CI)2 | |
|---|---|---|---|---|
| Weight SDS 4 wks PNA | 0.57 (0.37 to 0.72) | 0.75 (0.60 to 0.84) | 2.2 (0.4 to 4.0)a | −2.2 (−4.0 to −0.4)b |
| Weight SDS 36 wks PMA | 0.64 (0.46 to 0.78) | 0.82 (0.71 to 0.89) | 1.8 (0.5 to 3.1)c | −1.8 (−3.1 to −0.5)d |
| Weight SDS Term age | 0.62 (0.42 to 0.76) | 0.62 (0.42 to 0.76) | 1.7 (0.7 to 2.8)e | −1.7 (−2.8 to −0.7)f |
| Change in weight SDS birth— 4 wks PNA | −0.54 (−0.70 to −0.32) | −0.72 (−0.82 to −0.57) | −198.9 (−383.3 to −14.6)g | 200.9 (12.1 to 389.6)h |
| Change in weight SDS birth—36 weeks PMA | 0.57 (0.37 to 0.72) | 0.68 (0.51 to 0.80) | 32.3 (9.2 to 55.5) i | −32.9 (−56.6 to −9.1)j |
| Change in weight SDS from 30 weeks PMA onwards | 0.77 (0.65 to 0.86) | 0.72 (0.56 to 0.82) | 52.4 (34.7 to 70.1)k | −53.9 (−72.0 to −35.9)l |
All associations were statistically significant P < 0.05.
Abbreviations: PMA, postmenstrual age; PNA, postnatal age.
1 Correlations were controlled for gestational age at birth and postmenstrual age at time of body composition measurement
2 Gestational age at birth and postmenstrual age at the time of body composition measurement were entered in the regression model as covariates
a R2 0.176, P 0.015; b R2 0.179, P 0.013; c R2 0.199, P 0.009; d R2 0.202, P 0.008; e R2 0.210, P 0.008; f R2 0.210, P 0.008; g R2 0.151, P 0.028; h R2 0.154, P 0.026; i R2 0.193, P 0.008; j R2 0.197, P 0.007; k R2 0.438, P < 0.001; l R2 0.445, P < 0.001.
Table 6.
Predictive value of growth for fat free mass percentage at term equivalent age
| B (SE) | ß | P value | |
|---|---|---|---|
| Model 1 weight gain | |||
| Constant | 98.9 (9.8) | <0.001 | |
| PMA (weeks) | −0.4 (0.2) | −0.22 | 0.056 |
| Change in weight SDS from 30 weeks PMA onwards (SDS per week) | −55.7 (9.4) | −0.67 | <0.001 |
| Model 2 length gain | |||
| Constant | 87.0 (14.6) | <0.001 | |
| PMA (weeks) | −0.6 (0.3) | −0.29 | 0.031 |
| Gestational age (weeks) | 0.6 (0.3) | 0.25 | 0.064 |
| Length SDS at term equivalent age | −1.7 (0.6) | 0.58 | 0.005 |
| Model 3 head circumference growth | |||
| Constant | 106.1 (11.4) | <0.001 | |
| PMA (weeks) | −0.6 (0.3) | −0.28 | 0.033 |
| Head circumference SDS at 36 weeks PMA | −1.4 (0.6) | −0.31 | 0.020 |
| Change in head circumference SDS from 30 weeks PMA onwards | −23.1 (8.9) | −0.33 | 0.013 |
Abbreviation: PMA, postmenstrual age.
Model 1: R2 = 0.425, P < 0.001 Backward regression model. Variables not included in the final model: gestational age at birth, week 4 weight SDS, change in weight SDS from birth to week 4, weight SDS at 36 weeks PMA, change in weight SDS from birth to 36 weeks PMA, weight SDS at term equivalent age.
Model 2: R2 = 0.260, P 0.010 Backward regression model. Variables not included in the final model: Length z-score at 36 weeks postmenstrual age.
Model 3: R2 = 0.280, P 0.014 Backward regression model. Variables not included in the final model: gestational age at birth, head circumference SDS at term equivalent age.
Figure 3.
