Abstract
Only a handful of US Food and Drug Administration (FDA) Emergency Use Authorizations exist for drug and biologic therapeutics that treat severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) infection. Potential therapeutics include repurposed drugs, some with cardiac liabilities. We report on a chronic preclinical drug screening platform, a cardiac microphysiological system (MPS), to assess cardiotoxicity associated with repurposed hydroxychloroquine (HCQ) and azithromycin (AZM) polytherapy in a mock phase I safety clinical trial. The MPS contained human heart muscle derived from induced pluripotent stem cells. The effect of drug response was measured using outputs that correlate with clinical measurements, such as QT interval (action potential duration) and drug‐biomarker pairing. Chronic exposure (10 days) of heart muscle to HCQ alone elicited early afterdepolarizations and increased QT interval past 5 days. AZM alone elicited an increase in QT interval from day 7 onward, and arrhythmias were observed at days 8 and 10. Monotherapy results mimicked clinical trial outcomes. Upon chronic exposure to HCQ and AZM polytherapy, we observed an increase in QT interval on days 4–8. Interestingly, a decrease in arrhythmias and instabilities was observed in polytherapy relative to monotherapy, in concordance with published clinical trials. Biomarkers, most of them measurable in patients’ serum, were identified for negative effects of monotherapy or polytherapy on tissue contractile function, morphology, and antioxidant protection. The cardiac MPS correctly predicted clinical arrhythmias associated with QT prolongation and rhythm instabilities. This high content system can help clinicians design their trials, rapidly project cardiac outcomes, and define new monitoring biomarkers to accelerate access of patients to safe coronavirus disease 2019 (COVID‐19) therapeutics.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
As the global pandemic of coronavirus disease 2019 (COVID‐19) expanded, clinicians were pressed to treat patients with new drug combinations, in the absence of regulatory approval. Months after the first cases, the US Food and Drug Administration (FDA)‐approved medicines for the treatment of COVID‐19 have emerged with mixed results and safety concerns. There is a need for tools to rapidly screen for cardiac liability associated with potential COVID‐19 therapeutics.
WHAT QUESTION DID THIS STUDY ADDRESS?
Does hydroxychloroquine (HCQ) and azithromycin (AZM) COVID‐19 polytherapy show synergetic cardiac liability when compared to their monotherapy?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
In this in vitro safety “clinical trial,” HCQ or AZM alone showed significant 80% repolarization time (APD80; proxy for QT) prolongation and arrhythmia, whereas their combination polytherapy rescued instances of arrhythmias while increasing APD80.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study demonstrates that a complex in vitro tissue model (cardiac MPS) can predict arrhythmias and rhythm instabilities under experimental conditions mimicking safety clinical trials. We also identified biomarkers associated with cardiac injury, which can be used to design clinical trial monitoring protocols.
INTRODUCTION
When the World Health Organization declared a global pandemic on March 11, 2020, little was known about the pathogenesis of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). It was described and treated as a respiratory disease, in which the virus targeted the epithelial cells of the respiratory tract, resulting in alveolar damage, edema, and fibrosis. Now, with more than 140 million cases and 3 million deaths worldwide, there is clinical evidence that the virus also has non‐negligible long‐term effects on multiple organs, including the heart, kidneys, vasculature, liver, and even the brain. 1 , 2 , 3 , 4
With only a limited number of US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) drug and biological therapeutics available for the treatment of SARS‐CoV‐2 infection, clinicians have been pressed to treat patients in critical stages without FDA‐approved protocols. They have therefore relied on several small‐scale clinical studies to repurpose compounds approved by regulatory bodies as monotherapies in the hope of improving patient outcomes. Early clinical trials identified chloroquine (CQ), hydroxychloroquine (HCQ), and azithromycin (AZM) as promising drugs to help treat or reduce the effects of SARS‐CoV‐2. 5 , 6 , 7 , 8 , 9 A nonrandomized clinical trial in France identified HCQ, in combination with AZM, as being capable of significantly reducing respiratory viral loads. 5 This was confirmed by retrospective studies, 10 , 11 but was also heavily criticized and refuted by other recent studies employing a priori designs. 12 , 13 , 14 Several additional small clinical trials have shown mixed outcomes for HCQ treatment of patients with coronavirus disease 2019 (COVID‐19). 15
A serious concern with these studies is that patients were treated with drugs that have known cardiac complications, and their effects on the heart in polytherapy were unknown. HCQ inhibits hERG (IKr) potassium channels, it is known to increase in QT interval of cardiomyocytes, and can induce arrhythmias that are responsible for sudden death. 16 AZM is also associated with an increased risk of cardiovascular death, due to Torsade de Pointes (TdP) and polymorphic ventricular tachycardia. 17 With respect to polytherapy, clinical trials have demonstrated a synergistic effect of HCQ and AZM to prolong QT interval 18 , 19 , 20 ; however, alterations in arrhythmic event frequency were controversial when compared to HCQ or AZM alone.
In the absence of rapid clinical trials for polytherapy safety, there is an urgent need for screening tools to increase the speed at which potential therapeutics are evaluated for cardiac liabilities. Common in vitro systems used for cardiac drug screening include cell 2D monolayers and animal testing, which often fail to replicate human physiology, particularly electrophysiology and pharmacokinetic properties. 21 Engineered heart tissue, organoids, or microfluidic‐based microphysiological systems are emerging alternatives for state‐of‐the‐art drug screening. 22 With the rapid spread of COVID‐19, microphysiological systems (MPS) have recently shown to be a promising tool to study virus entry and replication mechanisms, subsequent cytokine production, as well as effects of existing and novel therapeutics or vaccines. 23 , 24
In this paper, we demonstrate the utility of a cardiac MPS (Figure 1) for determining the cardiac liability associated with repurposed drug polytherapy in an in vitro design analogous to a phase I safety clinical trial. Our cardiac MPS contains a three‐dimensional (3D) cell chamber in which human‐induced pluripotent stem cell‐derived cardiomyocytes (hiPSC‐CMs) are confined and self‐assemble to form uniaxially beating heart muscle. 21 , 25 The hiPSC‐CMs have been successfully used for in vitro assessment of drug‐induced arrhythmias, especially because they respond consistently to hERG channel block (QT prolongation and arrhythmias) and calcium channel block (action potential duration shortening and impaired contractile function). 26 , 27 It makes them excellent candidates to screen for cardiac liability of HCQ and AZM, both of which are known to block hERG channels, and also act upon other cardiac ion channels. 28 Combining hiPSC‐CMs with our physiologically relevant cardiac MPS creates a 3D aligned human heart muscle, which we have demonstrated is superior to conventional 2D assays in its ability to prognosticate on drug efficacy and toxicity in vitro. 21 , 25 The design aligns cells immediately upon loading, fostering rapid production of native cardiac extracellular matrix (ECM), and alignment of the cells’ contractile machinery fostering geometrically aligned contraction. The cardiac MPS model has been validated in a number of both experimental and computational studies. 21 , 25 , 29 , 30 , 31 By assaying hiPSC‐CMs expressing a genetically encoded calcium sensor (GcAMP6f), loaded with a voltage‐sensitive fluorescent probe (BeRST), we assessed the electrophysiology and calcium handling of the heart muscle during serial drug exposures. We showed that HCQ and AZM significantly increase 80% repolarization time (APD80) and rhythm instabilities, starting at clinically relevant exposure days, and were accompanied with early afterdepolarization (EAD) and TdP instances. The HCQ + AZM combination also showed a significant increase in APD80; however, few instabilities or arrhythmic events were observed. Finally, proteomics analysis of cell culture effluent enabled detection of biomarkers that were directly correlated with cardiotoxicity, apoptosis, and contraction mechanics alteration.
FIGURE 1.
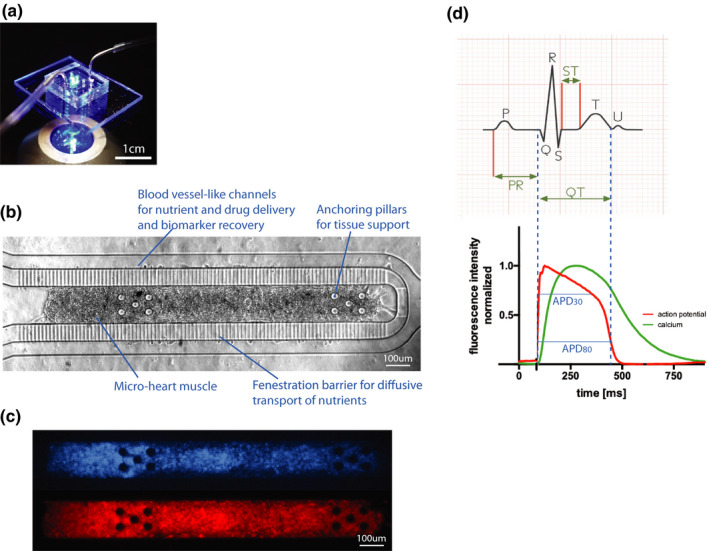
The cardiac microphysiological system. (a) Photograph of a cardiac MPS under fluorescent lighting with fluidic tubing to deliver media and drugs. (b) Brightfield image of a cardiac MPS containing ~ 2,500 human induced pluripotent stem cell‐derived cardiomyocytes. The cell chamber is separated from adjacent feeding channels via a fenestration barrier of 2 μm wide grooves allowing for nutrient diffusion while protecting the tissue in the cell chamber from media flow‐induced shear stress. The anchoring pillars on either side of the cell chamber help keep the heart muscle elongated and provide resistance for contraction. (c) Representative images of the same tissue under GFP fluorescence for calcium transient recordings (top) or FarRed voltage dye staining (bottom). (d) The top graph shows a typical ECG recording from which the clinical QT interval can be determined. We use APD80 as a proxy for QT duration, corresponding to the duration of the action potential at 80% of its repolarization (bottom). The APD (red) and Ca (green) waveforms are timestamped identifying temporal kinetics. APD80, 80% repolarization time; Ca, calcium; ECG, electrocardiogram; GFP, green fluorescent protein; MPS, microphysiological system
MATERIALS AND METHODS
Cell sourcing
The human hiPSC line wild type C (WTC) harboring a single‐copy of CAG‐driven GCaMP6f knocked into the first Exon of the AAVS1 “safe harbor” locus 32 was used for all experiments in this study. It is available from the Coriell Repository (#GM25256 hiPSC from Fibroblast). More detailed information can be found in ref. 32
Cardiomyocyte differentiation