Weight gain in relation to IGF-I and body composition in preterm infants. A, Change in IGF-I in relation to change in weight SDS up to 36 weeks PMA (r = 0.62 [95% CI, 0.46-0.74; P < 0.001]). B, Change in weight SDS from 30 weeks PMA onwards in relation to fat free mass and fat mass at term equivalent age. Partial correlation with fat free mass (r = 0.72 [95% CI, 0.56-0.82; P < 0.001]); partial correlation with fat mass (r = 0.77 [95% CI, 0.65-0.86; P < 0.001]) Correlations were controlled for gestational age at birth and postmenstrual age at time of body composition measurement. C, Change in weight SDS from 30 weeks PMA onwards in relation to fat free mass percentage at term equivalent age. Linear regression model: R2: 0.45 P < 0.001, B for change in weight SDS −54.0 (95% CI, −72.0 to −35.9, P < 0.001) (gestational age at birth and postmenstrual age at the time of body composition measurement were entered in the regression model as covariates). Abbreviations: PMA, postmenstrual age.
Correlations between length indices and fat free mass at term equivalent age showed a positive trend. Likewise, correlations between head circumference and fat free mass at term equivalent age showed a positive trend. Nevertheless, only a few potential critical windows had a statistically significant impact on body composition at term equivalent age (Tables 4 and 5). In particular, the change in length and head circumference SDS in the first 4 weeks of life did not significantly impact fat free mass percentage.
Table 4.
Associations between length gain and body composition at term age
| Fat mass term age, r (95% CI)1 | Fat free mass term age, r (95% CI)1 | Fat mass percentage term age, B (95% CI)2 | Fat free mass percentage term age, B (95% CI)2 | |
|---|---|---|---|---|
| Length SDS 4 wks PNA | 0.34 (0.06 to 0.56) | 0.44 (0.18 to 0.64) | 0.9 (−0.5 to 2.3)a | −0.9 (−2.4 to 0.5)b |
| Length SDS 36 wks PMA | 0.54 (0.29 to 0.72) | 0.72 (0.53 to 0.84) | 1.3 (0.2 to 2.4) c | −1.3 (−2.4 to −0.2) d |
| Length SDS Term age | 0.60 (0.39 to 0.75) | 0.82 (0.70 to 0.89) | 1.4 (0.4 to 2.4) e | −1.4 (−2.5 to −0.4) f |
| Change in length SDS birth—4 wks PNA | −0.073 (−0.33 to 0.20) | −0.03 (−0.30 to 0.24) | −6.2 (−32.6 to 20.2)g | 5.4 (−21.6 to 32.4)h |
| Change in length SDS birth—36 weeks PMA | 0.13 (−0.14 to 0.39) | 0.39 (0.13 to 0.60) | 0.2 (−12.9 to 13.4) i | −0.4 (−13.9 to 13.0)j |
| Change in length SDS from 30 weeks PMA onwards | 0.24 (−0.02 to 0.47) | 0.47 (0.23 to 0.65) | 13.5 (−16.9 to 43.8)k | −14.6 (−45.6 to 16.4)l |
Associations in bold were statistically significant P < 0.05.
Abbreviations: PMA, postmenstrual age; PNA, postnatal age.
1 Correlations were controlled for gestational age at birth and postmenstrual age at time of body composition measurement
2 Gestational age at birth and postmenstrual age at the time of body composition measurement were entered in the regression model as covariates
a R2 0.071, P 0.086; b R2 0.078, P 0.073; c R2 0.152, P 0.016; d R2 0.154, P 0.015; e R2 0.139, P 0.011; f R2 0.188, P 0.010; g R2 0.033, P 0.190; h R2 0.088, P 0.171; i R2 0.047, P 0.138; j R2 0.197, P 0.007; k R2 0.042, P 0.148; l R2 0.049, P 0.124.
Table 5.
Associations between head circumference gain and body composition at term age
| Fat mass term age, r (95% CI)1 | Fat free mass term age, r (95% CI)1 | Fat mass percentage term age, B (95% CI)2 | Fat free mass percentage term age, B (95% CI)2 | |
|---|---|---|---|---|
| Head circumference SDS 4 wks PNA | 0.23 (−0.05 to 0.47) | 0.46 (0.21 to 0.65) | 0.3 (−1.0 to 1.5)a | −0.2 (−1.5 to 1.0)b |
| Head circumference SDS 36 wks PMA | 0.51 (0.25 to 0.70) | 0.58 (0.35 to 0.75) | 1.4 (0.2 to 2.6) c | −1.4 (−2.7 to −0.2) d |
| Head circumference SDS Term age | 0.53 (0.31 to 0.70) | 0.55 (0.33 to 0.71) | 1.7 (0.6 to 2.7) e | −1.7 (−2.7 to −0.7) f |
| Change in head circumference SDS birth—4 wks PNA | −0.11 (−0.36 to 0.17) | 0.09 (−0.18 to 0.35) | −6.5 (−19.5 to 6.5)g | 5.8 (−7.5 to 19.2)h |
| Change in head circumference SDS birth—36 weeks PMA | 0.14 (−0.14 to 0.39) | 0.20 (−0.08 to 0.44) | 5.5 (−7.4 to 18.4) i | −6.3 (−19.4 to 6.9)j |
| Change in head circumference SDS from 30 weeks PMA onwards | 0.15 (−0.12 to 0.39) | 0.00 (−0.27 to 0.27) | 17.5 (−0.6 to 35.6)k | −19.1 (−37.5 to −0.7) l |
Associations in bold were statistically significant P < 0.05.
Abbreviations: PMA, postmenstrual age; PNA, postnatal age.
1 Correlations were controlled for gestational age at birth and postmenstrual age at time of body composition measurement
2 Gestational age at birth and postmenstrual age at the time of body composition measurement were entered in the regression model as covariates
a R2 0.038, P 0.178; b R2 0.099, P 0.152; c R2 0.129, P 0.029; d R2 0.188, P 0.026; e R2 0.186, P 0.003; f R2 0.193, P 0.002; g R2 0.047, P 0.136; h R2 0.048, P 0.131; i R2 0.069, P 0.079; j R2 0.197, P 0.007; k R2 0.138, P 0.042; l R2 0.104, P 0029.
Of the potential critical windows for length, the strongest predictor of fat free mass percentage was length SDS at term equivalent age: a higher length SDS was associated with a lower fat free mass percentage. For head circumference, the change in head circumference SDS from 30 weeks PMA to term equivalent age was the strongest predictor (Table 6). Similarly to weight gain, more gain in head circumference in this time frame was associated with a lower fat free mass percentage at term equivalent age (Table 6).
IGF-I in Relation to Growth and Body Composition
IGF-I levels are shown in Fig. 2. Between birth and term equivalent age, IGF-I showed a mean (SD) increase of 0.4 (0.2) nmol/L per week. The change in IGF-I over time was independent of gestational age at birth (r = 0.07; 95% CI, −0.27-0.16; P = 0.608).
The increase in IGF-I between birth and 36 weeks PMA positively correlated with the concurrent change in weight and head circumference SDS (respectively, r = 0.61; 95% CI, 0.45-0.73; P < 0.001 and r = 0.33; 95% CI, 0.11-0.52; P = 0.004), but not with the concurrent change in length SDS (r = 0.20; 95% CI, -0.02-0.41; P = 0.081).
Potential Critical Windows for IGF-I
From birth to 4 weeks PNA, a greater increase in IGF-I was associated with more fat free mass at term equivalent age. A similar association was seen for the change in IGF-I from birth to 36 weeks PMA (Table 7). Of the potential critical windows, the change in IGF-I from birth to 4 weeks PNA was the strongest predictor (Table 8). IGF-I levels were not associated with fat or fat free mass percentage (Table 7).
Table 7.
Associations between IGF-I and body composition at term age
| Fat mass term age, r (95% CI)1 | Fat free mass term age, r (95% CI)1 | Fat mass percentage term age, B (95% CI)2 | Fat free mass percentage term age, B (95% CI)2 | |
|---|---|---|---|---|
| IGF-I 4 wks PNA | 0.14 (−0.18 to 0.43) | 0.53 (0.27 to 0.72) | −0.2 (−1.0 to 0.5)a | 0.2 (−0.6 to 1.0)b |
| IGF-I 36 wks PMA | 0.18 (−0.18 to 0.48) | 0.16 (−0.19 to 0.47) | 0.4 (−0.4 to 1.1)c | 0.4 (−1.1 to −0.4)d |
| IGF-I Term age | 0.24 (−0.16 to 0.57)3 | 0.43 (0.06 to 0.69) 3 | 0.0 (−0.5 to 0.5)e | 0.0 (−0.5 to 0.5)f |
| Change in IGF-I birth—4 wks PNA | 0.22 (−0.12 to 0.51) | 0.59 (0.33 to 0.77) | −2.5 (−35.7 to 30.7)g | 2.2 (−32.1 to 36.5)h |
| Change in IGF-I birth—36 weeks PMA | 0.23 (−0.04 to 0.47)4 | 0.33 (0.07 to 0.55) 4 | 4.4 (−4.2 to 13.1) i4 | −4.6 (−13.5 to 4.2)j4 |
| Change in IGF-I from 30 weeks PMA onward | 0.06 (−0.21 to 0.32)4 | 0.03 (−0.24 to 0.30)4 | 2.0 (−3.0 to 7.1)k4 | −2.2 (−7.4 to 3.0)l4 |
Associations in bold were statistically significant P < 0.05.