The hiPSC stocks were thawed and plated on Matrigel hESC‐Qualified Matrix (Corning, Corning, NY) coated tissue culture plates in mTeSR‐1 (mTeSR‐1; StemCell Technologies, Vancouver, Canada) containing 10 µM of the Rho kinase inhibitor (RI) Y27632‐dihydrochloride (Peprotech, Rocky Hill, NJ). Media was exchanged the next day to remove the inhibitor, and hiPSC were split three times at a density of 20,000 cells/cm2 to allow for recovery from cryopreservation. After three splits, the hiPSCs were seeded in differentiation plates at 30–40,000 cells/cm2 to allow for full confluency. Initial seeding was considered as “day 3” of differentiation. At day 0, when hiPSC were greater than 90% confluent, differentiation was started following the protocol developed by Lian et al., 33 which exposes the cells to 8 µM CHIR99021 (Peprotech) in Roswell Park Memorial Institute Medium 1640 (RPMI) containing B‐27 supplement without insulin (RPMI‐I). At day 1, only RPMI‐I was added to the cells. On day 2, cells were exposed to 5 µM IWP‐4 (Peprotech) in RPMI‐I supplemented with 150 µg/mL L‐ascorbic acid (LAA) for 2 days. On day 4, only RPMI‐I was given to the cells for another 2 days. From then onward, RPMI containing standard B‐27 supplement with insulin (RPMI‐C) was added every second day. Once spontaneous contractions were observed around day 7 or 8 of differentiation, the hiPSC‐CMs were washed several times with dPBS‐Ca/Mg and left to soak for 10 min to disrupt cadherins. Cells were then exposed to 0.25% Trypsin (Thermo Fisher) for 10 min at 37°C, and were pipetted gently to break up tissue into single cells and collected. Cells were pelleted at 300 g for 5 min before being plated at a density of 100,000 cells/cm2 onto Matrigel, in RPMI‐C with 10 µM RI (“day 0” after replating). At day 1 after replating, medium was exchanged for RPMI‐C. At day 3, cells were washed with dPBS to remove glucose and were exposed to RPMI 1640 (no glucose, no pyruvate, supplemented with 23 mM sodium bicarbonate, and 5 mM Sodium L‐lactate) for 4 to 5 days (exchanged every other day) to select for cardiomyocytes only. At day 8, cells were washed with dPBS and exposed to RPMI‐C for 2 to 3 days to allow for recovery. Cardiomyocyte purity was characterized by flow cytometry with Cardiac Troponin T both before and after this procedure.
Preparation of maturation media
Details of the maturation media (MM) have been published previously. 25 Briefly, a base media was prepared from RPMI 1640 powder (Sigma, R1383‐10X1L) and supplemented with 0.5 g/L D‐glucose (Fisher, BP350‐1), 10 mM D‐galactose (Sigma, G5388) and 2 g/L sodium bicarbonate (Fisher, S233–500). A 9% bovine serum albumin (BSA; Fisher, BP1605‐100) solution was prepared in base media and adjusted to pH 7.4. Aliquots of fatty acid (FA) stocks were prepared from oleic acid (OA; Sigma, O1383‐5G) and palmitic acid (PA; Sigma, P0500‐10G; 100 mg/mL in DMSO) and stored at −20°C. OA was prediluted 1:10 in DMSO. PA and OA were added to the 9% BSA solution at 10.23 µg/mL for PA and 0.8 mM OA. To dissolve the FA, the solution was heated to 60°C and sonicated for 5 min. The final MM was prepared by mixing 1 part FA solution and 3 parts MM base media yielding a final concentration of 2.56 µg/mL PA and 0.2 mM OA. Finally, the media was supplemented with 2% B27 (Gibco, 17504‐044) and 150 μg/mL ascorbic acid (Fisher, AC105021000) and sterile filtered.
Fabrication and cell loading of cardiac MPS
The microfluidic design for each MPS consisted of four identical cell culture chambers (1300 by 130 µm) with media channels running parallel on either side of the cell culture chambers (Figure 1). The microfluidic devices were fabricated from polydimethylsiloxane (PDMS) using classic replica molding techniques. 21 , 25 For loading, ~ 4,000 lactate purified hiPSC‐CM were injected into each tissue chamber, with ~ 2,500 cells retained withing the cell chamber. The following day and every other day from then on, media was changed to our in‐house MM as described in ref. 25. MPS tissues were allowed to mature for at least 10 days before any subsequent experiments were performed.
Drug preparation for pharmacology studies
We based our study on the following protocols: clinical drug administration for HCQ is 400 mg twice per day followed by 200 mg twice per day for 4 days. AZM is 500 mg on day 1 followed by 250 mg per day for the following 4 days. 20 , 34 Based on clinical peak plasma concentration (Cmax) and area under curve for 24 h, 35 we exposed the MPS tissues to 0.24 μM HCQ at day 1 and 0.12 μM HCQ from day 2 to day 10; and to 0.15 μM AZM at day 1 and 0.075 μM AZM at day 2 to day 10.
Thorough action potential analysis as a proxy for clinical QT interval study and arrhythmia prediction
Clinically, drug‐induced QT prolongation is a strong predictor of arrhythmic cardiotoxicity in patients. At the cellular level, AP prolongation and increased AP triangulation indicate slowed repolarization and are strong markers of whole heart QT prolongation and arrhythmia, 36 translated by observations of EADs, 37 , 38 and subsequent TdP. 37 Large beat‐to‐beat variation in AP duration is a specific indicator of repolarization instability, which can be readily visualized by Poincaré plots, which we created by plotting CaD80 of each (nth) beat in a 30 s calcium transient recording, against CaD80 of the preceding beat (n‐1)th, normalized to the CaD80 mean. 36 We also performed a qualitative and nonparametric evaluation of drug arrhythmogenesis by categorizing arrhythmic behaviors present in the calcium time‐series.
Plasma protein profiling using Olink Proteomics multiplex panel
Effluents were sent to Olink Proteomics for quantification of proteins associated with toxicity and tissue damage. Olink Proteomics uses multiplex proximity extension assay (PEA) panels. 39 In this study, we have used the Organ Damage panel, which consists of 92 unique markers of toxicity and cellular damage.
Statistics
All statistics were calculated using GraphPad Prism. All electrophysiology data were analyzed with one‐way analysis of variance (ANOVA) repeated measures and Dunnett’s post hoc correction with multiple comparison to day 0 and to one another was run. If some values were missing, the mixed‐effects model was run. Nonparametric χ2 approximation was run for qualitative arrhythmic events assessment in a pairwise manner. Significance was determined with p value less than 0.05.