Abbreviations: PMA, postmenstrual age; PNA, postnatal age.
1 Correlations were controlled for gestational age at birth and postmenstrual age at time of body composition measurement
2 Gestational age at birth and postmenstrual age at the time of body composition measurement were entered in the regression model as covariates
3 Also corrected for postmenstrual age at blood sampling
4 Not controlled for gestational age at birth, because gestational age did not correlate with change in IGF-I across PMA nor did it correlate with body composition.
a R2 0.070, P 0.448; b R2 0.078, P 0.396; c R2 0.133, P 0.226; d R2 0.140, P 0.204; e R2 0.136, P 0.330; f R2 0.146, P 0.294; g R2 0.066, P 0.530; h R2 0.073, P 0.483; i R2 0.069, P 0.160; j R2 0.075, P 0.136; k R2 0.062, P 0.195; l R2 0.069, P 0.159.
Table 8.
Predictive value of IGF-I for fat free mass at term equivalent age
| B (SE) | ß | P value | |
|---|---|---|---|
| Included variables | |||
| Constant | −5204 (1370) | 0.001 | |
| PMA (weeks) | 146 (30) | 0.59 | <0.000 |
| Change in IGF-I up to week 4 (nmol/L per week) | 4652 (1247) | 0.44 | 0.001 |
R2 = 0.581, P < 0.001 Backward regression model. Variables not included in the final model: gestational age at birth, week 4 IGF-I, change in IGF-I from birth to 36 weeks PMA.
Abbreviation: PMA, postmenstrual age.
Potential Confounders
Total caloric intake (kcal/kg/day) up to 36 weeks PMA was associated with fat free mass at term equivalent age (r = 0.364, P = 0.044, corrected for PMA at the time of body composition measurement). In multivariate analysis, including the change in IGF-I up to 4 weeks PNA and the change in weight SDS from 30 weeks onwards, caloric intake was no longer a significant predictor. There was no association between caloric intake and fat free mass percentage. Protein, fat, and carbohydrate intake up to 36 weeks was not associated with body composition at term equivalent age.
Comorbidities (necrotizing enterocolitis, late-onset sepsis, bronchopulmonary dysplasia, retinopathy of prematurity, intraventricular hemorrhage, and patent ductus arteriosus) were not associated with body composition at term equivalent age.
Gender and ethnicity were not associated with body composition at term equivalent age.
Discussion
This study confirmed that, in preterm infants, the postnatal growth and IGF-I are associated with body composition at term equivalent age. In particular, higher IGF-I levels in the first month of life and more weight gain in that period were associated with a more favorable body composition at term equivalent age. Meanwhile a greater increase in weight, length, or head circumference SDS after this window was associated with a higher fat mass percentage at term equivalent age.
IGF-I and Growth in the First Month of Life
After preterm birth, nutrient supply is abruptly disrupted. Moreover, as the transplacental supply of essential growth factors stops, the preterm infant now solely relies on their own production of these factors. This leads to a drop in IGF-I and consequent decrease in the SDS for weight, length, and head circumference [14]. Accordingly, our population showed low IGF-I levels and a decline in growth rate in the first weeks of life.
In our study cohort, a greater increase in IGF-I in the first month of life was associated with more fat free mass at term equivalent age. In addition, our results showed that less decrease in weight SDS in the first month of life was associated with a higher fat free mass percentage at term equivalent age. The change in length and head circumference SDS were not associated with body composition. Nevertheless, while a higher length SDS at 4 weeks postnatal age was associated with both a higher fat mass as well as a higher fat free mass, a higher head circumference SDS at 4 weeks postnatal age was associated with higher fat free mass alone. In line with our findings, Hernandez and colleagues found higher IGF-I levels in the first week of life to be associated with increased lean mass at 2 years corrected age in small-for-gestational-age preterm infants [10].
Therefore, our results suggest that impaired growth in the first month of life, that is, lower IGF-I levels and weight and head circumference SDS, is associated with a less favorable body composition at term equivalent age.
Interestingly, we observed that the postnatal change in IGF-I was independent of gestational age at birth, that is, the rate of increase of IGF-I depended on PMA at the time of blood sampling regardless of the gestational age at birth. Likewise, Hansen-Pupp and colleagues found IGF-I to increase at 30 weeks gestational age, irrespective of gestational age at birth [14]. Accordingly, in our population, IGF-I showed an increase from 31 weeks PMA. Taking into account that our findings suggest that early IGF-I levels are important for a more favorable body composition, there may be a window of opportunity for interventions to increase IGF-I levels in this early phase. Indeed, trials with IGF-I administration between preterm birth and 30 weeks PMA show promising results with regards to the occurrence of major comorbidities such as bronchopulmonary dysplasia. However, IGF-I is expected to affect various organ systems. Current studies have not shown a decrease in other comorbidities such as retinopathy of prematurity and the effects of IGF-I administration on body composition are yet to be investigated [15]. Furthermore, IGF-I administration is an invasive and burdensome procedure, which might limit its implementation in clinical practice.
IGF-I Levels and Growth up to Term Equivalent Age
From birth to 36 weeks PMA and at term equivalent age there was still a positive, but weak, association between change in IGF-I and fat free mass. Nevertheless, there was no association with the change from 30 weeks PMA onwards. Taking into account the stronger association in the first month of life, it could be suggested that early changes in IGF-I are more important in influencing body composition at term equivalent age. Nevertheless, given the sample size and observational nature of this study, the evidence for this hypothesis is inconclusive and remains speculative. Moreover, this is in contrast to reports by Ruys and colleagues who found a positive association between IGF-I and fat free mass as well as fat mass measured in preterm infants at term equivalent age, implying that IGF-I relates to overall growth rather than body composition [11]. However, in relation to later measurements of body composition, others did find IGF-I to relate to body composition. For example, Cooper et al reported a higher increase in IGF-I between hospital discharge and 1 year corrected age to be associated with a concurrent higher increase in fat free mass in infants born prematurely [9].
After correcting for gestational age at birth, more weight gain between birth and 36 weeks PMA, and in particular from 30 weeks PMA onwards, was associated with a lower fat free mass percentage at term equivalent age. Hypothesizing this would imply that, in contrast to weight gain in the first month of life, high rates of weight gain are not desirable up to term equivalent age. In line with that, we found that a higher SD score for length and head circumference at 36 weeks PMA and term equivalent age was associated with a lower fat mass percentage. Furthermore, the increase in head circumference SDS from 30 weeks onwards was associated with a lower fat free mass percentage. Our data suggest that increased growth rates, in particular after the first month of life, are associated with a lower fat free mass percentage and thus a less favorable body composition. We speculate that it might be beneficial to prevent the decrease in SDS in the first weeks of life. Then there might be a less rapid increase in SDS afterwards and potentially a more favorable body composition at term equivalent age. Nevertheless, the fat deposition may be an adaption to extra-uterine life to enable adequate thermoregulation and provide energy stores [16]. In that light it might be valuable to develop normative data, with cutoff points where fat accumulation becomes undesirable, for example, based on later cardiometabolic outcomes.
Study Limitations
Our study cohort had a relatively low incidence of postnatal growth restriction and a relatively high mean fat mass percentage. Others previously showed that preterm infants with postnatal growth restriction (weight SD at term age < −2 SD), had a lower fat mass percentage compared with preterm infants without postnatal growth restriction [17, 18]. This could partially explain the higher fat mass percentage in our cohort. Yet, it makes our results less generalizable. In addition, our effect size was limited. Despite the sample size of this cohort, the number of IGF-I measurements per week was low. Blood sampling took place on alternating weeks, and combined with a relatively small number of extremely preterm infants, this resulted in a sample size ranging between 1 infant at 26 weeks PMA to 33 infants at 33 weeks PMA. These conditions may have contributed to our findings.
Conclusion
IGF-I plays an important role in growth and body composition in preterm infants. Higher IGF-I levels in the first month of life are associated with a more favorable body composition at term equivalent age. It remains to be elucidated what the optimal body composition would be at term equivalent age. Future studies taking into account long-term health outcomes are warranted to establish useful guidance for growth and body composition in early life.