RESULTS AND DISCUSSION
Chronic exposure to HCQ for 10 days resulted in QT prolongation and rhythm instabilities that correlated with arrhythmic events and clinical observations
Chronic exposure to an HCQ dose mimicking clinical protocols decreased the beat rate starting at day 4 with a statistically significant decrease on days 4, 7, and 8 (Figure 2a). APD80 increased markedly from day 7 onward (Figure 2b), with the maximum APD80 increase reaching 850 ms. All but one tissue exhibited an APD80 at least 400 ms longer on day 7 and day 9 than prior to HCQ exposure. These arrhythmogenic changes to the AP were tightly correlated with the appearance of instabilities in the HCQ tissues from day 5 onward (Figure 3a). APD80 was directly correlated to calcium transient duration (CaD) (Figure S1d), and an increase of CaD80 above 600 ms was observed at day 2, and increased over the duration of HCQ treatment. Arrhythmic events began appearing at day 4 in 50% of the tissues. At day 7, tissues exhibited both arrhythmogenic AP waveforms and CaD80 increase, and by days 9 and 10, 50% of tissues exhibited weak or no dynamic signal change, suggesting loss of resting membrane potential (Figure 3d). Representative traces of arrhythmic events are shown in Figure 3g–i, comparing 30 s calcium traces at day 0 and day 9 (Figure 3g). Late calcium peaks are a signature of EADs in membrane potential and were clearly observed (red arrows) in day 9 HCQ recordings, as were the marked increases in duration of the calcium transient, itself a marker for APD prolongation. Together these data reflect HCQ’s well known block of IKr, which prolongs the QT interval and its in vitro proxy APD, 18 , 40 which are associated with arrhythmic events. 16 , 41 , 42 Our AP data, which is consistent with the clinical literature, indicates that the cardiac MPS system is a good predictor of clinical cardiotoxicity of HCQ.
FIGURE 2.
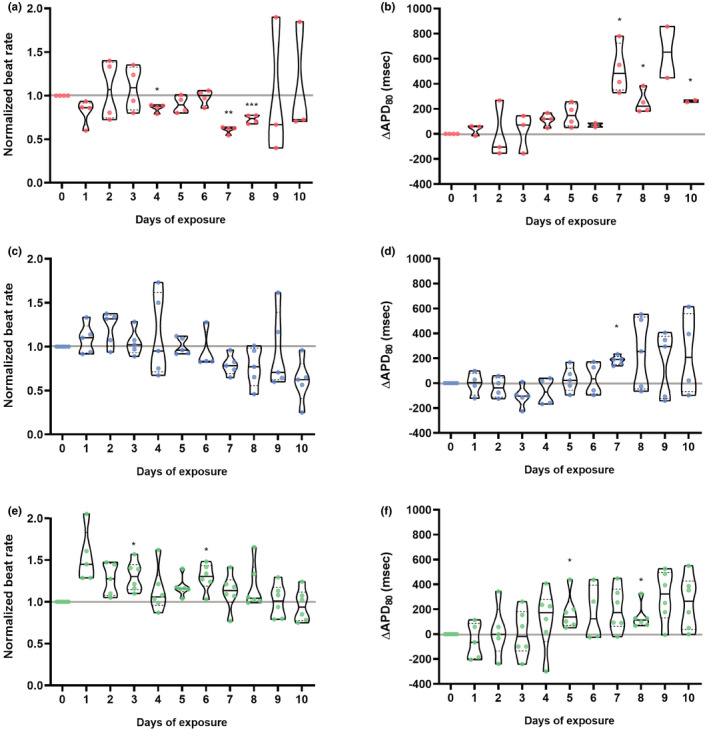
Electrophysiology analysis of chronic exposure to hydroxychloroquine (HCQ), azithromycin (AZM) or their polytherapy. Doses were chosen to closely mimic clinical trial drug prescription used for coronavirus disease 2019 (COVID‐19) treatment: 0.24 μM HCQ and 0.15 μM AZM on day1 followed by 0.12 μM and 0.075 μM AZM on day 2 to day 10. 19 , 20 Polytherapy was the combination of both monotherapy doses (Table S1). Violin plots demonstrate spontaneous beating during the therapy for HCQ (a), AZM (c) or polytherapy (e). Violin plots depicting the change in 80% repolarization time (APD80) during the therapy for HCQ (b), AZM (d) or polytherapy (f). APD80 values were calculated from spontaneous recording, corrected for beat rate using the Frederica correction. 48 In all graphs, the values were normalized to baseline at day 0 for each tissue. Each point corresponds to one heart muscle. The violin plots show the arithmetic median (solid line) and upper and lower quartile (dashed lines) as well as minimum and maximum values (truncation of violin shape). All tissues analyzed were within inclusion criteria of APD80 less than 500 ms at baseline. Statistics run were one‐way analysis of variance (ANOVA) repeated measures with multiple comparison to baseline day 0 and Dunnett’s post hoc correction. *p < 0.05; **p < 0.01; ***p < 0.001
FIGURE 3.
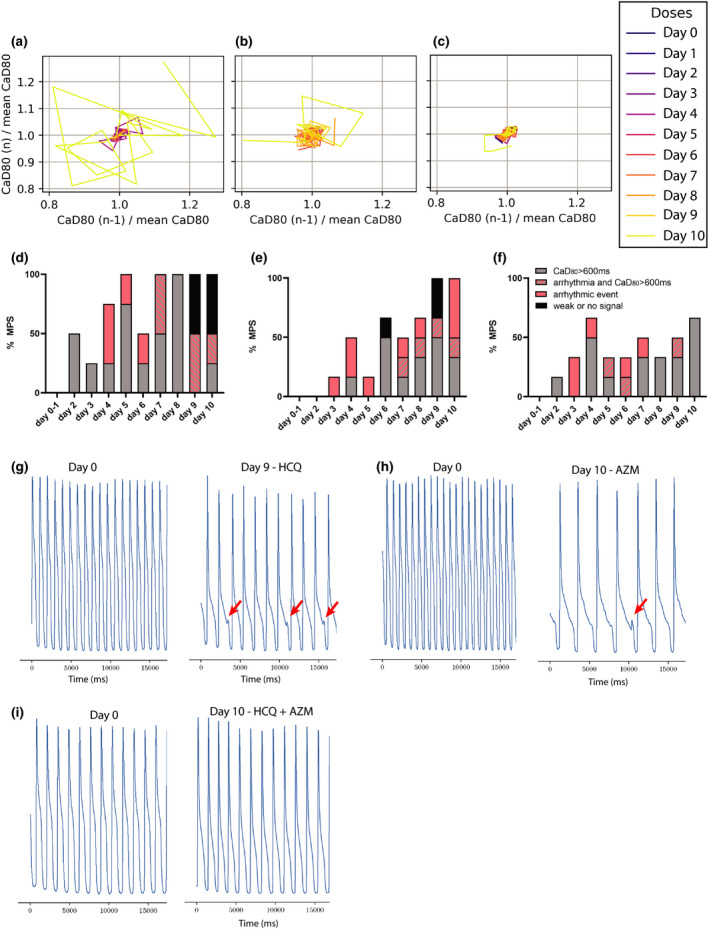
Instability and arrhythmic study of chronic exposure to hydroxychloroquine (HCQ), azithromycin (AZM) and their polytherapy. Poincaré plots 36 , 49 were used to visualize rhythm instabilities in different tissues. Where small clusters of daily traces represent minimal arrhythmic risk, and large complex polygons are indicative of rhythm instability and high arrhythmic risk. (a) Representative Poincaré graph of a tissue exposed to HCQ only. Disorganized polygons can be observed at days 5 and 10 indicative of drug‐induced arrhythmia. (b) Poincaré graph of AZM‐treated heart muscle where instabilities were observed starting on day 6. (c) Representative graph of polytherapy instabilities, which show fewer and smaller polygons when compared to monotherapy, indicative of reduced arrhythmic risk. (d–f) Histograms showing weak or no signal (black), CaD80 above 600 ms with (striated) or without (grey) early afterdepolarization (EAD) and arrhythmic event (pink) as percentage of total heart muscles (i.e., MPS). The analysis was performed for HCQ alone (d), AZM alone (e), and for polytherapy (f). Representative calcium transient trace at baseline and after exposure to 9 days of HCQ (g) or 10 days of AZM (h) where EAD instances can be observed (red arrows). (i) Representative calcium transient trace at 10 day exposure of polytherapy showing no EADs
Chronic exposure of AZM for 10 days showed QT prolongation and rhythm instabilities that correlated with arrhythmic events and clinical observations
Chronic AZM exposure did not significantly change beat rate (Figure 2c). However, as for HCQ, chronic AZM treatment increased APD80 by day 7, and a trend for persisting prolonged APD80 continued until day 10, albeit with large variation (Figure 2d). Triangulation also trended (p < 0.2) to increase from day 8 onward, although this did not reach significance (p = 0.07 at day 10; Figure S1b). Instabilities arose at day 6 and worsened over time (Figure 3b). Nonparametric analysis of instability showed a significant increase of chaotic polygons in AZM‐treated tissues when compared with HCQ (p < 0.05, not shown). CaD80 increased beyond 600 ms at day 4, and day 6 onward in 30%–50% of the tissues. Arrhythmic events were observed from day 3 onward, reaching 60% of tissues at day 10, with some tissues exhibiting both arrhythmia and CaD80 increase. Day 6 and 9 had, respectively, 16% and 33% of tissues with weak or no signal (Figure 3e). Figure 3 shows a representative trace of arrhythmic events at day 0 versus day 10 (Figure 3h) of AZM exposure. Overall, APD80 increased on all treatment days compared to day 0, and EADs appeared after day 3, and were most prevalent on day 10. Because clinical AZM application is typically limited to 5 days, the observed incidence of pro‐arrhythmic events during longer exposure times cannot be directly compared to clinical outcomes. However, AZM has been associated with increases in cardiovascular death, mostly through QT prolongation and arrhythmia, 17 , 43 and these outcomes are clearly indicated by our MPS measurements.