Acknowledgments
The authors would like to express their gratitude to all the infants and their families who participated in this study. We thank Femke Maingay, Sophie van der Schoor, and Dianne Maingay for their contribution to the data collection and Alexandra Calor, Khan Vu, Dide De Jongh, Fleur Walschot, and Isabelle Koster for data collection and processing.
Financial Support: An unrestricted research grant was provided by Danone Nutricia Research.
Clinical Trial Information: Dutch Trial Register no. NTR5311 (www.trialregister.nl).
Glossary
Abbreviations
- IGF-I
insulin-like growth factor I
- NICU
neonatal intensive care unit
- PMA
postmenstrual age
- PNA
postnatal age
- SDS
standard deviation score
Additional Information
Disclosures: The authors have nothing to disclose. There is no conflict of interest.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Horbar JD, Ehrenkranz RA, Badger GJ, et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000-2013. Pediatrics. 2015;136(1):e84-e92. [DOI] [PubMed] [Google Scholar]
- 2. Lee SM, Kim N, Namgung R, Park M, Park K, Jeon J. Prediction of postnatal growth failure among very low birth weight infants. Sci Rep. 2018;8(1):3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics. 2012;130(3):e640-e649. [DOI] [PubMed] [Google Scholar]
- 4. Cormack BE, Harding JE, Miller SP, Bloomfield FH. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: a narrative review. Nutrients. 2019;11(9):2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheurer JM, Zhang L, Plummer EA, Hultgren SA, Demerath EW, Ramel SE. Body composition changes from infancy to 4 years and associations with early childhood cognition in preterm and full-term children. Neonatology. 2018;114(2):169-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Euser AM, Finken MJ, Keijzer-Veen MG, Hille ET, Wit JM, Dekker FW; Dutch POPS-19 Collaborative Study Group . Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. Am J Clin Nutr. 2005;81(2):480-487. [DOI] [PubMed] [Google Scholar]
- 7. Nakano Y. Adult-onset diseases in low birth weight infants: association with adipose tissue maldevelopment. J Atheroscler Thromb. 2020;27(5):397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yumani DF, Lafeber HN, van Weissenbruch MM. Dietary proteins and IGF I levels in preterm infants: determinants of growth, body composition, and neurodevelopment. Pediatr Res. 2015;77(1-2):156-163. [DOI] [PubMed] [Google Scholar]
- 9. Cooper DM, Girolami GL, Kepes B, et al. Body composition and neuromotor development in the year after NICU discharge in premature infants. Pediatr Res. 2020;88(3):459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernandez MI, Rossel K, Peña V, et al. Leptin and IGF-I/II during the first weeks of life determine body composition at 2 years in infants born with very low birth weight. J Pediatr Endocrinol Metab. 2012;25(9-10):951-955. [DOI] [PubMed] [Google Scholar]
- 11. Ruys CA, van de Lagemaat M, Lafeber HN, Rotteveel J, Finken MJJ. Leptin and IGF-1 in relation to body composition and bone mineralization of preterm-born children from infancy to 8 years. Clin Endocrinol (Oxf). 2018;89(1):76-84. [DOI] [PubMed] [Google Scholar]
- 12. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53(3):486-492. [DOI] [PubMed] [Google Scholar]
- 14. Hansen-Pupp I, Löfqvist C, Polberger S, et al. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatr Res. 2011;69(5 Pt 1):448-453. [DOI] [PubMed] [Google Scholar]
- 15. Ley D, Hallberg B, Hansen-Pupp I, et al. ; study team . rhIGF-1/rhIGFBP-3 in preterm infants: a phase 2 randomized controlled trial. J Pediatr. 2019;206:56-65.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sauer PJ. Can extrauterine growth approximate intrauterine growth? Should it? Am J Clin Nutr. 2007;85(2):608S-613S. [DOI] [PubMed] [Google Scholar]
- 17. Roggero P, Giannì ML, Liotto N, et al. Rapid recovery of fat mass in small for gestational age preterm infants after term. PLoS One. 2011;6(1):e14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruckner M, Khan Z, Binder C, et al. Extremely preterm infants have a higher fat mass percentage in comparison to very preterm infants at term-equivalent age. Front Pediatr. 2020;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.