Chronic exposure to both HCQ and AZM for 10 days showed QT prolongation and rhythm instabilities that correlated with arrhythmic events and clinical observations
The beat rate increased at day 3 and 6; however, there was no clear overall trend (Figure 2e). APD80 was significantly increased on days 5 and 8, with a trend toward APD prolongation for all recordings after day 7 (p < 0.2; Figure 2f). This data set closely mimics clinical trials performed by Chorin et al., 19 , 20 where the QT interval increased starting at day 2 to day 5 with high variability in patient population. Triangulation was not significantly altered over the 10 days during our in vitro polytherapy trials, although there was a very slight trend toward increasing triangulation at days 4, 5, and 10 (p < 0.2; Figure S1c). Interestingly, instabilities were almost absent in this data set, when running nonparametric analysis on Poincaré plots from the combination study (Figure 3c) versus those for individual HCQ (significant, p < 0.05) or AZM (nonsignificant trend; Figure 3a,b). Although we observed a clear increase in APD80, and an increase of CaD80 above 600 ms at day 2 and days 4–10 (Figure 3f), chronic polytherapy resulted in fewer arrhythmic events and only mild instability compared to monotherapy (Figure 3i). At most, 33% of tissues showed arrhythmia at days 3 and 6, with 16% arrhythmias at days 4, 5, 7, and 9 (Figure 3f). Nonparametric contingency analysis showed a decrease between EAD instances in polytherapy versus HCQ (significant, p < 0.05) or AZM (trend, p < 0.1) monotherapy. No tissues had weak signal or stopped beating. At the pathophysiologic level, this data fit well with prior studies describing the important role of AP triangulation in the transition from benign AP prolongation to unstable repolarization. 36
Together, these observations suggest that polytherapy rescues arrhythmogenesis resulting from the individual drugs. Recent clinical studies demonstrated chronic exposure to combination of HCQ and AZM led to QT increases with few arrhythmic events. 12 , 34 , 43 The concordance of the cardiac MPS data to arrive at similar conclusions demonstrates its power in predicting cardiac liabilities for combination therapy of repurposed drugs to treat SARS‐CoV‐2. Mechanisms explaining how arrhythmic events are absent despite a significant increase of the QT interval, can be complex and additional studies would be required to elucidate HCQ and AZM polytherapy‐dependent mechanisms. However, based on the fact that HCQ and AZM have known multichannel blocking effects, and that ICaL and INa block is known to reduce IKr‐dependent arrhythmias, 41 we can hypothesize that the combination of both drugs can synergistically increase the multichannel block responsible for lower arrhythmic instances when compared to monotherapies.
Proteomics analysis of MPS effluent reveal candidate biomarkers for cardiotoxicity monitoring in patients treated with HCQ and AZM
For the polytherapy pharmacology study, media were analyzed for over 92 proteins as biomarkers of tissue injury (Figure 4a). The proteomics analysis of the MPS effluent included a wide array of biomarkers, most of them measurable in patients’ plasma, associated with different cardiac mechanisms, from morphology, cytoskeleton, mechanics to apoptosis, and stress response. Cardiac troponin I (TNNI3), is a well‐known biomarker of cardiac injury and increased risk in mortality, common in patients with COVID‐19 with underlying cardiovascular conditions. 44 Chronic exposure to the combination of AZM and HCQ showed no significant change in TNNI3 expression, suggesting that arrhythmic tissues are not undergoing major tissue damage. However, a clear decrease in erythropoietin (EPO) was observed. HPGDS, an intracellular enzyme that catalyzes the conversion of PGH2 to PGD2, was shown to decrease significantly. Interestingly, similar significant changes were also observed for carbonic anhydrase 14 (CA14) and tyrosine‐protein kinase Fes/Fps (FES). These intracellular proteins are typically not secreted, and therefore are not strong biomarkers unless cells were damaged. The fact that alterations in levels for these proteins were observed in our study suggests some degree of cell damage or stress, but not to the extent where troponin‐actin complexes break down. 45 It is known that CA14 facilitates lactic acid transport across the cardiac sarcolemma, 46 as well as improves myocardial energetics by facilitating mitochondria CO2 clearance. 47 We hypothesize the drug‐related change in CA14 expression is a mechanism for the muscle to adapt antioxidant, contraction, or waste management mechanisms to counter‐balance cardiotoxic effects. Identification of biomarkers in the context of HCQ and AZM, or other polytherapies, will be a valuable tool in the design of COVID‐19 therapeutics trials.
FIGURE 4.
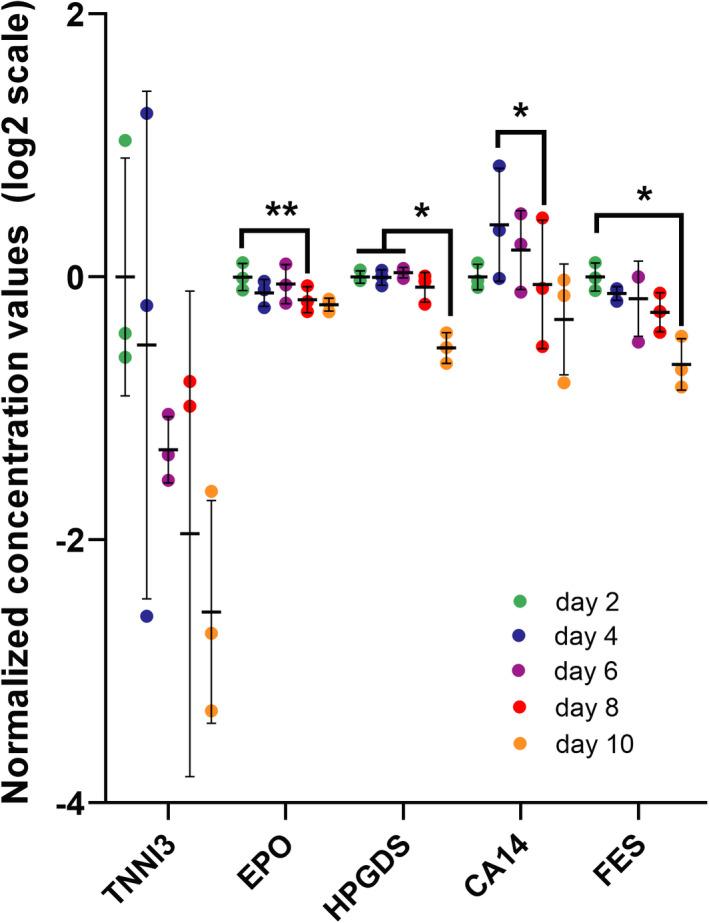
Proteomics analysis of microphysiological systems effluent during chronic exposure to hydroxychloroquine (HCQ) and azithromycin (AZM) polytherapy. Scatter plot of biomarkers showing significant changes with polytherapy in the chronic study. The effluent media was analyzed on days 2, 4, 6, 8, and 10. In the data postprocessing, we only included experiments with greater than 65% of samples above limit of detection. The limit of detection is set at three standard deviations above negative control values. Statistics run were one‐way analysis of variance (ANOVA) with multiple comparison to one another and Dunnett’s post hoc correction. *p < 0.05; **p < 0.01. TNNI3 (cardiac troponin I), EPO (erythropoietin), HPGDS (hematopoietic prostaglandin D synthase), CA14 (carbonic anhydrase 14), FES (FES proto‐oncogene, tyrosine kinase)
SUMMARY
The outcomes of this paper suggest that chronic drug exposure in this MPS format elicits arrhythmic outcomes similar to those observed in published clinical trials. 12 , 18 , 19 , 20 , 34 , 43 Specifically, the known arrhythmia risk of HCQ and AZM alone was recapitulated by our in vitro observation of APD80 increase in combination with arrhythmic events. Combination therapy also exhibited an increase in QT, but compared to monotherapy, was benign at inducing arrhythmogenic behaviors. This also corresponds with recent clinical findings. 19 , 20 , 34 Together, these data suggest that this our high content in vitro heart muscle model can aid clinicians in clinical trial design, rapidly predict the cardiac outcomes of polytherapy for SARS‐CoV‐2 treatment, and help to identify relevant biomarkers to monitor during clinical trials for potential COVID‐19 therapeutics.
LIMITATIONS
Clinical QT interval values represent the summation of all the electrical activity in the ventricles. We used APD80 as a proxy for clinical QT prolongation, which is a credible and common approach to compare directional effects and provide some mechanistic insight, but is not sufficiently sensitive for precise prediction of instability thresholds. 26 Patients with COVID‐19 with drug‐induced QT interval changes of greater than 60 ms or QT interval values above 500 ms are considered high risk, and treatment is suspended. 40 Our cardiac muscle exhibited unphysiologically high APD80 values in response to chronic drug exposure. The mechanism for these large responses is unclear, but likely related to a combination of the hiPSC source, well‐known modest maturity of hiPSC‐CMs relative to the adult human heart, and possibly due to the altered current source‐sink relationship in these very small tissues. Additionally, we have used a single patient line to perform this study, albeit with a significant number of replicates. By screening more patient lines, one can achieve a clinically relevant dataset; although anticipated patient variability will require further expansion of the data size. In future work, our approach for chronic drug testing can be extended to diseased cell lines to better understand the arrhythmic risk of patients with cardiovascular complications or comorbidities (i.e., diabetes) who are most likely to be seriously affected by SARS‐CoV‐2.
CONFLICT OF INTEREST
K.E.H., A.E., B.S., and H.F. have financial relationships with Organos Inc. and both they and the company may benefit from the results of this research. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
B.C., V.C., A.G.E., and K.E.H. wrote the manuscript. B.C., V.C., and K.E.H. designed the research. B.C., V.C., and B.S. performed the research. B.C., V.C., A.G.E., and K.E.H. analyzed the data. H.F. and E.M. contributed new reagents/analytical tools.
Supporting information
Figure S1‐Table S1
ACKNOWLEDGEMENTS
The authors thank Bruce Conklin (Gladstone Institutes, San Francisco, CA, USA) for technical advice on the WTC iPSC line. We thank the Marvel Nanofabrication laboratory (UC Berkeley, Berkeley, CA, USA) and their staff for assistance and technical support for the microfabrication.
Bérénice Charrez and Verena Charwat contributed equally to the work.
Funding information
This work was funded in part by the California Institute for Regenerative Medicine DISC2‐10090 and DISCP‐11946 (K.E.H.), NIH‐NHLBI HL130417 (K.E.H.), NIH‐NIGMS R35GM1195855 (E.W.M.), and the Jan Fandrianto and Selfia Halim Chair Fund (K.E.H.).
REFERENCES
- 1. Pérez‐Bermejo JA, Kang S, Rockwood SJ, et al. SARS‐CoV‐2 infection of human iPSC‐derived cardiac cells predicts novel cytopathic features in hearts of COVID‐19 patients [published online ahead of print September 12, 2020]. bioRxiv. https://doi.org/10.1101/2020.08.25.265561.
- 2. Lu Y, Li X, Geng D, et al. Cerebral micro‐structural changes in COVID‐19 patients – An MRI‐based 3‐month follow‐up study. EClinicalMedicine. 2020;25:100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lindner D, Fitzek A, Bräuninger H, et al. Association of cardiac infection with SARS‐CoV‐2 in confirmed COVID‐19 autopsy cases. JAMA Cardiol. 2020;5:1281‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farouk SS, Fiaccadori E, Cravedi P, Campbell KN. COVID‐19 and the kidney: what we think we know so far and what we don’t. J Nephrol. 2020;33:1213‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71(15):732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang M, Tang T, Pang P, et al. Treating COVID‐19 with chloroquine. J Mol Cell Biol. 2020;12(4):322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71:732‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID‐19. Int J Infect Dis. 2020;97:396‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lagier J‐C, Million M, Gautret P, et al. Outcomes of 3,737 COVID‐19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Travel Med Infect Dis. 2020;36:101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in‐hospital mortality in patients with COVID‐19 in New York State. JAMA. 2020;323:2493‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furtado RHM, Berwanger O, Fonseca HA, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID‐19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396:959‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID‐19 infection. Med Mal Infect. 2020;50:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyerowitz EA, Vannier AGL, Friesen MGN, et al. Rethinking the role of hydroxychloroquine in the treatment of COVID‐19. FASEB J. 2020;34:6027‐6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jankelson L, Karam G, Becker ML, Chinitz LA, Tsai MC. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID‐19: A systematic review. Heart Rhythm. 2020;17:1472‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID‐19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;5:1067‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chorin E, Dai M, Shulman E, et al. The QT interval in patients with COVID‐19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020;26:808‐809. [DOI] [PubMed] [Google Scholar]
- 20. Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID‐19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathur A, Loskill P, Shao K, et al. Human iPSC‐based cardiac microphysiological system for drug screening applications. Sci Rep. 2015;5:8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mathur A, Ma Z, Loskill P, Jeeawoody S, Healy KE. In vitro cardiac tissue models: current status and future prospects. Adv Drug Deliv Rev. 2016;96:203‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Si L, Bai H, Rodas M, et al. Human organs‐on‐chips as tools for repurposing approved drugs as potential influenza and COVID19 therapeutics in viral pandemics [published online ahead of print April 15, 2020]. bioRxiv. https://doi.org/10.1101/2020.04.13.039917.
- 24. Tang H, Abouleila Y, Si L, et al. Human organs‐on‐chips for virology. Trends Microbiol. 2020;28:934‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huebsch N, Charrez B, Siemons B, et al. Metabolically‐driven maturation of hiPSC‐cell derived cardiac chip. bioRxiv 10.1101/485169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blinova K, Dang Q, Millard D, et al. International multisite study of human‐induced pluripotent stem cell‐derived cardiomyocytes for drug proarrhythmic potential assessment. Cell Rep. 2018;24:3582‐3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharma A, Garcia G, Arumugaswami V, Svendsen NC. Human iPSC‐derived cardiomyocytes are susceptible to SARS‐CoV‐2 infection. Cell Rep Med. 2020;1:100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Z, Prinsen JK, Bersell KR, et al. Azithromycin causes a novel proarrhythmic syndrome. Circulation: Arrhythmia Electrophysiol. 2017;10:e003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tveito A, Jaeger KH, Huebsch N, et al. Inversion and computational maturation of drug response using human stem cell derived cardiomyocytes in microphysiological systems. Sci Rep. 2018;8:17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaeger KH, Charwat V, Charrez B, et al. Improved computational identification of drug response using optical measurements of human stem cell derived cardiomyocytes in microphysiological systems. Front Pharmacol. 2020;10:1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jæger KH, Charwat V, Wall S, Healy KE, Tveito A. Identifying drug response by combining measurements of the membrane potential, the cytosolic calcium concentration, and the extracellular potential in microphysiological systems. Front Pharmacol. 2021;11:569489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huebsch N, Loskill P, Mandegar MA, et al. Automated video‐based analysis of contractility and calcium flux in human‐induced pluripotent stem cell‐derived cardiomyocytes cultured over different spatial scales. Tissue Eng Part C Meth. 2015;21:467‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lian X, Zhang J, Azarin SM, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta‐catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cipriani A, Zorzi A, Ceccato D, et al. Arrhythmic profile and 24‐hour QT interval variability in COVID‐19 patients treated with hydroxychloroquine and azithromycin. Int J Cardiol. 2020;316:280‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu P, Allaudeen H, Chandra R, et al. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single‐dose extended‐release regimen versus a 3‐day immediate‐release regimen. Antimicrob Agents Chemother. 2007;51:103‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004‐2013. [DOI] [PubMed] [Google Scholar]
- 37. Qu Z, Xie LH, Olcese R, et al. Early afterdepolarizations in cardiac myocytes: beyond reduced repolarization reserve. Cardiovasc Res. 2013;99:6‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sato D, Xie LH, Sovari AA, et al. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc Natl Acad Sci USA. 2009;106:2893‐2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96‐plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Asensio E, Acunzo R, Uribe W, Saad EB, Saenz LC. Recommendations for the measurement of the QT interval during the use of drugs for COVID‐19 infection treatment. Updatable in accordance with the availability of new evidence. J Interv Card Electrophysiol. 2020;59:315‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blinova K, Stohlman J, Vicente J, et al. Comprehensive translational assessment of human‐induced pluripotent stem cell derived cardiomyocytes for evaluating drug‐induced arrhythmias. Toxicol Sci. 2017;155:234‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharma TS, Wasko MCM, Tang X, et al. Hydroxychloroquine use is associated with decreased incident cardiovascular events in rheumatoid arthritis patients. J Am Heart Assoc. 2016;5:e002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vouri SM, Thai TN, Winterstein AG. An evaluation of co‐use of chloroquine or hydroxychloroquine plus azithromycin on cardiac outcomes: A pharmacoepidemiological study to inform use during the COVID19 pandemic. Res Social Adm Pharm. 2021;17:2012‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sandoval Y, Januzzi JL, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID‐19. J Am Coll Cardiol. 2020;76:1244‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luciano Babuin ASJ. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ. 2005;173(10):1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hallerdei J, Scheibe RJ, Parkkila S, et al. T Tubules and surface membranes provide equally effective pathways of carbonic anhydrase‐facilitated lactic acid transport in skeletal muscle. PLoS One. 2010;5:e15137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47. Schroeder MA, Ali MA, Hulikova A, et al. Extramitochondrial domain rich in carbonic anhydrase activity improves myocardial energetics. Proc Natl Acad Sci USA. 2013;110:E958‐E967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vandenberk B, Vandael E, Robyns T, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc. 2016;5:e003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weiss JN, Garfinkel A, Karagueuzian HS, Qu Z, Chen P‐S. Chaos and the transition to ventricular fibrillation: a new approach to antiarrhythmic drug evaluation. Circulation. 1999;99:2819‐2826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐Table S1


